Abstract
The potential application of biological copper (Cu) nanoparticles (BCuNPs) as fungicides, insecticides, and fertilizers has piqued the interest of agricultural scientists. In the current study, BCuNPs were biosynthesized by an isolate of Bacillus, which was determined to be Bacillus subtilis subsp. subtilis. This strain can tolerate copper concentrations up to 12 mM and reduce copper by synthesizing BCuNPs. Ultraviolet-visible spectroscopy, dynamic light scattering (DLS), Zeta potential, Fourier transform infrared (FTIR), and transmission electron microscopy (TEM) equipment were used to characterize BCuNPs. As assessed by TEM and DLS, they ranged in size from 15 to 45 nm, with an average particle size of 23 nm. It was necessary to conduct both in vitro and in vivo studies to confirm the nematicidal activity of the fabricated BCuNPs on the infective second-stage juveniles (J2s) and eggs of Meloidogyne incognita the causal agent of root knot disease of cucumber. The in vitro application of different concentrations (100, 125, 150, 175, 200, 225, and 250 ppm) of BCuNPs significantly (P<0.05) increased the number of dead (J2s) of M. incognita, and significantly (P<0.05) decreased the percentage of egg hatching of M. incognita after 12, 24, 48, 72, and 96 h of application compared to the negative control (distilled water) with 250 ppm being the most effective dose of application after 96 h of application. Furthermore, the in vitro application of these different concentrations of BCuNPs combined with nematicide Tervigo® (abamectin, at the recommended application rate (RAR) of 0.1), significantly (P<0.05) increased the number of dead (J2s), and significantly (P<0.05) decreased the percentage of egg hatching of M. incognita compared to the negative control (distilled water), the positive control (Tervigo® alone), and compared to the BCuNPs alone, with 250 ppm being the most effective dose of application after 96 h of application. The number of galls, number of egg masses, root gall index, egg mass index of M. incognita on infected cucumber, the population density of M. incognita J2s 100 g soil-1, as well as the population of Tylenchorhynchus spp., Pratylenchus spp., and Helicotylenchus spp. in soil, were all significantly (P<0.05) reduced by 100, 150, 200, and 250 ppm of BCuNPs when applied under greenhouse conditions, with 250 ppm being the most toxic compared to the untreated of M. incognita treatment. When the nematicide abamectin (used at 0.1 RAR) and BCuNPs (used at 250 ppm) were used together in vivo, they had a synergistic effect on M. incognita. This treatment was found to be significantly (P<0.05) better than the nematicide abamectin alone in reducing the number of galls, the number of egg masses, the root gall index, the egg mass index of M. incognita on infected cucumber, and the population density of J2s 100 g soil-1. These findings indicate that the use of BCuNPs at 250 ppm either individually or combined with nematicide abamectin in integrated pest management systems for controlling nematodes infesting cucumber is recommended.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
More than 4,100 different species of plant-parasitic nematodes (PPNs) are known to infest a wide range of agricultural plants (Mesa-Valle et al., 2020). Loss of crop production due to PPNs costs the global economy an estimated $173 billion per year (Kumar et al., 2020). PPNs pose a significant risk to the production of vegetables and are responsible for significant reductions in crop quality and yield all over the world (Williamson & Kumar, 2006; Zasada et al., 2010; Jones et al., 2013; Zhang et al., 2022; El-Marzoky et al., 2023; Kassam et al., 2023).
Meloidogyne spp. (root-knot nematodes) are the major pests of vegetables especially in the tropics and subtropics (El-Saadony et al., 2022; Saad et al., 2022a; Zhang et al., 2022; Kassam et al., 2023), infecting the majority of commercially significant vegetable crops including tomatoes (Radwan et al., 2012; Daoush et al., 2023; Nie et al., 2023), eggplants (El-Ashry et al., 2022; Caliskan et al., 2023; Khan & Tanaka, 2023), potatoes (Getu et al., 2023), sugar beet (Gohar et al., 2023), and cucumbers (Zhang et al., 2022; Fu et al., 2023; Parsiaaref et al., 2023). Meloidogyne has over 90 species, with Meloidogyne incognita (Kofoid & White) Chitwood, Meloidogyne javanica (Treub) Chitwood, and Meloidogyne arenaria (Neal) Chitwood being particularly polyphagous apomictic species (Hunt & Handoo, 2009). In addition to their widespread distribution in tropical and subtropical regions, these three species can also be found in more temperate regions, particularly in sheltered cultivation (Hunt & Handoo, 2009).
M. incognita is the most common species of nematode that causes root knot disease on vegetable crops (El-Deeb et al., 2018; El-Eslamboly et al., 2019; El-Ashry et al., 2021; Zhang et al., 2022; Fu et al., 2023; Kassam et al., 2023). M. incognita frequently causes considerable damage to plant roots, which results in a reduction in the plant's ability to absorb both nutrients and water (Zhang et al., 2022). Growers, on the other hand, are not typically aware of M. incognita and the disastrous effects it produces because the early indications of the disease are not noticeable until after the population has established itself and caused economic losses (El-Ashry et al., 2021; Zhang et al., 2022).
These nematodes are obligate, endoparasites which produce galls in their hosts' roots (Noureddine et al., 2022; Khan & Tanaka, 2023). M. incognita-affected plants exhibit above-ground symptoms of water and nutritional stress, including yellowing, wilting, and stunting (Fernandez et al., 2015; Zhang et al., 2021). The most common symptom is below-ground galling on roots, bulbs, and tubers. Plant death may result if the infection level is high (Fernandez et al., 2015; Zhang et al., 2021). Root symptoms result from the stimulation of big cells that the worm uses as food sources at different times during its life cycle (Moens et al., 2009). Because of the formation of defective roots and root galls, they cause significant economic damage to the crop (Singh et al., 2019).
Depending on the species and climatic conditions, their life cycle might last from 3 to 6 weeks. Female adults lay 500 to 1,000 eggs in a gelatinous matrix produced on the root surface. Second-stage juveniles (pre-parasitic J2s) develop from eggs under favorable conditions and are the only infective stage that enters the plant roots at the root-tip. Once within, parasitic J2s travel through intercellular gaps to the root meristem (Jagdale et al., 2021). When parasitic J2s reach the protoxylem, they do a U-turn and move up the vascular cylinder, becoming stationary. At this point, they choose five to eight cells and infuse them with oesophageal gland-secreted effectors, resulting in cellular reprogramming and the development of large giant cells (Jagdale et al., 2021).
Cucumber (Cucumis sativus L.) is an economically important vegetable crop worldwide. China ranks first both in acreage and yield of cucumber. In China, >70,338,971 tons of cucumbers were harvested on 1,258,370 ha in 2019 (FAOSTAT, 2020). In Egypt, cucumber production reached to 519.858 tons ha-1 (FAOSTAT, 2016). Root damage and yield losses caused by M. incognita are serious problems for cucumber farmers around the world (El-Ashry et al., 2021; Zhang et al., 2022; Fu et al., 2023; Parsiaaref et al., 2023). Growers in Egypt face various obstacles that limit productivity and quality of cucumbers as a direct result of consecutive monocropping. These challenges pose a huge danger to growers. M. incognita limits cucumber production in Egypt, and gardeners must act quickly to manage this nematode pest (El-Eslamboly et al., 2019; El-Ashry et al., 2021)
To control root-knot nematodes in affected locations, several control techniques were used. The classic nematode control strategy relies heavily on chemical nematicides. However, due to the potential harmful effects on the environment and ineffectiveness after continuous usage, most chemical nematicides have been banned or limited, and there is an urgent need for safer and more effective replacements (Zukerman & Esnard, 1994; Li et al., 2018; Huang et al., 2019). Researchers from all over the world are working to standardize root-knot nematode management strategies through non-chemical and eco-friendly methods such as organic amendments, fertilization, soil management, sanitation, heat-based methods, production of resistant cultivars and biological control, to stabilize vegetable production (Collange et al., 2011; Radwan et al., 2012; Giné & Sorribas, 2017; Wang et al., 2021; Yin et al., 2021; Antil et al., 2023; Kassam et al., 2023; Khan & Tanaka, 2023; Park et al., 2023).
Research in nanotechnology has quickly become one of the most exciting subfields in the field of materials science (Kate et al., 2022). The importance of using environmentally friendly green synthesis methods for producing nanomaterials has increased significantly over the past few years, making this method one of the most prominent ways in the field of modern material sciences (Mahboub et al., 2022).
The characteristics of nanoparticles are determined by their dimensions, shapes, contents, crystallinity, and structures (Hidangmayum et al., 2022; Rajput et al., 2022). The traditional physical and chemical techniques for the synthesis of nanomaterials both have a number of drawbacks (Sukumar et al., 2020; Kate et al., 2022). According to Sukumar et al. (2020), some of these problems include settings of extremely high pressure and temperature, chemicals that are both expensive and hazardous, a longer reaction time, and the absorption of toxic byproducts on the surface of the nanomaterial. All of these aspects increase the difficulty of producing the nanomaterials (Sukumar et al., 2020). One of the most often used methods for avoiding the drawbacks of chemical or physical manufacturing is the environmentally friendly synthesis, which has been increasingly popular in recent years (Saad et al., 2022a, b; Alowaiesh et al., 2023).
There are numerous green synthesis techniques for converting metal salts to nanomaterials, such as the use of plant extracts, algal extracts, bacterial enzymes, extracellular polymeric molecules (El-Saadony et al., 2021; El-Saadony et al., 2022; Saad et al., 2022b; Elkobrosy et al., 2023). Metal-tolerant bacteria are critical nano-factories that collect and detoxify heavy metals (El-Saadony et al., 2022; Saad et al., 2022b). Synthesis of nanomaterials using plant extracts may be easier than synthesis using microorganisms; nevertheless, microbial production is more cost-effective and resistant to fluctuations in seasonality and plant growth stage (El-Saadony et al., 2021; El-Saadony et al., 2022).
Copper sulfate (CuSO4), the most basic component of the copper chemical family, possesses a wide range of physical properties that could be placed to beneficial use (Buazar et al., 2019). Because of its unique features, such as durability, conductivity, catalytic activity, and anticancer and antibacterial capabilities, copper sulfate has sparked more interest than other nanomaterials (Buazar et al., 2019). Copper nanoparticles, also known as CuNPs, are becoming increasingly popular for a number of reasons, including the fact that they are easily accessible and much less expensive than other noble metals, such as gold and silver, and that they have a significant amount of untapped potential for use in antimicrobial applications (Gkanatsiou et al., 2019; El-Saadony et al., 2020; Amin et al., 2021; Sathiyavimal et al., 2021). Due to their low cost, non-toxicity, and ease of production, CuNPs have gained a lot of interest in a variety of research domains, including solar cells, biofuels, photocatalytic degradation, water pollution remediation, supercapacitors, and electrocatalysis (Velsankar et al., 2021).
Because of its unique qualities, such as remarkable strength, increased chemical reactivity, and the production of nanoparticles with strong electrical conductivity, nanotechnology have rapidly emerged in agricultural applied sciences, including traditional and biocontrol of insect pests and plant-parasitic nematodes, primarily root-knot nematode species, Meloidogyne spp. (Sabry, 2019; Wallyn et al., 2019; El-Ashry et al., 2022; Ghareeb et al., 2022; Daoush et al., 2023; Elkobrosy et al., 2023).
There has been no research on the use of green CuNPs as a nematicidal agent to combat root-knot nematodes and improve the physiochemical and morphological features of root-knot nematodes-infected cucumber. In the current study, we used a newly isolated strain of Bacillus subtilis AM18 to successfully produce green biological Cu nanoparticles (BCuNPs), which we then optimized and characterized before testing for their larvicidal and ovicidal effects on the number, percentage mortality of M. incognita J2s larvae and the hatching percentage of M. incognita eggs in vitro, either individually or in combination with the nematicide Tervigo (abamectin, at the recommended application rate (RAR) of 0.1).
As a result, the present study also aimed to compare the effectiveness of different concentrations of BCuNPs on M. incognita larvae and eggs under in vivo greenhouse conditions, either alone or in combination with other biological amendments and abamectin, as well as the performance of cucumber plants infected with M. incognita. The objective was also to determine the optimal doses of BCuNPs for inhibiting the total population of M. incognita, Tylenchorhynchus spp., Pratylenchus spp., and Helicotylenchus spp. in soils where cucumbers were grown.
Materials and Methods
Collection of soil samples
Four soil samples (B11, B15, B20, and B30) were collected from cucumber rhizophore soils. The top 0–30 cm of soils were removed, dried, and then milled into a powder. Following the removal of any small stones or root pieces from the soil samples by means of a sieve with a mesh size of 5 mm, the samples were then placed in clean jars with screw-on lids and stored at 28°C in the dark for 7 days prior to being subjected to microbiological analysis.
Isolation and screening of CuSO4-resistant bacteria
The soil dilution plate method (Johnson & Curl, 1972) employing a nutrient agar medium was utilized in order to successfully isolate bacteria from each soil sample. Nutrient agar (Lab M Limited, Lancashire, United Kingdom) was supplemented with cycloheximide and nystatin (50 g mL-1 each; Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). After cooling to 45°C, these two antifungal antibiotics were added to the nutrient agar right before pouring the plates.
Ten g of each soil sample was combined with 100 mL of sterile deionized water in Erlenmeyer flasks. The soil suspension was then agitated for 30 min at 28°C using a rotary shaker (Model G76, New Brunswick Scientific, Edison, NJ, USA) at 250 revolutions per minute (rpm). After shaking the stock original flask, tenfold dilutions (10-2 – 10-5) were performed with sterile deionized water as indicated by Johnson & Curl (1972). Aliquots (0.2 mL) were then spread across the surface of nutrient agar in Petri plates using a sterile hockey-shaped glass rod. For each dilution, five plates were used, and after drying for 10 min in a laminar airflow cabinet, the plates were incubated for 3 days in the dark at 28°C.
Various bacteria were cultured and purified using nutrient agar plates. Color, texture, elevation, margin, and opacity were used to select representative colonies for collection. Colonies were purified and maintained at 4°C on nutrient agar slants.
Determination of bacterial tolerance to different concentrations of CuSO4
Nutrient agar plates containing deionized water and 2, 4, 6, 7, 10, and 12 mM CuSO4 (Saad et al., 2022b) were used to test the selected isolates for CuSO4 tolerance. After streaking the isolates onto plates, they were incubated at 28°C in the dark for 4 days (Williams et al., 1972). Isolates that continued to develop vigorously after being grown in nutrient agar plates supplemented with 12 mM CuSO4 were classified as being tolerant to the highest concentration of CuSO4 (i.e. CuSO4-tolerant bacteria). There were five replicates of each isolate.
Identification of CuSO4-tolerant bacteria
Only the strongest selected CuSO4-tolerant bacteria which grew up to 12 mM CuSO4 was putatively identified to species level using cultural, morphological, biochemical, and physiological tests as described by Logan & De Vos (2009). The identification was confirmed by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS, Bruker Daltonics GmbH & Co. KG, Bremen, Germany) (Schumaker et al., 2012). The Biotyper Compass Explorer (Bruker Daltonics) was used to assess and convert spectrum data, as described by Jadhav et al. (2021).
Fabrication and optimization of culture conditions to improve the production of BCuNPs
An aliquot (100 µL) of a bacterial suspension (1x108 colony forming units) (CFU) was homogenized into 100 mL of Luria-Bertani (LB) broth (Lab M) and cultured in an orbital shaker incubator at 200 rpm at 35°C for two days. Cultures were centrifuged at 12,000 x g for 30 min. The supernatant was collected and mixed with 100 mL of LB broth containing 12 mM CuSO4 (Saad et al. 2022a). The transformation of CuSO4 to BCuNPs by the selected isolate supernatant was represented by a shift in color from blue to pale green once the optimum circumstances have been determined. The control flasks containing only CuSO4 or the bacterial supernatant without CuSO4 (12 mM) were kept under same conditions (Saad et al., 2022a).
Several studies were conducted to establish the optimal culture conditions required to optimize BCuNP production. Freshly produced bacterial inoculum grown LB medium was added with CuSO4 under the following growth conditions specified below. The conditions tested were; reaction time (1, 2, 3, 4, 5, and 6 days), temperature (10, 20, 30, 40, 50, and 60°C), pH values (2, 4, 6, 8, 10, and 12), different concentrations of CuSO4 (2, 4, 6, 8, 10, ad 12 mM), different aliquots of bacterial supernatant (5, 10, 15, 20, 25, and 30 mL), and using different cultivation media (Mueller Hinton broth (MHB), LB broth, nutrient broth (NB), mineral salts medium (MSM), tryptic soy broth (TSB), and plate count broth (PCB)).
Using a shaking incubator, the flasks were incubated either under static conditions or agitated at different speeds (100, 150, 200, 250, 300, and 350) rpm. Optical density (OD) was measured at 300 nm (OD300) using a Zetasizer (Malvern Panalytical, Malvern, UK). These experiments were carried out according to El-Saadony et al. (2020). Except for one variable factor, all factors in each of these studies were held constant. There were five replicates for each experiment.
Characterization of BCuNPs produced by CuSO4-tolerant bacteria
After observing BCuNPs formation by the media changing color from blue to pale green, they were further characterized by Jenway ultraviolet (UV) visible spectroscopy (Cole-Parmer, IL, USA) to measure BCuNPs' UV absorption spectra in 200 - 1,000 nm. Fourier-transform infrared spectroscopy (FTIR; Bruker Tensor, Kaller, Germany) at 3,500 - 500 cm-1 was used to investigate the interaction between proteins detected in cell-free extract and BCuNPs (Chattopadhyay et al., 2013). Potential active substances in the cell-free extract of bacteria were also characterized using FTIR.
The particle size of nanoparticles in dispersals and their aggregation rate were determined by dynamic light scattering (DLS) (Malvern Panalytical) (Gunti et al., 2019). The polydispersity index (PDI) assesses the homogeneity of nanoparticles; the lower the PDI, the more homogeneous the nanoparticles (Gunti et al., 2019). At 272°C, the size distribution of BCuNPs was measured using a Zetasizer nano range (Malvern Panalytical).
The surface charge of the nanoparticles was determined using a zeta-potential analyzer (Malvern Panalytical) (Gunti et al., 2019). Transmission electron microscopy (TEM; JEOL Ltd., Tokyo, Japan) was used to determine the form and size of BCuNPs (Mendez et al., 2011).
Production of M. incognita inoculum
For the current research, a single egg mass was used to produce a population of M. incognita on tomato (Solanum lycopersicum L.) susceptible cultivar super strain B (El-Ashry et al. 2022). Nematode species were identified based on the perineal pattern region root rot nematode females and infective juveniles (J2s) measurements. Free eggs and J2s were collected from the infected roots for plant inoculation (El-Ashry et al. 2022). A 200-mesh filter was used to filter the egg suspension twice. In order to ensure that no sodium hypochlorite solution was left behind, the free eggs were collected using a 500-mesh filter and then rinsed thoroughly with distilled water before being placed in Petri plates and incubated at 25°C. According to El-Ashry et al. (2022), only recently hatched J2s were collected for in vitro testing.
In vitro assessment of BcuNPs on M. incognita J2s and percentage of egg hatching
BCuNPs assay on M. incognita J2s
The technique outlined below was utilized to evaluate the inhibitory impact of BCuNPs concentrations as larvicidal agent against M. incognita J2s. The nematicidal effect of BCuNPs concentrations (100, 125, 150, 175, 200, 225, and 250 ppm) was tested on J2 of M. incognita at 25˚C.
Aliquots (100 µL) of J2s suspension (200 J2s) was added to 10 mL of BCuNPs concentrations in petri plates. In addition, 10 mL of distilled water was placed in each of the control plates replacing BCuNPs. Juvenile mortality was assessed 12, 24, 48, 72, and 96 h after treatment (El-Ashry et al., 2022). J2s was judged dead if they maintained an inert straight posture or did not move after being prodded (De Nardo & Grewal, 2003). Over the indicated time intervals, mortality counts in 1 mL were recorded using a Hawksley counting slide, under 100 X magnification. Immobile juveniles were sieved, collected, and washed in distilled water for 5 h to allow them to recuperate (Saad et al., 2021).
Each treatment was replicated five times and the plates were incubated at 25°C (Saad et al., 2021) and the percentage of mortality was determined by using the following equation number 1:
BCuNPs assay on egg hatching percentage of M. incognita
Different concentrations of BCuNPs (100, 125, 150, 175, 200, 225, and 250 ppm) were tested in vitro to determine their toxicity on the hatching of free eggs. The free egg count was adjusted to 2000 eggs mL-1 by allowing the eggs to settle for 10 min. An aliquot of 0.1 mL containing approximately 200 eggs was pipetted onto 9 cm Petri plates with 10 mL of BCuNPs concentrations and stored at 25˚C and each treatment was replicated five times.
For 12, 24, 48, 72, and 96 h, Petri plates were checked daily for egg hatching inhibition. Live members were counted as hatching eggs or active J2 juveniles, while immotile eggs or J2s were given 5 h to recuperate in tap water before being counted again. In the control Petri plates, only 10 mL of distilled water was given to the hatching eggs without the application of BCuNPs. The number of hatching eggs was determined using a Nikon-Eclipse 50i light microscope (Nikon Instruments Inc., NY, USA), at 100X magnification.
Equation number 2 was used to determine the extent to which BCuNPs treatment inhibited egg hatching in comparison to the control treatment:
Interactive effects of BCuNPs and the nematicide Tervigo (abamectin) in vitro
BCuNPs and the nematicide Tervigo® 020SC (abamectin, at the recommended application rate (RAR) of 0.1; Syngenta, Basel, Switzerland) were tested for their combined effects on the hatching and mortality of M. incognita free eggs and juveniles.
Nematicidal effect of the mixture on the mortality of M. incognita J2s
M. incognita J2s were exposed to nematicide and BCuNPs and then left at room temperature (25˚C) to determine their impact on mortality. The positive control treatment consisted of only 10 mL of the nematicide Tervigo® 020SC (at RAR of 0.1) in 9-cm Petri plates with 200 J2s of M. incognita in 0.1 mL. The negative control treatment employed simply distilled water in 9-cm Petri plates containing 200 J2s.
The combination of BCuNPs tested concentrations (100, 125, 150, 175, 200, 225, and 250 ppm) and Tervigo® (abamectin, at RAR of 0.1) was used as tested treatments against M. incognita J2s by adding 5 mL of abamectin, at RAR of 0.1 containing 200 J2s in 0.1 mL and 5 mL from each BCuNPs concentration. The J2s mortality rate of the tested materials was checked every day. According to De Nardo and Grewal (2003), J2s who exhibited an inert straight posture or failed to demonstrate any movement in response to prodding were believed to be dead.
Over the given time intervals, mortality was observed under 100 X magnification in 1 mL. Percentages of mortality were determined using equation (1). Each treatment was repeated five times and the plates were incubated at 25°C (Saad et al., 2021).
Nematicidal effect of the mixture on the percentage egg hatching of M. incognita
The following experiments were used to assess the nematicidal interactive effect of 0.1 RAR of the tested nematicide Tervigo® (abamectin) mixed with BCuNPs tested concentrations (100, 125, 150, 175, 200, 225, and 250 ppm) compared to the nematicide alone on egg hatching of M. incognita.
The free eggs from infected roots were extracted by Hussey and Barker (1973). About 200 nematode eggs in 0.L ml of distilled water were added to 10 mL of 0.1 RAR of nematicide Tervigo® (positive control) or 5 mL of 0.1 RAR of nematicide combined with 5 mL of BCuNPs mixture (100, 125, 150, 175, 200, 225, and 250 ppm) to obtain 10 ml in 9-cm Petri plate. Eggs that showed no signs of life were considered to be dead. All Petri plates were kept at a temperature of 25°C and inspected daily for suppression of egg hatching at 12, 24, 48,72, and 96 h. The negative control treatment contained only free eggs in 10 mL of distilled water.
All treatments were replicated five times. Healthy eggs and active J2s were scored as living members. In contrast, immobile eggs or J2s were allowed to recover in tap water for 5 h, and the number of hatched J2s was represented as the cumulative number of viable J2s juveniles (Talavera-Rubia et al., 2020). Data collection included the number of hatched J2s from eggs and percentages of egg-hatching inhibition due to BCuNPs application in comparison with distilled water (control treatment). Percentages of egg hatching inhibition were calculated using equation (2).
Experimental design of in vivo BCuNPs activity against M. incognita infecting cucumber
Cucumber (Cucumis sativus L.) grown in a greenhouse at a temperature of 25°C, with a photoperiod of 18:6, D, L, and a relative humidity of 75%, cucumber plants were subjected to M. incognita inoculum or tested BCuNPs. Surface sterilized cucumber seeds were planted in plastic pots (15 cm in diameter and height of 21 cm) filled with sterilized soil (74.1% sand, 13.5% clay, 6.1% silt), 122 g peat moss, and 3 mg urea fertilizer per kilogram of soil, and standard agricultural practices, such as irrigation and fertilization, were used. The experiments were carried out at the beginning of summer 2020 and repeated again in summer 2021. No pesticides were employed, and each treatment and control were replicated five times, and all plants were irrigated at the same time.
After 3 weeks of sowing the seeds, three seedlings of the tested cucumber cultivar were transplanted into each plastic pot. After 5 days, each seedling was inoculated with 1000 newly hatched J2s from a pure culture of M. incognita by pipetting 5 mL of the inoculum suspension into five holes around the root system, which were directly covered with 5 g of moist sandy soil. All plastic pots treatments were arranged in a randomized complete block design (RCBD) with five replicates.
In vivo nematicidal activities of different doses of BCuNPs on M. incognita infecting cucumber
In the current investigation, different concentrations of BCuNPs were used (100, 150, 200, and 250 ppm). Aliquots of 40 mL of each BCuNPs concentration were incorporated with the upper 5 cm of soil around each plant. The healthy plants in the negative control group were not exposed to any nematodes or BCuNPs, while the infected plants in the positive control group were only exposed to J2s of M. incognita. Similar horticultural practices were applied to each plant in the greenhouse, which was incubated at a constant temperature of 25°C.
A total of three treatments were applied as the following: (i) BCuNPs with 100, 150, 200, and 250 ppm were added at the same time with nematode inoculation, (ii) BCuNPs with 100, 150, 200, and 250 ppm were added 3 days after nematode inoculation, and (iii) BCuNPs with 100, 150, 200, and 250 ppm were added 7 days after nematode inoculation.
After 60 days, all uprooted cucumbers were wrapped with tissue paper to avoid drying. Plant growth parameters (plant weight g-1) and the nematode parameters (number of galls, number of egg masses, root gall index, egg mass index, and number of J2s 100 g soil-1) were evaluated. Root gall index and egg mass index were calculated according to the scale given by Taylor & Sasser (1978), where 0: no galls or egg masses, 1: 1-2 galls or egg masses, 2: 3-10 galls or egg masses, 3: 11-30 galls or egg masses, 4: 31-100 galls or egg masses, and 5: more than 100 galls or egg masses. Samples of 100 g soil were processed for nematode extraction using a combination of sieving and Baermann trays techniques (Hooper et al., 2005). The percentage reduction in number of J2s 100 g soil-1 and the percentage increase of plant weight were assessed following equation 3, and 4, respectively.
In vivo nematicidal activities of different doses of BCuNPs on the population of PPNs infecting cucumber
At the end of the experiment described above (after 60 days), the population of PPNs (Tylenchorhynchus spp., Pratylenchus spp., and Helicotylenchus spp.) were counted. PPNs were collected from naturally infected cucumber plants. Subsamples were obtained with a hand trowel at a depth of 15-30 cm from the rhizosphere area of infected cucumber and then blended to make a composite sample of around 1 kg, which was stored in polyethylene bags.
The samples were stored in an icebox at 15°C until they were shipped off to the laboratory for nematode extraction. Using a combination of sieving and the Baermann trays method, about 250 g of soil and roots were removed (Hooper et al., 2005). Pipetting 1 mL of nematode suspension into a Hawksely counting slide and inspecting with Nikon-Eclipse 50i light microscope (Nikon) at 100X magnification enabled the identification of PPNs. Adult and juvenile nematode morphology was used to determine species according to Soetaert et al. (2002). Nematode reduction (%) was calculated according to the following equation:
Reproduction factor (RF) = Pf/Pi was calculated. Where Pf = final population, and Pi = initial population. Each treatment and measurement was replicated five times.
Effect of synergistic interactions of BCuNPs and some bioagents and a nematicide on cucumber performance infected with M. incognita under greenhouse conditions
Another in vivo greenhouse experiment was also carried out to study the effect of the application of BCuNPs concentrations (100, 150, 200, and 250) ppm individually and in combination with biological products (BECTO Grow Roots®, and Biozeid®), composted chicken manure, and the nematicide Tervogo on cucumber, cultivar Chief (SC 4145) infected with M. incognita.
In the individual treatments, BECTO Grow Roots®, and Biozeid®, were added as per the manufacturer recommended rate at 10 mL plant-1. Tervigo®, was added at RAR of 0.1, and composted chicken manure was added as 3 g plant -1. The additions were mixed with the upper 5 cm of soil around each plant.
Aliquots of 40 mL of BCuNPs concentration were incorporated with the upper 5 cm of soil around each plant, 7 days after nematode inoculation as described above. The healthy plants in the negative control group were not exposed to any nematodes or BCuNPs, while the infected plants in the positive control group were only exposed to J2s of M. incognita. Similar horticultural practices were applied to each plant in the greenhouse, which was incubated at a constant temperature of 25°C.
A total of 12 treatments were applied as the following: T1, M.incognita + 250 ppm BCuNPs; T2, M.incognita + 200 ppm BCuNPs; T3, M.incognita + 150 ppm BCuNPs; T4, M.incognita + 100 ppm BCuNPs; T5, M.incognita + composted chicken manure; T6, M.incognita + Biozeid®; T7, M.incognita + BECTO Grow Roots®; T8, M.incognita + nematicide Tervigo® (abamectin, at RAR of 0.1), T9, M.incognita + composted chicken manure + 250 ppm BCuNPs; T10, M.incognita + Biozeid® + 250 ppm BCuNPs; T11, M.incognita + BECTO Grow Roots® + 250 ppm BCuNPs; and T12, M.incognita + nematicide Tervigo® (abamectin, at RAR of 0.1) + 250 ppm BCuNPs. The concentration of BCuNPs at 250 ppm was exclusively employed for the combination application because it was proven to be the most effective dose.
After 60 days, all uprooted cucumbers were wrapped with tissue paper to avoid drying. Plant growth parameters (weight of fresh root and fresh shoot (g), and number of leaves) and the nematode parameters (number of galls, number of egg masses), were evaluated. Samples of 100 g soil were processed for nematode extraction using a combination of sieving and Baermann trays technique (Hooper et al., 2005) in order to determine the number of J2s 100 g soil-1. The percentage reduction in number of galls, number of egg masses, and number of J2s 100 g soil-1 and the percentage increase of weight of fresh root and fresh shoot (g), and number of leaves were assessed following equations 3, and 4, respectively.
Statistical analysis
All in vitro tests were carried out using a randomized design. RCBD was used in all of the greenhouse experiments in the present study. The same results were seen in a second round of the greenhouse experiment, and accordingly, all data were combined and analyzed using SAS Software version 9 ANOVA technique (SAS Institute Inc., NC, USA). Fisher's least significant difference (LSD) test was used at the P = 0.05 level to compare treatment means. Percentage data were arcsine transformed before analysis.
Results
Isolation and determination of CuSO4-resistant isolates
A total of 64 bacterial isolates were isolated from four cucumber rhizophore soils. To identify the most active CuSO4-resistant bacteria, all 64 pure cultures were cultivated on nutrient agar plates supplemented with 2, 4, 6, 8, 10, and 12 mM CuSO4. Only 30 of the tested isolates showed significant growth on CuSO4 plates, albeit their levels of tolerance varied. The remaining 34 isolates were eliminated from the present study because they failed to thrive on plates with 2, 4, 6, 8, 10, and 12 mM CuSO4 (Table 1).
Soil sample B11 yielded nine different CuSO4-resistant isolates (AM1, AM2, AM3, AM4, AM5, AM7, AM9, AM10, AM11), sample B15 yielded seven CuSO4-resistant isolates (AM12, AM15, AM18, AM19, AM22, AM23, AM24), sample B20 yielded ten different CuSO4-resistant isolates (AM25, AM27, AM29, AM32, AM36, AM40, AM44, AM47, AM50, AM52), and sample B30 yielded four different CuSO4-resistant isolates (AM56, AM59, AM60, AM6) (Table 1).
The selected 30 isolates were classified based on their tolerance to 2, 4, 6, 8, 10, and 12 mM CuSO4 (Table 2). All 30 isolates grew strongly on nutrient agar plates supplemented with 2 mM CuSO4, whereas 21 isolates grew strongly on nutrient agar plates provided with 4 mM CuSO4, and 18 isolates grew strongly on nutrient agar plates supplemented with 6 and 8 mM CuSO4 (Table 2). On nutrient agar plates enriched with 10 mM CuSO4, only five isolates (AM2, AM18, AM22, AM29, and AM47) grew vigorously; whilst, on plates with 12 mM CuSO4, only one isolate (AM18) grew vigorously (Table 2).
Only AM18, which showed robust growth on nutrient agar plates enriched with 2, 4, 6, 8, 10, and 12 mM CuSO4 (Table 2), was employed in this investigation due to its high CuSO4 tolerance.
Identification of the CuSO4-resistant strain AM18
Strain AM18 was demonstrated to be Gram-positive, rod-shaped, motile, and endospore-forming bacterium. On nutrient agar, colonies from strain AM 18 were spherical, flattened, homogeneous, clear, off-white, and undulating along the edges. Morphological and biochemical analyses suggested that our isolate may be classified as a subspecies of Bacillus.
The identity was confirmed using MALDI-TOF MS. The selected bacterium was found to be 99% identical to Bacillus subtilis subsp. subtilis.
Optimization of the production conditions of BCuNPs
The ideal conditions for Bacillus isolate AM18 to fabricate BCuNPs are depicted in Fig. 1 A-H. To control the size of the nanoparticles, the optimization of biosynthetic parameters such as reaction durations, incubation temperature, pH, CuSO4 concentrations, aliquots of bacterial supernatant added, agitation speed, types of culture medium, and incubation under static or shaken conditions, was investigated independently (Fig. 1 A-H).
Determination of the optimal conditions for the production of biological copper nanoparticles (BCuNPs) fabricated by Bacillus subtilis AM18. These conditions were reaction time (A), temperature (B), pH (C), concentration of copper sulfate (CuSO4) (D), aliquots of bacterial supernatant (E), agitation speed (F), type of culture medium (G), and movement (H). Mueller Hinton broth, MHB; Luria-Bertani broth, LB; nutrient broth, NB; mineral salts medium, MSM; tryptic soy broth, TSB; and plate count broth, PCB
The size of the BCuNPs decreased as the reaction duration and bacterial supernatant quantity increased (Fig. 1A, E). DLS analysis revealed that five days of incubation with 25 mL of bacterial supernatant resulted in the maximum output of nanoparticles with sizes of 35 and 45 nm (Fig. 1 A, E). The smallest BCuNPs can be produced at a temperature of 40°C, whereas at higher temperatures the nanoparticles' volume grows again (Fig. 1B), possibly because the accumulation of nanoparticles is facilitated by the higher temperature.
The smallest BCuNPs developed in a pH 6 media (Fig. 1C), where hydrogen ions altered the activity of bacterial enzymes. Furthermore, at 10 mM CuSO4 concentration, BCuNPs with a size of 41 nm were produced, which increases 7-fold at 12 mM concentration (Fig. 1D). Nanoparticle size was also affected by the medium used; with LB proved to be the most suitable (Fig. 1G). Furthermore, agitation was necessary to produce the nanoscale BCuNPs (Fig. 1H). BCuNPs measuring 33 nm in size was successfully produced by agitation at 200 rpm (Fig. 1F).
Characterization of BCuNPs produced by B. subtilis AM18
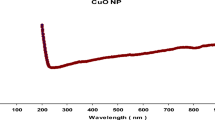
When B. subtilis AM18 supernatant was added to LB media enriched with CuSO4 solution, the color changes from blue to pale green, suggesting that CuSO4 was being biotransformed into BCuNPs. As shown in Fig. 2A, the generated BCuNPs exhibited UV absorption at 235 nm, proving the Cu transformation. BCuNPs were spherical with a size between 15 and 45 nm, as seen in the TEM image (Fig. 2B).
Characterization of biological copper nanoparticles (BCuNPs) fabricated by Bacillus subtilis AM18 supernatant. (A) UV absorbance at 235 nm, (B) transmission electron microscopy showing spherical nanoparticles, (C) size of 23 nm as measured by Zetasizer, (D) zeta potential showed nanoparticles charge of ‒16.26, (E) active groups surrounded BCuNPs, and (F) active groups in B. subtilis AM18 as detected by Fourier transform infrared (FTIR)
DLS, which was utilized to assess the size and charge of nanoparticles in suspension, indicated that the stable BCuNPs had a precise size of 23 nm (Fig. 2C) and a net negative charge of 16.26 mV (Fig. 2D). The FTIR spectrum of BCuNPs indicated 13 bands between 3500 and 500 cm-1 (Fig. 2E), whereas the FTIR spectrum of B. subtilis AM18 supernatant revealed eight bands (Fig. 2F). These bands suggested the presence of active groups such as alcohol, phenols, amides, and amines in the structure of BCuNPs (Fig. 2E, F). The OH and NH2 groups are represented by 3,419.39 cm-1, whilst the CH group was represented by 2,982.66 cm-1. The band at 2094 cm-1 corresponds to the alkyne group, whereas the bands at 1,650.09, 1,373, and 920.66 cm-1 were related to alkanes, alkenes, and aromatic chemicals, respectively (Fig. 2F). Esters were detected in the band (1150 cm-1), while halide compounds were detected at 617.39 cm-1 (Fig. 2F). These findings demonstrated that BCuNPs underwent monodisperse biotransformation.
Effect of different concentrations of BCuNPs on the mortality of M. incognita J2s in vitro
Table 3 shows the in vitro mortality of M. incognita J2s using different concentrations of BCuNPS. The in vitro application of different concentrations (100, 125, 150, 175, 200, 225, and 250 ppm) of BCuNPs significantly (P<0.05) increased the number of dead J2s of M. incognita after 12, 24, 48, 72, and 96 h of application compared to the negative control (distilled water) with 250 ppm being the most effective dose of application after 96 h of application (Table 3).
The number of dead J2s M. incognita increased significantly (P<0.05) with increasing time of exposure from 12 up to 96 h, with the maximum nematicidal activity in killing J2s of M. incognita occurring after exposure for 96 h (Table 3). Overall, based on all concentrations evaluated, the best nematicidal concentration of BCuNPs against J2s larvae was 250 ppm, resulting in the highest increase in the number of dead J2s of M. incognita (Table 3).
Effect of different concentrations of BCuNPs on egg hatching percentage of M. incognita in vitro
Table 4 displays the percentage of M. incognita eggs that hatched following 12, 24, 48, 72, and 96 h of in vitro treatment with varying doses of BCuNPS. After 12, 24, 48, 72, and 96 h of application, different concentrations (100, 125, 150, 175, 200, 225, and 250 ppm) of BCuNPs significantly (P<0.05) decreased the percentage of egg hatching of M. incognita compared to the negative control (distilled water), with 250 ppm being the most effective dose of application after 96 h of application (Table 4).
Hatching was significantly (P<0.05) decreased proportionally by increasing the concentrations of tested BCuNPs, and 100, 150, 200, and 250 ppm demonstrated distinguishable effects as ovicidal against free eggs of M. incognita in vitro (Table 4). Maximum nematicidal activity in killing M. incognita eggs occurred after exposure for 96 h (Table 4), with the proportion of M. incognita eggs that hatched decreasing significantly (P<0.05) with increasing period of exposure from 12 up to 96 h.
In vitro effect of varying doses of BCuNPs in combination with the nematicide Tervigo® on the number of J2s of M. incognita
Table 5 shows the effect of varying concentrations of BCuNPs combined with the nematicide Tervigo® on the number of dead J2s of M. incognita after 12, 24, 48, 72, and 96 h of treatment in vitro. The in vitro application of various concentrations of BCuNPs combined with the nematicide Tervigo® (abamectin, at the recommended application rate (RAR) of 0.1), significantly (P<0.05) increased the number of dead J2s of M. incognita compared to the negative control (distilled water) and the positive control (Tervigo® alone), with 250 ppm being the most effective dose of application after 96 h (Table 5).
The nematicide abamectin was utilized at a 0.1 RAR concentration, which resulted in the in vitro larvicidal activity of BCuNPs against J2s of M. incognita increasing significantly (P<0.05) (Table 5). Compared to 0.1 RC Tervigo alone, the nematicides combinations containing BCuNPs demonstrated a significantly (P<0.05) greater action against the number of J2s of M. incognita (Table 5). Maximum nematicidal activity of 250 ppm BCuNPs + Tervigo 0.1 RAR in killing J2s of M. incognita under in vitro conditions occurred after exposure for 96 h (Table 5), and the number of dead J2s M. incognita rose significantly (P<0.05) with increasing period of exposure from 12 up to 96 h (Table 5).
Effect of different concentrations of BCuNPs in combination with the nematicide Tervigo® on percentage of egg hatching of M. incognita in vitro
Table 6 illustrates the effect of increasing concentrations of BCuNPs mixed with the nematicide Tervigo® on the percentage of M. incognita eggs that hatched in vitro following treatment for 12, 24, 48, 72, and 96 h, respectively. The percentage of M. incognita eggs collected was significantly (P<0.05) lower after in vitro application of various concentrations of BCuNPs combined with the nematicide Tervigo® (abamectin, at the recommended application rate (RAR) of 0.1) compared to the negative control (distilled water) and the positive control (Tervigo® alone) (Table 6).
When evaluated against eggs of M. incognita, the combined effect of 0.1 RC Tervigo and BCuNPs exhibited a considerably superior significant effect (P<0.05) against the eggs of M. incognita compared to 0.1 RC rate alone (Table 6). In vitro, 250 ppm of BCuNPs + Tervigo 0.1 RAR showed maximum nematicidal activity against M. incognita eggs after 96 h of exposure (Table 6), and the percentage of M. incognita eggs that hatched decreased significantly (P<0.05) with increasing periods of exposure from 12 to 96 h (Table 6).
The results demonstrated in Tables 5 and 6 showed that the in vitro larvicidal (Table 5) and ovicidal (Table 6) activity of 250 ppm BCuNPs against J2s and eggs of M. incognita enhanced significantly (P<0.05) when the nematicide abamectin was employed at 0.1 RAR. These findings shed light on the synergistic effects that resulted from the combination of these two agents (Tables 5, 6).
Determination of nematicidal activities of varying doses of BCuNPs on M. incognita infecting cucumber under greenhouse conditions
Table 7 shows the impact that supplementing with various concentrations of BCuNPs had on cucumber performance (plant weight), nematode reproductive parameters (number of galls, number of egg masses, root gall index, and egg mass index of M. incognita), and the M. incognita soil population parameter (number of M. incognita J2s 100 g soil-1) after 60 days of planting cucumber in the presence of M. incognita under greenhouse conditions.
Under greenhouse conditions, 100, 150, 200, and 250 ppm of BCuNPs were significantly (P<0.05) more effective than the untreated control treatment (nematode treatment only) in reducing the number of galls, the number of egg masses, the root gall index, the egg mass index of M. incognita on infected cucumber, and the population density of M. incognita J2s 100 g soil-1 (Table 7). In comparison to the other concentrations of BCuNPs (100, 150, and 200), it was found that BCuNPs at a concentration of 250 ppm was the most successful treatment (Table 7).
In addition, the performance of cucumbers after 60 days, as measured by plant weight g-1, was significantly (P<0.05) improved by the application of various doses of BCuNPs (100, 150, 200, and 250 ppm) when the treatment was carried out in the greenhouse (Table 7). Table 7 further demonstrates that BCuNPs applied at a concentration of 250 ppm significantly enhanced the productivity of greenhouse-grown cucumber compared to other doses of BCuNPs (100, 150, and 200 ppm).
It is noteworthy to mention that the number of galls, the number of egg masses, the root gall index, the egg mass index of M. incognita on infected cucumber, and the population density of M. incognita J2s 100 g soil-1 were significantly (P<0.05) reduced more effectively by applying all four concentrations of BCuNPs (100, 150, 200, and 250 ppm) 7 days after soil inoculation with M. incognita (Table 7), as opposed to applying all four concentrations of BCuNPs 3 days after soil inoculation with M. incognita or adding the BCuNPs simultaneously with M. incognita (Table 7).
Similarly, BCuNPs (100, 150, 200, and 250 ppm) applied 7 days after soil inoculation with M. incognita resulted in greater cucumber growth after 60 days than BCuNPs added 3 days after soil inoculation with M. incognita or adding the BCuNPs simultaneously with M. incognita (Table 7).
Effect of different concentrations of BCuNPs on the population of PPNs infesting cucumber under greenhouse conditions
In addition to M. incognita, three species of PPNs associated with cucumber roots were also surveyed in the current investigation. These species were Tylenchorhynchus spp., Pratylenchus spp., and Helicotylenchus spp. (Table 8).
The natural population of Tylenchorhynchus spp., Pratylenchus spp., and Helicotylenchus spp. in cucumber infested soil under greenhouse conditions was significantly (P<0.05) reduced by 100, 150, 200, and 250 ppm of BCuNPs after 60 days of application, with 250 ppm being the most toxic compared to the untreated control treatment (Table 8). When applied under greenhouse circumstances, the RF for Tylenchorhynchus spp., Pratylenchus spp., or Helicotylenchus spp. were all significantly (P<0.05) lowered by 100, 150, 200, and 250 ppm of BCuNPs, with 250 ppm being the most harmful compared to the untreated control treatment (Table 8).
After 60 days, 100, 150, 200, and 250 ppm of BCuNPs significantly (P<0.05) increased the percentage reduction of Tylenchorhynchus spp., Pratylenchus spp., and Helicotylenchus spp. when applied under greenhouse conditions, with 250 ppm being the most toxic to the three PPNs compared to the untreated control treatment (Table 8). BCuNPs with different concentrations with their nematicidal action on PPNs other than M. incognita could be employed as a sustainable approach of pest management of nematodes attacking cucumber.
Effect of synergistic interactions of BCuNPs and some bioagents and a nematicide on cucumber performance infected with M. incognita under greenhouse conditions
Table 9 shows the effect of different concentrations of BCuNPs, composted chicken manure, bioagents (BECTO Grow Roots®, and Biozeid®), and the nematicide abamectin on cucumber growth (root and shoot fresh weight, and number of leaves), and M. incognita parameters (number of galls, number of egg masses, and the number of J2s 100 g soil-1) associated with cucumber plants infected with M. incognita after 60 days of application under greenhouse conditions.
The obtained results from Table (9) revealed that the individual application of 250 ppm of BCuNPs (treatment 1), 200 ppm of BCuNPs (treatment 2), 150 ppm of BCuNPs (treatment 3), and 100 ppm of BCuNPs (treatment 4) in soils artificially infested with M. incognita significantly (P<0.05) reduced the number of galls, number of egg masses of M. incognita on infected cucumber, the population density of M. incognita J2s 100 g soil-1, and significantly (P<0.05) improved the growth performance of cucumber (root and shoot fresh weight, and number of leaves) when applied under greenhouse conditions, with 250 ppm being the most effective treatment compared to the untreated control treatment which included only the presence of M. incognita without any curative or preventive treatment (no BCuNPs) (Table 9).
The percentage reduction of number of galls, number of egg masses of M. incognita on infected cucumber, the population density of M. incognita J2s 100 g soil-1 was found to be significantly (P<0.05) enhanced in treatment 1 with the BCuNPs at 250 ppm compared to other treatments and to the untreated control with only M. incognita (Table 9). In addition, the application of BCuNPs at 250 ppm (treatment 1) resulted in a significant (P<0.05) greatest percentage increase in root and shoot fresh weight, as well as the number of leaves, compared to the other treatments and to the untreated control with only M. incognita (Table 9).
The application of the nematicide Tervigo® (abamectin, at RAR = 0.1) (treatment 8), and the application of nematicide Tervigo® (abamectin, at RAR = 0.1) + 250 ppm BCuNPs (treatment 12) in soils artificially infested with M. incognita significantly (P<0.05) reduced the number of galls, number of egg masses of M. incognita on infected cucumber, the population density of M. incognita J2s 100 g soil-1, and significantly (P<0.05) improved the growth performance of cucumber (root and shoot fresh weight, and number of leaves) when applied under greenhouse conditions, compared to the untreated control treatment which included only the presence of M. incognita without any curative or preventive treatment (no BCuNPs) (Table 9).
When comparing treatments 8 (nematicide alone) and 12 (nematicide + 250 ppm BCuNPs), it was interesting to note that treatment 12 had a more effective and significant (P<0.05) growth promoting effect on cucumber performance and a more efficient and significant (P<0.05) inhibitory effect on M. incognita parameters in soils. When the nematicide was combined with BCuNPs at a concentration of 250 ppm (treatment 12), a synergistic effect was observed. Treatment 12 was found to be the most effective treatment in the current study and showed the highest significant (P<0.05) increase in the percentage of root and shoot fresh weight, and number of leaves, and showed the highest significant (P<0.05) decrease in the percentage of number of galls, number of egg masses of M. incognita on infected cucumber, the population density of M. incognita J2s 100 g soil-1, compared to untreated control with only M. incognita (Table 9).
The application of all the other tested treatments which included the addition of composted chicken manure (treatment 5), Biozeid® (treatment 6), BECTO Grow Roots® (treatment 7), or composted chicken manure + 250 ppm BCuNPs (treatment 9), Biozeid® + 250 ppm BCuNPs (treatment 10), BECTO Grow Roots® + 250 ppm BCuNPs (treatment 11), all significantly (P<0.05) reduced the number of galls, number of egg masses of M. incognita on infected cucumber, the population density of M. incognita J2s 100 g soil-1, and significantly (P<0.05) improved the growth performance of cucumber when applied under greenhouse conditions, compared to the untreated control treatment which included only the presence of M. incognita without any curative or preventive treatment (no BCuNPs) (Table 9). Table 9 also shows that the synergistic effect when BCuNPs were incorporated was most apparent in all the combined treatment, which comprised the integration of 250 ppm BCuNPs.
Discussion
It is estimated that root-knot nematodes (Meloidogyne spp.), one of the most economically destructive genera of PPNs, cause a loss of US$100 billion worldwide every year in horticulture and field crops (Oka et al., 2000; Zhang et al., 2022; Kassam et al., 2023).
Root-knot nematodes were managed through a combination of strategies implemented in affected areas (Elmer & White, 2016; Yeon et al., 2019; Shang et al., 2020). Chemical nematicides have long been the backbone of the conventional approach to nematode management. There is an urgent need for safe and more effective alternatives to chemical nematicides due to their potential detrimental influence on the environment and ineffectiveness after continuous usage (Zukerman & Esnard, 1994; Kassam et al., 2023).
Nanotechnology has become an advanced pest management approach, primarily for PPNs, due to novel characteristics such as their extraordinary strength, greater chemical reactivity, and nanoparticles with high electrical conductivity (Wallyn et al., 2019; El-Saadony et al., 2020; El-Saadony et al., 2022). In the current investigation, BCuNPs were biosynthesized by the bacterium isolate # AM18. Morphological, physiological and biochemical tests revealed that AM18 may be categorized as a Bacillus subspecies.
MALDI-TOF MS was used to validate the identity, and the chosen bacterium was determined to be 99% identical to B. subtilis subsp. subtilis. Our results agreed with previous studies in which MALDI-TOF MS can be used to identify bacteria. It is well known that MALDI-TOF MS technique is utilized for the purpose of identifying bacterial isolates on the genus, species, and subspecies levels. This method is quick, accurate, and dependable (Schumaker et al., 2012; Sauget et al., 2017; Jadhav et al., 2021). Our findings also agreed with those of Kuppamuthu et al. (2017), who isolated and identified distinct isolates from whey for lipase synthesis using MALDI-TOF MS technique.
This strain B. subtilis subsp. subtilis AM18 obtained in the current study was discovered to be CuSO4 tolerant up to 12 mM and to reduce copper by synthesizing BCuNPs. The current investigation is consistent with the findings of John et al. (2021), who isolated new isolates from the Antarctic consortium and examined their Cu-tolerance at 10 doses (0-5 mM) and they reported that all bacteria tolerated CuSO4 up to 3.5-4 mM (John et al., 2021). They also discovered that increasing the concentration to 4 mM inhibited bacterial growth, particularly in Pseudomonas ef1 and Bacillus ef1 isolates (John et al., 2021). Our isolate, B. subtilis subsp. subtilis AM18, on the other hand, was able to survive CuSO4 doses up to 12 mM, making it a superior CuSO4-tolerant bacterium.
Bacteria, molds, and yeasts play an important role in the production of metal and metal oxide nanoparticles. Several recent studies have used several microorganisms as models in the manufacturing of nanoparticles (El-Saadony et al., 2020; El-Ashry et al., 2022). To maximize nanoparticle yield, optimal conditions for nanoparticle production must be addressed (El-Saadony et al., 2021) and the optimal cultivation conditions needed to maximize nanoparticles production have been the subject of multiple investigations (El-Saadony et al., 2021; El-Saadony et al., 2022).
Different factors were investigated and tested in the present study to determine the optimal conditions to produce BCuNPs. These factors included reaction time, temperature, pH, CuSO4 concentration, aliquots of bacterial supernatant, cultivation medium, growth conditions under static versus shaken conditions, and agitation speed. Our optimization factors were found to be in agreement with the findings of Kathiresan et al. (2009), Jain et al. (2013), El-Saadony et al. (2020), El-Saadony et al. (2021), El-Ashry et al. (2022), and Singh et al. (2023) who used the same optimization conditions in order to maximize the production of their biological nanoparticles.
In addition, the characterization of biological nanoparticles is an essential stage in the process of ensuring the biotransformation of the nanoparticles (El-Saadony et al., 2021). In the current study we used UV visible spectroscopy, FTIR, DLS, Zeta potential, TEM to characterize BCuNPs. As assessed by TEM and DLS, BCuNPs ranged in size from 15 to 45 nm, with an average particle size of 23 nm. However, Singh et al. (2023) found that biogenic CuNPs generated by Serratia sp. ZTB29 had diameters ranging from 20 to 40 nm. The zeta potential of biogenic CuNPs produced by Serratia sp. ZTB29 was also determined to be 15.4 mV, with UV absorbance at 285 nm (Singh et al., 2023).
To confirm the nematicidal activity of the produced BCuNPs on J2s and eggs of M. incognita, the causal agent of cucumber root knot disease, both in vitro and in vivo investigations were carried out. After 96 h of in vitro exposure, BCuNPs at concentrations of 100, 125, 150, 175, 200, 225, and 250 ppm significantly (P<0.05) increased the number of dead J2s of M. incognita compared to the negative control and significantly (P<0.05) decreased the percentage of egg hatching of M. incognita compared to 12, 24, 48, 72, and 96 h of application. The results that were obtained from the BCuNPs showed that the mortality of M. incognita J2 gradually rose as the concentrations of BCuNPs increased. On the other hand, Al Banna et al. (2018) found that 50 nm 21.5 silicon carbide nanoparticles (at a concentration of 172 mg L-1) had no cytotoxic effect on J2 or egg hatching of M. incognita. Similarly, Ardakani (2013) found that silica oxide nanoparticles (SiO2NPs) did not kill M. incognita J2 in laboratory trials.
On the other hand, several studies have shown that a number of distinct nanoparticles have a beneficial effect against M. incognita. For instance, inactive J2 of M. incognita was produced by silver nanoparticles at concentrations ranging from 30 to 150 g mL-1 (Cromwell et al. 2014). The larvicidal activity of a high concentration of silver nanoparticles (1500 ppm) attained 96.5% mortality after 72 h, as demonstrated by Taha and Abo-Shady (2016). These inconsistent results may be attributed to nanoparticle toxicity depending on the physicochemical properties (Pourchez et al. 2012) and the origin of the nanoparticles, whether chemical or biological synthesis (El-Saadony et al. 2020).
Furthermore, the agglomeration of chemically generated nanoparticles may be involved in the nanoparticles' lack of deadly efficacy (Gudikandula & Charya Maringanti, 2016). CuNPs have transgenerational consequences in animals, according to in vitro testing (Poma et al., 2014), and epigenetic alterations are impacted by nanoparticle exposure, resulting in reproductive abnormalities in later generations (Poma et al., 2014). Wei et al. (2020) reported that exposure to BCuNPs (150 mg L-1) significantly decreased the mRNA levels of met-2 and spr-5 genes in Caenorhabditis elegans P0 and F1 generations compared to the control group, and that high concentrations (250 ppm) of BCuNPs had a negative effect on reproduction and population density of M. incognita in treated pot soils (Wei et al., 2020). Similar findings have been reported regarding the nematicidal action of BCuNPs against J2 of M. incognita at various BCuNPs doses compared to the non-treated control (Al Banna et al., 2020). According to our current findings, BCuNPs may give alternative solutions for controlling M. incognita due to their nematicidal action.
The in vitro nematicidal properties of BCuNPs in the present study are consistent with the findings of Ch et al. (2019), and Ahamad & Siddiqui (2021). Furthermore, when CuNPs were combined with other materials, such as Fe oxides and chlorides, a synergistic effect was seen (Wang et al., 2020). In a study comparing the toxicity of CuONPs to that of other silver nanoparticles, it was shown that after three days of treatment, 200 ppm of silver nanoparticles caused 52% death of J2 (Shoaib et al., 2022), but CuNPs caused 98.50% after two days and 100% in the current study. While DNA damage is a possible cause of the toxicity of CuNPs, even a moderate effect of nanoparticles is dependent on its ability to disrupt a wide variety of cellular mechanisms, including ATP synthesis, membrane permeability, and the response to oxidative stresses in prokaryotes (Elkobrosy et al., 2023) and eukaryotes (Mohamed et al., 2019).
The toxicity of CuNPs against M. incognita has been reported in a number of studies, as has the toxicity of CuNPs to several species of bacteria and yeast (Smaoui et al., 2023), annelids (Velicogna et al., 2021), and human cells (Smaoui et al., 2023). This depends on a number of aspects, including aggregation (Fernando & Zhou, 2019), particle size (Zhang et al., 2019), solubility (Corsi et al., 2022), and the presence of organic material in the test medium (Zhang et al., 2019).
CuNPs aggregates have the potential to persist in a nanostructured state, making them more highly reactive than CuNPs alone. This increases the likelihood that they will have a detrimental effect on fish (Gupta et al., 2022). Other research has concluded that the CuNPs component itself may work to block essential cellular activities (Gupta et al., 2022).
CuNPs have the potential to disrupt many cellular processes, including membrane permeability, ATP production, and the oxidative stress response, which can have a nematode-toxic effect on M. incognita (Ma et al., 2009). This can occur via a number of different mechanisms. If it is anticipated that J2 will die, nanoparticles can affect the cellular architecture of nematodes (Ma et al., 2009). Heavy metals were found to be harmful to C. elegans by compromising the integrity of cell membranes and changing the cations connected to proteins, according to Ma et al. (2009). Because of condensed cellular energy, copper ion and other metals affect neuron function by modifying mitochondrial activity, raising stress through the formation of reactive oxygen species (ROS), and activating cell death pathways such as apoptosis and necrosis (Chen et al., 2013). Our results showed that biosynthesized BCuNPs have the potential for direct or indirect usage in the fight against root-knot nematodes, which result in decreased crop yields.
Our research showed that after 96 h of in vitro application, the combination of BCuNPs at 250 ppm and Tervigo® at a RAR of 0.1 significantly (P<0.05) increased the number of dead (J2s) and significantly (P<0.05) decreased the percentage of egg hatching of M. incognita compared to the negative control, the positive control (Tervigo® alone), and the BCuNPs alone. Our findings agreed with those of previous similar studies by Hassan et al. (2016), Rastogi et al. (2019), and El-Ashry et al. (2022). The results obtained by El-Ashry et al. (2022) showed that combining silicon nanoparticles with half-recommended doses (0.5) of commercial nematicides, specifically fenamiphos, nemathorin, and fosthiazate, increased egg hatching inhibition and J2 mortality of M. incognita after exposure to silicon nanoparticles (100 ppm) mixed with 0.5 recommended doses of synthetic nematicides. According to Rai and Ingle (2012), the biosynthesized BCuNPs could improve the efficacy of synthetic nematicides and facilitate their distribution. When silver nanoparticles were coupled with the commercial nematicides fenamiphos and oxamyl, a similar inhibitory action was found (Hassan et al., 2016). In addition, the effectiveness and durability of pesticide active components may be improved by using nano-pesticides, as suggested by Zhai et al. (2020).
Under greenhouse conditions in the present investigation, the number of galls, number of egg masses, root gall index, egg mass index of M. incognita on infected cucumber, and population density of M. incognita J2s 100 g soil-1 as well as the population of Tylenchorhynchus spp., Pratylenchus spp., and Helicotylenchus spp. in soil, were all significantly (P<0.05) reduced by 100, 150, 200, and 250 ppm of BCuNPs, with 250 ppm being the most inhibitory compared to the untreated of M. incognita treatment. In addition, we also found that the effectiveness of the combination of BCuNPs and the nematicide on the reduction of root infection parameters (such as the number of galls, the number of egg masses, the root gall index, and the egg mass index of M. incognita) and soil infection parameters (such as the J2s population per 100 g of soil-1) was synergistically improved.
Cromwell et al. (2014) and Hassan et al. (2016) reported similar findings in which silver nanoparticles reduced root galling and J2 population in infected soil. Hassan et al. (2016) revealed that the combination of fenamiphos and silver nanoparticles resulted in a significant boost in tomato seedling development parameters due to a reduction in J2 population and reproduction factor. Shekoohi et al. (2021) also observed that the combination usage of silicon nanoparticles concentrations increased the efficacy of the nematicide and decreased root infection by M. incognita, as well as M. incognita reproduction in field tests. Our findings suggest that combining BCuNPs with 0.1 RAR of nematicide better reduced nematode reproduction, gall formation, egg masses on roots, and the final population of J2 in the soil, and as a result, the plant growth parameters are improved by lowering the M. incognita population.
It is worth noting that applying all four concentrations of BCuNPs (100, 150, 200, and 250 ppm) 7 days after soil inoculation with M. incognita reduced the number of galls, the number of egg masses, the root gall index, the egg mass index of M. incognita on infected cucumber, and the population density of M. incognita J2s 100 g soil-1 significantly (P<0.05) more effectively than applying all four concentrations of BCuNPs 3 days after soil inoculation with M. incognita or adding the BCuNPs concurrently with M. incognita. Our results also agreed with previous studies in which Eloh et al. (2016) found an EC50 value of 48.6 g mL-1 for CuSO4 against M. incognita. In addition, Yeon et al. (2019) showed that CuSO4 reduced root knot nematode disease in tomatoes in a pot experiment, repressed gall formation on melon, and lowered nematode population density in the soil. Ch et al. (2019) also reported that CuFeNPs increased fresh shoot and root weight in tomato plants while decreasing the root-knot nematode Meloidogyne spp. In addition to this, it is essential to have a solid understanding of the time-resolved interactions that take place between the studied materials, rhizosphere bacterial communities, and nano-pesticides as suggested by Zhang et al. (2020).
Conclusion
The BCuNPs fabricated from B. subtilis subsp. subtilis AM18 in the current study demonstrated significant anti-PPNs activity against M. incognita, Tylenchorhynchus spp., Pratylenchus spp., and Helicotylenchus spp. infecting cucumber under in vitro and in vivo conditions. BCuNPs have shown protective properties that imply they may represent a novel way in preventing the spread of diseases caused by PPNs and, at the same time, can increase crop yields. To maintain or boost crop output while reducing negative environmental impacts, one viable strategy is to optimize the delivery efficiency of BCuNPs with typical pesticide application rates. This can be accomplished by making the appropriate amendments to nanoparticle concentrations and the timing of treatments. Understanding the methods by which BCuNPs protect their host plants against PPNs is a crucial step toward creating effective nanotechnology-enabled disease management plans for sustainable farming. By combining the low application rate of traditional pesticides with nano-pesticides, the efficacy and effectiveness of pesticide active components can be increased, making BCuNPs as bio-pesticides potentially revolutionary for diseases caused by PPNs. However, intensive treatment may change the community of PPNs with other beneficial rhizosphere organisms due to the lack of clarity surrounding the method of action and interaction with other soil species. This study found that BCuNPs are effective nematicides that can be employed alone or to boost the efficiency and ease of delivery of commercial nematicides. However, additional research is required to validate these findings, as well as additional research to understand the unique mode of action of BCuNPs against M. incognita.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
References
Ahamad, L., & Siddiqui, Z. A. (2021). Effects of silicon dioxide, zinc oxide and titanium dioxide nanoparticles on Meloidogyne incognita, Alternaria dauci and Rhizoctonia solani disease complex of carrot. Experimental Parasitology, 230, 108176. https://doi.org/10.1016/j.exppara.2021.108176
Al Banna, L., Salem, N., Ghrair, A. M., & Habash, S. S. (2018). Impact of silicon carbide nanoparticles on hatching and survival of soil nematodes Caenorhabditis elegans and Meloidogyne incognita. Applied Ecology and Environmental Research, 16, 2651–2662. https://doi.org/10.15666/aeer/1603_26512662
Al Banna, L. S., Salem, N. M., Jaleel, G. A., & Awwad, A. M. (2020). Green synthesis of sulfur nanoparticles using Rosmarinus officinalis leaves extract and nematicidal activity against Meloidogyne javanica. Chemistry International, 6, 137–143. https://doi.org/10.5281/zenodo.3528019
Alowaiesh, B. F., Alhaithloul, H. A. S., Saad, A. M., & Hassanin, A. A. (2023). Green biogenic of silver nanoparticles using polyphenolic extract of olive leaf wastes with focus on their anticancer and antimicrobial activities. Plants, 12, 1410. https://doi.org/10.3390/plants12061410
Amin, F., Khattak, B., Alotaibi, A., Qasim, M., Ahmad, I., Ullah, R., Bourhia, M., Gul, A., Zahoor, S., & Ahmad, R. (2021). Green synthesis of copper oxide nanoparticles using Aerva javanica leaf extract and their characterization and investigation of in vitro antimicrobial potential and cytotoxic activities. Evidence-Based Complementary and Alternative Medicine, 18, 2021. https://doi.org/10.1155/2021/5589703
Antil, S., Kumar, R., Pathak, D. V., & Kumari, A. (2023). Recent advances in utilizing bacteria as biocontrol agents against plant parasitic nematodes emphasizing Meloidogyne spp. Biological Control, 183, 105244. https://doi.org/10.1016/j.biocontrol.2023.105244
Ardakani, A. S. (2013). Toxicity of silver, titanium and silicon nanoparticles on the root-knot nematode, Meloidogyne incognita, and growth parameters of tomato. Nematology, 15, 671–677. https://doi.org/10.1163/15685411-00002710
Buazar, F., Sweidi, S., Badri, M., & Kroushawi, F. (2019). Biofabrication of highly pure copper oxide nanoparticles using wheat seed extract and their catalytic activity: A mechanistic approach. Green Processing and Synthesis, 8, 691–702. https://doi.org/10.1515/gps-2019-0040
Caliskan, S., Toppino, L., Boyaci, H. F., Rotino, G. L., & Cebeci, E. (2023). Assessment of new genetic resources to uncover potential nematode resistance traits for eggplant (Solanum melongena) improvement. Phytoparasitica, 1-14. https://doi.org/10.1007/s12600-023-01081-y
Ch, G., Ntalli, N., Menkissoglu-Spiroudi, U., & Dendrinou-Samara, C. (2019). Essential metal-based nanoparticles (copper/iron NPs) as potent nematicidal agents against Meloidogyne spp. Journal of Nanotechnology Research, 1, 044–058. https://doi.org/10.26502/jnr.2688-8521004
Chattopadhyay, S., Dash, S. K., Ghosh, T., Das, D., Pramanik, P., & Roy, S. (2013). Surface modification of cobalt oxide nanoparticles using phosphonomethyl iminodiacetic acid followed by folic acid: A biocompatible vehicle for targeted anticancer drug delivery. Cancer Nanotechnology, 4, 103–116. https://doi.org/10.1007/s12645-013-0042-7
Chen, P., Martinez-Finley, E. J., Bornhorst, J., Chakraborty, S., & Aschner, M. (2013). Metal-induced neurodegeneration in C. elegans. Frontiers in Aging Neuroscience, 5, 18. https://doi.org/10.3389/fnagi.2013.00018
Collange, B., Navarrete, M., Peyre, G., Mateille, T., & Tchamitchian, M. (2011). Root knot nematode (Meloidogyne) management in vegetable crop production: The challenge of an agronomic system analysis. Crop Protection, 30, 1251–1262. https://doi.org/10.1016/j.cropro.2011.04.016
Corsi, I., Desimone, M. F., & Cazenave, J. (2022). Building the bridge from aquatic nanotoxicology to safety by design silver nanoparticles. Frontiers in Bioengineering and Biotechnology, 10, 836742. https://doi.org/10.3389/fbioe.2022.836742
Cromwell, W. A., Yang, J., Starr, J. L., & Jo, Y. K. (2014). Nematicidal effects of silver nanoparticles on root-knot nematode in bermudagrass. Journal of Nematology, 46, 261.
Daoush, A. S. M., Hendawey, M. H., Yaseen, R., El-Nuby, A. S. M., Bedair, T. M., Alwutayd, K. M., Al-Hoshani, N., Shaaban, A., Bashir, A., & Li, L. (2023). Effect of biosynthesized nanoselenium on controlling tomato root-knot nematode Meloidogyne incognita. Agronomy, 13, 1668. https://doi.org/10.3390/agronomy13071668
De Nardo, E. A., & Grewal, P. S. (2003). Compatibility of Steinernema feltiae (Nematoda: Steinernematidae) with pesticides and plant growth regulators used in glasshouse plant production. Biocontrol Science and Technology, 13, 441–448. https://doi.org/10.1080/0958315031000124495
El-Ashry, R. M., Youssif, M. A. I., Elsobki, A. E. A. M., & Helaly, S. M. (2021). Effect of different control agents on Meloidogyne incognita kofoid infesting cucumber plants. Journal of Plant Protection and Pathology, 12, 259–268. https://doi.org/10.21608/jppp.2021.66215.1019
El-Ashry, R. M., El-Saadony, M. T., El-Sobki, A. E., El-Tahan, A. M., Al-Otaibi, S., El-Shehawi, A. M., Saad, A. M., & Elshaer, N. (2022). Biological silicon nanoparticles maximize the efficiency of nematicides against biotic stress induced by Meloidogyne incognita in eggplant. Saudi Journal of Biological Sciences, 29, 920–932. https://doi.org/10.1016/j.sjbs.2021.10.013
El-Deeb, A., El-Ashry, R. M., & El-Marzoky, A. M. (2018). Nematicidal activities of certain animal manures and biopesticides against Meloidogyne incognita infecting cucurbit plants under greenhouse conditions. Journal of Plant Protection and Pathology, 9, 265–271. https://doi.org/10.21608/JPPP.2018.41405
El-Eslamboly, A. A. S. A., Abd El-Wanis, M. M., & Amin, A. W. (2019). Algal application as a biological control method of root-knot nematode Meloidogyne incognita on cucumber under protected culture conditions and its impact on yield and fruit quality. Egyptian Journal of Biological Pest Control, 29, 18. https://doi.org/10.1186/s41938-019-0122-z
Elkobrosy, D., Al-Askar, A. A., El-Gendi, H., Su, Y., Nabil, R., Abdelkhalek, A., & Behiry, S. (2023). Nematocidal and bactericidal activities of green synthesized silver nanoparticles mediated by Ficus sycomorus leaf extract. Life, 13, 1083. https://doi.org/10.3390/life13051083
El-Marzoky, A. M., Elnahal, A. S. M., Jghef, M. M., Abourehab, M. A. S., El-Tarabily, K. A., & Ali, M. A. (2023). Purpureocillium lilacinum strain AUMC 10620 as a biocontrol agent against the citrus nematode Tylenchulus semipenetrans under laboratory and field conditions. European Journal of Plant Pathology. https://doi.org/10.1007/s10658-023-02684-1
Elmer, W. H., & White, J. C. (2016). The use of metallic oxide nanoparticles to enhance growth of tomatoes and eggplants in disease infested soil or soilless medium. Environmental Science Nano, 3, 1072–1079. https://doi.org/10.1039/C6EN00146G
Eloh, K., Demurtas, M., Mura, M. G., Deplano, A., Onnis, V., Sasanelli, N., Maxia, A., & Caboni, P. (2016). Potent nematicidal activity of maleimide derivatives on Meloidogyne incognita. Journal of Agricultural and Food Chemistry, 64, 4876–4881. https://doi.org/10.1021/acs.jafc.6b02250
El-Saadony, M. T., Abd El-Hack, M. E., Taha, A. E., Fouda, M. M. G., Ajarem, J. S., Maodaa, S. N., Allam, A. A., & Elshaer, N. (2020). Ecofriendly synthesis and insecticidal application of copper nanoparticles against the storage pest Tribolium castaneum. Nanomaterials, 10, 587. https://doi.org/10.3390/nano10030587
El-Saadony, M. T., Desoky, E. M., Saad, A. M., Eid, R. S. M., Selem, E., & Elrys, A. S. (2021). Biological silicon nanoparticles improve Phaseolus vulgaris L. yield and minimize its contaminant contents on a heavy metals-contaminated saline soil. Journal of Environmental Sciences, 106, 1–14. https://doi.org/10.1016/j.jes.2021.01.012
El-Saadony, M. T., Saad, A. M., Soliman, S. M., Salem, H. M., Desoky, E. M., Babalghith, A. O., El-Tahan, A. M., Ibrahim, O. M., Ebrahim, A. A. M., Abd El-Mageed, T. A., Elrys, A. S., Elbadawi, A. A., El-Tarabily, K. A., & AbuQamar, S. F. (2022). Role of nanoparticles in enhancing crop tolerance to abiotic stress: A comprehensive review. Frontiers in Plant Science, 13, 946717. https://doi.org/10.3389/fpls.2022.946717
FAOSTAT. (2016). FAOSTAT data sheet cucumbers production in Egypt. Rome, Italy: Food and Agriculture Organization of the United Nations. Accessed 22 Dec 2016.
FAOSTAT. (2020). Data on crop production. Rome, Italy: Food and Agriculture Organization of the United Nations. http://www.fao.org/faostat/en/#home. Accessed 24 Dec 2020.
Fernandez, D., Petitot, A. S., Grossi de Sá, M., Nguyên, V. P., de Almeida Engler, J., & Kyndt, T. (2015). Recent advances in understanding plant-nematode interactions in monocots. Advances in Botanical Research, 73, 189–219. https://doi.org/10.1016/bs.abr.2014.12.006
Fernando, I., & Zhou, Y. (2019). Concentration dependent effect of humic acid on the transformations of silver nanoparticles. Journal of Molecular Liquids, 284, 291–299. https://doi.org/10.1016/j.molliq.2019.04.027
Fu, G., Liu, H., Li, Y., Liu, B., Zhang, S., Ji, X., & Qiao, K. (2023). Evaluation of the biocontrol potential of a natural extract from Paecilomyces variotii against Meloidogyne incognita in cucumber. Plant and Soil, 1-11. https://doi.org/10.1007/s11104-023-05982-z
Getu, T., Mohammed, W., Seid, A., Mekete, T., & Kassa, B. (2023). Evaluation of the reaction of various potato (Solanum tuberosum L.) cultivars to the Meloidogyne incognita and Ralstonia solanacearum disease complex under field conditions. Potato Research, 1-19. https://doi.org/10.1007/s11540-023-09624-w
Ghareeb, R. Y., Shams El-Din, N. G. E. D., Maghraby, D. M. E., Ibrahim, D. S., Abdel-Megeed, A., & Abdelsalam, N. R. (2022). Nematicidal activity of seaweed-synthesized silver nanoparticles and extracts against Meloidogyne incognita on tomato plants. Scientific Reports, 12, 3841. https://doi.org/10.1038/s41598-022-06600-1
Giné, A., & Sorribas, F. J. (2017). Effect of plant resistance and BioAct WG (Purpureocillium lilacinum strain 251) on Meloidogyne incognita in a tomato-cucumber rotation in a greenhouse. Pest Management Science, 73, 880–887. https://doi.org/10.1002/ps.4357
Gkanatsiou, Ch., Ntalli, N., Menkissoglu-Spiroudi, U., & Dendrinou-Samara, C. (2019). Essential metal-based nanoparticles (copper/iron NPs) as potent nematicidal agents against Meloidogyne spp. Journal of Nanotechnology Research, 1, 44–58. https://doi.org/10.26502/fjnr.004
Gohar, I. M. A., Alyamani, A., Shafi, M. E., Mohamed, E. A. E., Ghareeb, R. Y., Desoky, E. M., Hasan, M. E., Zaitoun, A. F., Abdelsalam, N. R., El-Tarabily, K. A., & Elnahal, A. S. M. (2023). A quantitative and qualitative assessment of sugar beet genotype resistance to root-knot nematode. Meloidogyne incognita. Frontiers in Plant Science, 13, 966377. https://doi.org/10.3389/fpls.2022.966377
Gudikandula, K., & Charya Maringanti, S. (2016). Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. Journal of Experimental Nanoscience, 11, 714–721. https://doi.org/10.1080/17458080.2016.1139196
Gunti, L., Dass, R. S., Kalagatur, & N. K., (2019). Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Frontiers in Microbiology 30, 931. https://doi.org/10.3389/fmicb.2019.00931
Gupta, G., Cappellini, F., Farcal, L., Gornati, R., Bernardini, G., & Fadeel, B. (2022). Copper oxide nanoparticles trigger macrophage cell death with misfolding of Cu/Zn superoxide dismutase 1 (SOD1). Particle and Fibre Toxicology, 19, 33. https://doi.org/10.1186/s12989-022-00467-w
Hassan, M. E., Zawam, H. S., El-Nahas, S. E., & Desoukey, A. F. (2016). Comparison study between silver nanoparticles and two nematicides against Meloidogyne incognita on tomato seedlings. Plant Pathology Journal, 15, 144–151.
Hidangmayum, A., Debnath, A., Guru, A., Singh, B., Upadhyay, S., & Dwivedi, P. (2022). Mechanistic and recent updates in nano-bioremediation for developing green technology to alleviate agricultural contaminants. International Journal of Environmental Science and Technology, 1–26. https://doi.org/10.1007/s13762-022-04560-7
Hooper, D. J., Hallmann, J., & Subbotin, S. A. (2005). Methods for extraction, processing and detection of plant and soil nematodes. In M. Luc, R. A. Sikora, & J. Bridge (Eds.), Plant Parasitic Nematodes in Subtropical And Tropical Agriculture. (2nd ed., pp. 53–86). Wallingford, UK: CABI Publishing. https://doi.org/10.1079/9780851997278.0053
Huang, B., Wang, Q., Guo, M., Fang, W., Wang, X., Wang, Q., Yan, D., Ouyang, C., Li, Y., & Cao, A. (2019). The synergistic advantage of combining chloropicrin or dazomet with fosthiazate nematicide to control root-knot nematode in cucumber production. Journal of Integrative Agriculture, 18, 2093–2106. https://doi.org/10.1016/S2095-3119(19)62565-7
Hunt, D. J., & Handoo, Z. A. (2009). Taxonomy, identification and principal species. In R. N. Perry, M. Moens, & J. L. Starr (Eds.), Root-knot Nematodes (pp. 55–97). Cambridge, MA (USA): CABI International.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter, 57, 1025–1028.
Jadhav, S. R., Shah, R. M., & Palombo, E. A. (2021). MALDI-TOF MS: A rapid methodology for identifying and subtyping Listeria monocytogenes. In E. M. Fox, H. Bierne & B. Stessl (Eds.), Listeria monocytogenes. Methods in Molecular Biology (vol. 2220, pp. 17–29). New York, NY: Humana. https://doi.org/10.1007/978-1-0716-0982-8_2
Jagdale, S., Rao, U., & Giri, A. P. (2021). Effectors of root-knot nematodes: An arsenal for successful parasitism. Frontiers in Plant Sciences, 12, 800030. https://doi.org/10.3389/fpls.2021.800030
Jain, N., Bhargava, A., Tarafdar, J. C., Singh, S. K., & Panwar, J. (2013). A biomimetic approach towards synthesis of zinc oxide nanoparticles. Applied Microbiology and Biotechnology, 97, 859–869. https://doi.org/10.1007/s00253-012-3934-2
John, M. S., Nagoth, J. A., Zannotti, M., Giovannetti, R., Mancini, A., Ramasamy, K. P., Miceli, C., & Pucciarelli, S. (2021). Biogenic synthesis of copper nanoparticles using bacterial strains isolated from an Antarctic consortium associated to a psychrophilic marine ciliate: Characterization and potential application as antimicrobial agents. Marine Drugs, 19, 263. https://doi.org/10.3390/md19050263
Johnson, L. F., & Curl, E. A. (1972). Methods for Research on the Ecology of Soil-Borne Plant Pathogens (p. 247). Minneapolis, MN: Burgess Publishing Company.
Jones, J. T., Haegeman, A., Danchin, E. G., Gaur, H. S., Helder, J., Jones, M. G., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J. E., Wesemael, W. M., & Perry, R. N. (2013). Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology, 14, 946–961. https://doi.org/10.1111/mpp.12057
Kassam, R., Jaiswal, N., Hada, A., Phani, V., Yadav, J., Budhwar, R., Godwin, J., Chatterjee, M., Bhar, C. G., Mishra, J., Rana, V. S., Kundu, A., Chawla, G., Somvanshi, V. S., & Rao, U. (2023). Evaluation of Paecilomyces tenuis producing Huperzine A for the management of root-knot nematode Meloidogyne incognita (Nematoda: Meloidogynidae). Journal of Pest Science, 96, 723–743. https://doi.org/10.1007/s10340-022-01521-4
Kate, A., Sahu, L. K., Pandey, J., Mishra, M., & Sharma, P. K. (2022). Green catalysis for chemical transformation: The need for the sustainable development. Current Research in Green and Sustainable Chemistry, 5, 100248. https://doi.org/10.1016/j.crgsc.2021.100248
Kathiresan, K., Manivannan, S., Nabeel, M., & Dhivya, B. (2009). Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids and Surfaces B: Biointerfaces, 71, 133–137. https://doi.org/10.1016/j.colsurfb.2009.01.016
Khan, M., & Tanaka, K. (2023). Purpureocillium lilacinum for plant growth promotion and biocontrol against root-knot nematodes infecting eggplant. PLOS One, 18, e0283550. https://doi.org/10.1371/journal.pone.0283550
Kumar, V., Khan, M. R., & Walia, R. (2020). Crop loss estimations due to plant-parasitic nematodes in major crops in India. National Academy Science Letters, 43, 409–412. https://doi.org/10.1007/s40009-020-00895-2
Kuppamuthu, K., Soundiraraj, S., & Palanisamy, K. (2017). Utilization of whey as a cheap substrate for the optimization of lipase production by Bacillus subtilis B10 isolated from dairy industry. Journal of Microbiology, Biotechnology and Food Sciences, 7, 193–198. https://doi.org/10.15414/jmbfs.2017.7.2.193-198
Li, B., Ren, Y., Zhang, D. X., Xu, S., Mu, W., & Liu, F. (2018). Modifying the formulation of abamectin to promote its efficacy on southern root-knot nematode (Meloidogyne incognita) under blending-of-soil and root-irrigation conditions. Journal of Agricultural and Food Chemistry, 66, 799–805. https://doi.org/10.1021/acs.jafc.7b04146
Logan, N. A., & De Vos, P. (2009). Genus I Bacillus Cohn 1872, 174AL. In P. De Vos, G. M. Garrity, D. Jones, N. R. Krieg, W. Ludwig, F. A. Rainey, K. H. Schleifer, & W. B. Whitman (Eds.), Bergey’s Manual of Systematic Bacteriology. The Firmicutes (2nd ed., Vol. 3, pp. 21–128). New York, USA: Springer.
Ma, H., Bertsch, P. M., Glenn, T. C., Kabengi, N. J., & Williams, P. L. (2009). Toxicity of manufactured zinc oxide nanoparticles in the nematode Caenorhabditis elegans. Environmental Toxicology and Chemistry, 28, 1324–1330. https://doi.org/10.1897/08-262.1
Mahboub, H. H., Rashidian, G., Hoseinifar, S. H., Kamel, S., Zare, M., Ghafarifarsani, H., Algharib, S. A., Moonmanee, T., & Van Doan, H. (2022). Protective effects of Allium hirtifolium extract against foodborne toxicity of zinc oxide nanoparticles in common carp (Cyprinus carpio). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 257, 109345. https://doi.org/10.1016/j.cbpc.2022.109345
Mendez, M. A. A., Martinez, E. S. M., Arroyo, L. O., Portillo, G. C., & Espindola, E. S. (2011). Synthesis and characterization of silver nanoparticles: Effect on phytopathogen Colletotrichum gloeosporioides. Journal of Nanoparticle Research, 13, 2525–2532. https://doi.org/10.1007/s11051-010-0145-6
Mesa-Valle, C. M., Garrido-Cardenas, J. A., Cebrian-Carmona, J., Talavera, M., & Manzano-Agugliaro, F. (2020). Global research on plant nematodes. Agronomy, 10, 1148. https://doi.org/10.3390/agronomy10081148
Moens, M., Perry, R. N., & Starr, J. L. (2009). Meloidogyne species - a diverse group of novel and important plant parasites. In R. N. Perry, M. Moens, & J. L. Starr (Eds.), Root-Knot Nematodes (pp. 1–17). Wallingford, UK: CABI. https://doi.org/10.1079/9781845934927.0001
Mohamed, E. A., Elsharabasy, S. F., & Abdulsamad, D. (2019). Evaluation of in vitro nematicidal efficiency of copper nanoparticles against root-knot nematode Meloidogyne incognita. South Asian Journal of Parasitology, 2, 1–6. https://doi.org/10.9734/SAJP/2019/46065
Nie, W., Liu, L., Chen, Y., Luo, M., Feng, C., Wang, C., Yang, Z., & Du, C. (2023). Identification of the regulatory role of SlWRKYs in tomato defense against Meloidogyne incognita. Plants, 12, 2416. https://doi.org/10.3390/plants12132416
Noureddine, Y., Mejias, J., da Rocha, M., Thomine, S., Quentin, M., Abad, P., Favery, B., & Jaubert-Possamai, S. (2022). Copper microRNAs modulate the formation of giant feeding cells induced by the root knot nematode Meloidogyne incognita in Arabidopsis thaliana. New Phytologist, 236, 283–295. https://doi.org/10.1111/nph.18362
Oka, Y., Koltai, H., Bar-Eyal, M., Mor, M., Sharon, E., Chet, I., & Spiegel, Y. (2000). New strategies for the control of plant-parasitic nematodes. Pest Management Science, 56, 983–988. https://doi.org/10.1002/1526-4998(200011)56:11%3c983::AID-PS233%3e3.0.CO;2-X
Park, E.-J., Jang, H.-J., Park, J.-Y., Yang, Y. K., Kim, M. J., Park, C. S., Lee, S., Yun, B.-S., Lee, S.-J., Lee, S. W., & Rho, M.-C. (2023). Efficacy evaluation of Streptomyces nigrescens KA-1 against the root-knot nematode Meloidogyne incognita. Biological Control, 179, 105150. https://doi.org/10.1016/j.biocontrol.2023.105150
Parsiaaref, S., Cao, A., Li, Y., Ebadollahi, A., Parmoon, G., Wang, Q., Yan, D., Fang, W., & Zhang, M. (2023). Nematicidal and toxicity effects of Eupatorium adenophorum Spreng against the root-knot nematode Meloidogyne incognita in soil producing cucumber. Agriculture, 13, 1109. https://doi.org/10.3390/agriculture13061109
Poma, A., Colafarina, S., Fontecchio, G., & Chichiriccò, G. (2014). Transgenerational effects of NMs. Advances in Experimental Medicine and Biology, 811, 235–254. https://doi.org/10.1007/978-94-017-8739-0_12
Pourchez, J., Forest, V., Boumahdi, N., Boudard, D., Tomatis, M., Fubini, B., Herlin-Boime, N., Leconte, Y., Guilhot, B., Cottier, M., & Grosseau, P. (2012). In vitro cellular responses to silicon carbide nanoparticles: impact of physico-chemical features on pro-inflammatory and pro-oxidative effects. Journal of Nanoparticle Research, 14, 1143. https://doi.org/10.1007/s11051-012-1143-7
Radwan, M. A., Farrag, S. A. A., Abu-Elamayem, M. M., & Ahmed, N. S. (2012). Biological control of the root-knot nematode, Meloidogyne incognita on tomato using bioproducts of microbial origin. Applied Soil Ecology, 56, 58–62. https://doi.org/10.1016/j.apsoil.2012.02.008
Rai, M., & Ingle, A. (2012). Role of nanotechnology in agriculture with special reference to management of insect pests. Applied Microbiology and Biotechnology, 94, 287–293. https://doi.org/10.1007/s00253-012-3969-4
Rajput, V. D., Minkina, T., Upadhyay, S. K., Kumari, A., Ranjan, A., Mandzhieva, S., Sushkova, S., Singh, R. K., & Verma, K. K. (2022). Nanotechnology in the restoration of polluted soil. Nanomaterials, 12, 769. https://doi.org/10.3390/nano12050769
Rastogi, A., Tripathi, D. K., Yadav, S., Chauhan, D. K., Živčák, M., Ghorbanpour, M., El-Sheery, N. I., & Brestic, M. (2019). Application of silicon nanoparticles in agriculture. 3 Biotech, 9, 90. https://doi.org/10.1007/s13205-019-1626-7
Saad, A. M., El-Saadony, M. T., El-Tahan, A. M., Sayed, S., Moustafa, M. A., Taha, A. E., Taha, T. F., & Ramadan, M. M. (2021). Polyphenolic extracts from pomegranate and watermelon wastes as substrate to fabricate sustainable silver nanoparticles with larvicidal effect against Spodoptera littoralis. Saudi Journal of Biological Sciences, 28, 5674–5683. https://doi.org/10.1016/j.sjbs.2021.06.011
Saad, A. M., Salem, H. M., El-Tahan, A. M., El-Saadony, M. T., Alotaibi, S. S., El-Shehawi, A. M., Abd El-Mageed, T. A., Taha, A. E., Alkahtani, M. A., Ezzat Ahmed, A., & Ahmed, A. E. (2022a). Biological control: An effective approach against nematodes using black pepper plants (Piper nigrum L.). Saudi Journal of Biological Sciences, 29, 2047–2055. https://doi.org/10.1016/j.sjbs.2022.01.004
Saad, A. M., Sitohy, M. Z., Sultan-Alolama, M. I., El-Tarabily, K. A., & El-Saadony, M. T. (2022b). Green nanotechnology for controlling bacterial load and heavy metal accumulation in Nile tilapia fish using biological selenium nanoparticles biosynthesized by Bacillus subtilis AS12. Frontiers in Microbiology, 13, 1015613. https://doi.org/10.3389/fmicb.2022.1015613
Sabry, A. K. H. (2019). Role of Nanotechnology applications in plant-parasitic nematode control. In: K. Abd-Elsalam, R. Prasad (Eds.), Nanobiotechnology applications in plant protection. Nanotechnology in the life sciences (pp. 223–240). Cham: Springer. https://doi.org/10.1007/978-3-030-13296-5_12
Sathiyavimal, S., Vasantharaj, S., Veeramani, V., Saravanan, M., Rajalakshmi, G., Kaliannan, T., Al-Misned, F. A., & Pugazhendhi, A. (2021). Green chemistry route of biosynthesized copper oxide nanoparticles using Psidium guajava leaf extract and their antibacterial activity and effective removal of industrial dyes. Journal of Environmental Chemical Engineering, 9, 105033. https://doi.org/10.1016/j.jece.2021.105033
Sauget, M., Valot, B., Bertrand, X., & Hocquet, D. (2017). Can MALDI-TOF mass spectrometry reasonably type bacteria? Trends in Microbiology, 25, 447–455. https://doi.org/10.1016/j.tim.2016.12.00
Schumaker, S., Borror, C. M., & Sandrin, T. R. (2012). Automating data acquisition affects mass spectrum quality and reproducibility during bacterial profiling using an intact cell sample preparation method with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Communications in Mass Spectrometry, 26, 243–253. https://doi.org/10.1002/rcm.5309
Shang, H., Ma, C., Li, C., White, J. C., Polubesova, T., Chefetz, B., & Xing, B. (2020). Copper sulfide nanoparticles suppress Gibberella fujikuroi infection in rice (Oryza sativa L.) by multiple mechanisms: contact-mortality, nutritional modulation and phytohormone regulation. Environmental Science: Nano, 7, 2632–2643. https://doi.org/10.1039/D0EN00535E
Shekoohi, S. S., Charehgani, H., Abdollahi, M., & Rajabi, H. R. (2021). Combined effect of β-aminobutyric acid and silver nanoparticles on eggplants, Solanum melongena, infected with Meloidogyne javanica. Nematology, 50, 1–16.
Shoaib, R. M., Abdel-Razik, A., Ebeed, N. M., Esmail, R. M., & Taha, E. H. (2022). Impact of engineered nano silver on root-knot nematode, Meloidogyne incognita and on DNA damage in tomato plants under screen house conditions. Egyptian Journal of Chemistry, 65, 171–187. https://doi.org/10.21608/EJCHEM.2021.97189.4547
Singh, D., Jain, D., Rajpurohit, D., Jat, G., Kushwaha, H. S., Singh, A., Mohanty, S. R., Al-Sadoon, M. K., Zaman, W., & Upadhyay, S. K. (2023). Bacteria assisted green synthesis of copper oxide nanoparticles and their potential applications as antimicrobial agents and plant growth stimulants. Frontiers in Chemistry, 11, 1154128. https://doi.org/10.3389/fchem.2023.1154128
Singh, A., Sharma, P., Kumari, A., Kumar, R., & Pathak, D. V. (2019). Management of root-knot nematode in different crops using microorganisms. In A. Varma, S. Tripathi & R. Prasad (Eds.), Plant Biotic Interactions (pp. 85–99). Springer Cham, Switzerland. https://doi.org/10.1007/978-3-030-26657-8_6
Smaoui, S., Chérif, I., Hlima, H. B., Khan, M. U., Rebezov, M., Thiruvengadam, M., Sarkar, T., Shariati, M. A., & Lorenzo, J. M. (2023). Zinc oxide nanoparticles in meat packaging: A systematic review of recent literature. Food Packaging and Shelf Life, 36, 101045. https://doi.org/10.1016/j.fpsl.2023.101045
Soetaert, K., Muthumbi, A., & Heip, C. (2002). Size and shape of ocean margin nematodes: morphological diversity and depth-related patterns. Marine Ecology Progress Series, 242, 179–193. https://doi.org/10.3354/meps242179
Sukumar, S., Rudrasenan, A., & Padmanabhan Nambiar, D. (2020). Green-synthesized rice-shaped copper oxide nanoparticles using Caesalpinia bonducella seed extract and their applications. ACS Omega, 5, 1040–1051. https://doi.org/10.1021/acsomega.9b02857
Taha, E. H., & Abo-Shady, N. M. (2016). Effect of silver nanoparticles on the mortality pathogenicity and reproductivity of entomopathogenic nematodes. International Journal of Zoological Research, 12, 47–50. https://doi.org/10.3923/ijzr.2016.47.50
Talavera-Rubia, M., Vela-Delgado, M. D., & Verdejo-Lucas, S. (2020). Nematicidal efficacy of milbemectin against root-knot nematodes. Plants, 9, 839. https://doi.org/10.3390/plants9070839
Taylor, A., & Sasser, J. (1978). Biology, identification and control of root-knot nematodes. In International Nematology Project. North Carolina State University Graphics (vol. 111). Raleigh, NC: North Carolina State University.
Velicogna, J. R., Schwertfeger, D., Jesmer, A., Beer, C., Kuo, J., DeRosa, M. C., Scroggins, R., Smith, M., & Princz, J. (2021). Soil invertebrate toxicity and bioaccumulation of nano copper oxide and copper sulphate in soils, with and without biosolids amendment. Ecotoxicology and Environmental Safety, 217, 112222. https://doi.org/10.1016/j.ecoenv.2021.112222
Velsankar, K., Suganya, S., Muthumari, P., Mohandoss, S., & Sudhahar, S. (2021). Ecofriendly green synthesis, characterization and biomedical applications of CuO nanoparticles synthesized using leaf extract of Capsicum frutescens. Journal of Environmental Chemical Engineering, 9, 106299. https://doi.org/10.1016/j.jece.2021.106299
Wallyn, J., Anton, N., & Vandamme, T. F. (2019). Synthesis, principles, and properties of magnetite nanoparticles for in vivo imaging applications—A review. Pharmaceutics, 11, 601. https://doi.org/10.3390/pharmaceutics11110601
Wang, D., Zhang, H., Fan, Y., Cao, R., Gao, Y., & Chen, J. (2020). Synergistic effect of mixed Cu and Fe oxides and chlorides on electrophilic chlorination of dibenzo-p-dioxin and dibenzofuran. Science of the Total Environment, 721, 137563. https://doi.org/10.1016/j.scitotenv.2020.137563
Wang, J., Guo, C., Zhao, P., Yu, F., Su, Y., Qu, J., Wang, J., Lin, R., Wang, B., Gao, Z., Yang, Z., & Zhou, B. (2021). Biocontrol potential of Bacillus altitudinis AMCC1040 against root-knot nematode disease of ginger and its impact on rhizosphere microbial community. Biological Control, 158, 104598. https://doi.org/10.1016/j.biocontrol.2021.104598
Wei, C. C., Yen, P. L., Chaikritsadakarn, A., Huang, C. W., Chang, C. H., & Liao, V. H. (2020). Parental CuO nanoparticles exposure results in transgenerational toxicity in Caenorhabditis elegans associated with possible epigenetic regulation. Ecotoxicology and Environmental Safety, 203, 111001. https://doi.org/10.1016/j.ecoenv.2020.111001
Williams, S. T., Shameemullah, M., Watson, E. T., & Mayfield, C. I. (1972). Studies on the ecology of actinomycetes in soil - VI. The influence of moisture tension on growth and survival. Soil Biology and Biochemistry, 4, 215–225. https://doi.org/10.1016/0038-0717(72)90014-4
Williamson, V. M., & Kumar, A. (2006). Nematode resistance in plants: the battle underground. Trends in Genetics, 22, 396–403. https://doi.org/10.1016/j.tig.2006.05.003
Yeon, J., Park, A. R., Kim, Y. J., Seo, H. J., Yu, N. H., Ha, S., Park, H. W., & Kim, J.-C. (2019). Control of root-knot nematodes by a mixture of maleic acid and copper sulfate. Applied Soil Ecology, 141, 61–68. https://doi.org/10.1016/j.apsoil.2019.05.010
Yin, N., Liu, R., Zhao, J. L., Khan, R. A. A., Li, Y., Ling, J., Liu, W., Yang, Y. H., Xie, B. Y., & Mao, Z. C. (2021). Volatile organic compounds of Bacillus cereus strain Bc-cm103 exhibit fumigation activity against Meloidogyne incognita. Plant Disease, 105, 904–911. https://doi.org/10.1094/PDIS-04-20-0783-RE
Zasada, I. A., Halbrendt, J. M., Kokalis-Burelle, N., LaMondia, J., McKenry, M. V., & Noling, J. W. (2010). Managing nematodes without methyl bromide. Annual Review of Phytopathology, 48, 311–328. https://doi.org/10.1146/annurev-phyto-073009-114425
Zhai, Y., Monikh, F. A., Wu, J., Grillo, R., Arenas-Lago, D., Darbha, G. K., Vijver, M. G., & Peijnenburg, W. J. (2020). Interaction between a nano-formulation of atrazine and rhizosphere bacterial communities: atrazine degradation and bacterial community alterations. Environmental Science: Nano, 7, 3372–3384. https://doi.org/10.1039/D0EN00638F
Zhang, L., Wu, L., Mi, Y., & Si, Y. (2019). Silver nanoparticles induced cell apoptosis, membrane damage of Azotobacter vinelandii and Nitrosomonas europaea via generation of reactive oxygen species. Bulletin of Environmental Contamination and Toxicology, 103, 181–186. https://doi.org/10.1007/s00128-019-02622-0
Zhang, X., Xu, Z., Qian, X., Lin, D., Zeng, T., Filser, J., Li, L., & Kah, M. (2020). Assessing the impacts of Cu (OH) 2 nanopesticide and ionic copper on the soil enzyme activity and bacterial community. Journal of Agricultural and Food Chemistry, 68, 3372–3381. https://doi.org/10.1021/acs.jafc.9b06325
Zhang, M., Zhang, H., Tan, J., Huang, S., Chen, X., Jiang, D., & Xiao, X. (2021). Transcriptome analysis of eggplant root in response to root-knot nematode infection. Pathogens, 10, 470. https://doi.org/10.3390/pathogens10040470
Zhang, W., Sun, W., Wang, Y., Liu, H., Zhang, S., Dong, B., Ji, X., & Qiao, K. (2022). Management of Meloidogyne incognita on cucumber with a new non fumigant nematicide fluopimomide. Plant Disease, 106, 151–155. https://doi.org/10.1094/PDIS-05-21-0943-RE
Zukerman, B. M., & Esnard, J. (1994). Biological control of plant nematodes current status and hypothesis. Japanese Journal of Nematology, 24, 1–13.
Acknowledgment
This project was funded by the Abu Dhabi Award for Research Excellence, Department of Education and knowledge (Grant #: 21S105) to K. A. El-Tarabily. The authors gratefully acknowledge the support of the Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R31), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. In addition, the authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for supporting this work under the grant number (R.G.P.2/121/44).
Author information
Authors and Affiliations
Contributions
Conceptualization, R.M.E.-A., and K.A.E.-T., formal Analysis, M.M.N., A.S., A.M.A., A.E.A., investigation, R.M.E.-A., K.A.E.-T., F.S.A., A.M.A., A.I.A., M.A.A.H., and S.E.A., data curation, M.M.N., A.S., A.M.A., and A.E.A., writing original draft preparation S.A.A., A.A., F.S.A., A.M.A., A.I.A., and K.A.E.-T., writing final manuscript and editing, R.M.E.-A., and K.A.E.-T., Visualization and Methodology, R.M.E.-A., K.A.E.-T, M.M.N., A.S., A.M.A., A.E.A., S.A.A., A.A., F.S.A., A.M.A., A.I.A., M.A.A.H., and S.E.A., All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
El-Ashry, R.M., Nader, M.M., Shami, A. et al. Ecofriendly synthesis and nematicidal application of copper nanoparticles fabricated from Bacillus subtilis AM18, against root-knot nematode of cucumber. Eur J Plant Pathol 168, 53–81 (2024). https://doi.org/10.1007/s10658-023-02727-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-023-02727-7