Abstract
Most strawberry growers in Brazil use imported transplants due to their higher quality in terms of plant health and productivity. However, there is a risk of entry of quiescently infecting pathogens such as Botrytis species and/or isolates resistant to different fungicides. In this work, we investigated the incidence of Botrytis in imported transplants, and identified the species. In addition, isolates were characterized for their pathogenicity in fruit and sensitivity to seven fungicides (procymidone, fludioxonil, iprodione, cyprodinil, pyrimethanil, boscalid and fluazinam). The average incidence of Botrytis spp. in transplants imported from Chile, Argentina, and Spain was 43.5%. A total of 79 isolates were identified molecularly as B. cinerea and the pathogenicity in fruit was confirmed for a subsample of 14 isolates. To evaluate sensitivity to fungicides, the following discriminatory doses were used: procymidone (P) (10 µg/mL), fludioxonil (F) (0.5 µg/mL), iprodione (I) (10 µg/mL), cyprodinil (C) (10 µg/mL), pyrimethanil (PY) (10 µg/mL), boscalid (B) (50 µg/mL) and fluazinam (FL) (1 µg/mL). As a result, 24 resistant phenotypes were identified with the most frequent being the phenotype with resistance to three fungicides (C-PY-B). The isolates with low sensitivity to cyprodinil and pyrimethanil fungicides, which are not yet widely used in Brazil, represent a risk for strawberry production and should be considered in disease management and future fungicide monitoring programs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In strawberry production, transplants are usually replaced every year for healthier plants and for the reduction of diseases and pests (Antunes et al., 2015). Therefore, it is important to have good quality transplants for yield and healthy fruit. Some essential characteristics to determine transplant quality are healthy leaf area with two to three leaves, no symptoms of pests and diseases, and transplant size of approximately 15 cm (Rufato et al., 2023). Although Brazil has been advancing in the production of transplants, there are still phytosanitary problems, and growers end up opting for imported transplants (Antunes & Cocco, 2012). In Brazil, the transplants imported by strawberry growers are mainly from Argentina, Chilean Patagonia and Spain (Brandt et al., 2022; Costa et al., 2018). Imported transplants have a higher cost due to the phytosanitary inspections carried out to prevent new diseases from entering the national territory and costly transportation (Nunes et al., 2018; Schmitt et al., 2016;). As the production of bare-root transplants in Argentina and Chile occurs during the summer, when the average temperatures are about 20 °C, with low rainfall, incidence of diseases is usually low (Rufato et al., 2023). In Spain, strawberry mother plants are grown in high-altitude nurseries in northern Spain, mainly in the provinces of Segovia, Ávila and Valladolid, where ideal agro-climatic conditions prevail from March to September (Pastrana et al., 2017).
One of the main diseases that cause losses to strawberry growers is gray mold, caused by Botrytis spp., a pathogen capable of infecting various parts of the plant (Williamson et al., 2007). The fungus can remain latent for a long period, which offers Botrytis spp. an essential mechanism for survival. Within the living epidermal cell, the pathogen is protected from adverse weather conditions, interference from other pathogens and the effects of protective fungicides, lasting up to eight months in leaves (Braun & Sutton, 1988). Thus, transplants can serve as an important source of primary inoculum and transport the pathogen through latent infections to commercial strawberry fields, as has already been reported in the USA (Oliveira et al., 2017). Moreover, different subpopulations of Botrytis spp. may be selected in transplant nurseries under different fungicide spraying conditions (Amiri et al., 2018).
The genus Botrytis has more than 30 phytopathogenic species (Fillinger & Elad, 2015). Among these, B. cinerea, B. fragariae (Rupp et al., 2017), B. caroliniana (Li et al., 2012), B. mali (Dowling & Schnabel, 2017), B. pseudocinerea (Plesken et al., 2015) and Botrytis Group S (Leroch et al., 2013) are capable of infecting strawberry plants. In Brazil, B. cinerea is the species reported so far infecting strawberries (Lopes et al., 2017; Maia et al., 2021). Even though there are no reports of these other species infecting strawberries in countries from which Brazil has imported transplants (Farr & Rossman, 2021), B. pseudocinerea has been reported causing gray mold in vineyards in Spain (Acosta Morel et al., 2018). In Chile, the presence of B. pseudocinerea in peonies was reported (Muñoz et al., 2016). This species has also been reported causing gray mold on strawberries in Germany (Leroch et al., 2013; Plesken et al., 2015).
In addition to the entry of new species when importing transplants, there is the possibility of entry of isolates with resistance to the main fungicides used in the country. In the United States, isolates of B. cinerea from strawberry transplants showed, for the most part, resistance to four fungicides used in the management of strawberry diseases (Oliveira et al., 2017). Studies on the incidence of Botrytis in imported strawberry transplants have not yet been performed in Brazil. In a study in peach fruit imported from Spain, Chile, United States and Argentina, isolates of Monilinia spp. with resistance to the fungicides azoxystrobin, tebuconazole, iprodione and thiophanate methyl, which are widely used to control brown rot in Brazil, were found (Pereira et al., 2018).
In strawberry, the lack of sensitivity of isolates from transplants can directly interfere with disease management during the season. If these isolates are as competitive as field isolates and adapt to adverse conditions, the efficacy of fungicides may be impaired. This shows the importance of monitoring the strawberry transplants that are being imported for the entry of possible new species of Botrytis and their resistance to the fungicides used to manage the disease in the country, especially those that still perform well against gray mold. Therefore, the objectives of this study were to: i) evaluate the incidence of Botrytis from imported strawberry transplants, ii) identify the Botrytis species of the isolates recovered from transplants, and iii) evaluate the sensitivity of the isolates to the fungicides procymidone, fludioxonil, iprodione, cyprodinil, pyrimethanil, boscalid and fluazinam.
Material and methods
Assessment of Botrytis spp. incidence in transplants, isolate recovery, and confirmation of pathogenicity.
In 2021 and 2022, transplants imported from Chile, Argentina and Spain were purchased to detect Botrytis spp. (Fig. 1). The cultivars used were based on the availability of importers, namely: Albion, Aromas, Camino Real, Fronteras, Monterey, Portola, and San Andreas. Fifty transplants per cultivar/country were obtained, totaling 1000 transplants.
The transplants were surface disinfected with 0.5% sodium hypochlorite for 2 min and then washed twice with sterile distilled water. They were then frozen overnight at -18 °C in a freezer to accelerate tissue death and then incubated inside a gerbox over a wire mesh, with distilled water at the bottom to maintain high humidity. The boxes were incubated for seven days at 22 °C (Oliveira et al., 2017). The incidence of Botrytis spp. in the transplants was observed under a stereoscopic microscope (40x). Seventy-nine Botrytis spp. isolates were obtained by placing the pathogen structures onto plates containing PDA medium. To prevent them from losing characteristics after successive replications, all isolates were preserved in silica gel using filter paper disks and in 30% glycerol, both at -180C in a freezer. The isolates were deposited in a collection at the Laboratory of Epidemiology for Integrated Management of Diseases (LEMID) at the Federal University of Paraná.
For the evaluation of pathogenicity, 14 isolates, from Argentina (1), Chile (8) and Spain (5), were selected. Eight replicates were used for each isolate. Each replicate consisted of three strawberry fruit inside a plastic pot of 15 cm in diameter and 4.5 cm in height with the presence of cotton and filter paper sterilized and moistened with distilled water. Albion cultivar fruit at the green to red maturation stage, without fungicide residues, was used. The fruit were disinfested with 0.5% sodium hypochlorite for 2 min, washed three times in distilled water and dried at room temperature. The fruit were placed inside disinfested plastic containers and 50 μL of suspension at 1 × 105 conidia/mL were deposited on their surfaces in a previously determined location on each non-wounded fruit. The control treatment consisted of applying 50 μL of distilled water to non-wounded fruit. The fruit were incubated at 22ºC with a 12 h photoperiod and evaluated at intervals of 24 h up to five days for the presence of symptoms and signs of the pathogen.
Botrytis species identification
All 79 isolates obtained were molecularly identified. For this, the DNA of each isolate was extracted using the CTAB protocol, with some modifications (Doyle & Doyle, 1987; Pereira et al., 2019). Species identification was performed using PCR with the forward primers G3PDH-F1 and G3PDH-F2 together with the reverse G3PD-R to identify B. caroliniana and B. cinerea, respectively (Li et al., 2012). The reaction was run in a total volume of 12.5 µL, consisting of 6.25 µL of PCR Master Mix 2x (Promega), 10 µM of each primer and 1.5 µL of DNA. Amplification consisted of an initial denaturation at 94 °C for 3 min followed by 32 cycles at 94 °C for 30 s, 56 °C for 30 s and 72 °C for 1 min and a final extension at 72 °C for 5 min. The PCR products were analyzed on a 1% agarose gel in 0.5X TBE buffer, stained with gelRed (Biotium) and photo documented on an ultraviolet transilluminator.
Sensitivity to fungicides
The sensitivity of the 79 Botrytis spp. isolates to the fungicides procymidone (Sumilex 500 WP®, Sumitomo), fludioxonil (Maxim®, Syngenta), iprodione (Rovral SC®, Basf), cyprodinil (Unix 750 WG®, Syngenta), pyrimethanil (Mythos®, Bayer), boscalid (Cantus ®, Basf) and fluazinam (Frowncide 500 SC®, Ihara) was evaluated by mycelial growth or spore germination, depending on the fungicide, using discriminatory dose inhibition assays..
For procymidone, iprodione, fludioxonil, cyprodinil and pyrimethanil, the discriminatory dose was evaluated by mycelial growth inhibition assays. The doses used to discriminate the sensitivity of the isolates were: I) 10 µg/mL for procymidone and iprodione, II) 0.5 µg/mL for fludioxonil, and III) 10 µg/mL for cyprodinil and pyrimethanil. Commercial fungicides were diluted with sterilized distilled water to obtain stock solutions, which were later added to autoclaved potato-dextrose-agar (PDA) medium and cooled down to 60 °C. The medium was added to 90 mm Petri dishes and 5 mm diameter mycelium discs, obtained from seven-day-old colonies, were deposited on the surface of the culture medium. For cyprodinil and pyrimethanil, sensitivity was evaluated in mycelial growth inhibition assay on L-asparagine-based agar medium (ASP-agar) (Hilber and Schuepp, 1996). The control treatment consisted of placing the mycelium discs from the isolates on PDA or ASP-agar medium without fungicide. Two plates were used per isolate/discriminating dose. Plates were incubated at 22 °C with a 12-h photoperiod for two days for PDA and five days for ASP-agar. Colony diameter was obtained by averaging two perpendicular measurements of the colony using a digital caliper.
For boscalid and fluazinam, which act on germination, the discriminatory dose was determined by spore germination. The doses used were I) 50 µg/mL for boscalid and II) 1 µg/mL for fluazinam. The stock solutions were added to the autoclaved water-agar (WA) medium and kept at 60 °C. The medium with fungicide was added to 90 mm diameter Petri dishes. Mycelium discs 5 mm in diameter from the isolates were transferred to canned peaches for inoculum production. Sporulation on the surface of canned fruit was used to produce spore suspension. Aliquots of 100 μl of the suspension containing 105 conidia/ml of each isolate were added to the surface of the culture media and spread with the aid of a Drigalski loop. Plates were incubated at 22 °C for 14 h in the dark. After this period, germination was stopped by adding lactophenol with Amann blue dye to the plates. Two plates were used per isolate/discriminatory dose. One hundred conidia per plate were evaluated. Conidia were considered germinated when germ tubes were at least twice their size.
The mycelial growth inhibition percentage ratio was calculated using the formula %ICM = [(C-T) /C] × 100, where C refers to the diameter of the control and T to the average diameter of the treatment with fungicide. The inhibition conidial germination was calculated using the formula %IGC = [(C-T)-C] × 100, where C is the number of conidia germinated in the control and T the number of conidia germinated in the treatment. The experiments were performed twice. The phenotypic classification of isolates was performed according to Table 1. The discriminatory doses recommended for cyprodinil and pyrimethanil were initially 4 µg/mL (Fernandez-Ortuño et al., 2014) and 5 µg/mL (Amiri et al., 2013) respectively. However, with these doses it was not possible to discriminate our isolates since all isolates were growing at these doses. Then other doses were tested until reaching dose 10 µg/mL, which was used as discriminate dose for both fungicides.
Potential cross-resistance between pyrimethanil and cyprodinil was evaluated using the mycelial growth inhibition value to determine the phenotypes for cyprodinil and pyrimethanil, for the same 79 isolates, at a dose of 10 µg/ml. Cross-resistance analysis was performed by calculating Pearson's correlation coefficient (r).
Results
Incidence, identification of Botrytis spp. in transplants and pathogenicity of isolates
The average incidence of B. cinerea in transplants was 43.5% and a total of 79 isolates were obtained. San Andreas and Monterey cultivars imported from Spain in 2022 had the highest incidences, with 88 and 90%, respectively (Table 2). Cultivar Albion from Chile had 68% incidence of B. cinerea in 2022, and San Andreas cultivar from Argentina had 64% incidence (Table 2). Fragment analysis of the Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene showed the presence of a fragment of 238 base pairs in all isolates, identifying them as Botrytis cinerea. The 14 selected isolates were pathogenic to fruit, with the presence of symptoms and signs 36 h after inoculation. On the fifth day, the final incidence was above 75% for all isolates.
Fungicide sensitivity
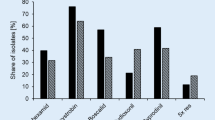
Discriminatory doses allowed to distinguish different B. cinerea sensitivity patterns to fungicides. For the seven fungicides used in the sensitivity tests, 24 different phenotypes were observed. Of the 79 isolates, two isolates were sensitive to all fungicides (Fig. 2). On opposite, two isolates were resistant to six of the seven evaluated fungicides. The most frequent phenotype was resistant to three fungicides, C-PY-B, with a frequency of 24.1% (Fig. 2). The percentage of isolates sensitive to procymidone, fludioxonil, iprodione, cyprodinil, pyrimethanil, boscalid, and fluazinam was 86.1, 89.9, 81.0, 21.5, 21.5, 21.5, 38.0 and 94.9%, respectively (Fig. 3A). No major differences were observed between the sensitivity frequencies of isolates from Chile and Argentina compared to isolates from Spain (Fig. 3B and C), only boscalid, isolates from Chile and Argentina showed a higher number of resistant compared to isolates from Spain. The correlation between the percent mycelial growth inhibition of pyrimethanil and cyprodinil was significantly positive (r = 0.76, P = 0.000).
Phenotypic classification of the sensitivity of Botrytis cinerea isolates to the fungicides procymidone, fludioxonil, iprodione, cyprodinil, pyrimethanil, boscalid and fluazinam. S- Sensitive, P- procymidone, F- fludioxonil, I- iprodione, C- cyprodinil, PY- pyrimethanil, B- boscalid, FL- fluazinam. 0R- without resistance to the fungicide,1R-resistant to a fungicide, 2R-resistant to two fungicides, 3R-resistant to three fungicides, 4R-resistant to four fungicides, 5R-resistant to five fungicides and 6R-resistant to six fungicides
Discussion
Our study shows that transplants are an important source of primary inoculum for gray mold due to their latent infection by B. cinerea. This inoculum is being introduced every year from different sources around the world, representing a risk of entry of new species. In this study, only B. cinerea was detected, but the isolates showed reduced sensitivity to multiple fungicides, including fungicides recently registered to control the disease in Brazil, to which our local population is still highly sensitive (Maia et al., 2023b).
In Brazil, no species other than B. cinerea has yet been found causing gray mold on strawberries (Lopes et al., 2017; Maia et al., 2021). However, one of the ways that new species might enter the country is through transplants. Our study confirmed that, so far, only B. cinerea has been introduced with imported transplants. This is important to report since in a study carried out in the USA, a small frequency of Botrytis group S isolates was found coming from nurseries in the USA and Canada (Amiri et al., 2018).
The high incidence of B. cinerea observed entering the country with the transplants opens the possibility of entry of isolates with reduced sensitivity to the main fungicides used in Brazil since several fungicides sprayed by the growers can also be used in nurseries to produce healthy transplants (Oliveira et al., 2017). In Spain, among the fungicides tested in this study, fludioxonil, pyrimethanil and the mixture fludioxonil + cyprodinil are registered for use on strawberry (MAPA, 2023). In Argentina and Chile, the fungicides procymidone, iprodione, and the mixtures fludioxonil + cyprodinil and boscalid + pyraclostrobin are registered for strawberry, with pyrimethanil registered only in Chile (CASAFE, 2023; SAG, 2023) (Table 3).
Historically, there are many reports of resistance of B. cinerea isolates to several fungicides in the world, including azoxystrobin in Japan (Ishii et al., 2009), boscalid in Greece, USA and China (Bardas et al., 2010; Fernandez-Ortuño et al. 2012; Cui et al., 2021), procymidone in China (Liu et al., 2016; Sun et al., 2010), iprodione in USA (Cosseboom & Hu, 2021; Grabke et al., 2014), cyprodinil in USA (Fernández-Ortuño et al., 2013; Avenot et al., 2018; Cosseboom & Hu, 2021) and fludioxonil in China and USA (Dowling et al., 2021; Sang et al., 2018; Zhou et al., 2020). In Brazil, resistant isolates have already been found for azoxystrobin, boscalid, iprodione, procymidone, thiophanate methyl, pyrimethanil and cyprodinil (Lopes et al., 2017; Baggio et al., 2018; Maia et al., 2021; 2023ab).
The frequency of 86.1% of isolates sensitive to procymidone was higher in relation to a previous study carried out with isolates of B. cinerea from the state of Paraná, where 57% of the isolates were classified as sensitive (Maia et al., 2021). The same occurred with iprodione, where 81% of isolates were sensitive which is higher than the 56% found by Maia et al., 2021.
Fluazinam showed 61.9% efficacy in controlling gray mold in the state of Paraná in ex vitro fruit assay (Maia et al., 2021). Therefore, the frequency of 96.3% of fluazinam-sensitive isolates is a good indicator since the entry of B. cinerea isolates through transplants does not represent a great risk for the efficacy of this fungicide, but alternating between FRAC groups is still recommended so the proportion of resistant isolates does not grow. Fluazinam is not registered for use on strawberries in Argentina, Chile, and Spain, which explains the low frequency of resistant isolates (MAPA, 2023).
Fludioxonil was recently registered in Brazil in a mixture with cyprodinil for the management of strawberry diseases and proved to be the most effective in controlling gray mold (Maia et al., 2023b). Thus, it is important to avoid the entry of isolates with reduced sensitivity to this fungicide. In this study, 10.1% of the isolates showed resistance to fludioxonil. Although this is not as high as the previous fungicides mentioned, the alert remains as in Spain, Argentina, and Chile this fungicide is already registered and can be used in the production of transplants. In Spain, in addition to the fludioxonil + cyprodinil mixture, fludioxonil is also registered as a solo product for use in strawberries (MAPA, 2023). Therefore, fungicides containing fludioxonil in their composition should be avoided in the control of diseases in transplant production nurseries.
For boscalid, cyprodinil and pyrimethanil fungicides, most isolates showed low sensitivity with 59.3, 78.5 and 78.5% of isolates classified as resistant, respectively. The frequency of boscalid-resistant isolates in this study was higher than that found in a study with isolates collected in the state of Paraná, where the frequency of boscalid-resistant isolates was 45.3% (Maia et al., 2021). The high frequency of pyrimethanil-resistant isolates is concerning, as the fungicide has not yet been widely used in the country to control gray mold and showed an efficacy of 84.61% in controlling the disease in a study carried out in Brazil (Maia et al., 2023a). In another study also carried out in Brazil with isolates of B. cinerea from strawberry, only 12.5% of the isolates were classified as highly resistant to pyrimethanil (Baggio et al., 2018). This shows that within the country the frequency of pyrimethanil-resistant isolates is still low, contrary to other areas where this fungicide has been used for much longer and pyrimethanil-resistant isolates are frequently found (Korolev et al., 2011; Myresiotis et al., 2007; Zhao et al., 2010). Furthermore, pyrimethanil is registered for use on strawberry in Chile and Spain with a higher recommended dose than that used in Brazil (SAG, 2023; MAPA, 2023; AGROFIT, 2023). Cyprodinil was registered in Brazil only in 2019 in a mixture with fludioxonil and demonstrated an efficacy of 85.7% in the control of gray mold in strawberry, being an important fungicide in the management of strawberry diseases (Maia et al., 2023b). Pyrimethanil and cyprodinil have similar chemical structures since they belong to the same chemical group, anilinopyrimidines, and cross-resistance may occur between the two fungicides (Leroux et al., 1999). When evaluating the cross-resistance between cyprodinil and pyrimethanil, a positive correlation of 0.76 was observed. Positive correlations have already been found in Brazil and Greece, with values of 0.82 and 0.71, respectively (Maia et al., 2023a; Myresiotis et al., 2007). This reinforces the risk of selecting isolates resistant to both fungicides.
The establishment of fungicide-resistant B. cinerea isolates in Brazil coming from imported transplants is concerning, as it may interfere with the management of the disease in the future, especially if these isolates can adapt and compete with other isolates. In peach fruit imported from Chile, USA, and Argentina, many Monilinia isolates were resistant to iprodione, and there are no reports of isolates with resistance to this fungicide in Brazil, which could impair disease control in the country (Pereira et al., 2018). In the USA, studies have shown that fungicide-resistant populations of B. cinerea were introduced into strawberry fields through transplants (Amiri et al., 2018; Oliveira et al., 2017).
In our study, resistance to up to six fungicides was observed from transplants and the most frequent phenotype (24.1%) was resistant to cyprodinil, pyrimethanil, and boscalid. In the USA, phenotype resistant to three fungicides from transplants was also the most frequent (42.1%) and isolates with resistance to up to six fungicides found simultaneously (Oliveira et al., 2017).
Strawberry growers in Brazil are receiving transplants already infected with B. cinerea isolates resistant to several fungicides, some of which do not yet represent a risk of control failure in the field in the country. This discovery is relevant and should alert nurseries and strawberry growers to adopt new strategies in the management of strawberry diseases.
For growers, according to the data obtained in this work, it is recommended to reduce the primary inoculum of B. cinerea by immersing the transplants in fungicides before or at the time of planting (Oliveira et al., 2017), using heat treatment at 44° C for 4 h (Zuniga et al., 2023) and alternating fungicides between FRAC groups. In future studies, the adaptability and competitiveness of these isolates can be verified. Furthermore, monitoring the sensitivity of B. cinerea to fungicides in nurseries can help in disease management, preventing resistant isolates from being selected and subsequently disseminated through transplants.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
AGROFIT. Sistema de agrotóxicos fitossanitários. Retrieved April 7, 2023, from http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons
Amiri, A., Heath, S. M., & Peres, N. A. (2013). Phenotypic characterization of multifungicide resistance in Botrytis cinerea isolates from strawberry fields in Florida. Plant Disease, 97(3), 393–401. https://doi.org/10.1094/PDIS-08-12-0748-RE
Amiri, A., Zuniga, A. I., & Peres, N. A. (2018). Prevalence of Botrytis cryptic species in strawberry nursery transplants and strawberry and blueberry commercial fields in the eastern United States. Plant Disease, 102(2), 398–404. https://doi.org/10.1094/pdis-07-17-1065-re
Antunes, L. E. C., & Cocco, C. (2012). Tecnologia apara a produção de frutas e mudas do morangueiro. Agropecuária Catarinense., 25(2), 61–65.
Antunes, L. E. C., ReisserJúnior, C., Vignolo, G. K., & Gonçalves, M. A. (2015). Morangos do jeito que o consumidor gosta. Campo Lavoura, Anuário HF, 1, 64–72.
Avenot, H. F., Quattrini, J., Puckett, R., & Michailides, T. J. (2018). Different levels of resistance to cyprodinil and iprodione and lack of fludioxonil resistance in Botrytis cinerea isolates collected from pistachio, grape, and pomegranate fields in California. Crop Protection, 112, 274–281. https://doi.org/10.1016/j.cropro.2018.06.005
Baggio, J. S., Peres, N. A., & Amorim, L. (2018). Sensitivity of Botrytis cinerea isolates from conventional and organic strawberry fields in Brazil to azoxystrobin, iprodione, pyrimethanil, and thiophanate-methyl. Plant Disease, 102, 1803–1810. https://doi.org/10.1094/pdis-08-17-1221-re
Bardas, G. A., Veloukas, T., Koutita, O., & Karaoglanidis, G. S. (2010). Multiple resistance of Botrytis cinerea from kiwifruit to SDHIs, QoIs and fungicides of other chemical groups. Pest Management Science, 66, 967–973. https://doi.org/10.1002/ps.1968
Brandt, G. Q., Silva, L. F. L., Souza, D. C., Resende, L. V., & Nunes, N. S. (2022). Productivity and analysis of morphological characters of experimental strawberry genotypes. Horticultura Brasileira, 40, 426–431. https://doi.org/10.1590/s0102-0536-20220411
Braun, P. G., & Sutton, J. C. (1988). Infection cycles and population dynamics of Botrytis cinerea in strawberry leaves. Canadian Journal of Plant Pathology, 10(2), 133–141.
CASAFE. Cámara de Sanidad Agropecuaria y fertilizantes. Retrieved April 7, 2023, from https://www.casafe.org/publicaciones/guia-de-productos-fitosanitarios/
Cosseboom, S., & Hu, M. (2021). Identification and characterization of fungicide resistance in Botrytis populations from small fruit fields in the mid-atlantic United States. Plant Disease, 105, 2366–2373. https://doi.org/10.1094/PDIS-03-20-0487-RE
Costa, R. C., Calvete, E. O., DeNardi, F. S., Pedersen, A. C., Chiomento, J. L. T., Trentin, N. S. (2018). Quality of strawberry transplants can determine precocity. Australian J Crop Sci, 81–86. https://doi.org/10.21475/ajcs.18.12.01.pne710
Cui, K., He, L., Li, T., Mu, W., & Liu, F. (2021). Development of boscalid resistance in Botrytis cinerea and an efficient strategy for resistance management. Plant Disease, 105, 1042–1047. https://doi.org/10.1094/pdis-05-20-1009-re
Dowling, M. E., & Schnabel, G. (2017). First report of Botrytis mali causing gray mold on strawberry in the United States. Plant Disease, 101, 1034. https://doi.org/10.1094/PDIS-01-17-0054-PDN
Dowling, M., Gelain, J., May De Mio, L. L., & Schnabel, G. (2021). Characterization of high fludioxonil resistance in Botrytis cinerea isolates from calibrachoa flowers. Phytopathology, 111, 478–484. https://doi.org/10.1094/phyto-07-20-0268-r
Doyle, J. J., & Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, 19, 11–15.
Farr, D. F., & Rossman, A. Y. Fungal Databases. U.S. National Fungus Collections, Ars, Usda. Disponível Em: <https://Nt.Ars-Grin.Gov/Fungaldatabases/>. Acesso Em 01/06/2021.
Fernández-Ortuño, D., Chen, F., & Schnabel, G. (2012). Resistance to pyraclostrobin and boscalid in Botrytis cinerea isolates from strawberry fields in the Carolinas. Plant Disease, 96, 1198–1203. https://doi.org/10.1094/pdis-12-11-1049-re
Fernández-Ortuño, D., Chen, F., & Schnabel, G. (2013). Resistance to cyprodinil and lack of fludioxonil resistance in Botrytis cinerea isolates from strawberry in North and South Carolina. Plant Disease, 97, 81–85. https://doi.org/10.1094/pdis-06-12-0539-re
Fernández-Ortuño, D., Grabke, A., Bryson, P. K., Amiri, A., Peres, N. A., & Schnabel, G. (2014). Fungicide resistance profiles in Botrytis cinerea from strawberry fields of seven southern U.S. states. Plant Disease, 98(6), 825–833. https://doi.org/10.1094/PDIS-09-13-0970-RE
Fillinger, S., Elad, Y. (2015). Botrytis – the fungus, the pathogen and its management in agricultural systems. Springer
Grabke, A., Fernández-Ortuño, D., Amiri, A., Li, X., Peres, N. A., Smith, P., & Schnabel, G. (2014). Characterization of iprodione resistance in Botrytis cinerea from strawberry and blackberry. Phytopathology, 104, 396–402. https://doi.org/10.1094/phyto-06-13-0156-r
Hilber, U. W., & Schüepp, H. (1996). A reliable method for testing the sensitivity of Botryotinia fuckelianato anilinopyrimidines in vitro. Pesticide Science, 47(3), 241–247. https://doi.org/10.1002/(Sici)1096-9063(199607)47:3%3c241:Aid-Ps410%3e3.0.Co;2-6
Ishii, H., Fountaine, J., Chung, W. H., Kansako, M., Nishimura, K., Takahashi, K., & Oshima, M. (2009). Characterisation of QoI-resistant field isolates Botrytis cinerea from citrus and strawberry. Pest Management Science, 65, 916–922. https://doi.org/10.1002/ps.1773
Korolev, N., Mamiev, M., Zahavi, T., & Elad, Y. (2011). Screening of Botrytis cinerea isolates from vineyards in Israel for resistance to fungicides. European Journal of Plant Patholology, 129, 591–608. https://doi.org/10.1007/s10658-010-9723-9
Leroch, M., Plesken, C., Weber, R. W. S., Kauff, F., Scalliet, G., & Hahn, M. (2013). Gray mould populations in german strawberry fields are resistant to multiple fungicides and dominated by a novel clade closely related to Botrytis cinerea. Applied and Environmental Microbiology, 79, 159–167. https://doi.org/10.1128/AEM.02655-12
Leroux, P., Chapeland, F., Desbrosses, D., & Gredt, M. (1999). Patterns of cross-resistance to fungicides in Botryotinia fuckeliana (Botrytis cinerea) isolates from French vineyards. Crop Protect, 18, 687–697. https://doi.org/10.1016/S0261-2194(99)00074-5
Li, X., Kerrigan, J., Chai, W., & Schnabel, G. (2012). Botrytis caroliniana, a new species isolated from blackberry in South Carolina. Mycologia, 104, 650–658. https://doi.org/10.3852/11-218
Liu, S., Che, Z., & Chen, G. (2016). Multiple-fungicide resistance to carbendazim diethofencarb, procymidone, and pyrimethanil in field isolates of Botrytis cinerea from tomato in Henan Province, China. Crop Protectection, 84, 56–61. https://doi.org/10.1016/j.cropro.2016.02.012
Lopes, U. P., Zambolim, L., Capobiango, N. P., Gracia, N. A. O., & Freitas-Lopes, R. L. (2017). Resistance of Botrytis cinerea to fungicides conttrolling gray mold on strawberry in Brazil. Bragantia, 76, 266–272. https://doi.org/10.1590/1678-4499.055
Maia, J. N., Beger, G., Pereira, W. V., May De Mio, L. L., & Duarte, H. S. S. (2021). Gray mold in strawberries in the Paraná state of Brazil is caused by Botrytis cinerea and its isolates exhibit multiple-fungicide resistance. Crop Protection, 140, 105415. https://doi.org/10.1016/j.cropro.2020.105415
Maia, J.N., Beger, G., Boldrini, I.B., Peres, N.A., May De Mio L. L., Duarte, H. S. S, (2023a). Sensitivity of Brazilian Botrytis cinerea isolates from strawberry to pyrimethanil and its control efficacy. Tropical Plant Pathology. https://doi.org/10.1007/s40858-023-00586-5
Maia, J.N., Beger, G., Boldrini, I.B., Peres, N.A., May De Mio L. L., Duarte, H. S. S, (2023b). Baseline sensitivity of Botrytis cinerea strawberry isolates from Brazil to fludioxonil and cyprodinil and disease control efficacy. Tropic Plant Pathology. https://doi.org/10.1007/s40858-023-00588-3
MAPA. Ministerio de agricultura pesca y alimentación. Retrieved April 7, 2023, from https://servicio.mapa.gob.es/regfiweb#
Muñoz, G., Campos, F., Salgado, D., Galdames, R., Gilchrist, L., Chahin, G., & Andrade, O. (2016). Molecular identification of Botrytis cinerea, Botrytis paeoniae and Botrytis pseudocinerea associated with gray mould disease in peonies (Paeonia lactiflora Pall.) in Southern Chile. Revista Iberoamericana de Micología, 33(1), 43–47. https://doi.org/10.1016/j.riam.2015.02.002
Myresiotis, C. K., Karaoglanidis, G. S., & Tzavella-Klonari, K. (2007). Resistance of Botrytis cinerea isolates from vegetable crops to anilinopyrimidine, phenylpyrrole, hydroxyanilide, benzimidazole, and dicarboximide fungicides. Plant Disease, 91, 407–413. https://doi.org/10.1094/pdis-91-4-0407
Oliveira, M. S., Amiri, A., Zuniga, A. I., & Peres, N. A. (2017). Sources of primary inoculum of Botrytis cinerea and their impact on fungicide resistance development in commercial strawberry fields. Plant Disease. https://doi.org/10.1094/Pdis-02-17-0203-Re
Pastrana, A. M., Basallote-Ureba, M. J., Aguado, A., & Capote, N. (2017). Potential Inoculum Sources and Incidence of Strawberry Soilborne Pathogens in Spain. Plant Disease, 101(5), 751–760. https://doi.org/10.1094/pdis-08-16-1177-re
Pereira, W. V., Bertolini, E., Cambra, M., & Junior, N. S. M. (2019). Multiplex real-time PCR for detection and quantification of Colletotrichum abscissum and C. gloeosporioides on Citrus leaves. European Journal of Plant Pathology, 155(4), 1047–1059. https://doi.org/10.1007/s10658-019-01831-x
Pereira, W. V., Padilha, A. C. N., Kaiser, J. A. O., Nesi, C. N., Fischer, J. M. M., & May-De-Mio, L. L. (2018). Monilinia spp. from imported stone fruits may represent a risk to Brazilian fruit production. Tropical Plant Pathology, 44, 120–127. https://doi.org/10.1007/s40858-018-0243-z
Plesken, C., Weber, R. W. S., Rupp, S., Leroch, M., & Hahn, M. (2015). Botrytis pseudocinerea is a significant pathogen of several crop plants but susceptible to displacement by fungicide resistant B Cinerea Stains. Applied and Environmental Microbiology, 81, 7048–7056. https://doi.org/10.1128/AEM.01719-15
Rufato, L., Kretzschmar, A. A., Fagherazzi A. F., Nerbass, F. R., Lima, J. M., Costa, B. M., Pereira, F. (2023) Produção de matrizes e mudas, viveiros e legislação. In F. O. G. Menezes Jr, & P. F. Silva (Eds.), Cultivo do morangueiro em Sistema semi-hidropônico (1st pp. 101–119). Epagri.
Rupp, S., Plesken, C.; Rumsey, S., Dowling, M., Schnabel, G., Weber, R. W. S., Hahn, M. (2017). Botrytis Fragariae, a new species causing gray mold on strawberries, shows high frequencies of specific and efflux-based fungicide resistance. Applied And Environmental Microbiology, 83(9). https://doi.org/10.1128/AEM.00269-17
SAG- Servicio Agrícola y Ganadero. Retrieved April 7, 2023, from https://www.sag.gob.cl/ambitos-de-accion/insumos-y-productos-silvoagricolas/registros
Sang, C., Ren, W., Wang, J., Xu, H., Zhang, Z., Zhou, M., et al. (2018). Detection and fitness comparison of target-based highly fludioxonil-resistant isolates of Botrytis cinerea from strawberry and cucumber in China. Pesticide Biochemistry Physiology, 147, 110–118. https://doi.org/10.1016/j.pestbp.2018.01.012
Schmitt, O. J., Andriolo, J. L., Schultz, E., Lerner, M. A., Souza, J. M., & Dal Picio, M. (2016). Produção de estolhos de cultivares de morangueiro em função da condutividade elétrica da solução nutritiva. Horticultura Brasileira, 34(2), 294–301.
Sun, H. Y., Wang, H. C., Chen, Y., Li, H. X., Chen, C. J., & Zhou, M. G. (2010). Multiple resistance of Botrytis cinerea from vegetable crops to carbendazim, diethofencarb, procymidone, and pyrimethanil in China. Plant Disease, 94, 551–556. https://doi.org/10.1094/PDIS-94-5-0551
Williamson, B., Tudzynski, B., Tudzynski, P., & Van Kan, J. A. (2007). Botrytis cinerea: The cause of grey mould disease. Molecular Plant Pathology., 8, 561–580.
Zhao, H., Kim, Y. K., Huang, L., & Xiao, C. L. (2010). Resistance to thiabendazole and baseline sensitivity to fludioxonil and pyrimethanil in Botrytis cinerea populations from apple and pear in Washington State. Postharvest Biology Technology, 56, 12–18. https://doi.org/10.1016/j.postharvbio.2009.11.013
Zhou, F., Hu, H. Y., Song, Y. L., Gao, Y. Q., Liu, Q. L., Song, P. W., et al. (2020). Biological characteristics and molecular mechanism of fludioxonil resistance in Botrytis cinerea from Henan Province of China. Plant Disease, 104, 1041–1047. https://doi.org/10.1094/PDIS-08-19-1722-RE
Zuniga, A. I., Wang, N. Y., & Peres, N. A. (2023). Heat treatment as a possible means to reduce Botrytis inoculum on strawberry transplants. Plant Health Progress. https://doi.org/10.1094/PHP-08-22-0078-RS
Acknowledgements
This study was financed in part by the ‘Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil’ (CAPES) – Finance Code 001. The fourth and fifth authors thanks the ‘Conselho Nacional de Desenvolvimento Científico e Tecnológico’ (CNPq)/Brazil for the research fellowship. We are grateful to BIOAGRO and Maxx Mudas (Heitor Pagan) for the strawberry transplants used in this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Juliana Nicolau Maia, Giovana Beger, Denise Silva da Rosa, Natalia Aparecida Peres, Louise Larissa May De Mio and Henrique da Silva Silveira Duarte. The first draft of the manuscript was written by Juliana Nicolau Maia and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors declare that this material has not been published in whole or in part elsewhere; the manuscript is not currently being considered for publication in another journal; all authors have been personally and actively involved in substantive work leading to the manuscript and will hold themselves jointly and individually responsible for its content. We declare that our manuscript complies with all ethical rules applicable for this journal and that there are no potential conflicts of interest or even research involving human participants and/or animals.
Conflicts of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maia, J.N., Beger, G., Rosa, D.S. et al. Detection of Botrytis cinerea in strawberry transplants imported into Brazil and fungicide sensitivity characterization of the isolates. Eur J Plant Pathol 169, 669–680 (2024). https://doi.org/10.1007/s10658-024-02863-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-024-02863-8