Abstract
The Trans-Amazonian Highway (TAH) is located in the northern region of Brazil, comprising a border region where agricultural, mining, and logging activities are the main activities responsible for fostering economic development, in addition to large hydroelectric plants. Such activities lead to environmental contamination by potentially toxic elements (PTEs). Environmental monitoring is only possible through the determination of element contents under natural conditions. Many extraction methods have been proposed to determine PTEs’ bioavailability in the soil; however, there is no consensus about which extractor is most suitable. In this study, we determined the contents of PTEs in soils in the surroundings of TAH after mineral extraction with diethylenetriaminepentaacetic acid-triethanolamine (DTPA-TEA), Mehlich I, and Mehlich III solutions. Soil samples were collected in areas of natural vegetation in the vicinity of TAH in the state of Pará, Brazil. Chemical attributes and particle size were determined, besides concentrations of Fe, Al, Mn, and Ti by sulfuric acid digestion, Si after alkaline solution attack, and poorly crystalline Fe, Al, and “free” Fe oxides. Mehlich III solution extracted greater contents from Fe, Al, and Pb as compared to Mehlich I and DTPA-TEA and similar contents from Cd, Mn, Zn, and Cu. Significant correlations were found between concentrations of PTEs and the contents of Fe and Mn oxides as well as organic carbon and soil cation exchange capacity. Contents of Cu, Mn, Fe, and Zn by the three methods were positively correlated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Potentially toxic elements (PTEs) occur naturally in the soils as a result of weathering. By definition, they have atomic density greater than 6 g cm−3 and atomic number greater than 20 (Alloway 1995). The natural concentrations of PTEs in the soils are generally low, but the intensive use of fertilizers and pesticides, along with increased industrial and mining activities, has contributed significantly to increase PTEs’ contents in the soils. Because of these polluting sources, farmable areas have been reduced (Cancela et al. 2002; Kabata-Pendias and Pendias 2010).
Soil contamination by PTEs depends on the chemical species of the element and its concentration in the soil. PTEs are found in soils as soluble, exchangeable, occluded, precipitated, or complex forms (Luo et al. 2005). Ba, Mn, Fe, As, Cd, Pb, Hg, Co, Cu, Cr, Ni, Se, and Zn are potentially toxic to organisms, and most are commonly found in contaminated Brazilian soils (CONAMA 2009).
Nowadays, studies have been carried out taking PTEs’ total or pseudo-total contents into account. Such contents, however, do not provide adequate information about the potential risk of contamination, because not all forms of metals present in the soil are available for plant uptake (Zhang et al. 2010; Luo et al. 2012). The availability depends on soil characteristics such as organic matter content, cation exchange capacity (CEC), pH, texture, clay fraction composition and content, and biological activity (Basar 2009), in addition to the characteristics of each metal such as ionic radius, chemical speciation, and metal content in the solution.
There are several methods to extract contents of PTEs in the soil, and the mean values of extracted contents by each method are heterogeneous. Variations occur mainly due to soil diversity (Fadigas et al. 2002) and due to various extraction solutions. So, it is difficult to extrapolate or to compare the values from one to another region (Biondi et al. 2011).
Monitoring the forms of available PTEs depends, in part, on an efficient chemical method to measure available fractions of these elements. Among the extractors used to evaluate availability of PTEs for plants are diluted acidic solutions, such as Mehlich I (Mehlich 1953), and chelators, DTPA (Lindsay and Norvell 1978), and mixed solutions such as Mehlich III (Mehlich 1984).
Comparisons between extraction solutions are complex because of the different soil conditions where they are evaluated and the principle of each extraction method (Disla et al. 2010). Acidic extractors remove elements present in the exchangeable forms of the solid phase, solution, and part of the complexes. Complexants extract, predominantly, labile forms and forms preferably associated with organic matter (Abreu et al. 2002; Menezes et al. 2010).
Pará State is the second largest state in the Brazilian Amazon with a total area of 1,247,950.003 km2. It presents great diversity of soils and holds intense framing and mining activities. These activities are concentrated along federal and state highways. Nevertheless, uncontrolled exploitation has contributed to ecosystem changes with biodiversity loss (Fearnside and Millikan 2012). The Trans-Amazonian Highway (BR-230) is among the most important highways in the region, which is approximately 5,000 km long and runs through the Brazilian states of Paraíba, Ceará, Piaui, Maranhão, Tocantins, Pará, and Amazonas.
The Trans-Amazonian Highway crosses the state of Pará from east to west, connecting it to the states of Tocantins and Amazonas. This is a border region that, since 1970, has received investment from the federal government for the installation of large projects such as hydroelectric plants, mining, vegetal extractivism, land reform settlement, agriculture, and livestock, which comprise its main economic activity. These activities have contributed to high rates of deforestation (Tourneau and Bursztyn 2010; Fearnside and Millikan 2012) and soil pollution by PTEs.
The state of Pará and the Amazon as a whole present no studies on PTEs, as well as a definition of the most appropriate method to estimate availability. Thus, the objective of this study was to determine the contents of potentially toxic elements in the soils in the surroundings of the Trans-Amazonian Highway through diethylenetriaminepentaacetic acid-triethanolamine (DTPA-TEA), Mehlich I, and Mehlich III extraction solutions and to correlate physical and chemical soil attributes.
Materials and methods
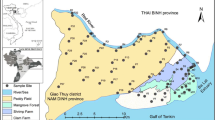
Soil sampling was performed in the vicinity of the Trans-Amazonian Highway since the municipality of São Domingos do Araguaia, on the border with Tocantins State, until the municipality of Jacareacanga, bordering Amazonas State (Fig. 1), in order to compose a heterogeneous set of physical chemical soil attributes.
According to Köppen classification, the climate is equatorial, Am-Aw type, with minor variations in the whole state. Annual average temperatures range from 25 to 27 °C. Annual rainfall varies between 1,400 and 2,800 mm, with a dry period (August to December) and a rainy season (January to July), which concentrates more than 70 % of the annual precipitation (Falesi 1986; Rodrigues et al. 2007). Geologically, the area of influence of the Trans-Amazonian Highway in Pará State is represented by lithologies from the Archean and Pre-Cambrian, Ordovician, Carboniferous, Jurassic, Cretaceous/Tertiary, and Quaternary periods (Rodrigues et al. 2007).
The soils studied were classified as Oxisols (60 % of the total area), Ultisols (27 %), Alfisols (2 %), Entisols (4 %), and subgroup Plinthic (7 %). These soil classes were mapped and characterized based on the criteria and differential characteristics established to fit them in the USDA Soil Taxonomy (USDA-NRCS 1999). Soil classification was estimated using the ArcGIS 10.1 software program by overlapping information plans related to soils in the region, based on the work developed by the Ecological Economic Zoning project of the area of influence of BR-163 (Cuiabá-Santarém) and BR-230 (Trans-Amazonian) highways in the state of Pará (Rodrigues et al. 2007) and the Trans-Amazonian Highway soils (Falesi 1986). Samples were collected in areas of primary vegetation, at least 100 m away from the main highway and secondary roads.
Samples were collected at a 0.0–0.2-m-depth layer by taking ten subsamples every 70 m to obtain a composite sample. Homogeneity among samples was considered regarding color, texture, topography, drainage, and vegetation, according to the methodology proposed by Silva (1999). Three composite samples were collected in each area, totaling 135 samples in 45 equidistant areas, approximately between 20 and 40 km. Samples were collected with a stainless steel auger according to the procedures adopted by CETESB (2001) to avoid contamination. Samples were air dried, crumbled, homogenized, and sieved through a 2.0-mm mesh, resulting in air-dried fine earth (ADFE).
Particle size analysis was performed by the pipette method using 0.1 M NaOH solution as a chemical dispersant and high-speed mechanical stirring for 10 min. Clay fraction was separated by sedimentation, sand was separated by sieving, and silt was calculated by difference (Gee and Or 2002).
pH was determined potentiometrically using 1:2.5 soil/water ratio. Calcium, magnesium, and aluminum were extracted with 1 M KCl solution. Al3+ was quantified by titration with 0.025 M NaOH and Ca2+ and Mg2+ by atomic absorption spectrophotometry (Anderson and Ingram 1992). K+ and P were extracted by Mehlich I (0.05 M HCl + 0.0125 M H2SO4) (Mehlich 1953). K+ was determined by flame photometry and P by colorimetry.
Potential acidity (H+Al3+) was extracted from 1 M calcium acetate buffered solution (pH 7.0) and determined by titration with NaOH (Anderson and Ingram 1993). Organic carbon (OC) was measured by colorimetry, according to Raij et al. (2001). Sum of bases (SB), CEC, base saturation (V%), and aluminum saturation (m%) were then calculated.
Contents of Al, Fe, Mn, and Ti oxides expressed as oxides (Fe2O3AS, Al2O3AS, MnOAS, and TiOAS) were extracted by the sulfuric acid digestion method using H2SO4 (1:1). Contents of Si oxide (expressed as SiO2AS) were extracted by 1 M NAOH solution. Contents of Fe, Al, and Mn were determined by atomic absorption spectrophotometry, Ti by colorimetry, and Si by gravimetry, as described by EMBRAPA (1997). The weathering indices Ki (SiO2/Al2O3, moles per mole) and Kr [SiO2/(Al2O3 + Fe2O3), moles per mole] were calculated. The Ki index is used to assess the degree to which tropical soils have been altered or weathered. For highly weathered Brazilian Oxisols, Ki is an indirect measure of the relative proportions of kaolinite and gibbsite. Poorly crystalline oxides (“amorphous”) of Fe2O3 (FeOX), Al2O3 (AlOX), MnO (MnOX) were extracted by an oxalic acid and ammonium oxalate solution, while “free” or crystalline iron oxides (FeDCB) were extracted with sodium dithionite-citrate-bicarbonate (Mehra and Jackson 1960; Loeppert and Inskeep 1996).
Available contents of Cd, Cr, Pb, Co, Cu, Fe, Mn, Al, Ba, and Zn were extracted in triplicate using three extractants: Mehlich I (already described); DTPA-TEA containing 0.005 M DTPA, 0.01 M CaCl2, and 0.1 M TEA, pH 7.3 Lindsay and Norvell (1978); and Mehlich III extraction solution containing 0.2 M of CH3COOH + 0.25 M of NH4NO3 + 0.015 M NH4F + 0.001 M EDTA (Mehlich 1984). Determinations were made by flame atomic absorption spectrophotometry (AAS). Limits of quantification (LOQs) were calculated using the means of each element analytical blanks over the sample standard deviation (ICH 1996).
Descriptive data analysis was used to determine the measures of central tendency and variability. Normality tests of Shapiro-Wilk were carried out. Pearson correlation was obtained between contents of PTEs, soil attributes, and the methods used. Exploratory analysis was conducted with contents of PTEs by composition of box-plot graphs to identify anomalous values. Kruskal-Wallis and kruskalmc significance test (p < 0.01) were applied for comparison of elements between the methods. Principal component analysis was used, in the group of soils, to summarize the values of physical and chemical properties between extraction solutions.
Principal components analysis (PCA) was used to visually explore the principal features of the analytical data and the distribution of the PTE contents in the soils. PCA is an unsupervised multivariate technique in which new variables (called principal components or PCs) are calculated as linear combinations of the original variables (soil attributes and PTEs contents). The essential characteristics of the multidimensional dispersion are obtained when most of the variation is explained by graph grid in the first, second, third, or more component of coordinate of plotted points. It was thus possible to display the information graphically using the first, second, third, or more PCs (in this case, PCs typically had eigenvalues ≥1). The grid values allow us to identify samples or groups with similar results and the correlations between the original variables. PCA was performed with Statistica 7.0 software (StatSoft 2005).
Results and discussions
The soils are acidic with mean pH in H2O of 4.2 (Table 1). OC contents presented an average of 5.9 g kg−1 and varied from 1.4 to 17.1 g kg−1. Distribution was not normal due to a wide variation, which can be attributed to soil variability. Organic matter is one of the soil components that mostly contribute to CEC and metal retention, while increased acidity provides the presence of free cationic forms, which are more prone to leaching (Campos 2010). Mean base saturation (BS) was 9.7 millimoles of charge (mmolc kg−1, which is considered low, with an amplitude of 2.9 to 76.9 mmolc kg−1. This suggests low nutrient availability and high levels of exchangeable Al (14.3 mmolc kg−1) mainly caused by the high weathering degree (Fageria and Moreira 2011).
Soil particle size distribution showed predominance of sand fraction in relation to fine, clay, and silt fractions. The dominant textural class ranged from clayey sandy to sandy clay loam.
Mean contents of Fe oxides extracted by DCB were higher than those extracted by ammonium oxalate, highlighting the predominance of crystalline forms as compared to the amorphous ones. Oxides and hydroxides are among the variables that mostly influence the mobility of PTEs in soils from humid tropics (Alleoni et al. 2005). Low Ki and Kr values characterized the soils as highly weathered with predominance of clay minerals of the kaolinite group (Campos et al. 2012). Mean contents of PTEs presented a wide variation, indicating asymmetric distribution of elements and lack of data normality (Table 2), which may be related to heterogeneity in soil physical chemical properties.
Cr content was below LOQ for all extractors. Cr content below LOQ may be indicative of geochemical affinity for iron oxides, which limits the mobility in the system (Adriano 2001). Andrade et al. (2009) assessed the concentration of PTEs in mining areas of the state of Paraná, Brazil and observed Cr contents extracted by DTPA-TEA to be below the limit of detection (LOD). Otero et al. (2012) investigated the availability of toxic elements in Spain and also reported Cr levels extracted by Mehlich III to be below LOD.
Al > Fe > Mn were the elements with the highest contents regardless of the extractor (Table 2). Rodrigues et al. (2001) evaluated extraction solutions for available contents of Fe and Mn in Ultisols and Oxisols of the Brazilian Amazon region and also noted higher levels of Fe compared to Mn regardless of the extractor used; the following decreasing order of contents was verified: Mehlich III > Mehlich I > DTPA-TEA. High levels of these elements reflect abundance and dynamics in the soil, as a result of the high degree of weathering. Contents depend on redox potential, chemical speciation, and oxidation of soil and reflect the predominance of oxides in the clay fraction of the soil (Biondi et al. 2011).
Cu and Zn contents had higher coefficients of variation (CVs) than the other elements (Table 2). Heterogeneity in soil physicochemical characteristics (Table 1) affects metal dynamics and availability and contributes to raise the coefficient of variation (Basar 2009). Samples collected in Alfisols, Oxisols, and Entisols with anthropic A horizon showed high contents of Cu and Zn. On the other hand, 70 % of Oxisols samples presented contents smaller than 3 mg kg−1. Heterogeneity between soil classes and between soil samples of the same class contributes to data dispersion (Fig. 2). The low contents of extracted Cu and Zn, in most soils (70 %), may be related to the strong adsorption of PTEs to Fe, Al, Mn oxides/hydroxides, and OC, in addition to intensive leaching McBride et al. (2004), which is common in acidic soils such as those of the Amazon. In Spain, Cancela et al. (2002) evaluated the availability of Cu, Mn, Fe, and Zn in soils under natural conditions and detected greater variations of Cu and Zn when compared to Fe and Mn for the DTPA-TEA and Mehlich III extractors.
Pb mean contents were low, with the following order of extraction: Mehlich III > Mehlich I > DTPA-TEA (Table 2). The highest CV in Mehlich III (108 %) is related to samples collected in areas with high concentration of OC. Pb is strongly associated with organic compounds. As Mehlich III is an acidic solution combined with a complexing agent (EDTA), it is efficient in extracting the contents of metals associated with the organic fraction (Abreu et al. 1998). Furthermore, EDTA increases Pb solubility because of its high equilibrium constant and strong chemical affinity (Luo et al. 2005). OC contents varied between soil samples of the same class and of different classes (Table 1), and this may have contributed to the heterogeneity of Pb contents extracted by DTPA-TEA, Mehlich I, and Mehlich III (Fig. 2). Abreu et al. (1998) found higher Pb contents in surface horizons of soils in the state of São Paulo extracted by DTPA-TEA and Mehlich III than in subsurface samples. Abreu et al. (1998) attributed this variation to the heterogeneous distribution of OC in the soil profile.
Other factors contributing to the low contents of Pb in soils are the low solubility of the element and the strong adsorption to Fe and Mn oxides (Abreu et al. 2004). In Oxisols and Ultisols under natural conditions in the state of São Paulo, Abreu et al. (1995) reported high amplitudes in the contents extracted by DTPA-TEA (0.6 to 5.9 mg kg−1), Mehlich I (1.1–2.6 mg kg−1), and Mehlich III (4.6 to 11.0 mg kg−1) and attributed this variation to the variation in oxide contents.
Cd had the lowest variation among the elements analyzed for all extraction solutions (Table 2). However, contents did not present a normal distribution between soil samples (Fig. 2). This heterogeneity could be attributed to parent material diversity. In the study area, 87 % are Oxisols and Ultisols predominantly of “Alter do Chão” Formation and 2 % are Alfisols of Penatecaua Formation (Rodrigues et al. 2007). Soils formed from basic rocks exhibit higher levels of PTEs in relation to soils developed under the influence of acidic rocks (Kabata-Pendias and Pendias 2010).
The low levels of Cd may be related to high mobility due to low affinity with Al and Fe oxides (Kabata-Pendias and Pendias 2010), which are predominant in the soils of the state of Pará (Falesi 1986). Birani (2010) found available contents of PTEs in soils of the state of Pará and reported lower means and ranges for Cd compared to the elements Ba, Cr, Cu, Fe, Mn, Ni, Pb, and Zn, attributing these low concentrations to high soil weathering degree and acidic conditions that favor solubility.
Ba contents were the fourth highest, averaging 3.3, 4.5, and 7.0 mg kg−1 for DTPA-TEA, Mehlich I, and Mehlich III, respectively. Higher values of Ba, compared to other PTEs, may be related to its higher contents in the parent material and to the replacement of K+ in the feldspar structure, which can be mobilized under different environmental conditions (Biondi et al. 2011). Additionally, contents vary according to soil characteristics such as pH, clay, and OM (Merlino et al. 2010). In soils from another region of Pará, Birani (2010) reported lower contents of PTEs compared to those found in the soils of this study, averaging 0.1, 2.9, and 0.2 mg kg−1 for Mehlich I, Mehlich III, and DTPA-TEA, respectively.
The state of Pará does not have studies to determine classes of interpretation and definition of extractors to assess contents of available micronutrients in the soil. We compared the values from our study with classes of micronutrient interpretation in use in different Brazilian states. Due to lack of data normality, comparison was performed by the median contents.
Cu, Zn, and Mn contents found in soils in the surroundings of the Trans-Amazonian Highway were below the minimum sufficient for nutritional requirements of the main crops, according to the classes of interpretation for micronutrients (Raij 2011; Alvarez Venegas et al. 1999; EMBRAPA 2007). Low contents of Cu, Zn, and Mn are indicators of deficiency and the need of fertilization with micronutrients.
In general, Fe concentration was higher than the average reported in other Brazilian regions and another region of Pará State for all extractors (Raij 2011; Alvarez Venegas et al. 1999; EMBRAPA 2007). High levels may be related to genesis and weathering degree, with predominance of secondary minerals such as hematite and goethite in the clay fraction (Burak et al. 2010). High Fe contents can cause toxicity and affect the availability of other nutrients such as P (Farella et al. 2007). Contrasts between extractors and classes of interpretation emphasize the importance of determining available contents at the regional level and, from these, establishing reference values. The extrapolation of values to regions different from those of data collection or for comparison of extraction solutions can cause inadequate intervention and changes in soil quality and human health.
Pearson correlations (p < 0.05 and p < 0.01) were established for metals between extraction solutions (Table 3), except for Pb and Cd, which showed no significant correlations. Lack of correlation might be related to the low contents extracted. Positive correlations (p < 0.05 and p < 0.01) were found among the DTPA-TEA, Mehlich I, and Mehlich III methods regarding extraction of Mn, Zn, and Cu (Table 3). Sarto et al. (2011) reported a significant correlation for Mn, Cu, and Zn contents by Mehlich I and by Mehlich III in the soils of Paraná. Brennan et al. (2008) also found positive correlations between Cu and Zn concentrations extracted by Mehlich III and DTPA-TEA on Irish soils.
Al and Co contents extracted by Mehlich I and Mehlich III were positively correlated (Table 3). Significant correlations between Mehlich I and Mehlich III are assigned to the intimate relation between extraction solutions, because the chemical extraction principle is similar, with high acidity, providing high element solubilization (Sarto et al. 2011). Ba and Fe contents extracted by DTPA-TEA correlated positively with those extracted by Mehlich III (Table 3). Similarity between extractors can be related to the presence of the EDTA and DTPA complexing agents in the extraction solutions (Brennan et al. 2008), because they present the amino and carboxylic groups, which have affinity for Ba and Fe. Abreu et al. (2004) found a positive correlation coefficient of 0.82 for Fe contents extracted by Mehlich III and TEA-DTPA in the soils of the state of São Paulo, Brazil.
Significant positive correlations between the contents of most PTEs extracted by DTPA-TEA, Mehlich I, and Mehlich III show similarity and suitability of extractants to estimate these metal concentrations, which are available to plant uptake or to be leached into underground water (Kabata-Pendias and Pendias 2010). Dispersion of points can be attributed to heterogeneity in physical and chemical properties and high weathering degree of soils, mostly Oxisols and Ultisols. Furthermore, solutions act differently according to the soil fractions where the elements are adsorbed or complexed (Bortolon and Gianello 2009), justifying different extractions between extractants for the same soil sample.
Pearson correlation between contents of PTEs and physical chemical attributes of the soil can be considered low (Table 4). OC concentration was positively correlated with Zn, Mn, Ba, and Cd contents extracted by Mehlich I and Al, Cd, Ba, and Zn contents extracted by DTPA-TEA. Correlations between the contents of OC and PTEs are indicative of complexation/adsorption of these elements by organic anions derived from humic and fulvic acids by binding to oxygen in carboxylic and phenolic groups (Nascimento et al. 2002), which reinforces the importance of OM in the retention of PTEs in Amazonian acidic soils. Roca et al. (2012) found a positive correlation between Cu, Cd, Mn, and Zn contents extracted by DTPA-TEA and organic carbon in the soils from semiarid Argentina.
Cu and Zn contents were positively correlated with CEC, clay, and OC contents regardless of the extractor. In highly weathered Amazonian soils, CEC is dependent on both content and type of clay as well as on organic carbon content (Nogueirol et al. 2010). Organic carbon is one of the variables that mostly influence exchangeable cations and availability of PTEs in the soil (Otero et al. 2012).
pH was negatively correlated with Al and Co contents extracted by Mehlich I and Mehlich III and with Fe contents extracted by DTPA-TEA and Mehlich I. On the other hand, it was positively correlated with Cu, Mn, and Zn contents for the three extractors and with Ba when extracted by DTPA-TEA and Mehlich III (Table 4). Correlations between pH and metals suggest that the mobility of these elements is linked to the acid-base conditions of the soil. In the soils of Ireland, Brennan et al. (2008) reported a positive correlation between Zn and Cu contents extracted by Mehlich III and DTPA-TEA and pH, both in calcareous and sandstone parent soils. In Spain, Otero et al. (2012) reported a positive correlation between pH and Cu, Pb, and Zn contents extracted by Mehlich III.
Lack of correlation between some PTEs and pH may be related to low element content in acidic soils (Zhang et al. 2010) of humid tropical regions, due to intense leaching and microbial activity, which promotes solubilization and losses by leaching (Fonseca et al. 2010). Fonseca et al. (2010) reported that pH was the main factor affecting Cu, Fe, Mn, and Zn availability in soils from Paraná State.
Clay contents showed positive correlations with Ba, Cu, and Al contents extracted by Mehlich I, Cu by DTPA-TEA and Cu and Zn by Mehlich III (Table 4). Clay concentration is one of the most important factors when evaluating the contents of PTEs in the soil because of the association between PTEs and clay minerals (Alleoni et al. 2005). Correlation between clay and only a few metals extracted by solutions can be associated with sand fraction predominance in the upmost surface layer, with low sorptive capacity. Roca et al. (2012) observed a positive correlation between clay and Cu, Zn, Mn, and Fe contents extracted by DTPA-TEA in the soils of Argentina. Moreover, Birani (2010) compared the availability of EPTs by DTPA, Mehlich I, and Mehlich III in uncultivated soils of Para State and did not obtain a positive correlation between the contents of PTEs and clay concentration in the 0.0–0.2-m-depth layer.
Oxide contents were the variables that correlated with fewer concentrations of PTEs in all extraction solutions (Table 4). Cd contents extracted by DTPA-TEA and Mehlich I correlated with amorphous Fe, Mn, and “free” Fe oxides and Ti extracted by sulfuric acid digestion. Zn-DTPA-TEA and Zn-Mehlich I contents correlated negatively with the content of amorphous Al. Co contents extracted by Mehlich I and Mehlich III were negatively correlated with the content of Al extracted by sulfuric acid digestion. Among oxides, the amorphous form was the one that best correlated with the contents of PTEs, probably due to smaller dimensions and high specific surface area, which greatly influence the physicochemical properties of the soil (He et al. 2010).
There was no difference (p > 0.05) between the three extraction solutions in extraction capacity of Cd, Cu, Zn and Mn (Fig. 2), whose contents were very low. This result may reflect the geochemical behavior of these elements, which present similar atomic mass, ionic radius, and oxidation state (Paye et al. 2012; Burak et al. 2010). During the pedogenetic processes, these elements are not incorporated to the structures of minerals due to imbalance of charges, being easily leached (Marques et al. 2004), presenting very low levels in most soils.
The DTPA-TEA and Mehlich III solutions extracted Ba contents similar or greater than those extracted by Mehlich I (Fig. 2). These higher contents occur due to the presence of the EDTA and DTPA complexing agents, which are efficient in extracting heavy metals associated with exchangeable fraction, organic carbon, and carbonate, although with lower efficiency in extracting metals bound to oxides (Elliott and Shastri 1999; Abreu et al. 2002, 2004). Ba contents extracted by DTPA-TEA in the soils of Para State correlated positively with OC contents. In addition, the correlation between Ba contents extracted by Mehlich I and OC contents was negative, suggesting that much of the Ba may be linked to OC.
Differences (p < 0.05) were found between solutions for extraction from Fe, Pb, and Al, whose contents were higher in Mehlich I and Mehlich III followed by DTPA-TEA. The highest levels extracted by the Mehlich III solution may be associated with the combination of chelating agents and acids, favoring the extraction of metals bound to different soil fractions. Complexing solutions extract more from the labile fractions while acidic solutions extract more elements in the solid phase of the soil (Andrade et al. 2009).
The higher capacity of the Mehlich III solution in extracting Fe was proven by Rodrigues et al. (2001) comparing the DTPA-TEA, Mehlich I, and Mehlich III solutions in soils of the Amazon region, by Sarto et al. (2011) in the soils of the state of Paraná, and by Abreu et al. (2004) in the soils of the state of São Paulo. Assessing the availability of Cd, Pb, Cu, and Zn in the soils of southwestern China with the accumulation of these elements in rice plants, Zhang et al. (2010) reported greater extraction of Pb by Mehlich III compared to Mehlich I and DTPA-TEA.
The acidic extractants Mehlich I and Mehlich III extracted similar concentrations of Co, greater than with DTPA-TEA (Fig. 2). However, both extracted low contents of the element, 2 and 7 mg kg−1, respectively. The low Co extraction may be related to the high concentration of Fe and Al oxide contents in the soils studied (Table 1). During weathering, in acidic and oxidized environments, Co is relatively mobile, but it exhibits low mobility if adsorbed at the surface of Fe, Mn, and Al oxides and silicate minerals (Kabata-Pendias and Pendias 2010). On the other hand, low Co contents extracted by DTPA-TEA may be connected to poor extractant efficiency at the extraction of oxidic forms (Andrade et al. 2009).
The levels of PTEs extracted by DTPA-TEA, Mehlich I, and Mehlich III and soil attributes were used for PCA. From the eigenvalues, three principal components explained 65, 63, and 61 % of the total variance for DTPA-TEA, Mehlich I, and Mehlich III, respectively (Table 5 and Figs. 3a and 4a). But for Mehlich III, five principal components were used that explained 84 % of the total variance (eigenvalues ≥1) (Fig. 5a). The discriminatory attributes were those with the largest eigenvectors (in magnitude) (Table 6). The grid of sample distribution between the first two factors for PTEs extracted by DTPA-TEA, Mehlich I, and Mehlich III is shown in Figs. 3b, 4b, and 5b.
The arrangement of data points along the horizontal axis (factor 1), from the origin (0.0) to 1.0, shows the direct correlation of Mn, Co, Ba, and Zn for DTPA-TEA with pH and Mn oxide contents (Table 6 and Fig. 3c). The correlation between Al, Cd, and Cu with OC, effective cation exchange capacity (CECe), and clay can be seen on the vertical axis (factor 2), from the origin (0.0) to 1.0 (Fig. 3c). The correlation between Zn and Pb with CECe can be seen on the vertical axis (factor 3), from the origin (0.0) to 1.0 (Fig. 3d).
Mn oxides are present in the soils and sediments and play a role in several chemical reactions that affect the composition of groundwater and soils (Rout et al. 2009). Considered as “electron pumps” due to the high reactivity of their internal region (interlayers), Mn oxides represent the primary sinks for heavy metals and metalloids (Violante et al. 2010). Cerqueira et al. (2011a), b) observed that Cu and Pb were more adsorbed than Cd in from moderately acidic to lightly alkaline Aridisols, Alfisols, and Inceptisols and were linearly correlated with Mn oxide content. Anderson and Christensen (1988) and Fageria et al. (2002) reported that Co adsorption is directly related to Mn oxide contents and its availability varies with pH.
The levels of Mn, Co, Ba, and Zn, extracted by Mehlich I, along the horizontal axis (factor 1), from the origin (0.0) to −1.0, show the direct correlation with pH and Mn oxide (Fig. 4c). The correlation between Pb and Cu with OC, CECe, and clay can be seen on the vertical axis (factor 2), from the origin (0.0) to −1.0 (Fig. 4c). However, factor 3 is influenced only by the correlation of Cd with the Fe oxide (Fig. 4d). The correlation between PTEs can be related to the lithology of the study area, the origin of these metals, and the similar geochemical behavior (Gupta and Sinha 2007; Burak et al. 2010; Krishna, et al. 2011; Yu et al. 2012).
pH and Mn oxide also strongly influence the PCA for PTEs extracted by Mehlich III. The arrangement of data points along the horizontal axis (factor 1), from the origin (0.0) to 1.0, shows the direct correlation of Mn, Co, Cu, and Zn (Fig. 5c). Factor 2 is influenced only by the correlation of Al with the CECe (Fig. 5c and Table 6). The levels of Zn and Cu extracted by Mehlich III with Ki, CECe, and OC can be seen on the vertical axis (factor 3), from the origin (0.0) to 1.0 (Fig. 5d). In summary by PCA, pH and Mn oxide are the most important soil attributes for levels of PTEs extracted by DTPA-TEA, Mehlich I, and Mehlich III.
Conclusions
The evaluation of methods for extracting available contents of potentially toxic elements available in the soil from the humid tropics contributes to proper management, reducing production risks by limitations of the essential elements or excessive soil contamination. The extraction solutions showed different recoveries to the elements, and Mehlich III extracted the highest contents of Fe, Al, and Pb as compared to Mehlich I and DTPA-TEA and similar contents for Cd, Mn, Zn, and Cu. The TEA-DTPA, Mehlich I, and Mehlich III extraction solutions presented a positive correlation with Cu, Mn, Fe, and Zn contents. It is important to note that the extractors did not show the same behavior for all of the soil classes. Principal component analysis showed that available contents of metals were related to pH and Mn oxide contents, which in combination was robust to explain the variation of data. Significant correlations were found between the contents of potentially toxic elements and organic carbon, pH, cation exchange capacity, and Fe and Mn oxide contents. Cu and Zn correlated with most of the attributes analyzed.
References
Abreu, C. A., Abreu, M. F., Raij, B. V., & Santos, W. R. (1995). Comparação de métodos de análise para avaliar a disponibilidade de metais pesados em solos. Revista Brasileira de Ciência do Solo, 9, 463–468 (In Portuguese with English abstract).
Abreu, C.A., Abreu, M.F., & Andrade, J.C. (1998). Distribution of lead in the soil profile evaluated by DTPA and Mehlich-3 solutions. Bragantia, vol. 57, no. 1. (In Portuguese with English abstract).
Abreu, C. A., Raij, B. V., Gabe, U., Abreu, M. F., & Paz-González, A. (2002). Efficiency of multinutrient extractants for the determining of available zinc in soils. Communications in Soil Science and Plant Analysis, 33, 3313–3324.
Abreu, C. A., Raij, B. V., Abreu, M. F., & Paz-González, A. (2004). Evaluation of manganese and iron availability in soils by a modified ion exchange resin method. Revista Brasileira de Ciência do Solo, 28, 579–584 (In Portuguese with English abstract).
Adriano, D. (2001). Trace elements in terrestrial environments: biogeochemistry, bioavailability and risks of metals. New York: Springer.
Alleoni, L.R.F., Borba, R.P., & Camargo, O.A. (2005). Heavy metals: from cosmogony to Brazilian soils. In P. Torrado-Vidal, L.R.F., Alleoni, M., Cooper., & A.P., Silva (Eds.), Topics in soil science. Viçosa: Soc. Bras. Ciênc. Solo (vol. IV, pp. 1–42). (In Portuguese).
Alloway, B. J. (1995). The origins of heavy metals in soil. In B. J. Alloway (Ed.), Heavy metals in soils (2nd ed., pp. 38–57). London: Blackie Academic.
Alvarez Venegas, V.H.R.F., Novais, N.F., Barros, R.B., Catarutti, A., & Lopes, S. (1999). Interpretation of soil analysis results. In A.C., Ribeiro, P.T.G. Guimarães., & V.H. Alvarez Venegas, Recommendations for use of amendments and fertilizers in Minas Gerais, Brazil (5th ed., pp. 25–32). Viçosa: SFSEMG (In Portuguese).
Anderson, P. R., & Christensen, T. H. (1988). Distribution coefficients of Cd, Co, Ni and Zn in soils. Journal of Soil Science, 39, 15–22.
Anderson, J. M., & Ingram, J. S. I. (1992). Tropical soil biology and fertility: handbook of methods. Wallingford: CAB International.
Anderson, J. M., & Ingram, J. S. I. (1993). Tropical soil biology and fertility: a handbook of the methods (2nd ed.) (p. 221). Wallingford: C.A.B. International.
Andrade, M. G., Melo, V. F., Souza, L. C. P., Gabardo, J., & Reissmann, C. B. (2009). Heavy metals in soils of a lead mining and metallurgy area. II—forms and plant availability. Revista Brasileira de Ciência do Solo, 33, 1889–1897. In Portuguese with English abstract.
Basar, H. (2009). Estimation methods for metals in phytoavailable soils. Communications in Soil Science and Plant Analysis, 40, 1087–1105.
Biondi, C. M., Nascimento, C. W. A., Fabricio Neta, A. B., & Ribeiro, M. R. (2011). Concentrations of Fe, Mn, Zn, Cu, Ni and Co in benchmark soils of Pernambuco, Brazil. Revista Brasileira de Ciência do Solo, 35, 1057–1066 (In Portuguese with English abstract).
Birani, S.M. (2010). Available metal contents in soils and chemical attributes of the Pará state. Dissertation. Federal Rural University of Amazon. (In Portuguese).
Bortolon, L., & Gianello, C. (2009). Availability of copper and zinc in soils from southern Brazil. Revista Brasileira de Ciência do Solo, 33, 647–658 (In Portuguese with English abstract).
Brennan, D., Coulter, B., Mullen, G., & Courtney, R. (2008). Evaluation of Mehlich 3 for extraction of copper and zinc from Irish grassland soils and for prediction of herbage content. Communications in Soil Science and Plant Analysis, 39, 1943–1962.
Burak, D. L., Fontes, M. P. F., Santos, N. T., Monteiro, L. V. S., Martins, E. S., & Becquer, T. (2010). Geochemistry and spatial distribution of heavy metals in Oxisols in a mineralized region of the Brazilian Central Plateau. Geoderma, 160, 131–142.
Campos, M. C. C. (2010). Soil attributes and risk of leaching of heavy metals in tropical soils. Ambiência, 6, 547–565 (In Portuguese with English abstract).
Campos, M. C. C., Ribeiro, M. R., Souza, V. S. J., Ribeiro, M. R. F., & Almeida, M. C. (2012). Relations of the soil with the geomorphic surface in a “Várzea-Terra Firme” Topossequence in the Humaitá Region, Amazonian. Revista Brasileira de Ciência do Solo, 36, 325–336 (In Portuguese with English abstract).
Cancela, R. C., Abreu, C. A., & Paz-González, A. (2002). DTPA and Mehlich micronutrient extractability in natural soils. Communications in Soil Science and Plant Analysis, 33, 2879–2893.
Cerqueira, B., Covelo, E. F., Andrade, M. L., & Vega, F. A. (2011a). Retention and mobility of copper and lead in soils as influenced by soil horizon properties. Pedosphere, 21(5), 603–614.
Cerqueira, B., Covelo, E. F., Andrade, M. L., & Vega, F. A. (2011b). The influence of soil properties on the individual and competitive sorption and desorption of Cu and Cd. Geoderma, 162, 20–26.
CETESB. (2001). Environmental Agency of the State of Sao Paulo, report establishment of guiding values for soils and groundwater of the State of Sao Paulo, São Paulo, Brazil (p. 247). São Paulo: CETESB (In Portuguese).
CONAMA. (2009). National Council for the Environment, Brazil. Resolution n° 420/2009. Available online at < http://www.mma.gov.br/port/conama/legiabre.cfm?codlegi=620>. Accessed on 16 Jan 2012. (In Portuguese).
Disla, J. M. S., Speir, T. W., Gómez, I., Clucas, L. M., McLaren, R. G., & Pedreño, J. N. (2010). Evaluation of different extraction methods for the assessment of heavy metal bioavailability in various soils. Water, Air, and Soil Pollution, 213, 471–483.
Elliott, H. A., & Shastri, N. L. (1999). Extractive decontamination of metal-polluted soils using oxalate. Water, Air, and Soil Pollution, 110, 335–346.
EMBRAPA. (1997). Manual of chemical analysis of soils plants and fertilizers. Brasilia: EMBRAPA. (In Portuguese).
EMBRAPA. (2007). Recommendations for liming and fertilization of the Pará state, Brazil (p. 262). Brasilia: EMBRAPA. (In Portuguese).
Fadigas, F. S., Sobrinho, N. M. B. A., Mazur, N., Anjos, L. H. C., & Freixo, A. A. (2002). Natural contents of heavy metals in some Brazilian soil classes. Bragantia, 61, 151–159. In Portuguese with English abstract.
Fageria, N.K., & Moreira, A. (2011). The role of mineral nutrition on root growth of crop plants. In D.L., Sparks (Ed.) Adv. agron. (vol. 110, pp. 251–331). Burlington: Academic.
Fageria, N. K., Baligar, V. C., & Clark, R. B. (2002). Micronutrients in crop production. Advances in Agronomy, 77, 185–267.
Falesi, I.C. (1986). Soils of the Trans-Amazonian Highway Bulletin technical. No. 55, pp. 196. (In Portuguese).
Farella, N., Davidson, R., Lucotte, M., & Daigle, S. (2007). Nutrient and mercury variations in soils from family farms of the Tapajos region (Brazilian Amazon): recommendations for better farming. Agriculture, Ecosystems and Environment, 120, 449–462.
Fearnside, P.M., & Millikan, B. (2012). Hydropower in the Amazon: source of clean energy? In P.F. Moreira (Ed.). The Brazilian Electricity Sector and Sustainability in the 21st century: opportunities and challenges (2nd ed., pp. 100). Rios Internacionais. (In Portuguese).
Fonseca, A. F., Caires, E. F., & Barth, G. (2010). Extraction methods and availability of micronutrients for wheat under a no-till system with a surface application of lime. Science in Agriculture, 67, 60–70.
Gee, G.W., & Or, D. (2002). Particle-size analysis. In J.H. Dane & G.C. Toop (Eds.), Methods of soil analysis. Madison: American Society of Agronomy and Soil Science Society of America.
Gupta, A. K., & Sinha, S. (2007). Assessment of single extraction methods for the prediction of bioavailability of metals to Brassica juncea L. Czern. (var. Vaibhav) grown on tannery waste contaminated soil. Journal of Hazardous Materials, 149, 144–150.
He, J. Z., Meng, Y. T., Zheng, Y. M., & Zhang, L. M. (2010). Cr (III) oxidation coupled with Mn(II) bacterial oxidation in the environment. Journal of Hazardous Materials, 10, 767–773.
ICH (1996) ICH topic Q-2b. Validation of analytical procedures: methodology. London: , European Agency for the Evaluation of Medicinal Products.
Kabata-Pendias, A., & Pendias, H. (2010). Trace elements in soil and plants (4th ed.). Boca Raton: CRC
Krishna, A. K., Mohan, K. R., & Murthy, N. N. (2011). A multivariate statistical approach for monitoring of heavy metals in sediments: a case study from Wailpalli Watershed. Nalgonda District, Andhra Pradesh, IndiaRes. Journal of Environment and Earth Science, 3, 103–113.
Lindsay, W. L., & Norvell, W. A. (1978). Development of a DTPA soil test for zinc iron, manganese, and copper. Soil Science Society of America Journal, 42, 421–428.
Loeppert, R.L., & Inskeep, W.P. (1996). Iron. In J.M., Bigham (Ed.) Methods of soil analysis (pp. 639–664). Madison: Soil Science Society of America.
Luo, A., Zhang, S., & Shan, X. (2005). Time effect on the fractionation of heavy metals in soils. Geoderma, 125, 225–234.
Luo, X. S., Yu, S., & Li, X. D. (2012). The mobility, bioavailability, and human bioaccessibility of trace metals in urban soils of Hong Kong. Applied Geochemistry, 27, 995–1004.
Marques, J. J., Schulze, D. G., Curi, N., & Mertzman, S. A. (2004). Trace element geochemistry in Brazilian Cerrado Soils. Geoderma, 121, 31–43.
McBride, M. B., Richards, B. K., & Steenhuis, T. (2004). Biovailability and crop uptake of trace elements in soil columns amended with sewage sludge products. Plant and Soil, 262, 71–84.
Mehlich, A. (1953). Determination of P, Ca, Mg, K, Na and NH 4 (p. 23). Raleigh: North Carolina Soil Testing Division.
Mehlich, A. (1984). Mehlich-3 soil test extractant: a modification of Mehlich-2 extractant. Communications in Soil Science and Plant Analysis, 15, 1409–1416.
Mehra, J. A., & Jackson, M. L. (1960). Iron oxides removal from soils and clays by dithionite–citrate system buffered with sodium bicarbonate. Clays and Clay Minerals, 5, 317–327.
Menezes, A. A., Dias, L. E., Neves, J. C. L., & Silva, J. V. O. (2010). Zinc availability to maize in different soils with and without liming determined by Mehlich-1, Mehlich-3 and DTPA. Revista Brasileira de Ciência do Solo, 34, 417–424 (In Portuguese with English abstract).
Merlino, L. C. S., Melo, W. J. F., Macedo, G., Guedes, A. C. T. P. M., Ribeiro, H., Melo, V. P., & Melo, G. M. P. (2010). Barium, cadmium, chromium and lead in maize plants and in an oxisol after eleven years of sewage sludge applications. Revista Brasileira de Ciência do Solo, 34, 2031–2039. In Portuguese with English abstract.
Nascimento, C. W. A., Fontes, R. L. F., Neves, J. C. L., & Melício, A. C. F. D. (2002). Fractioning, desorption, and chemical extraction of zinc from oxisols. The Revista Brasileira de Ciência do Solo, 26, 599–606. In Portuguese with English abstract.
Nogueirol, R. C., Alleoni, L. R. F., Nachtigall, G. R., & Melo, G. W. (2010). Sequential extraction and availability of copper in Cu fungicide-amended vineyard soils from Southern Brazil. Journal of Hazardous Materials, 181, 1931–1937.
Otero, X. L., Álvarez, E., Fernández-Sanjurjo, M. J., & Macías, F. (2012). Micronutrients and toxic trace metals in the bulk and rhizospheric soil of the spontaneous vegetation at an abandoned copper mine in Galicia (NW Spain). Journal of Geochemical Exploration, 112, 84–92.
Paye, H. S., Mello, J. W. V., & Melo, S. B. (2012). Métodos de análise multivariada no estabelecimento de valores de referência de qualidade para elementos-traço em solos. Revista Brasileira de Ciência do Solo, 36, 1031–1042 (In Portuguese with English abstract).
Raij, B.V. (2011). Soil fertility and nutrient management (p. 420). Norcross: International Plant Nutrition Institute. (In Portuguese).
Raij, B. V., Andrade, J. C., Cantarella, H., & Quaggio, J. A. (2001). Chemical analysis to evaluate fertility of tropical soils (p. 285). São Paulo: Agronomic Institute of Campinas (In Portuguese).
Roca, N., Pazos, M. S., & Bech, J. (2012). Background levels of potentially toxic elements in soils: a case study in Catamarca (a semiarid region in Argentina). Catena, 92, 5–66.
Rodrigues, M. R. L., Malavolta, E., & Moreira, A. (2001). Comparação de soluções extratoras de ferro e manganês em solos da Amazônia. Pesquisa Agropecuária Brasileira, 36, 143–149 (In Portuguese with English abstract).
Rodrigues, T.E., Silva, R.C., Silva, B.N.R., Silva, J.M.L., Valente, M.A., Dariva, T.A., Souto de Jesus, A.A., & Venturieri, A. (2007). Characterization, mapping and classification of soils of the area of influence of the BR-163 (Santarém-Cuaba) and BR-230 (Trans-Amazonian Highway), in the Pará state, Brazil. Zoning ecological-economic of the area of influence of the BR-163 (Cuiabá-Santarém) (pp.403–571). Brasilia: EMBRAPA. (In Portuguese).
Rout, K., Mohapatra, M., Mohapatra, B. K., & Anand, S. (2009). Pb(II), Cd(II) and Zn(II) adsorption on low grade manganese ore. International Journal of Engineering, Science and Technology, 1(1), 106–122.
Sarto, M. V. M., Steiner, F., & Lana, M. C. (2011). Assessment of micronutrient extractants from soils of Paraná, Brazil. Revista Brasileira de Ciência do Solo, 35, 2093–2103 (In Portuguese with English abstract).
Silva, F.C. (1999). Manual of chemical analyses of soil and fertilizer plants (p. 370). Brasilia: EMBRAPA. (In Portuguese).
StatSoft. (2005). Statistica 7.0 software. Tucksa: StatSoft.
Tourneau, F. M., & Bursztyn, M. (2010). Rural settlements in the Amazon: contradictions between the agrarian policy and environmental policy. Ambiente & Sociedade, 1, 111–130 (In Portuguese with English abstract).
USDA-NRCS. (1999). Soil taxonomy—a basic system of soil classification for making and interpreting soil surveys (2nd ed., p. 869). Washington, D.C.: U.S. Government Printing Office. Agriculture Handbook: 436.
Violante, A., Cozzolino, V., Perelomov, L., Caporale, A. G., & Pigna, M. (2010). Mobility and bioavailability of heavy metals and metalloids in soil environments. Journal of Soil Science and Plant Nutrition, 10(3), 268–292.
Yu, J., Huang, Z., Chen, T., Qin, D., Zeng, X., & Huang, Y. (2012). Evaluation of ecological risk and source of heavy metals in vegetable-growing soils in Fujian province, China. Environmental Earth Sciences, 65, 29–37.
Zhang, M. K., Liu, Z., & Wang, H. (2010). Use of single extraction methods to predict bioavailability of heavy metals in polluted soils to rice. Communications in Soil Science and Plant Analysis, 41, 820–831.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Souza, E.S., Fernandes, A.R., de Souza Braz, A.M. et al. Potentially toxic elements (PTEs) in soils from the surroundings of the Trans-Amazonian Highway, Brazil. Environ Monit Assess 187, 4074 (2015). https://doi.org/10.1007/s10661-014-4074-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-014-4074-1