Abstract
Abundance of CaCO3 rich soil dust is a typical feature of atmospheric environment in the Indian region. During prevailing dry weather conditions, dustfall is deposited onto the foliar surfaces of plant affecting their morphology, stomata and the levels of biochemical constituents. This study reports the chemical characteristics of dustfall, its effect on foliar morphology and biochemical constituents of a medicinal plant (Morus alba) at two sites which are differentiated on the basis of landuse pattern, viz., (i) residential, Jawaharlal Nehru University (JNU), and (ii) industrial, Sahibabad (SB), located in the National Capital Region (NCR) of Delhi. Dustfall was characterized for major anions (F−, Cl−, NO3 − and SO4 −−) and cations (Na+, NH4 +, K+, Mg++ and Ca++). Biochemical parameters such as chlorophyll a, chlorophyll b, total chlorophyll, carotenoid, proline and ascorbic acid were determined in foliar samples. The results showed that the dustfall fluxes of all the major ions were found to be higher at the industrial site (SB) as compared to the residential site (JNU). Foliar analysis revealed that the levels of biochemical parameters were more affected at SB site due to higher levels of dust SO4 −− contributed by various anthropogenic sources resulting in more stressful conditions affecting the biochemistry of the plant. The possible entry pathways for dust SO4 −− into foliar cells are also discussed in the paper. It was noticed that the deposition of urban dust was responsible for the damage of trichome, epidermis, cuticle and stomatal guard cells significantly affecting foliar morphology. SB exhibited more damage to these morphological parts suggesting that industrial dust is harmful to the plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dust is considered as one of the most widespread air pollutants predominantly found in African and Asian regions (Tegen and Fung 1995). Very high loadings of atmospheric dust in the Indian region are responsible for high levels of particulate matter. Often these levels are recorded higher than the prescribed limits of National Ambient Air Quality Standards (NAAQS) of the Central Pollution Control Board (CPCB 2013; Kulshrestha et al. 1999, 2003; Kulshrestha 2013). Atmospheric deposition of particles to the earth’s surface takes place via wet and dry deposition processes. Wet deposition generally takes place in the form of precipitation while dry deposition includes the uptake of gases at the surface as well as settling and impaction of particles. Dustfall which is an important phenomenon in the Indian region occurs mainly through sedimentation and impaction processes (Morselli et al. 1999; Yun et al. 2002; Kulshrestha 2013). Dust is primarily a mixture of suspended soil, road dust and other particulate matter. Dustfall deposition plays a significant role in the transfer of aerosols from the atmosphere to the earth’s surface during dry weather conditions (Freer-Smitha et al. 2005). The dust deposited onto the foliar surfaces affecting their surface, stomata and biochemical constituents (Stevovic et al. 2010; Pourkhabbaz et al. 2010). Selected plant species absorb, detoxify and tolerate high levels of pollution (Nivane et al. 2001; Kapoor et al. 2009; Verma and Singh 2006) by taking up gases and particles. Air pollutants are generally removed by the plants through three basic processes, viz, (i) absorption of pollutants, (ii) deposition onto the leaves and (iii) the fallout of the particulates on the leeward side of the plant (Thambavani et al. 2014). Hence, trees play an important role in improving air quality in urban environments (Woo and Je 2006; Simon et al. 2011). Monitoring of air pollution using plants as indicators is called biomonitoring. It is a low-cost method for air pollution impact assessment (Posthumus 1983; Klumpp et al. 1994; Wolterbeek 2002). A number of studies on plants serving as a sink of NO2, SO2 and atmospheric aerosols have been reported (Ashenden 1979; Ali 1992; Broadmeadow and Freer-smith 1996). However, there are very limited studies which have reported the role of dustfall deposition on foliar surface in controlling biochemical parameters (Mandre and Tuulmets 1997; Prusty et al. 2005; Mandre and Lukjanova 2011).
Considering the importance of dustfall deposition in India, the present study was carried out to measure dustfall deposition fluxes of ionic species along with the change in concentrations of biochemical constituents of the plant. The possible pathways of transport of dust SO4 −− into the foliar cells have also been discussed. An attempt has also been made to study the morphological changes in relation to dustfall deposition.
Methodology
Site description
The study was carried out at two sites located in the National Capital Region of Delhi, (i) Jawaharlal Nehru University (JNU) and (ii) Sahibabad (SB) which represent residential and industrial characteristics, respectively. The location of both the sampling sites is shown in Fig. 1.
Jawaharlal Nehru University
The campus of JNU is located extreme south of Delhi (28° 53′ N, 77° 34′ E) away from any industrial activities (Fig. 1). The campus has a mini forest area on the ridge in its surrounding. The Ridge forest is dominated by Prosopis (Prosopis juliflora) plant species. Other tropical plant species such as Morus (Morus alba), Acacia (Acacia nilotica), Arjun (Terminalia arjuna), Amaltas (Casia fistula), Leucaena (Leucaena leucocephala), Bauhinia (Bauhinia variegate), Pongamia (Pongamia pinnata), Ashoka (Polyalthia longifolia), etc. are also found in this forest. There is no major pollution source within the JNU campus except vehicular traffic and construction of the buildings. Hence, most of the atmospheric dust at JNU site is contributed by the suspension of soil, road dust and construction activities.
Sahibabad
Around 35 km easterly from central Delhi, SB site (28° 67′ N, 77° 34′ E) is located in Ghaziabad District of Uttar Pradesh state (Fig. 1). The site is located near an important juncture of two national highways (NH 24 and NH 58) that give access to Delhi City where diesel emissions from heavy-duty vehicles add a significant contribution to air pollution in the city. Sahibabad is an industrial area having large number of steel, electrical, paint and plastic industries. The population density of the area is very high as compared to the JNU campus. Apart from road dust, residential and commercial activities, significant atmospheric dust is contributed by industrial smoke at this site. Alstonia (Alstonia scholaris), Bauhinia (B. variegate), Ficus (Ficus religiosa), Shisham (Dalbergia sissoo), Ashoka (P. longifolia), Arjun (T. arjuna), etc. are the common plants found in the locality.
Sample collection and analysis
Collection of foliar dustfall and other atmospheric constituents
Dustfall deposition samples were collected on the Morus or Mulberry (M. alba) leaves selected at around 3 m height from the road side. Samples were collected on a 10-day exposure basis during winter seasons (November to January) 2012–2013. Prior to sample collection, leaves were tagged, cleaned and properly washed with distilled-deionized water using a sprayer and then these leaves were air-dried. Each time after 10 days, the leaves were plucked and washed with 50-ml distilled-deionized water using surface washing method (Davidson and Wu 1990). Samples of fine aerosols (Teflon filters) and SO2 were also collected during this period by using a handy sampler (Envirotech) at a flow rate of 1 LPM. Details of the method have been given elsewhere (Singh et al. 2014). A total of six samples of dustfall, aerosols and SO2 were collected representing the duration of foliar sample. Major anions (Cl−, F−, NO3 − and SO4 −−) and cations (Na+, K+, NH4 +, Ca++ and Mg++) were determined in the aqueous extract of the collected samples of aerosols and dustfall by using ion chromatograph (Metrohm 883 Basic IC Plus).
Estimation of total dustfall fluxes on foliar
Total dustfall fluxes were estimated by using gravimetric method. Using similar collection procedure as mentioned in the “Collection foliar dustfall and other atmospheric constituents” section, the selected leaves were immersed in minimum quantity of distilled-deionized water in a preweighed petri dish (m 1) for about 20 min to leach out the deposited material from the leaf. The adaxial and abaxial surfaces of leaves were cleaned with a spray of water using a no-hair-loss paint brush. Then, the water was evaporated by putting the petri dish on a hot plate for about 20–30 min at 110–120 °C. After cooling, the petri dish was weighed (m 2). The total dustfall weight was calculated by the difference of m 1 and m 2, and the dustfall fluxes were calculated by using the following formula:
where DF is total dustfall fluxes (mg/cm2/day), m 1 is the initial weight of petri dish, m 2 is the final weight of the petri dish, A is the surface area of the selected leaves (cm2) and d is the number of days. Area of the leaf was calculated by the graph sheet drawing method.
Biochemical analysis
As mentioned earlier, on each tree, one branch was tagged from which foliar samples were collected. Only healthy and fully expanded foliar samples were collected after an exposure of 10 days. The foliar samples were analysed in triplicate to ensure authenticity of the results. These foliar samples were processed and analysed for the chlorophyll a, chlorophyll b and total chlorophyll, carotenoids, proline amino acid and ascorbic acid content by using the respective methods as given in Table 1.
Foliar morphology analysis
Selected numbers of leaves were studied for their morphological characteristics by using scanning electron microscopic (SEM) (Carl Zeiss EVO 40, Germany) studies at the Advance Instrumentation Research Facility (AIRF), JNU. The collected foliar samples were washed with distilled-deionized water using a soft hair brush followed by gently wiping with tissue paper. A piece of the area was cut from this leaf and fixed in 2.5 % glutaraldehyde (in phosphate buffer, pH 7.2). Further, it was dehydrated twice in 50, 70, 90 and 100 % ethanol and placed in hexamethyldisilazane (HMDS) for 5 min. Then, the sample was mounted on the aluminum stubs with carbon tape, which was dried overnight with CO2 in a critical point dryer. After drying, it was sputter-coated with a thin layer of gold (Sputter coater-Polaron SC7640) by placing the sample specimen in a high vacuum evaporator and vaporizing the metal held in a heated tungsten basket. The coated samples were then observed under scanning electron microscope.
Statistical analysis
In order to know the distribution of data points and the relationship of various components between and within the site, statistical analyses were performed using Statistical Package for Social Sciences (SPSS Ver.16). Data normality distribution was checked by One-Sample Kolmogorov–Smirnov test. The correlation and regression analyses were attempted to estimate relationship, cause and effects of the variation in concentrations of total dustfall and its ionic components and biochemical constituents of the plant.
Results and discussion
Dust deposition fluxes on foliar surfaces
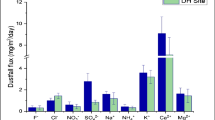
On an average, total dust deposition flux on foliar was recorded as 344 mg/m2/day at SB site which was almost three times higher than that of JNU (130 mg/m2/day) site. Similarly, average deposition fluxes of ionic components (Cl−, F−, NO3 −, SO4 −− , Na+, NH4 +, K+, Ca++ and Mg++) were found to be higher at SB as compared to JNU (Fig. 2). The order of ionic species at JNU was Ca++ > SO4 −− > NO3 − > Cl− > K+ > Mg++ > NH4 + > F− > Na+, while at SB, the order of ionic species was SO4 −− > Ca++ > K+ > Cl− > NO3 − > Mg++ > Na+ > NH4 + > F−. These ionic fluxes were found to have similar ranges as reported by Kumar et al. (2003) for vegetative surfaces in this region. SB being an industrial location was expected to show higher emissions of pollutants from industries. In addition, road dust and resuspension of soil can also contribute to higher fluxes of these ionic components at SB. Among all ions, SO4 −− fluxes are significantly higher at SB. SO2 emitted by diesel-driven vehicles, electricity generators, industries and other sources contributes very high fluxes of SO4 −− at SB. Oxidation of SO2 further gives rise to higher SO4 −− fluxes which has been discussed in the “Deposition fluxes of dust sulphate on foliar surfaces” section.
Deposition fluxes of dust sulphate on foliar surfaces
As shown in Fig. 2, the average dustfall fluxes of SO4 −− on the foliar surfaces were observed to be 4.1 ± 0.7 and 18.0 ± 3.0 mg/m2/day at JNU and SB sites, respectively. SO4 −− fluxes at SB were around four times higher as compared to that of JNU. Such fluxes of SO4 −− are attributed to the oxidation of SO2 (Seinfeld and Pandis 1998; Finlayson-Pitts and Pitts 1986). Being an Industrial site, various industries and vehicles especially diesel-driven vehicles and power generators are the major sources of SO2. Dry deposition of SO2 as well as SO2 adsorption onto the dust particle settled on the foliar can give rise to CaSO4 (Kulshrestha 2013). Soil dust is highly rich in CaCO3 in India which has been found to be a significant scavenger of atmospheric SO2 (Kulshrestha et al. 2003). This is corroborated by the fact that SB had high concentrations of SO4 −− aerosols as well as having a pattern very similar to its dustfall fluxes. Figure 3 shows the ratio of SO4 −− (flux)/Ca(flux) which is an indicator of predominant sulphur vs. crustal sources was also found to be higher at SB as compared to JNU. Similarly, the ratio of SO4 −− (aerosols)/Ca++ (aerosols) and SO2(air)/Ca++ (flux) was higher at SB than that of JNU (Fig. 3). The higher values of these ratios at SB indicated a higher level of acidity due to dust SO4 −−. Higher acidity causes a higher degree of stress (Varshney and Garg 1980; Mansfield 1998). This feature related to acidity was reflected by the pH values of water-soluble extract of the dustfall on the foliar at JNU (7.41) and SB (7.28) also corroborated higher stress at SB. A similar pattern was observed in case of acidity of leaf extract. The pH of leaf extract at SB was recorded as 7.70 as compared to 8.35 at JNU. This is in accordance with the concentration of SO4 −− in air, the flux of SO4 −− on the foliar and ambient SO2 (Table 2).
Higher acidity of foliar extract along with higher ratios of SO2(air)/Ca++ (flux) indicated that air was more polluted at SB having greater risks of foliar damage. Probably, due to this effect, remarkable changes in the foliar morphology and biochemical constituents of the plant have been observed at SB as discussed in the next sections.
Entry pathways of dust SO4 −− and SO2-SO4 −− inside the leaves
When stomata are open, inward diffusion of gases takes place as diffusion resistance is minimum during the opening of stomata (Bache 1979; Georgiadis and Rossi 1989). Sometimes, particles of submicron size can also enter through stomata, cuticular breaks and wounds depending upon the solubility of particulates and affinity of cuticular compounds (Guderian 1980; Kerstiens et al. 1992; Tomasevic et al. 2004). Figure 4 shows the possible entry pathways for SO2 or particulate SO4 −− to the foliar cells. These steps can be summarized as follows:
-
(i)
Stomatal/cuticular uptake of gaseous SO2 or dust SO4 −− to mesophyll cells and the vacuole through plasma membrane where sulphate/proton (SO4 −−/3H+) acts as transporter (Kaiser et al. 1989; Smith et al. 1997; Buchner et al. 2004). According to reports, after absorption, SO2 is readily dissolved in the intercellular or intracellular water to form HSO3 − and SO3 −− ionic species which are further converted to SO4 −−.
-
(ii)
Remobilization of both particulate SO4 −− as well as SO2-derived SO4 −− efflux from mesophyll cell cytoplasm and vacuole to vascular tissues (xylem and phloem) (Takahashi et al. 2000; Yoshimoto et al. 2003).
-
(iii)
Vascular downloading of SO4 −− and its transport to sink cells for assimilation (Hartmann et al. 2000).
-
(iv)
Finally, excess SO4 −− affects the formation and accumulation of biochemical constituents (Plsenicar 1983). Such translocation of SO4 −− generates reactive oxygen species (ROS) having detrimental effects on the levels of biochemical constituents further affecting photosynthesis, transpiration and respiration processes (Linzon et al. 1979; Huttunen et al. 1985; Dmuchowski and Bytnerowicz 1995; Xu et al. 1996).
Dustfall fluxes of SO4 −− and its correlation with biochemical parameters
Photosynthetic pigment levels vs. SO4 −− fluxes
Chlorophyll (Chl) a and b and carotenoids (Car) are found in the chloroplasts which are essential for the photosynthetic activity of green plants. Measurements of these pigments have primary importance to assess the impact of air pollutants on the plants. Chlorophyll a and b plays an important role in plant metabolism. In this study, average concentrations of Chl a, Chl b, total Chl and Car were recorded as 2.5 ± 0.2, 0.6 ± 0.04, 3.0 ± 0.2 and 1.2 ± .1 mg/g f.w., respectively, at JNU and 1.8 ± 0.1, 0.3 ± 0.07, 2.1 ± 0.13 and .09 ± 0.1 mg/g f.w., respectively, at SB. As shown in Fig. 5a–h, the concentrations of Chl a, Chl b, total Chl and Car at SB were decreasing with an increase in SO4 −− fluxes. Trendlines in theses plots are more negative, clear and prominent for the SB site clearly showing a reduction in pigment levels. The most significant decrease was seen in the case of total chlorophyll and carotenoids (Fig. 5g, h). The reduction in the chlorophyll content is considered as an indicator of pollution (Pandey and Pandey 1994; Al Sayegh Petkovsek et al. 2007). Lowering of chlorophyll indicates stressful conditions which harm the plants (Agrawal et al. 2003). Photosynthesis process is known to be sensitive to SO4 −− concentrations as it is a competitive inhibitor of ribulose-1,5-bisphosphate carboxylase and inhibits the photophosphorylation process also (Kaiser et al. 1986; Ryrie and Jagendorf 1971). This effect is clearly noticed in our study.
Carotenoids (Car) are lipid-soluble antioxidants and play multiple roles in plant metabolism. These are responsible for carrying out three major functions in plants: (i) absorption of light between 400 and 550 nm, which is transferred to the Chl (Sieferman-Harms. 1987); (ii) protection of photosynthetic apparatus by quenching a triplet sensitizer (Chl3), 1O2 and other harmful free radicals formed during photosynthesis (Collins. 2001); and (iii) establishment of light harvesting complex proteins and thylakoid membrane (Niyogi et al. 2001; Gill et al. 2011). Similar to Chl a and b and total Chl, the concentrations of Car are reduced with the increase in SO4 −− fluxes at both the sites. The reduction of Car concentrations is more dominating at SB (Fig. 5h) where SO2 levels are much higher than that of JNU. Higher SO4 −− fluxes play a destructive role which either catalyses the breakdown of Car or does not allow its accumulation in the foliar. It also indicates greater oxidative stress on the plant (Tiwari et al. 2006; Mandre and Tuulmets 1997).
Ascorbic acid vs. SO4 −− fluxes
Ascorbic acid (AsA) is a natural antioxidant which is known to provide stability to the cell membranes during pollution stress. It scavenges cytotoxic free radicals which can otherwise cause lipid peroxidation and destruction of membranes (Halliwell and Gutteridge 1989; Smirnoff 1996). AsA is a strong reductant which also activates biochemical and physiological activities of the cell such as cell wall synthesis and cell division (Conklin 2001; Raza and Murthy 1988). The average AsA content of foliar was estimated to be 1.2 ± 0.1 and 1.95 ± 0.2 mg/g f.w. at JNU and SB site, respectively. Results showed that AsA content was increased with the increase in SO4 −− fluxes at both the sites (Fig. 6). Increase in ascorbic acid content of the plant species may be due to increased rate of production of reactive oxygen species (ROS) during photooxidation of SO2 to SO3 −, where SO3 − ions are generated from SO2 absorbed (Smirnoff 2005; Athar et al. 2008). Scholz and Reck (1997) have reported that in the presence of an acidic pollutant, the leaf pH is lowered and the decline is greater in sensitive species. Higher ascorbic acid content of the plant has higher tolerance against SO2 pollution (Chaudhary and Rao 1977). The relationship of dust SO4 −− with AsA noticed in this study can also be explained in the same manner.
Proline amino acid vs. SO4 −− fluxes
Proline (Pro) is an osmolyte in plants which controls osmotic adjustment. It is responsible for stabilizing sub-cellular structures (e.g. membranes and proteins), scavenging free radicals and buffering cellular redox potential under stressful conditions ((Hasegawa et al. 2000; Zhu 2001). It can also play a role as a protein-compatible hydrotrope (Srinivas and Balasubramanian 1995) alleviating cytoplasmic acidosis and maintaining appropriate NADP+/NADPH ratios compatible with metabolism (Hare and Cress 1997). On an average, foliar samples collected had 4.2 ± 0.2 and 5.6 ± 0.2 mg/g f.w. Pro content at JNU and SB, respectively. As shown in Fig. 7, Pro content is seen increasing with the increase in the fluxes of SO4 −− at both the sites. In response to environmental stresses, Pro is normally accumulated in the large quantities in the plants, especially the higher plants (Rains 1989; Ashraf 1994; Ali et al. 1999; Rhodes et al. 1999; Ozturk and Demir 2002; Hsu et al. 2003; Kishore et al. 2005; Wang et al. 2009). Higher level of Pro having a positive correlation with SO4 −− fluxes suggests that the Pro is acting as an inhibitor of air pollution-induced lipid peroxidation. Strong positive correlation of Pro with SO4 −− flux at SB might be due to higher SO2 stress contributed by diesel-driven traffic and other anthropogenic activities. Pro accumulation in leaves of plants has been reported during in situ exposure to SO2 fumigation (Tankha and Gupta 1992), heavy metals (Wang et al. 2009) and salt stress (Woodward and Bennett 2005). Accumulation of Pro is also reported during ex situ exposure to air pollution (Seyyednejad and Koochak 2011).
Changes in foliar morphology
Morphology of the collected foliar samples was studied using a scanning electron microscope (SEM). It is well established that the interception of aerosols and deposition of gaseous and particulate pollutants is greater in woody plants than in shorter vegetation (Fowler et al. 1989). In this study, SEM results showed a remarkable difference in size of the stomatal pores, ruptured guard cells, damage of cuticle and epidermal cell at both the sites at the abaxial surface (Fig. 8f, h). Ruptured trichomes on the adaxial surface are shown in Fig. 8a, b. As shown in Fig. 8c, d, the dustfall deposition at SB is seen higher as compared to JNU. Figure 8e, f shows damaged cuticle, epidermis and guard cells. Dust particles are deposited in and around stomatal pores on abaxial and adaxial leaf surfaces (Fig. 8a–h). Clogging of the stomatal pores is clearly depicted in Fig. 8h. The size of stomata is enlarged and having ruptured guard cells at SB. It is reported that the deposition of fine and coarse particles increases leaf temperature and decreases light absorption, finally affecting photosynthesis process (Tomasevic and Anicic 2010). This effect is probably responsible for negative correlation of pigment levels with SO4 −− levels as mentioned in the “Photosynthetic pigment levels vs. SO4 −− fluxes” section. Clogging of stomata leads to an increase in stomatal conductance which might further influence the water regime and photosynthetic rate (Farmer 1993; Hirano et al. 1995). Sometimes, stomatal cavities are partially blocked due to excess deposition of a nutritional element (Rai et al. 2010). It hinders the opening and closing of stomata and also affects the plant physiology (Mankovska et al. 2004). Majernik and Mansfield (1970, 1971) and Black and Black (1979) found that the normal diurnal cycle of stomatal opening and closing was not affected but the apertures observed during the day time were higher in SO2-exposed plants. Further, Black and Black (1979) also noticed an enhanced opening which was associated with damage to the epidermal cells adjacent to the stomata. They also recorded 20–25 % increase in conductance, when Vicia faba was exposed to 17 ppb SO2 level.
Conclusions
The foliar of M. alba plant experienced daily deposition of dustfall at the rate of 344 and 130 mg/m2 at SB (industrial site) and JNU (residential site), respectively, suggesting around three times higher deposition at the industrial site. Similarly, average deposition fluxes of different ions in aqueous extract of dustfall were recorded higher at industrial site as compared to residential site. Among anions, SO4 −− was found as prominent ion which had significantly higher (~4 times) fluxes at industrial site as compared to residential site due to higher SO2 levels contributed by various industries and transports especially the diesel-driven vehicles and power generators at SB. SO4 −− in foliar is contributed by settling dust SO4 −− which is formed in the atmosphere by the oxidation SO2 onto the CaCO3-rich particulate matter forming particulate CaSO4. This is apart from direct uptake of SO2 through stomata. Higher level of stress was noticed at industrial site due to uptake of higher amount of gaseous dust SO4 −− and SO2 as indicated by lower pH of leaf extract at this site. Higher pollution stress at industrial site resulted in decreasing levels of Chl a, Chl b, total chlorophyll and carotenoids with an increase in SO4 −− fluxes. However, ascorbic acid content was increased with the increase in SO4 −− fluxes at both the sites. But the rate of increase was higher at SB. This might be due to higher rate of production of reactive oxygen species (ROS). Proline content was also noticed to be increased with an increase in SO4 −− fluxes because proline controls osmotic adjustment and acts as an inhibitor of air pollution-induced lipid peroxidation. Morphological study revealed that foliar damage was more at industrial site due to deposition of dust SO4 −− and uptake of SO2 which resulted in the more damaged cuticle, the epidermal cell and ruptured guard cells along with clogged stomata.
References
Agrawal, M., Singh, B., Rajput, M., Marshall, F., & Bell, J. N. B. (2003). Effect of air pollution on peri-urban agriculture: a case study. Environmental Pollution, 126, 323–329.
Al Sayegh Petkovsek, S., Batic, F., & Ribaric Lasnik, C. (2007). Norway spruce needles as bioindicator of air pollution in the area of influence of the Šoštanj Thermal Power Plant. Slovenia. Environmental pollution, 151, 287–291.
Ali, A. E. (1992). Damage to plants due to industrial pollution and their use as bioindicators in Egypt. Environmental Pollution, 81, 251–255.
Ali, G., Srivastava, P. S., & Iqbal, M. (1999). Proline accumulation, protein pattern and photosynthesis in regenerants grown under NaCl stress. Biologia Plantarum, 42, 89–95.
Ashenden, T. W. (1979). a). The effects of long-term exposures to SO2 and NO2 pollution on the growth of Dactylis glomerata L. and Poa pratensis L. Environmental Pollution, 18, 249–258.
Ashraf, M. (1994). Breeding for salinity tolerance in plants. Critical Reviews in Plant Sciences, 13, 17–42.
Athar, H. R., Khan, A., & Ashraf, M. (2008). Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Env. Exp. Bot, 63, 224–231.
Bache, D. H. (1979). Particle transport within plant canopies. I. Framework for analysis. Atmospheric Environment, 13, 1257–1262.
Bates, L. S., Waldren, R. P., & Tear, I. D. (1973). Rapid determination of free proline for water stress studies. Plant and Soil, 39, 205–207.
Black, C. R., & Black, V. J. (1979). The effects of low concentrations of sulphur dioxide on stomatal conductance and epidermal cell survival in field bean (Vicia fiba L.). Journal of Experimental Botany, 30, 291–298.
Broadmeadow, M. S. J., & Freer-Smith, P. H. (1996). Urban woodland and the benefits for local air quality (DOE Research for Amenity tree Series No. 5). London: The Stationery Office.
Buchner, P., Takahashi, H. M., & Hawkesford, J. (2004). Plant sulphate transporters: co-ordination of uptake, intracellular and long distance transport. Journal of Experimental Botany, 55, 1765–1773.
Chaudhary, C. S., & Rao, D. N. (1977). Study of some factors in plants controlling their susceptibility to sulphur dioxide pollution. Proceedings of the Indian National Science Academy, 43, 236–241.
Collins, A. (2001). Carotenoids and genomic stability. Mutation Research, 475, 21–28.
Conklin, P. L. (2001). Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Enviroment, 24, 383–394.
CPCB (2013). ww.cpcb.nic.in/national_ambient_air_quality_standards.php. Annual report, Ministry of Environment & forests, Government of India.
Davidson, C. I., & Wu., Y. L. (1990). In: Dry deposition of particles and vapors Lindberg, S.E., Page, A.L., Norton, S.A. (Eds.), Acidic precipitation vs 3 sources, deposition and canopy interactions, vol. 3. Springer Verlag, New York, 103–216.
Dmuchowski, W., & Bytnerowicz, A. (1995). Monitoring environmental pollution in Poland by chemical analysis of Scots pine (Pinus sylvestris L.) needles. Environmental Pollution, 87, 87–104.
Farmer, A. M. (1993). The effects of dust on vegetation. A review. Environmental Pollution, 79, 63–75.
Finlayson-Pitts, B. J., & Pitts, J. N. (1986). Atmospheric chemistry: fundamentals and experimental techniques. New York: Wiley.
Fowler, D., Cape, J. N., & Unsworth, M. H. (1989). Deposition of atmospheric pollutants on forests. Philosophical Transactions of the Royal Society of London B, 324, 247–265.
Freer-Smitha, P. H., Beckettb, K. P., & Taylor, G. (2005). Deposition velocities to Sorbus aria, Acer campestre, Populus deltoides X trichocarpa ‘Beaupré’, Pinus nigra and X Cupressocyparis leylandii for coarse, fine and ultra-fine particles in the urban environment. Environmental Pollution, 133, 157–167.
Georgiadis, T., & Rossi, F. (1989). Dry deposition of pollutants and effects on vegetation. Aerobiologia, 5, 111–121.
Gill, S. S., Khan, N. A., Anjum, N. K., & Tuteja, N. (2011). Amelioration of cadmium stress in crop plants by nutrients management: morphological, physiological and biochemical aspects. In: Anjum NA, Lopez-Lauri F (Eds) Plant nutrition and abiotic stress tolerance III. Plant Stress, 5(Special Issue 1), 1–23.
Guderian, R. (1980). Terrestrial vegetation–air pollutant interaction: non-gaseous pollutants. In: Air pollutants and their effects on the terrestrial ecosystem (edited by Krupa S.V and Legge A.H) John Wiley and Sons, New York (proceeding held at Banff, Canada, May 1980).
Halliwell, B., & Gutteridge, J. M. C. (1989). Free radicals in medicine and biology (2nd ed., pp. 277–289). Oxford: Clarendon Press.
Hare, P. D., & Cress, W. A. (1997). Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regulation, 21, 79–102.
Hartmann, T., Mult, S., Suter, M., Rennenberg, H., & Herschbach, C. (2000). Leaf age-dependent differences in sulphur assimilation and allocation in poplar (Populus tremula × P. alba) leaves. Journal of Experimental Botany, 51, 1077–1088.
Hasegawa, P. M., Bressan, R. A., Zhu, J. K., & Bohnert, H. J. (2000). Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology, 51, 463–499.
Hirano, T., Kiyota, M., & Aiga, I. (1995). Physical effects of dust on leaf physiology of cucumber and kidney bean plants. Environmental Pollution, 89, 255–261.
Hiscox, J. D., & Israelstam, G. F. (1979). A method for the extraction of chlorophyll from leaf tissue without maceration. Canadian Journal of Botany, 57, 1332–1334.
Hsu, S. Y., Hsu, Y. T., & Kao, C. H. (2003). The effect of polyethylene glycol on proline accumulation in rice leaves. Biologia Plantarum, 46, 73–78.
Huttunen, S., Laine, K., & Torvela, H. (1985). Seasonal sulphur contents of pine needles as indices of air pollution. Ann. Bot. Fennici, 22, 343–359.
Kaiser, W. M., Schroppel-Meier, G., & Wirth, E. (1986). Enzyme activities in an artificial stroma medium. An experimental model for studying effects of dehydration on photosynthesis. Planta, 161, 292–299.
Kaiser, G., Martinoia, E., Schröppel-Maier, G., & Heber, U. (1989). Active transport of sulfate into the vacuole of plant cells provides halotolerance and can detoxify SO2. Journal of Plant Physiology, 133, 756–763.
Kapoor, C. S., Kapasya, V., Bamniya, B. R., & Kapoor, K. (2009). Physiological and biochemical studies on some common tree species in Udaipur city under pollution stress. Journal of Current Science, 14, 181–186.
Keller, T., & Schwager, H. (1977). Air pollution and ascorbic acid. European Journal of Forest Pathology, 7, 338–350.
Kerstiens, G., Federholzner, R., & Lendzian, K. J. (1992). Dry deposition and cuticular uptake of pollutant gases. Agriculture, Ecosystems & Environment, 42, 239–253.
Kishore, P. B. K., Sangam, S., Amrutha, R. N., Laxmi, P. S., Naidu, K. R., Rao, K. R. S. S., Rao, S., Reddy, K. J., Theriappan, P., & Sreenivasulu, N. (2005). Regulation of proline biosynthesis, degradation, uptake and transport in higher plants its implications in plant growth and abiotic stress tolerance. Current Science, 88, 424–438.
Klumpp, A., Klumpp, G., & Domingos, M. (1994). Plants as bioindicators of air pollution at the Serra do Mar near the industrial complex of Cubatão, Brazil. Environmental Pollution, 85, 109–116.
Kulshrestha, U. (2013). Acid rain: In Encyclopedia of environmental management; S.E. Jorgensen, ed. Taylor & Francis: New York, Vol. I, 8–22.
Kulshrestha, U. C., Jain, M., Mandal, Gupta, T. K., Prabhat, Sarkar, A. K., Parashar, D. C. (1999). Measurement of acid rain over Indian Ocean and surface measurements of atmospheric aerosols at New Delhi during INDOEX pre-campaigns, 76, 968–972.
Kulshrestha, M. J., Kulshrestha, U. C., Parashar, D. C., & Vairamani, M. (2003). Estimation of SO4 contribution by dry deposition of SO2 onto the dust particles in India. Atmospheric Environment, 37, 3057–3063.
Kumar, R., Rani, A., Kumari, K. M, Srivastva, S. S. (2003). Direct measurement of atmospheric dry deposition to natural surfaces in a semiarid region of north central India. J. Geophysical Research. 108, doi:10.1029/2002JD003194.
Linzon, S. N., Temple, P. J., & Pearson, P. J. (1979). Sulfur concentrations in plant foliage and related effects. J. Air Pollut. Contr. Assoc., 29(1979), 520–525.
Majernik, O., & Mansfield, T. A. (1970). Direct effect of SO2 pollution on the degree of opening of stomata. Nature, 227, 377–378.
Majernik, O., & Mansfield, T. A. (1971). Effects of SOz pollution on stomatal movements in Vicia faba. Phytopathologische Zeitschrift, 71, 123–128.
Mandre, M., & Lukjanova, A. (2011). Biochemical and structural characteristics of Scoots pine (Pinus sylvestris L.) in an alkaline environment. Estonian Journal of Ecology. doi:10.3176/eco.2011.4.02.
Mandre, M., & Tuulmets, L. (1997). Pigment changes in Norway spruce induced by dust pollution. Water Air and Soil Pollution, 94, 247–258.
Mankovska, B., Godzik, B., Badea, O., Shiparyk, Y., & Moravcik, P. (2004). Chemical and morphological characteristics of key tree species of the Carpathian Mountains. Environmental Pollution, 130, 41–54.
Mansfield, T. A. (1998). Stomata and plant water relations: does air pollution create problems? Environmental Pollution, 101, 1–11.
Morselli, L., Cecchini, M., Grandi, E., Iannuccilli, A., Barilli, L., & Olivieri, P. (1999). Heavy metals in atmospheric surrogate dry deposition. Chemosphere, 38(4), 899–907.
Nivane, S. Y., Chaudhari, P. R., Gajghate, D. G., & Tarar, J. L. (2001). Foliar biochemical features of plants as indicators of air pollution. Bulletin of Environment Contamination and Toxicology, 67, 133–140.
Niyogi, K. K., Shih, C., Chow, W. S., Pogson, B. J., DellaPenna, D., & Bjorkman, O. (2001). Photoprotection in a zeaxanthin-and lutein-deficient double mutant of Arabidopsis. Photosynthetic Res., 67, 139–145.
Ozturk, L., & Demir, Y. (2002). In vivo and in-vitro protective role of proline. Plant Growth Regulation, 38, 259–264.
Pandey, J., & Pandey, U. (1994). Evaluation of air pollution phytotoxicity in a seasonally dry tropical urban environment. Environmental Monitoring and Assessment, 33, 195–213.
Plsenicar, M. (1983). Study of sulfur dioxide effects on phosphorus metabolism in plants using 32P as an indicator. Int. J. Appl. Radiation. Isat., 34, 833–835.
Posthumus, A. C. (1983). Higher plants as indicators and accumulators of gaseous air pollution. Ecological Indicators for the Assessment of the Quality of Air, Water, Soil, and Ecosystems, 263–272.
Pourkhabbaz, A., Rastin, N., Olbrich, A., Langenfeld-Heyser, R., & Polle, A. (2010). Bulletin of Environment Contamination and Toxicology, 85, 251–255.
Prusty, B. A. K., Mishra, P. C., & Azeez, P. A. (2005). Dust accumulation and leaf pigment content in vegetation near the national highway at Sambalpur, Orissa, India. Ecotoxicology and Environmental Safety, 60, 228–235.
Rai, A., Kulshreshtha, K., Srivastava, P. K., & Mohanty, C. S. (2010). Leaf surface structure alterations due to particulate pollution in some common plants. Environmentalist, 30(1), 18–23.
Rains, D. W. (1989). Plant tissue and protoplast culture: application to stress physiology and biochemistry. In H. G. Jones, T. J. Flowers, & M. B. Jones (Eds.), Plant under stresses: biochemistry, physiology and ecology and their application to plant improvement (pp. 181–196). Cambridge: Cambridge University Press.
Raza, S. H., & Murthy, M. S. R. (1988). Air pollution tolerance index of certain plants of Nacharam Industrial area. Hyderabad. Indian J. Bot, 11(1), 91–95.
Rhodes, D., Verslues, P. E., & Sharp, R. E. (1999). Role of amino acids in abiotic stress resistance. In B. K. Singh (Ed.), Plant amino acids: biochemistry and biotechnology (pp. 319–356). New York: Marcel Dekker.
Ryrie, I. J., & Jagendorf, A. T. (1971). Inhibition of photophosphorylation in chloroplasts by inorganic sulfate. Journal of Biological Chemistry, 246, 582–588.
Scholz, F., & Reck, S. (1997). Effects of acids on forest trees as measured by Titration in vitro. www.springerlink.com/index/739LH291.
Seinfeld, J. H., & Pandis, S. N. (1998). Atmospheric chemistry and physics. New York: Wiley-Interscience Press.
Seyyednejad, S. M., &Koochak, H. (2011). A study on air pollution effects on Eucalyptus camaldulensis. International Conference on Environmental, Biomedical and Biotechnology IPCBEE, IACSIT Press, Singapoore, 16.
Sieferman-Harms, D. (1987). The light harvesting function of carotenoids in photosynthetic membrane. Plant Physiology, 69, 561–568.
Simon, E., Braun, M., Vidic, A., Bogyó, D., Fábián, I., & Tóthmérész, B. (2011). Air pollution assessment based on elemental concentration of leaves tissue and foliage dust along an urbanization gradient in Vienna. Environmental Pollution, 159, 1229–1233.
Singh, S., Gupta, G. P., Kumar, B., & Kulshrestha, U. C. (2014). Comparative study of indoor air pollution using traditional and improved cooking stoves in rural households of Northern India. Energy for Sustainable Development, 19, 1–6.
Smirnoff, N. (1996). The function and metabolism of ascorbic acid in plants. Annals of Botany, 78, 661–669.
Smirnoff, N. (2005). Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions in: N. Smirnoff (Ed.), Antioxidants and reactive oxygen species in plants, Blackwell Publishing Ltd., Oxford, UK, 53–86.
Smith, F. W., Hawkesford, M. J., Ealing, P. M., Clarkson, D. T., Vanden, B. P. J., Belcher, A. R., & Warrilow, A. G. (1997). Regulation of expression of a cDNA from barley roots encoding a high affinity sulphate transporter. Plant Journal, 12, 875.
Srinivas, V., & Balasubramanian, D. (1995). Proline is a protein-compatible hydrotrope. Langmuir, 11, 2830–2833.
Stevovic, S., Mikovilovic, V. S., & Calic, D. D. (2010). Environmental impact on morphological and anatomical structure of Tansy. African Journal of Biotechnology, 9(16), 2413–2421.
Takahashi, H., Watanabe-Takahasi, A., Smith, F. W., Blake-Kalf, M., Hawkesford, M. J., & Saito, K. (2000). The roles of three functional sulfate transporters involved in uptake and translocation of sulfate in Arabidopsis thaliana. The Plant Journal, 23, 171–182.
Tankha, K., & Gupta, R. K. (1992). Effect of water deficit and sulphur dioxide on total soluble proteins, nitrate reductase activity and free proline content in sunflower leaves. Biologia Plantarum, 34, 305–310.
Tegen, I., & Fung, I. (1995). Contribution to the mineral aerosol load from land surface modification. Journal of Geophysical Research, 100, 18707–18726.
Thambavani, D., Sarala, & Maheswari, J. (2014). Response of native tree species to ambient air quality. Chemical Science Transactions, 3(1), 438–444.
Tiwari, S., Agrawal, M., & Marshall, F. M. (2006). Evaluation of ambient air pollution impact on carrot plants at a suburban site using open top chambers. Environmental Monitoring and Assessment, 119(1–3), 15–30.
Tomasevic, M., & Anicic, M. (2010). Trace element content in urban tree leaves and SEM-EDX characterization of deposited particles. Physics, Chemistry and Technology, 8, 1–13.
Tomasevic, M., Rajšić, Đorđević, D., Tasić, M., Krstić, J., & Novaković, V. (2004). Heavy metals accumulation in tree leaves from urban areas. Environmental Chemistry Letters, 2(3), 151–154.
Varshney, C. K., & Garg, K. K. (1980). Significance of leaf surface characteristics in plant responses to air pollution. Water, Air, and Soil Pollution, 14, 429–433.
Verma, A., & Singh, S. N. (2006). Biochemical and ultrastructural changes in plat foliage exposed to auto pollution. Environmental Monitoring and Assessment, 120, 585–602.
Wang, F., Zeng, B., Sun, Z., & Zhu, C. (2009). Relationship between proline and Hg2+ induced oxidative stress in a tolerant rice mutant. Archives of Environment Contamination and Toxicology, 56, 723–731.
Wolterbeek, B. (2002). Biomonitoring of trace element air pollution: principles, possibilities and perspectives. Environmental Pollution, 120, 11–21.
Woo, S. Y., & Je, S. M. (2006). Photosynthetic rates and antioxidant enzyme activity of Platanus occidentalis growing under two levels of air pollution along the streets of Seoul. Journal of Plant Biology, 49, 315–319.
Woodward, A. J., & Bennett, I. J. (2005). The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulensis. Plant Cell Tissue Organ Culture, 82, 189–200.
Xu, H. L., Lopez, J., Rachidi, F., Tremblay, N., Gauthier, L., Desjardins, Y., & Gosseli, A. (1996). Effect of sulphate on photosynthesis in greenhouse-grown tomato plants. Physiologia Plantarum, 96, 722–726.
Yoshimoto, N., Inoue, E., Saito, K., Yamaya, T., & Takahashi, H. (2003). Phloem-localizing sulfate transporter, Sultr1;3, mediates re-distribution of sulfur from source to sink organs in Arabidopsis. Plant Physiology, 131, 1511–1517.
Yun, H. J., Yi, S. M., & Kim, Y. P. (2002). Dry deposition fluxes of ambient particulate heavy metals in a small city, Korea. Atmospheric Environment, 36, 5449–5458.
Zhu, J. K. (2001). Plant salt tolerance. Trends in Plant Science, 6, 66–71.
Acknowledgments
We sincerely thank the financial support received from University Grant Commission (UGC), New Delhi, to conduct this research work. Analytical assistance provided by Advance Instrumentation Research Facility (AIRF), JNU is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, G.P., Singh, S., Kumar, B. et al. Industrial dust sulphate and its effects on biochemical and morphological characteristics of Morus (Morus alba) plant in NCR Delhi. Environ Monit Assess 187, 67 (2015). https://doi.org/10.1007/s10661-015-4301-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-015-4301-4