Abstract
This study evaluates plant growth promotion and the suppressive effect of the burrowing nematode (Radopholus similis) in banana Musa AAA cv Grande Naine, by two ensilaged biostimulants (EBSs) in two greenhouse trials and in two different commercial farms. Conductive (CS) and suppressive (SS) soils to plant parasitic nematodes were used for EBSs production. The EBSs were incorporated in the growth substrate at 10% w/w before planting the in vitro banana plants and before R. similis inoculation in the greenhouse trials. In commercial banana plantations, the treatments were applied every four months by incorporating 500 g of the EBS into the soil in front of the successional sucker of each banana plant. The results showed that both EBSs were effective, stimulating the root growth and reducing R. similis. The EBS with CS reduced R. similis consistently in greenhouse and field evaluations. The data suggests the potential of EBSs to promote unfavorable conditions for the burrowing nematode reproduction and more favorable conditions to the development and production of the crop, which could contribute to promote more sustainable banana production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to the Food and Agriculture Organization of the United Nations [FAO] (2020), bananas are the fruits with the biggest international consumption. During 2020, the global banana market generated more than US$22.8 billion. However, this crop faces serious phytosanitary problems that place its global production at risk. Such is the case of the new variant of “fusariosis” whose causal agent is the phytoparasitic fungus Fusarium oxysporum f. sp. cubense tropical race 4 (Dita et al., 2018). Banana is also affected by plant parasitic nematodes, such as Radopholus similis, Helicotylenchus multicinctus, Meloidogyne spp. and Pratylenchus coffeae (Araya, 2004). These pathogens could destroy the banana root system with their stylet, causing plant collapse or severely reducing bunch weight (Araya & Vargas, 2018; Bechem et al., 2018; Jaramillo et al., 2019; Thammaiah et al., 2019). Chemical nematicides are the most common tools to control nematodes in this crop (Vargas et al., 2006; Aguirre et al., 2016). Currently, the international banana market demands fruits with less chemical residues, grown more environmentally friendly and with safer conditions for labor workers. In the medium long term, these commercial and environmental requirements could partially or totally restrict the use of nematicides (Das and Borgoain, 2018).
Most of the banana-producing areas in the world have favorable or conductive soil conditions for the reproduction and establishment of R. similis (Aguirre et al., 2016). On the other hand, in some banana-producing regions, farms have also been found where throughout the year the populations of R. similis remain well below the economic threshold of 8000 individuals per 100 g of root (Zum Felde et al., 2005; Vargas et al., 2012; Montenegro, 2013). The type of soil in these areas is called “suppressive”, since plant parasitic nematodes do not survive in them despite the existence of favorable environmental conditions and the presence of both a susceptible host and a virulent inoculum (Cook & Baker, 1983). Such suppressiveness can be attributed to biotic or abiotic factors associated with the rhizosphere (Akhtar et al., 2015, Steinberg et al., 2019). An important characteristic of these soils is that, when sterilized, they lose their suppressiveness (Mendes et al., 2011; Vargas et al., 2012). In some cases, suppressiveness could be transferred to conductive zones by removal and transport of soils between areas (Westphal, 2005); however, for banana producers, this would not be profitable. On the other hand, detecting and extracting biological control agents (BCA) from suppressive soils is limited, because the majority of soil microbiota (99%) cannot be cultivated in artificial media (Paul, 2015).
Currently, the main integrated nematode management strategy is based on increasing local biological diversity to stimulate the natural suppressive potential present in most agricultural soils (Stirling 2014., Akhtar et al., 2015, Steinberg et al., 2019). It is assumed that greater biological diversity leads to greater natural suppressiveness, since pathogens face greater antagonism, predation, and competition with other local biotic communities seeking nutrients and energy (Westphal, 2005). The stimulation of suppressiveness could be obtained through the addition of different organic amendments and carbon sources enriched with BCA (Stirling, 2014). In this sense, various cultural practices, including the use of cover and rotation crops, organic and mineral fertilizers, dolomitic lime, as well as reduced tillage systems, could improve the quality and health of the soil and its potential capacity to suppress root pathogens (Abawi & Widmer, 2000; Segura et al., 2021; Segura-Mena et al., 2021). The use of amendments with higher concentrations of beneficial microbes produced by fermentation during a silage or composting process, could help control soil-borne diseases including fusarium wilt disease in banana (Shen et al., 2013; Zhang et al., 2014). During the silage process, organic acids are synthesized by fermentation of the sugar present in the molasses, which reduces the pH. This low acidity preserves the biostimulant, stimulates beneficial microbiota and reduces pathogens, especially phytopathogenic bacteria. Besides the potential multiplication of biocontrol agents, this type of fermentation induces the multiplication of lactic bacteria, recently detected as helpful in the control of plant-endoparasitic sedentary nematodes such as Meloidogyne spp. (Seo et al., 2019). However, many of these ecological-friendly measures have not been studied in detail to control species with mobile endoparasitic ecology such as R. similis.
The incorporation of EBSs near the rhizosphere in conductive soils, could increase microbiological diversity and suppression of R. similis in the rhizoplane, while increases the organic matter content closer to the roots. These organic fermentation techniques are currently used in banana organic farming in Costa Rica with successful results in the reduction of diseases and an increase in growth of banana plants. These favorable results in organically cultivating farms, prompted us to study this technique in two identical experiments in greenhouse and two identical experiments in commercial plantations, in order to determine if the application of suppressive or conductive soils from the ensilage processes, reduce R. similis populations and improve plant growth in banana crops.
Materials and methods
Ensilaged bio-stimulant preparation for greenhouse and field experiments
Both EBSs were developed through an adaptation of the methodology of Torres Pérez et al. (2022). Said adaptation consisted of replacing the forest mulch that the researchers used as a biological additive for silage fermentation, with filtered aqueous suspensions 1/7 v/v of native soil (conductive or suppressant) and water. The remaining procedure was followed according to the indicated methodology.
Nematode inoculum preparation for greenhouse experiments
The population of R. similis were isolated in a commercial banana farm from the Siquirres Canton, Costa Rica. The roots were liquefied and sifted according to Taylor and Loegering (1953). A sample of the liquefied material (200 g) was taken to extract R. similis using the modified Baermann funnel technique (Hallmann & Subbotin, 2018). Slides with nematodes were checked using a binocular microscope and the morphological description of Sikora et al., (2018) was used for R. similis identification. The collected nematodes were reproduced in carrot disks according to the protocol of Speijer & De Waele (1997). To preserve active R. similis specimens, individuals were transferred to new carrot discs every 3 months and kept at 25 °C for reproduction.
Greenhouse experiments
The experiments were established in a greenhouse of the Research Center at the National Banana Corporation (CORBANA), Costa Rica. (10° 15′ 54″N and 83° 46′ 26″W). The first experiment was established between January and April 2020, with potting soil from Guácimo Canton, Limón Province (Soil 1). The second was carried out between April and July 2020 with soil from Siquirres Canton, Limón Province (Soil 2). Soil 1 consisted in 78% sand, 4% silt and 18% clay. Each 100 g of Soil 1 contained 250 R. similis, 1250 H. multicinctus, 500 Megalaima incognita and 250 P. coffeae individuals. The Soil 2 had 38% sand, 24% silt, and 38% clay. Each 100 g of this soil contained 125 R. similis, 125 H. multicinctus, 125 M. incognita, and no P. coffeae was found. The texture of the substrates was measured by an adaptation of the Bouyoucos methodology (Beretta et al., 2014). The Baermann funnel was used for soil nematodes extraction (Cesarz et al., 2019).
The treatments were as follows: T1: Untreated control, T2: Nematicide Oxamyl 24% of a.i. in liquid suspension (LS) (100 ppm a.i. /plant, applied 15 days after transplant), T3: EBS with SC (128 g of DM/plant) and T4: EBS with SS (128 g of DM/plant). The experimental design was completely randomized with 15 replications per treatment. To apply EBSs treatments, the unsterilized soil substrate was mixed with EBS CS or EBS SS at 10% w/w, respectively. Seven days after, the plastic pots of 1.8 L were filled with the prepared substrates and a phase IV in vitro banana plant (plant 10 cm high and three developed leaves) was planted in each pot (Fig. 1A). Each pot was inoculated 15 days after transplanting with approximately 500 nematodes (adults and juveniles) of R. similis.
Twenty days after planting, as preventive control of black sigatoka (Pseudocercospora fijiensis Morelet) Mancozeb at 60% of a.i. in encapsulated suspension (ES) (30 g a.i./L) was applied every 15 days. Fertilization was done once a week with Hoagland solution (100 ml/pot).
The experiments ended 90 days after sowing. For data collection, fresh weight (g) of root, pseudostem and foliage was measured using an electronic balance as well as the height (cm) from the base to the point of intersection between the sheaths of the last two leaves and the diameter of the pseudostem (cm) at the base of each plant. Also, the number of R. similis, H. multicinctus, M. incognita, P. coffeae were counted. Thereafter, total nematodes (sum of the four genera of nematodes) in 100 g per roots was calculated. Nematodes were extracted and quantified following Taylor and Loegering (1953).
Field experiment
Two identical experiments were set up in two commercials bananas fields. The first experiment was established between August 2020 and January 2021 in Guácimo Canton (10° 15′ 52″ N and 83° 38′ 15″ W) in soil with 78% sand, 4% silt and 18% clay of a 6-years-old banana crop. During the experimental period, this area registered a monthly average precipitation of 279.2 mm, 25.5 °C and a relative humidity of 87.4%. The second experiment was carried out between March and August 2021 in Siquirres Canton (10° 6′ 56″ N and 83° 29′ 25″ W) in soil with 51% sand, 42% silt and 7% clay of a 10-year-old banana crop. During the experimental period, this zone registered a monthly average precipitation of 316.4 mm, 25.8 °C of temperature and 90.5% of relative humidity. The texture of the soils was measured by an adaptation of the Bouyoucos methodology (Beretta et al., 2014). Both experimental areas: a) were planted with the Grande Naine cultivar (Musa AAA Cavendish subgroup) at a density of 1700 plants/ha, b) were divided in rectangular sections of land of approximately 25 × 50 m separated by drainage canals, where banana was planted.
The treatments consisted of T1: Untreated control, T2: EBS with SC (250 g of DM/plant) and T3: EBS with SS (250 g of DM/plant). The experiment was established under a randomized complete block design with 6 replicates. The experimental unit was formed in a plot with approximately 80 plants. One month before starting the experiment, all plants were injected with 1.2 g a.i. of Oxamyl at 24% in LS to standardize nematode populations throughout the experimental area. During the experimental period of 6 months, a total of two applications were made, one at the beginning of the experiment and one four months later. EBSs were incorporated into the soil near the root system of the youngest plant (sucker) (Fig. 1B). The total area of each experiment was 1.2 ha with 1440 plants. No nematicide molecules were included in either of the two field evaluations.
For data collection, repeated measurements were made each month for a total of 6 samplings for the monitoring of nematode populations and biometric variables of the root. The fresh weight of the total and functional root (g), percentage of functional root (%), which consists of the proportion of the weight in grams of the healthy root tissue with respect to the weight of the necrotic root, were measured. Root sampling was performed as follows: 4 recently flowered plants were selected from each plot. Roots of 6750 mL of soil collected in front of the sucker of each productive unit were subtracted. The production unit is made up of the pseudostem of three plants united in successional phenological phases. The roots collected from the 4 selected plants were pooled to form a composite sample per plot. The number of R. similis, H. multicinctus, M. incognita and total nematodes (summatory of the individuals of R. similis, H. multicinctus and M. incognita) in 100 g of root were also evaluated. In order to quantify the weight variables and the number of root nematodes, the method of Taylor & Loegering (1953) was used.
Statistical analysis
For the data from the greenhouse experiments, the biometric variables were analysed using analysis of variance (ANOVA) after normality evaluation. The number of each particular nematode genera was studied by analysis of deviance (McCullagh & Nelder, 1989) with log(x + 1) as transformation function, where x is the dependent variable, and errors are assumed to be negative binomially distributed. For the analysis of the field experiments, average per plot across the monthly evaluations were obtained for every measured variable (including the nematode variables) and compared with ANOVA after normality evaluation. Treatments means were compared with the Tukey test for all the variables of all experiments. All the analyses were performed in R version 4.0.2 (R Core Team, 2014) with its “lm” function to perform the LM analyses, “glm.nb” function of the “MASS” package (Venables & Ripley, 2002) to perform the GLM analysis, and “emmeans” package (Rusell, 2021) to compare treatment means.
Results
Greenhouse experiment
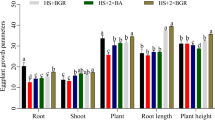
In the first experiment, both EBSs showed a growth-promoting effect (P ≤ 0.0014, Table 1) in plants inoculated with R. similis, which was observed with 32 and 36% more fresh foliar weight, 12 and 13% more height and 12 and 15% more pseudo stem diameter in the plants applied with CS and SS EBS, respectively, when compared to the control (P ≤ 0.05, Table 1). The EBSs promoted greater growth in fresh foliar weight and height when compared to the nematicide (P ≤ 0.0500, Table 1). In the second experiment, only EBS with CS promoted growth in all its variables with respect to the control, nematicide and EBS with SS (P ≤ 0.0075, Table 1).
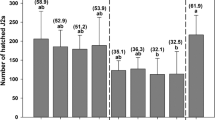
EBS with CS showed a suppressive effect on all nematodes reducing levels of R. similis between 76 and 57%, H. multicinctus between 71 and 69% and total nematodes between 73 and 64% in both repeated experiments, in comparison to the control (P ≤ 0.0500, Table 2). While EBS with SS reduced H. multicinctus by 79%, and total nematodes by 61% in the first experiment, only R. similis was reduced by 60% in the second experiment when compared to the control (P ≤ 0.0500, Table 2).
Field experiment
None of the EBSs promoted root growth expressed as fresh weigh or increased the percentage of functional roots in both experiments (P ≤ 0.7439, Table 3). However, both EBSs showed nematode suppression of R. similis under field conditions. This is supported by the reduction of 48 to 53% and 31 to 43% of R. similis in the first and second experiment in comparison to the control (P ≤ 0.0500, Table 4). The responses to H. multicinctus and total nematodes were variable depending on the site and the EBSs used. In Guácimo, the density of H. multicinctus were only reduced significantly under SS inoculated fields, but both EBSs reduced total nematodes. While in Siquirres, both EBSs were not effective to reduce density of H. multicinctus while EBSs with SS was able to reduce total nematodes.
Discussion
R. similis is one of the plant parasitic pathogens most difficult to control because its migratory endoparasitic behavior, which provides it with a type of armor within the root tissue. On the other hand, the semi perennial behavior of its host (banana), favors its constant reproduction and dissemination (Volcy, 2011; Cobon et al., 2019). This study showed that EBSs with CS had a suppressive effect on R. similis both at the two greenhouse experiments and at the two field evaluations. On the other hand, EBSs with SS were effective in reducing the endoparasite only at one of the two greenhouse evaluations, but they were effective in both field evaluations. In this sense, the native soil in EBSs (CS or SS) and/or the remaining microbiota after the silage process, showed an important interaction with the microbiota present at the soil manipulated for pot experiments (as it is the case for Guácimo soil for R. similis or Siquirres soil for H. multicinctus). Additionally, the inoculation levels of EBSs in all pot was 10% v/v, while at the field, soil levels are far lower and only for the sucker under each production unit. From the side of plant growth stimulation, the EBSs are made up of a diverse group of soil microbiota in which native microorganisms with biostimulant, biocontrol or biofertilizing qualities could be present. Such argument was evidenced on growth variables of the greenhouse evaluation, probably by the quantity of EBSs proportion with respect to the total soil (10% v/v). However, already under field conditions where there are multiple factors of production, the beneficial effects of EBSs on banana growth were probably dispersed.
To elucidate the mechanism that explains the EBSs nematode suppressive effect, it is necessary to consider the changes experienced by the native microbiota of SC and SS during their exposure time to three consecutive stages: a) during the silage process, b) in the rhizosphere after the incorporation of EBSs into the soil, and c) during their subsequent colonization of the rhizoplane, where the highest concentration of potentially nematode-suppressing and radical-stimulating microbiota is presumed to be present. Likewise, once the EBSs were incorporated into the soil, near the banana rhizosphere, a rapid biochemical interaction between the silage microbiota and its metabolites, with the biotic and abiotic factors of the soil, possibly began.
Since R. similis remains sheltered within the root environment, it seems likely that the suppression of the endoparasite is due to the activation of defensive mechanisms of the plant due to the effect of EBSs, or to some biological control agent present in the EBS that could act as an endophyte limiting the access or reproduction of the nematode within the intraradical environment (Poveda et al., 2020). The EBSs raw materials included melina sawdust (Gmelina arborea), carpet grass (Axonopus compressus), semoline, and molasses, which, once fermented, were converted into sugars, amino acids, enzymes, cellulose, lignin, and organic acids. The elicitors of the activation of these defense mechanisms could come from prebiotic molecules synthesized from the biological decomposition of raw materials formed at the EBSs process in the soil. Selby et al., (2016) presented evidence, that aqueous extracts of four ray grass (Lolium perenne) cultivars showed elicitor qualities in annual crops, demonstrating that such metabolites may affect soil-friendly environment for nematodes.
On the other hand, during the biological decomposition of EBSs, constituting materials near the root, native microbiota could have synthesized a series of by-products, metabolites, elicitors-effectors with prebiotic qualities near the rhizoplane, which could have promoted the suppression of R. similis (Bonanomi et al., 2020; Trabelsi & Mhamdi, 2013; Xue et al., 2015). Likewise, during the decomposition of these EBSs at the soil, different fungal growth was also observed at the applied area, which seems to be the interaction between the microbiota of the EBSs and the native microbiota of the soil. Besides, EBSs probably promoted phytohormone-producing microbiota, phosphorus and potassium solubilizers, as well as free-living nitrogen fixers with biofertilizing or biostimulant qualities that promoted healthier root tissue in the plant (Bonanomi et al., 2010). Even thought, EBSs with SC reduced H. multicinctus at the two greenhouse evaluations, the same were not effective at the field level. In contrast, EBSs with SS reduced H. multicintus in both greenhouse and field experiments. This effect on different plant-parasitic nematode species gave us the point that different mechanisms could be involved in the control of these nematodes with different ecological strategies (R. similis as migratory endoparasite and H. multicintus as migratory ectoparasite).
To understand in greater depth the mechanisms associated with the suppression and bio-stimulation of EBSs, new research phases are proposed. For example, include metabarcoding studies of the constitutive taxa of the biostimulants and their effect in the rhizoplane microbiome at the field, during a defined period and in the presence of R. similis. Likewise, the study of different control mechanisms against the endoparasite as direct antimicrobial effect or the action of metabolites of the soil microbiota would be important. The search for alternatives to chemical control of the burrowing nematode in banana cultivation is urgently needed and must continue in near future in order to implement a sustainable crop production system.
References
Abawi, G. S., & Widmer, T. L. (2000). Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Applied Soil Ecology, 15, 37–47.
Aguirre, O., Chávez, C., Giraud, A., & Araya, M. (2016). Frequencies and population densities of plant-parasitic nematodes on banana (Musa AAA) plantations in Ecuador from 2008 to 2014. Agronomía Colombiana, 34(1), 61–73. https://doi.org/10.15446/agron.colomb.v34n1.53915
Akhtar, M. S., Panwar, J., Abdullah, S. N. A., Siddiqui, Y., Swamy, M. K., & Ashkani, S. (2015). Biocontrol of plant parasitic nematodes by fungi: Efficacy and control strategies. En M. K. Meghvansi & A. Varma (Eds.), Organic amendments and soil suppressiveness in plant disease management (Vol. 46, pp. 219–247). Springer International Publishing. https://doi.org/10.1007/978-3-319-23075-7_11.
Araya, M. (2004). Current situation of nematode management in banana (Musa AAA) and plantain (Musa AAB) in the American tropics. En in: Rivas, G. and F. Rosales (eds.). Conventional and alternative management of black Sigatoka, nematodes and other pests associated with Musaceae cultivation in the tropics. Tropics. August 11-13, 2003. International plant genetics resources institute, Guayaquil, Ecuador. (pp. 79-102.).
Araya, M., & Vargas, R. (2018). Frecuency and population densities of parasitic nematodes in commercial banana (Musa AAA) plantations sampled between the interspace of the mother plant and its follower sucker and in front of the follower sucker. CORBANA, 44(64), 71–96.
Bechem, E. T., Wapouo, S. F., & Loubana, P. M. (2018). Nematicidal properties of endophytic fungi isolated from some Musa species in Cameroon, for the management of Radopholus similis and Pratylenchus coffeae. Journal of Advances in Biology and Biotechnology, 19(4), 1–19. https://doi.org/10.9734/JABB/2018/45952
Beretta, A. N., Silbermann, A. V., Paladino, L., Torres, D., Bassahun, D., Musselli, R., & García-Lamohte, A. (2014). Soil texture analyses using a hydrometer: Modification of the Bouyoucos method. Ciencia e Investigación Agraria, 41(2), 25–26. https://doi.org/10.4067/S0718-16202014000200013
Bonanomi, G., Antignani, V., Capodilupo, M., & Scala, F. (2010). Identifying the characteristics of organic soil amendments that suppress soilborne plant diseases. Soil Biology and Biochemistry, 42(2), 136–144. https://doi.org/10.1016/j.soilbio.2009.10.012
Bonanomi, G., Zotti, M., Idbella, M., Di Silverio, N., Carrino, L., Cesarano, G., Assaeed, A. M., & Abd-ElGawad, A. M. (2020). Decomposition and organic amendments chemistry explain contrasting effects on plant growth promotion and suppression of Rhizoctonia solani damping off. PLoS One, 15(4), e0230925. https://doi.org/10.1371/journal.pone.0230925
Cesarz, S., Schulz, A. E., Beugnon, R., & Eisenhauer, N. (2019). Testing soil nematode extraction efficiency using different variations of the Baermann-funnel method. https://doi.org/10.25674/SO91201.
Cobon, J. A., Pattison, A. B., Penrose, L. D. J., Chandra, K. A., O’Neill, W. T., & Smith, M. K. (2019). Comparison of the reproduction and pathogenicity of isolates of Radopholus similis (burrowing nematode) from Australia and Fiji on ginger (Zingiber officinale) and banana (Musa spp.). Australasian Plant Pathology, 48(5), 529–539. https://doi.org/10.1007/s13313-019-00656-w
Cook, R. J., & Baker, K. F. (1983). The nature and practice of biological control of plant pathogens American Phytopathological Society.
Das, D., & Borgohain, N. (2018). Biological approach for management of nematodes in banana. Annals of Plant Protection Sciences, 26(2), 374. https://doi.org/10.5958/0974-0163.2018.00085.X
Dita, M., Barquero, M., Heck, D., Mizubuti, E. S. G., & Staver, C. P. (2018). Fusarium wilt of banana: Current knowledge on epidemiology and research needs toward sustainable disease management. Frontiers in Plant Science, 9, 1468. https://doi.org/10.3389/fpls.2018.01468
Food and Agriculture Organization of the United Nations [FAO]. (2020). Statistical Yearbook 2021/World food and agriculture. https://www.fao.org/3/cb4477en/cb4477en.pdf
Hallmann, J., & Subbotin, S. (2018). Methods for extracting, processing and detection of plant and soil nematodes. En Plant parasitic nematodes in subtropical and tropical agriculture (3.a Ed.). CABI.
Jaramillo, J., Vintimilla, M., Rubio, D., Soto, G., Tobar, M., Salas, E., & Araya, M. (2019). Effect of nematicide rotation on banana (Musa AAA cv. Williams) root nematode control and crop yield. Agronomía Colombiana, 37(2), 153–165. https://doi.org/10.15446/agron.colomb.v37n2.79099
McCullagh, P., & Nelder, J. A. (1989). Generalized linear models: Monograph on statistics and applied probability 37. Chapman and Hall.
Mendes, R., Kruijt, M., de Bruijn, I., Dekkers, E., van der Voort, M., Schneider, J. H. M., Piceno, Y. M., DeSantis, T. Z., Andersen, G. L., Bakker, P. A. H. M., & Raaijmakers, J. M. (2011). Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science, 332(6033), 1097–1100. https://doi.org/10.1126/science.1203980
Montenegro, E. (2013). Caracterización físico-química y molecular de suelos supresivos al nematodo parásito del banano Radopholus similis. Universidad de Costa Rica.
Paul, E. A. (2015). Soil microbiology, ecology, and biochemistry. En soil microbiology, ecology and biochemistry (pp. 1-14). Elsevier. https://doi.org/10.1016/B978-0-12-415955-6.00001-3.
Poveda, J., Abril-Urias, P., & Escobar, C. (2020). Biological control of plant-parasitic nematodes by filamentous fungi inducers of resistance: Trichoderma, mycorrhizal and endophytic fungi. Frontiers in Microbiology, 11, 992. https://doi.org/10.3389/fmicb.2020.00992
R Core Team. (2014). R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. URL [http://www.R-project.org/].
Rusell, L. (2021). Emmeans: Estimated marginal means, aka least-squares means. R Package Version, 1(5), 4 https://CRAN.R-project.org/package=emmeans.
Segura, R. A., Stoorvogel, J. J., & Sandoval, J. A. (2021). The effect of soil properties on the relation between soil management and fusarium wilt expression in Gros Michel bananas. Plant and Soil, 471, 89–100. https://doi.org/10.1007/s11104-021-05192-5
Segura-Mena, R. A., Stoorvogel, J. J., García-Bastidas, F., Salacinas-Niez, M., Kema, G. H. J., & Sandoval, J. A. (2021). Evaluating the potential of soil management to reduce the effect of fusarium oxysporum f. Sp. Cubense in banana (Musa AAA). European Journal of Plant Pathology, 160(2), 441–455. https://doi.org/10.1007/s10658-021-02255-2
Selby, C., Carmichael, E., & Sharma, H. S. S. (2016). Bio-refining of perennial ryegrass (Lolium perenne): Evaluation of aqueous extracts for plant defence elicitor activity using French bean cell suspension cultures. Chemical and Biological Technologies in Agriculture, 3(1), 11. https://doi.org/10.1186/s40538-016-0061-9
Seo, H. J., Park, A. R., Kim, S., Yeon, J., Yu, N. H., Ha, S., Chang, J. Y., Park, H. W., & Kim, J.-C. (2019). Biological control of root-knot nematodes by organic acid-producing lactobacillus brevis WiKim0069 isolated from kimchi. The Plant Pathology Journal, 35(6), 662–673. https://doi.org/10.5423/PPJ.OA.08.2019.0225
Shen, Z., Zhong, S., Wang, Y., Wang, B., Mei, X., Li, R., Ruan, Y., & Shen, Q. (2013). Induced soil microbial suppression of banana fusarium wilt disease using compost and biofertilizers to improve yield and quality. European Journal of Soil Biology, 57, 1–8.
Sikora, R., Coyne, D., Hallmann, J., & Timper, P. (2018). Plant parasitic nematodes in subtropical and tropical agriculture (3.a Ed.). CAB international.
Speijer, P., & Waele, D. (1997). Screening of Musa germoplasm for resistanse and tolerance to nematodes Inibap Technical Guidelines.
Steinberg, C., Edel-Hermann, V., Alabouvette, C., & Lemanceau, P. (2019). Soil suppressiveness to plant diseases. En J. D. van Elsas, J. T. Trevors, A. S. Rosado, & P. Nannipieri (Eds.), Modern soil microbiology (3.a Ed., pp. 343–359). CRC Press. https://doi.org/10.1201/9780429059186-21.
Stirling, G. (2014). Biological control of plant-parasitic nematodes: Soil ecosystem management in sustainable agriculture. (2.a Ed.). CABI.
Taylor, A., & Loegering, A. (1953). Nematodos asociados a lesiones radiculares en abacá. Turrialba, 3(1–2), 8–13.
Thammaiah, N., Shirol, A., Prakash, P., & Praveen, J. (2019). Management of burrowing nematode, Radopholus similis in banana by using biocontrol agents. Journal of Entomology and Zoology Studies, 7, 985–989.
Torres Pérez, J. C., Aguilar Jiménez, C. E., Vázquez Solís, H., Solís López, M., Gómez Padilla, E., & Aguilar Jiménez, J. R. (2022). Evaluation of the use of activated mountain microorganisms in the cultivation of roses, Zinacantán, Chiapas. Siembra, 9(1), e3500. https://doi.org/10.29166/siembra.v9i1.3500
Trabelsi, D., & Mhamdi, R. (2013). Microbial inoculants and their impact on soil microbial communities: A review. BioMedicine Research International, 2013, 1–11. https://doi.org/10.1155/2013/863240
Vargas, R., Calvo, C., Collado, M., & Araya, M. (2006). Evaluation of strategies for the chemical control of nematodes in banana plantations renewed with in vitro plants. CORBANA, 32(59), 51–65.
Vargas, R., Solórzano, C., Salas, E., Quesada, M., Quirós, O., & Araya, M. (2012). Evaluación de un suelo supresor a Radopholus similis en una plantación comercial de banano (Musa AAA). In Memorias IV Congreso Internacional sobre Banano. CR: Heredia.
Venables, B., & Ripley, B. (2002). Modern applied statistics with S. En Springer. https://doi.org/10.1007/b97626
Volcy, C. (2011). Past and present of the nematode Radopholus similis (cobb) Thorne with emphasis on Musa: A review. Agronomía Colombiana, 29(3), 433–440.
Westphal, A. (2005). Detection and description of soils with specific nematode suppressiveness. Journal of Nematology, 37(1), 121–130.
Xue, C., Ryan Penton, C., Shen, Z., Zhang, R., Huang, Q., Li, R., Ruan, Y., & Shen, Q. (2015). Manipulating the banana rhizosphere microbiome for biological control of Panama disease. Scientific Reports, 5(1), 11124. https://doi.org/10.1038/srep11124
Zhang, N., He, X., Zhang, J., Raza, W., Yang, X.-M., Ruan, Y.-Z., Shen, Q.-R., & Huang, Q.-W. (2014). Suppression of fusarium wilt of banana with application of bio-organic fertilizers. Pedosphere, 24(5), 613–624. https://doi.org/10.1016/S1002-0160(14)60047-3
Zum Felde, A., Pocasangre, L. E., & Sikora, R. A. (2005). The potential use of microbial communities inside suppressive Banana plants for banana root protection. En banana root system: Towards a better understanding for its productive management. (pp. 169-177).
Acknowledgments
This research is part of the Ph D. study in Natural Sciences for Development (DOCINADE) of first author. We are also grateful to DOCINADE, the MICITT PINN scholarship program and CORBANA for the support to carry out this research project.
Funding
This research was financed with the joint support of the PINN scholarship program of the Ministry of Science, Technology and Telecommunications (MICITT) and the National Banana Corporation (CORBANA), of Costa Rica.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torres-Asuaje, P.E., Cotes-Prado, A.M., Echeverría-Beirute, F. et al. Ensilaged biostimulants promoting root health and control of Radopholus similis in banana (Musa AAA) cv. Grande Naine. Eur J Plant Pathol 165, 465–474 (2023). https://doi.org/10.1007/s10658-022-02617-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-022-02617-4