Abstract
The mining district of El Triunfo (ET-MD) has an estimated 800,000 t of mine wastes scattered in the environment, contaminating the sediment with potentially toxic elements such as As, Cd, Pb, and Zn. In order to estimate the toxicity of the sediment to the adjacent biota, the aims of our study are to calculate the mortality and inhibition through bioassays, using sediment, and test organisms such as Daphnia magna and Selenastrum capricornutum (Pseudokirchneriella subcapitata), respectively. The D. magna mortality was 31 ± 12 % and the S. capricornutum growth inhibition was 53 ± 24 %. The contamination of the sediment determines the high mortality of D. magna and the high inhibition of S. capricornutum in the system, indicating risk for the biota in the contaminated system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bioassays have been developed to evaluate the presence of toxic substances in water, sediment, soils, and anthropogenic wastes. The toxicity increases as the microbial growth rate decreases (Gabrielson et al. 2002). There are many physiological responses such as growth rate, inhibition, and mortality that have been monitored as toxicity criteria in water, sediment, soil, and tissue of organisms. Pseudokirchneriella subcapitata, known also as Selenastrum capricornutum, is a unicellular organism belonging to the group of green algae found in eutrophic and oligotrophic aquatic systems (Pica-Granados et al. 2004). These algae are organisms that are sensitive to pollutants which contain metals, and are used to evaluate phytotoxicity (Komjarova and Blust 2008). The algae could also be used to identify the sites impacted by the drainage of the mine effluent, and their incorporation into ecological risk-assessment studies has been recommended (Moreira-Santos et al. 2004; Antunes et al. 2007). Daphnia magna is a zooplankton organism with a high sensitivity to environmental changes (Guan and Wang 2004). Several studies have demonstrated the response of D. magna to essential and nonessential metals, showing a variable resistance to trace metals (Barata et al. 1998). This organism plays an important role in the food chain because of the link it creates by grazing on primary producers and being food for many fish species (Komjarova and Blust 2008). A combination of element analysis and ecotoxicity tests with D. magna was shown to be a potential tool for monitoring and mapping the levels of pollution in aquatic bodies and their sediments (He et al. 1998; Antunes et al. 2007; Lattuada et al. 2009).
Large quantities of metals (Cd, Hg, Pb, Zn) and metalloids (As, Sb), which are potentially toxic elements (PTEs) for adjacent biota, are released to the environment by mining activities. PTEs can be bioaccumulated and biomagnified through the trophic levels, causing irreversible damages (Merian 1991). Those toxic effects are proportional to the exposure period, element concentration, and exposure pathway (Komjarova and Blust 2009).
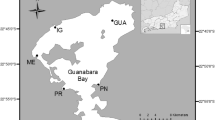
The El Triunfo mining district (ET-MD) has an area of ~200 km2 (Romero-Guadarrama 2011) and is located in the mountainous portion (490 m above sea level) of the El Carrizal hydrological basin, which has an area of ~1200 km2 (Fig. 1). Artisanal gold exploitation started in 1748 and continued intermittently until the end of the last century. Geochemical studies showed high concentrations of As in the tailings (Carrillo 1996; Volke-Sepúlveda et al. 2003) and in the ash and sediments (Marmolejo-Rodríguez et al. 2011). In this desert zone, the arroyo is a dry river which has water for only a few days when a hurricane arrives every 4 or 5 years. The distribution and accumulation of some elements along the arroyo was analyzed before (Romero-Guadarrama et al. 2010; Romero-Guadarrama 2011; Marmolejo-Rodríguez et al. 2011; Sánchez-Martínez et al. 2013). However, the toxicity of this natural sediment was not evaluated.
In addition, the sum of the metals and metalloids in the natural sediment generates different effects from bioassays of an individual element. Therefore, the aims of our study are to evaluate the toxicity of the natural sediment of the Hondo–Las Gallinas–El Carrizal arroyo (H-G-C), which is influenced by the abandoned tailings of the ET-MD enriched with PTEs, using D. magna and S. capricornutum.
Material and methods
Twenty-one samples were collected in August 2009 from the ET-MD along the 49 km of the arroyo (H-G-C) to its discharge into the Pacific Ocean (Fig. 1). The sediment was sampled in polyethylene bottles cleaned with HNO3 (15 %) and HCl (10 %). The samples were dried and pulverized using an agate mortar. For determination of the elements, 0.25 g of the sediment was digested with HF and then with an HNO3 and HClO4 acid mixture, and then heated in a controlled program with ramping heat cycles to dry the sample. After drying, the samples were reconstituted with HCl and HNO3. The elements were determined using a PerkinElmer ELAN 9000 inductively coupled plasma mass spectrometer (ICP-MS; Actlabs, Ontario, Canada). Validation of the methods was done using certified reference materials (PACS-2, MESS-3; National Research Council Canada Institute for National Measurement Standards). Blanks and duplicates of each ten samples were realized. Results of this validation are presented in Table 1.
The toxicity test was realized with elutriates obtained from the sediment samples according with USEPA (2001). Aliquots of 6–8 g were introduced in beakers of 250 mL, and reconstituted water (MgSO4 NaHCO3; KCl and CaSO4 2H2O, with hardness of 160 mg L−1; NMX-AA-087-1995-SCFI) was added at a 4:1 ratio (water to sediment) stirring the solutions during 1 h; after this process, the solutions were centrifuged obtaining the elutriates.
The toxicity test with D. magna was done according to Díaz-Báez et al. (2004). Ten neonates of D. magna (from the UAM-I stock) were introduced into each beaker (50 mL of capacity with 30 mL of each elutriate) in triplicate tests with one negative control (blank: water reconstituted), and one positive control of a reference (potassium dichromate LC50 = 0.14 mg L−1). The conditions during the experiment were a temperature of 20 ± 2 °C and a photoperiod of 16 h in the light and 8 h in the dark. Oxygen was measured at >7.8 mg L−1. After 24, and 48 h of exposure to the sediment samples, the surviving organisms of each test were evaluated. With the results obtained, we calculated the percentage mortality at 48 h in each bioassay to determine the toxicity of the sediment.
The bioassays with the microalgae S. capricornutum were done according Pica-Granados et al. 2004. The elutriates (2.6 mL) were used in quintuple tests. For each test, we inoculated microalgae (~10,000 cells per milliliter) taken from a stock concentrated of S. capricornutum. The solution for the control group was using reconstituted water (for negative control), and CuSO4 (EC50 = 0.404 mg L−1; positive control). The conditions maintained in the development of the bioassays were continuous light of 1000 lx and a temperature 22 ± 2 °C. At 72 h after the initiation of the experiment, an aliquot of 0.1 mL was taken to count the cell number in the test using a hematocytometer with an optical microscope to calculate the inhibition percentage (%) of each elutriate. This was calculated using the formula:
Results and discussion
The element contents, the mortality of D. magna, and the inhibition of S. capricornutum for each sample, are presented in Table 2, including the average of the Upper Continental Crust (UCC, Wedepohl 1995). In the arroyo sediment, the enrichment of elements such as As, Cd, Cu, Pb, and Zn is evident. The enrichment is both natural for the enrichment of elements associated to the gold ore mine, and anthropogenic for the scattered tailings produced with the gold obtaining. The influence of some potentially toxic elements was evaluated before (Marmolejo-Rodríguez et al. 2011; Sánchez-Martínez et al. 2013).
Sediment toxicity and their ratio with the TEC and PEC criteria
The results about the toxicity of PTEs in sediment is evaluated in this study with the reference values for freshwater sediment criteria threshold effect concentration (TEC) and probable effects concentration (PEC) (MacDonald et al. 2000). The TEC and PEC values have also been used as composite quotients that account for the presence of mixtures of contaminants in different concentrations that may have additive toxicity or other biological effects. The quotients of the elements average/PEC of this system for As, Cd, Pb, and Zn are 17, 3.8, 37, and 6, respectively. According to the reference values, As, Cd, Pb, and Zn are the most enriched in the sediments studied in this system and harmful effects are likely to be observed. Cu is enriched compared with the TEC limits. However, near the abandoned installations, the maximum contents are high for As, Cd, Cu, Pb, and Zn. Here, high toxicity was detected compared with the quality guidelines for metals in freshwater sediment (MacDonald et al. 2000) and are up to 500 times higher compared with the average for the Earth’s crust (Wedepohl 1995; Table 2). Chromium is less enriched in the system compared to the TEC and PEC, though if it is compared with the average for the Earth’s crust (Wedepohl 1995), it shows enrichment. Cobalt is not enriched in the system. Neither element represents a risk in the system. The contents of the LC50 of D. magna and EC50 of S. capricornutum are presented (Table 2; Ecotox database 2004) to be compared with the contents obtained in this study. The contents of As, Cd, Cu, Pb, and Zn of this study are more concentrated than the LC50 and EC50 compared with them.
PTEs concentration and their ratio with mortality of D. magna and inhibition of S. capricornutum
The results of the element concentrations in the sediments of each sample and the mortality of D. magna and inhibition of S. capricornutum are in Table 2. The mortality of D. Magna was 10 to 70 % (Table 2; Fig. 2). Despite the great quantities of PTEs, in some cases, they are not toxic for these organisms. This could be due to the chemical species of the PTEs and also because other materials in the sediment may modify the toxic effect of the elements, in some cases inhibiting their toxicity. This is documented in the samples evaluated. The sediments affected by the acidic mine drainage (AMD) discharge, which had a high concentration of metals, were shown to be toxic to the D. magna (Lattuada et al. 2009). The depuration time evaluated for the PTEs added to D. magna in the laboratory shows that Cd is not eliminated by the organism, unlike Zn, which has a significant depuration time of 6 days (Guan and Wang 2004).
Bioassays result vs distance and vs concentration of PTEs
As a general behavior, the sum of the PTEs shows a decrement from the source to destination (Table 2; Fig. 3). The anthropogenic influence and the dilution from the source to the destination are evident.
Sum of the trace elements (PTEs: mg kg−1) in sediments from the source to the destination. White circles inside the prediction intervals are the station results that were used to determine the equation and their correlation of potential toxic elements vs. distance. The squares are the percentages of mortality of D. magna, and the triangles are the percentages of inhibition of S. capricornutum
The results of the mortality and inhibition of D. magna and S. capricornutum were relatively high in all the samples; their association with the concentration of PTEs (Fig. 4) shows direct ratio (r 2 = 0.45, p < 0.005; Fig. 4) D. magna mortality and the PTEs. However, S. capricornutum did not show the same behavior (r 2 = 0.10, p = 0.405; Fig. 4). This could be caused because the oxidation states of the PTEs have different effects with the organisms studied here. In PTEs such as As, its natural form is mineralized into arsenopyrite, which includes high concentrations of S and Fe and is less toxic than the PTEs in tailings. In the tailings, it forms oxides, and in the ash, arsenolite, which is the most toxic form because of its high solubility in water. Thus, the toxicity depends on the process. In this study area, high concentration of As, Cd, Sb, Hg, Pb, Zn, and other potentially toxic elements to the gold mining are associated. The total concentration of PTEs in sediment is not an indicator of toxicity (MacDonald et al. 2000); they can be associated with stable mineral phases, or by the contrary, bioavailable in the environment (López-González et al. 2006). Although the bioavailability of PTEs in those sediments is different than in controlled conditions, the bioassays with S. capricornutum provide information about the bioavailability of PTEs. Thus, the results of the mortality and inhibition of the organism in ratio with the PTEs show synergy (sample 21; Table 2), and antagonism processes (sample 9; Table 2) probably are occurring. However, the low inhibition (sample 9) also could be due the elements are in stable mineral phases.
Studies of S. capricornutum to evaluate anthropogenic effects show that the growth inhibition test with S. capricornutum occurs on dilution of the sample; therefore, it is suitable for determination of heavy metal toxicity of a polluted matrix (Ivanova and Groudeva 2006). More studies on the natural sediments impacted by mining activities are necessary to evaluate the bioaccumulation of the PTEs in organisms and to determine which of them are concentrated in the food chain.
Conclusions
The variability of the concentration and the complex association of the potentially toxic elements are different in each environment, which is why the bioassays are necessary applied directly to evaluate the sediment toxicity in a determined system. In this study area, As, Cd, Cu, Pb, and Zn are more enriched than PEC in the sediments along the arroyo (Table 3). Those elements are more concentrated in sediments near the abandoned installations of the mining district, representing more risk in the system. Copper is more enriched than the TEC in the whole of the system, and also represents a risk. D. magna had percentage mortality in all the samples, with the maximum percentage mortality occurring in the sediments of the mining district. The S. capricornutum showed different sensitivities in the stations close to the tailings, decreasing along the 49 km of the arroyo, with the lowest inhibition of diluting toward the discharge of the arroyo; however, an accumulation is reflecting in the arroyo mouth indicating a risk in the sediments. The ratio between S. capricornutum with PTEs indicate that antagonism and synergy processes are involved. Those non-predictable behaviors indicate that the bioassays tests in this study are suitable for determination of toxicity of the natural sediments influenced by mine waste.
References
Antunes, S. C., Figueiredo, D. R., Marques, S. M., Castro, B. B., Pereira, R., & Goncalves, F. (2007). Evaluation of water column and sediment toxicity from an abandoned uranium mine using a battery of bioassays. Science of the Total Environment, 374, 252–259.
Barata, C., Baird, D. J., & Markich, S. J. (1998). Influence of genetic and environmental factors on the tolerance of Daphnia magna Straus to essential and non-essential metals. Aquatic Toxicology, 42, 115–137.
Carrillo, A. (1996). Environmental geochemistry of the San Antonio – El Triunfo mining area, southernmost Baja California Peninsula Mexico. PhD Thesis. Department of Geology and Geophysics, University of Wyoming, Laramie, Wyoming, USA, 186p.
Díaz-Báez, M.C., Pica-Granados, Y., & Ronco, A. (2004). Ensayo de toxicidad aguda con Daphnia magna. In: (Ensayos toxicológicos y métodos de evaluación de calidad de aguas. Estandarización, intercalibración, resultados y aplicaciones IDRC, IMTA, Canadá). 52–63.
Ecotox database (2004). US Environmental Protection Agency. http://cfpub.epa.gov/ ecotox/quick_query.htm.
Gabrielson, J., Hart, M., Jarelöv, A., Kühn, I., McKenzie, D., & Möllby, R. (2002). Evaluation of redox indicators and the use of digital scanners and spectrophotometer for quantification of microbial growth in microplates. Journal of Microbiological Methods, 50(1), 63–73.
Guan, R., & Wang, W. X. (2004). Cd and Zn uptake kinetics in Daphnia magna in relation to Cd exposure history. Environmental Science & Technology, 38, 6051–6058.
He, M., Wang, Z., & Tang, H. (1998). The chemical toxicological and ecological studies in assessing the heavy metal pollution in Le An river, China. Water Research, 32(2), 510–518.
Ivanova, I., & Groudeva, V. (2006). Use of Selenastrum capricornutum growth inhibition test for testing toxicity of metal ions in soil and water. Biotechnology and Biotechnological Equipment, 20(1), 179–183.
Komjarova, I., & Blust, R. (2008). Multi-metal interactions between Cd, Cu, Ni, Pb and Zn in water flea Daphnia magna, a stable isotope experiment. Aquatic Toxicology, 90, 138–144.
Komjarova, I., & Blust, R. (2009). Effect of Na, Ca and pH on simultaneous uptake of Cd, Cu, Ni, Pb, and Zn in the water flea Daphnia magna measured using stable isotopes. Aquatic Toxicology, 94, 81–86.
Lattuada, R. M., Menezes, C. T. B., Pavei, P. T., Peralba, M. C. R., & Dos Santos, J. H. Z. (2009). Determination of metals by total reflection X-ray fluorescence and evaluation of toxicity of a river impacted by coal mining in the south of Brazil. Journal of Hazardous Materials, 163, 531–537.
López-González, N., Borrego, J., Morales, J.A., Carro, O., & Lozano-Soria, O. (2006). Metal fracctionation in oxic sediments of an estuary affected by acid mine drainage (south-western Spain). Estuarine Coastal and Shelf Science, 68, 297–304.
MacDonald, D. D., Ingersoil, C. G., & Berger, T. A. (2000). Development and evaluation of Consensus-Based Sediment Quality Guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Marmolejo-Rodríguez, A. J., Sánchez-Martínez, M. A., Romero-Guadarrama, J. A., Sánchez-González, A., & Magallanes-Ordóñez, V. R. (2011). Migration of As, Hg, Pb and Zn in arroyo sediments from a semiarid coastal system influenced by the abandoned gold mining district at El Triunfo, Baja California Sur, Mexico. Journal of Environmental Monitoring, 13, 2182–2189.
Merian, E. (1991). Metals and their compounds in the environment: occurrence, analysis and biological relevance. Weinheim: Basel-Cambridge.
Moreira-Santos, M., Soares, A. M. V. M., & Ribeiro, R. (2004). An in situ bioassay for freshwater environments with the microalga Pseudokirchneriella subcapitata. Ecotoxicology and Environmental Safety, 59, 164–173.
NMX-AA-087-1995-SCFI. Análisis de agua – Evaluación de toxicidad aguda con Daphnia magna Status (Crustacea – Cladocera). Published November 14th, 1995 (in Spanish).
Pica-Granados, Y., Ronco, A., & Díaz-Báez, M.C. (2004). Ensayo de toxicidad crónica con el alga Selenastrum capricornutum (Pseudokirchneriella subcapitata) porel método de enumeración celular basado en el uso de Hemocitómetro Neubauer. In: Ensayos toxicológicos y métodos de evaluación de calidad de aguas. Estandarización, intercalibración, resultados y aplicaciones IDRC, IMTA, Canadá, (pp. 69–87).
Romero-Guadarrama, J.A. (2011). Geoquímica de As, Hg, Pb y Zn, y mineralogía en sedimentos superficiales de la cuenca de drenaje del distrito minero El Triunfo, B.C.S., México. Master’s Thesis. CICIMAR-IPN, México. pp. 98.
Romero-Guadarrama, J. A., Marmolejo-Rodríguez, A. J., Sánchez-Martínez, M. A., Sánchez-González, A., & Magallanes-Ordóñez, V. R. (2010). Mercury in sediments from drainage catchment basin of the Au mining district of El Triunfo, BCS, Mexico. In I. S. Torres-Alvarado (Ed.), Water–rock interaction XIII (pp. 551–554). London: CRC Press.
Sánchez-Martínez, M. A., Marmolejo-Rodríguez, A. J., Sánchez-González, A., & Magallanes Ordóñez, V. R. (2013). Vertical accumulation of potential toxic elements in a semiarid system that is influenced by an abandoned gold mine. Estuarine, Coastal and Shelf Science, 130, 42–53.
USEPA (2001). Methods for collection storage and manipulation of sediments for chemical and toxicological analyses: Technical manual. EPA 823-B-01-002. U.S. Environmental Protection Agency, office of water, Washington DC.
Volke-Sepúlveda, T., Solórzano-Ochoa, G., Rosas-Domínguez, A., Izumikawa, C., Aguilar, G.E., Velasco-Trejo, J.A., & Flores-Martínez, S. (2003). Remediación de sitios contaminados por metales provenientes de jales mineros en los distritos de El Triunfo-San Antonio y Santa Rosalía, Baja California Sur. Dirección de Investigación en Residuos y Proyectos Regionales. Centro Nacional de Investigación y Capacitación Ambiental Instituto Nacional de Ecología (SEMARNAT). Technical Report.pp.1-37.
Wedepohl, K. H. (1995). The composition of the continental crust. Geochimica et Cosmochimica Acta, 59, 1217–1232.
Acknowledgments
This research is a contribution to projects SIP-IPN “Efectos toxicológicos de los sedimentos en un sistema fluvial semiárido, influenciado por una mina de oro abandonada: El Triunfo, BCS Mexico SIP-20131030,” in collaboration with UAM-I “Evaluación del estado de salud de organismos de importancia ecológica y/o económica presentes en sistemas acuáticos.” We would like to thank the Consejo Nacional de Ciencia y Tecnología, Comisión de Operaciones y Fomento de las Actividades Académicas del Instituto Politécnico Nacional. In memoriam of Dr. Ellis Glazier (R.I.P.), who helps to edit this English-language text. The authors thank Horalia Arce laboratory assistant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sobrino-Figueroa, A.S., Becerra-Rueda, O.F., Magallanes-Ordóñez, V.R. et al. Toxicity in semiarid sediments influenced by tailings of an abandoned gold mine. Environ Monit Assess 187, 4158 (2015). https://doi.org/10.1007/s10661-014-4158-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4158-y