Abstract
Corn stunt disease is a disease that extends from the southern United States to Argentina and is one of the most important diseases in warm subtropical areas of the continent, periodically causing yield reductions in maize. The aims of this study were to develop a severity scale, estimate the effect of corn stunt caused by Spiroplasma kunkelii in maize hybrids, and identify attributes related to yield loss that would be useful to evaluate germplasms. Under artificial inoculation conditions with infective and non-infective insects, symptoms were grouped to develop and validate a scale and a severity index. Disease severity, moment of appearance of the characteristic symptom, pathogen concentration, and effect on yield were correlated for eight temperate and temperate x tropical commercial and precommercial hybrid maize germplasms. We developed and validated a 7-score severity scale. The characteristic symptom of S. kunkelii infection occurs earlier in the temperate germplasm than in the tropical x temperate germplasm. The performance of two of the four temperate germplasms was remarkable compared to the hybrids. Severity and pathogen concentration were correlated with each other, and both correlated with yield and with yield reduction. The moment of appearance of the characteristic disease symptom did not correlate with the other attributes. The severity index and the pathogen concentration may be useful to evaluate germplasm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Corn stunt disease is one of the most important diseases in warm subtropical areas of the Americas, extending from the southern United States to Argentina and periodically causing reduced yields (Carpane et al., 2012; E. De Oliveira et al., 2003; Galvão et al., 2021; Gimenéz Pecci et al., 2014; Pérez López et al., 2016).
The disease is caused by three agents, Maize rayado fino virus (MRFV), and the mollicutes Maize bushy stunt phytoplasma (MBSP) and Spiroplasma kunkelii Whitcomb et al., and these may act alone or in association. All three are transmitted in a persistent propagative manner by the insect Dalbulus maidis De Long and Wolcott (Hemiptera: Cicadellidae).
S. kunkelii was first detected in Argentina in 1991 (Lenardon et al., 1993), and knowledge of the disease, its vectors and hosts have advanced since then (Carloni et al., 2013; Carpane et al., 2012; Giménéz Pecci et al., 2002; Oleszczuk et al., 2020). The characteristic symptom caused by S. kunkelii consists of chlorotic stripes beginning in the base of leaf blades and progressively extending to the apex, usually followed by leaf redness (Carpane et al., 2006; Nault, 1980).
Research in different production cycles and crop regions of Argentina with hybrids and experimental lines concluded that there was a differential response to the disease among the hybrids assayed (Caro et al., 2008; Carpane et al., 2006; Díaz et al., 2005; Virla et al., 2004). Antibiosis and antixenosis studies in tropical and temperate maize also showed that genotypes affected the behavior of the D. maidis (Oleszczuk et al., 2020). In temperate hybrids, resistance to the disease is quantitative (Márquez Sánchez, 1982), but additive effects were observed in tropical hybrids (Silva et al., 2003). Subsequently, dominant rather than additive effects were found to be more important for resistance (Mendoza et al., 2002; Silva et al., 2003).
The aim of this study was to develop and validate a severity scale, analyze the behavior of eight maize hybrids, and identify attributes related to the effect of S. kunkelii on yield that would be useful for evaluating germplasms in maize breeding programs.
Materials and methods
Obtaining inoculum and vector insects
The experiments were conducted in Cordoba (-31.467980, -64.147594, Córdoba Province, Argentina) in 2014 and 2015.
The inoculum of S. kunkelii was obtained from maize plants with stunting and chlorotic stripes on leaves, collected in Charata (-27.202917, -61.162753, Chaco Province, Argentina) in the 2012/13 growing season (Fig. 1). The presence of S. kunkelii and the absence of MRFV was later confirmed by DAS-ELISA, using antisera developed at IPAVE-CIAP-INTA (Giménéz Pecci et al., 2009). The absence of MBSP was verified by PCR with the specific primers R16F2, 5’-ACGACTGCTGCTAAGACTGG-3’ and R16R2, 5’-TGACGGGCGGTGTGTACAAACCCCG-3’ (Lee, 1993).
Healthy insect vectors of the species D. maidis were reared in a controlled breeding room at a mean temperature of 25 °C, on healthy maize plants grown in greenhouses. To obtain S. kunkelii-carrying leafhoppers, D. maidis from the colony were left for 3 days on symptomatic plants to acquire the pathogen. These were used in S. kunkelii transmission experiments after three weeks, the latency period for S. kunkelii in D. maidis, when they become infective (Carpane et al., 2006; Nault, 1980).
Development and validation of scale and severity index
The infective insects were put to feed for 3 days on 525 maize plants grown in single pots, at a rate of three individuals/plant, in V4 stage (15–20 days after germination) (Alivizatos & Markham, 1986). Each plant was covered with a plastic bottle with ventilation openings protected with voile fabric.
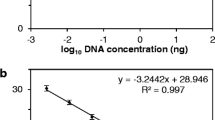
Symptoms associated with S. kunkelii were recorded twice a week and, when the plants reached the R4 stage (Ritchie & Hanway, 1982), samples, consisting of the basal part of one metabolically active leaf from the upper third of the plant, were collected to diagnose S. kunkelii. These were stored at -20 °C until serological analysis by DAS-ELISA, according to Giménéz Pecci et al. (2009). The pathogen concentration was measured as antigen concentration, indirectly evaluated through relative absorbance. This was measured through the optical density of the reaction under a wavelength of 405 nm. The samples with an absolute absorbance value higher than 0.1, determined by the serological test of DAS-ELISA at one hour reaction time, and also higher or equal to the mean of six healthy controls plus three times its standard deviation (cut-off value), were considered positive (Sutula, 1986). The relative absorbance value (quotient between absolute absorbance and cut-off value) was taken so as to perform statistical correlations.
All the maize ears of every plant were collected when these reached physiological maturation. These were threshed, and the grains conditioned in a stove until they reached 14% humidity. They were weighed to obtain the yield per plant (g/plant).
To study the degrees of damage caused by the pathogen in the plant, a severity scale was developed, based on studies and previous experience of symptom expression in temperate climate (Caro et al., 2006, 2008), and validated using relative absorbance and yield (g/plant) records (Pearson's correlation).
Subsequently, an index was elaborated to quantify the severity of each analyzed hybrid, considering a 25% increase in severity for each grade of the scale. The disease severity index expressed by the hybrids under treatment with infective insects was analyzed following the linear model, and the DGC test performed to establish possible differences between means with InfoStat v.2020p (Di Rienzo et al., 2020).
Hybrid evaluation
The treatments arose from a factorial combination of two temperate x tropical and four temperate commercial and precommercial hybrid maize germplasms, plus two controls (tropical germplasm: Experimental 110 and temperate germplasm: Opaco INTA sweet maize), in two association states of D. maidis and S. kunkelii (infective and non-infective insects). A control without insects was not included. The maize hybrids used in the experiment are listed in Table 1. A completely randomized three-block design was used (sowing dates: Nov 28, Dec 09, and Dec 30, 2014) with eight maize hybrids in each block. Five were used for the experimental unit, at a density of 4 plants/m2.
The same procedure as the one mentioned in the development and validation of scale and severity index was carried out. Subsequently, plants were transplanted to soil with a 0.52 m spacing between furrows, 0.33 m between plants for the same hybrid, and 1 m between hybrids (4 plants/m2). The blocks (sowing date) were separated by 1.5 m and the whole trial was covered by voile fabric to avoid external infection. Fifteen days before transplanting, the soil was prepared with an application of pre-sowing herbicide, consisting of 3.5 l/ha glyphosate, 1.5 kg/ha atrazine, and 0.8 l/ha acetochlor.
Disease severity
When the plants reached the R4 state, the symptoms were recorded according to the scale developed as above and used to estimate the severity index.
Appearance of the characteristic symptom
The time since transmission and the appearance of the characteristic disease symptom (leaf chlorotic stripes) was determined. The hybrids were compared considering the average day of appearance of the characteristic symptom of S. kunkelii after transmission, for each of the three sowing dates.
Pathogen concentration
When the different hybrids reached the R4 stage, the basal part of one metabolically active leaf was taken from the upper third of the plant, to diagnose S. kunkelii by DAS-ELISA, as previously mentioned. The pathogen was considered absent when the absolute absorbance value was below the cut-off value.
Yield and yield reduction
To study the yield and yield reduction between treatments for the same hybrid, considering only the effect of S. kunkelii since the control treatment was carried out with healthy insects, the ears of plants in physiological maturation were harvested, conditioned to 14% humidity, and weighed to obtain the yield per plant (g/plant). The yield reduction was estimated, and a Mixed Linear Model was adjusted for the yield reduction per hybrid with VarIdent variance structure.
Identification of useful attributes for germplasm evaluations
To identify useful attributes in maize breeding programs, Pearson’s correlation coefficients were estimated pairwise for: a) disease severity, b) moment of appearance of the characteristic symptom, c) pathogen concentration (antigen concentration indirectly evaluated through relative absorbance in the DAS-ELISA serological test), d) yield, and e) yield reduction.
All statistical analyses were performed with InfoStat v.2020p (Di Rienzo et al., 2020).
Results and discussion
Development and validation of scale and severity index
The study enabled R4 to be identified as the optimal state for registering the symptoms, because up to this phenological moment the symptoms increased in number and severity, without any of those previously registered disappearing, except for the death of the plant. The symptoms registered were grouped according to the severity observed in the plants on a scale of seven scores (Table 2), which was validated by Pearson's correlation (P<0.001) between the relative absorbance and yield of 525 maize plants.
Achieving a symptom severity scale that enables the different degrees observed in the field to be expressed is complicated in systemic diseases such as the one we are analyzing, and even more so in temperate climates where the lower temperature conceals the expression of symptoms. Factors such as the germplasm and phenology of the crop, inoculum pressure, high commercial hybrid turnover rate, among others, add difficulty to the development of this type of scales. The one used in this work reflects, with high correlation, the effect of pathogen on yield, and incorporates premature plant death as a symptom, which is not considered in other scales. It does not include the percentage of decrease in height as a symptom to be evaluated, because this can be subjective, depending on each observer.
To make the scale, we recorded the symptoms up to R4, in agreement with De Oliveira et al. (2002), who found that the expression of foliar symptoms varies between yellowing, redness and intensity of redness according to genotype and phenological state, with the greatest expression being at R4, the time of grain filling. The 5-score scale created by Carpane et al. (2006) was based on color changes but included the height of the plants with respect to the uninfected ones, a criterion that we included in score 5 (internode shortening), where easily observed serious symptoms are expressed. Although Caro et al. (2008) developed a practical 4-score scale, according to the concentration range of the pathogen, this groups the more visible symptoms only in one degree and the differences between the other degrees of the scale are very subtle, making it difficult to observe them. De Oliveira et al.( 2010) established a 5-score scale based on the percentage of leaves with symptoms associated with the disease, which is probably useful in tropical climates, but difficult to apply in temperate or subtropical climates where the expression and observation of disease symptoms is more difficult in the field.
Using the 7-score scale, each score was weighted by an increasing value, forming the following severity index, useful for quantifying the severity of the disease in different germplasms.
where (Sint n) is the number of plants with the same score. This methodology, consisting of developing an index based on a severity scale that evaluates symptoms in the entire plant, was used in temperate germplasm by Di Renzo et al. (2002) to evaluate Mal de Río Cuarto virus (MRCV), a disease also transmitted by hoppers. For Corn stunt, De Oliveira et al. (2010) in Brazil also created a disease index based on a scale of degrees of severity.
Hybrid evaluation
Disease severity
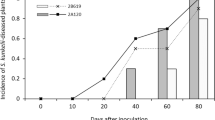
The disease severity index of each hybrid identified two significantly different groups: a) Te 3, Te 1, TrxTe 1, TrxTe 2, Opaco INTA and Te 4, which registered the highest disease severity index values, and b) Te 2 and Experimental 110, which registered the lowest disease severity index values (Fig. 2).
Appearance of the characteristic symptom
The second attribute evaluated was the moment of expression of the characteristic symptom. Te 1, Te 2 and Te 3, of temperate germplasm, were the first to express the symptom, 22 days after transmission. Te 4 and TrxTe 2 did so at 27 days and, finally, Opaco INTA, TrxTe 1 and Experimental 110 showed the leaf chlorotic stripes at 35, 38 and 40 days respectively. Thus, temperate germplasm expressed the symptom earlier. Different authors reported temporal variation in the moment of expression of the characteristic symptom, including Nault (1980) and Sabato et al. (2020) who indicated that high temperatures favor its early appearance. Bradfute et al. (1981) and Bajet (1989), in experiments carried out at high temperatures, also used this attribute to characterize the disease, reporting a period until the appearance of the characteristic symptom of between 15 and 30 days after inoculation. Carpane et al.( 2006), in turn, used this attribute to characterize isolates from different regions, finding their appearance from 40 to 70 days, while in our study, under the same experimental conditions, the symptom appeared in all germplasms before day 40.
Pathogen concentration
The DAS-ELISA serological test confirmed the presence of the pathogen in all of the plants under infective treatment and its absence in plants under non-infective treatment. The analysis of the differences among the mean pathogen concentration for each hybrid identified three groups: a) Te 3, TrxTe 1, Te 1 and TrxTe 2 with the higher relative absorbance mean values, 6.85, 6.35, 5.93 and 5.88, respectively, followed by b) Opaco INTA and Te 4, with 4.61 and 3.13, and c) Experimental 110 and Te 2 with 2.64 and 2.03, which recorded the lowest relative absorbance. Along with Experimental 110 (control), Te 2 and Te 4 showed the best performance against the multiplication of S. kunkelii.
Yield and yield reduction
Yield (Fig. 3) and Yield reduction (Fig. 4) confirmed, for all the analyzed germplasms, that plants treated with infective insects always yielded less than the control with non-infective insects. In addition, the differences observed in the behavior of the maize genotypes agree with Da Costa et al. (2019), De Oliveira et al. (2002), Sierra Macías et al. (2010), and with Virla et al. (2004) who found, in assays with natural infections carried out in Tucuman (Argentina), differences in grain production per plant with values of 67.59 g/plant to 117.06 g/plant when comparing serologically positive plants to negative plants for S. kunkelii.
The experiment aimed to suppress the effect of the damage produced by the feeding and oviposition of the insect, which made it possible to analyze the reduction in yield of each hybrid that was due exclusively to S. kunkelii. Two groups were identified, in decreasing order of yield reduction: a) Opaco INTA, Te 3, Te 1, TrxTe 1 and TrxTe 2, which recorded the highest yield losses, and b) Te 4, Te 2 and Experimental 110 with the lowest yield losses (Fig. 4). Because yield losses vary between hybrids (De Oliveira et al., 2007; Teixeira et al., 2013), this attribute is more useful than yield estimation to identify germplasms with good performance against infection with S. kunkelii.
Considering the severity index, pathogen concentration and yield reduction, hybrid Te 2, independently of Experimental 110, proved to be the most tolerant germoplasm to infection with S. kunkelii. In terms of the time of appearance of the characteristic symptom, hybrid Te 4 performs best against the disease.
Identification of useful attributes for germplasm evaluations
Under the conditions evaluated, there was no correlation between disease severity and the moment of appearance of the characteristic symptom. This indicates that the early appearance of symptoms, as recorded in the temperate germplasm evaluated, is not necessarily associated with evolution toward more severe symptoms.
A significant correlation (P <0.001) of 0.88 was observed between disease severity and pathogen concentration for treatments with infective insects. Although Oleszczuk et al. (2020) reported a rather inconsistent relationship between symptom severity and S. kunkelii accumulation in temperate and tropical x temperate germplasms, our results agree with Virla et al. (2004) and Gussie (1995) who observed a high accumulation of S. kunkelii in plants with severe disease symptoms. Carpane et al. (2006) also reported that symptom severity is directly related to S. kunkelii concentration in the plant.
The correlation between disease severity and yield, and that between disease severity and yield reduction, were significant (P <0.001) with values of -0.73 and 0.64, respectively. Similar results were indicated by Oleszczuk et al. (2020), who found that yield reductions are directly related to the severity of symptoms. Virla et al. (2004) also reported a strong relationship between symptom severity and reduced performance. On the other hand, Hidalgo et al. (1998) and De Oliveira et al. (2002) indicated that hybrids with similar symptom severity levels can present different yields, which highlights the importance of the genetic component in maize against corn stunt by S. kunkelii.
Under the conditions evaluated, no correlation was observed between the time of appearance of the characteristic symptom and the other attributes (disease severity, pathogen concentration, yield, and yield reduction), and therefore this would not serve as a parameter or indicator of the subsequent evolution of the disease and the damage it could cause. We evaluated the time of appearance of the characteristic symptom and not that of any other associated with the disease, because, in temperate zones, the other symptoms can be confused with nutritional deficiencies, environmental and other effects. However, some authors have found correlations for the time of appearance of symptoms other than the characteristic symptom. Massola et al. (1999b), Scott et al. (1977) and Hogenboom (1993) reported yield reductions that can reach 100% when S. kunkelii infection occurs in the early stages of the crop. Also, Scott et al. (1977) obtained a regression line for the moment of appearance of symptoms and yield in different hybrids, in assays with natural infections at successive crop dates. When the symptoms appeared before Day 52 after sowing, the yield was null, after Day 52 and for every subsequent day until Day 107 the yield gain was 1.82%, and after Day 107 there was no yield loss at all. Likewise, Massola et al. (1999a) detected a correspondence between the appearance of early symptoms, like reddish and yellowish leaf margins and the characteristic symptom, and yield reduction.
The correlations between pathogen concentration and yield, and between pathogen concentration and yield reduction were significant (P <0.001) but moderate, with values of -0.67 and 0.55, respectively. In all the hybrids analyzed, the pathogen concentration had a negative effect on yield. Because this is a quantitative attribute, it constitutes an important tool in breeding programs to early identify hybrids with good performance against the disease, enabling breeding to become less subjective.
We observed no correlation between yield and yield reduction, indicating that there is no direct relationship between potential hybrid yield and its genetic component in response to S. kunkelii.
The use of resistant and/or tolerant hybrids is always the most recommended strategy, along with other disease management tools such as sowing date management, seed treatment with insecticides, chemical or biological control of D. maidis, and elimination of volunteer maize plants in harvested fields.
Conclusions
-
A scale and severity index, suitable for the expression of symptoms by S. kunkelii in temperate germplasm currently cultivated in Argentina was developed and validated.
-
The characteristic symptom of S. kunkelii infection (chlorotic stripes at the base of the leaf) occurs earlier in temperate germplasm than in tropical x temperate germplasm.
-
The severity index and pathogen concentration increase as yield decreases; they are therefore suggested for evaluating maize germplasms, suitable for production in Argentina, against infection by S. kunkelii.
-
Although the main temperate germplasm used currently in Argentina is susceptible to S. kunkelii, under the evaluated conditions, the temperate germplasm hybrids Te 2 and Te 4 showed the best performance against S. kunkelii.
References
Alivizatos, A. S., & Markham, P. G. (1986). Acquisition and transmission of corn stunt spiroplasma by its leafhopper vector Dalbulus maidis. Annals of Applied Biology, 108(3), 535–544. https://doi.org/10.1111/j.1744-7348.1986.tb01992.x
Bajet, N. B. (1989). Occurrence of Corn Stunt Spiroplasma at different elevations in Mexico. Plant Disease, 73(11), 926. https://doi.org/10.1094/PD-73-0926
Bradfute, O. E., Tsai, J. H., & Gordon, D. T. (1981). Corn Stunt Spiroplasma and viruses associated with a maize disease epidemic in Southern Florida. Plant Disease, 65(10), 837. https://doi.org/10.1094/PD-65-837
Carloni, E., Carpane, P., Paradell, S., Laguna, I., & Giménez Pecci, M. P. (2013). Presence of Dalbulus maidis (Hemiptera: Cicadellidae) and of Spiroplasma kunkelii in the temperate region of Argentina. Journal of Economic Entomology, 106(4), 1574–1581. https://doi.org/10.1603/EC12323
Caro, L., Carpane, P., Carloni, E., Santa, M., Laguna, I. G., & Giménez Pecci, M. P. (2006). Relación entre síntomas y serología y plantas de Maíz (Zea mays L.) infectadas experimentalmente con Spiroplasma kunkelii Whitcomb (pp. 304–305). XII Jornadas Fitosanitarias Argentinas.
Caro, L., Laguna, I. G., & Giménez Pecci, M. P. (2008). Ajuste de la escala que asocia síntomas y serología en maíz experimentalmente infectado con Spiroplasma kunkelii (p. 327). 1o Congreso Argentino de Fitopatología.
Carpane, P., Laguna, I. G., Virla, E. G., Paradell, S., Murua, L., & Giménéz Pecci, M. P. (2006). Experimental transmission of corn stunt spiroplasma present in different regions of Argentina. Maydica, 51, 461–468.
Carpane, P., Melcher, U., Wayadande, A., Giménez Pecci, M. P., Laguna, G., Dolezal, W., & Fletcher, J. (2012). An Analysis of the Genomic Variability of the Phytopathogenic Mollicute <i>Spiroplasma kunkelii<i/>. Phytopathology, 103(2), 120926072324005. https://doi.org/10.1094/PHYTO-07-12-0158-R
Da Costa, R., Da Silva, D. D., Cota, L., Campos, L., Almeida, R., & Bernardes, F. (2019). Incidence of corn stunt disease in off-season corn hybrids in different sowing seasons. Pesquisa Agropecuária Brasileira, 54. https://doi.org/10.1590/s1678-3921.pab2019.v54.00872
De Oliveira, C. M., Lopes, J. R. S., Camargo, L. E. A., Fungaro, M. H. P., & Nault, L. R. (2007). No TitlGenetic diversity in populations of Dalbulus maidis (DeLong and Wolcott) (Hemiptera: Cicadellidae) from distant localities in Brazil assessed by RAPD-PCR Markers. Environmental Entomology, 36, 204–212.
De Oliveira, E., De Oliveira, C. M., Souza, I. R. P., Magalhães, P. C., & Cruz, I. (2002). Corn stuns: expression of leaf symptoms, detection of mollicutes and interactions with genotypes. Revista Brasileira de Milho e Sorgo, 1, 53–62.
De Oliveira, E., Landau, E., Guimarães, P. E., & Guimarães, L. J. M. (2010). Resistance of corn to stunting caused by spiroplasm and to stunting caused by phytoplasma. Asociación Brasileña de Maíz y Sorgo, 226.
De Oliveira, E., Resende, R., Giménéz Pecci, M. P., Laguna, I. G., Herrera, P., & Cruz, I. (2003). Incidência de viroses e enfezamentos e estimativa de perdas causadas por molicutes em milho no Paraná. Pesquisa Agropecuária Brasileira, 38(1), 19–25. https://doi.org/10.1590/S0100-204X2003000100003
Di Renzo, M. A., Bonamico, N. C., Díaz, D. D., Salerno, J. C., Ibañez, M. M., & Gesumaria, J. J. (2002). Inheritance of resistance to Mal de Río Cuarto (MRC) disease in Zea mays (L.). The Journal of Agricultural Science, 139(1), 47–53. https://doi.org/10.1017/S0021859602002241
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., & Robledo, C. W. (2020). InfoStatversión 2020. Centro de Transferencia (No. 2020). Centro de Transferencia InfoStat, FCA, Universidad Nacioinal de Córdoba, Argentina. www.infostat.com.ar
Díaz, C. G., Virla, E. G., Carloni, E., Giménéz Pecci, M. P., & Laguna, I. G. (2005). Evaluación preliminar del efecto de la fecha de siembra sobre la incidencia del corn stunt spiroplasma (CSS) (pp. 277–288). VIII Congreso Nacional de Maíz.
Galvão, S. R., Sabato, E. O., & Bedendo, I. P. (2021). Occurrence and distribution of single or mixed infection of phytoplasma and spiroplasma causing corn stunting in Brazil. Tropical Plant Pathology, 46(2), 152–155. https://doi.org/10.1007/s40858-020-00381-6
Giménéz Pecci, M. P., Carpane, P., Carloni, E., Nome, C. F., Fiorona, M., & Laguna, I. G. (2009). Técnicas empleadas en la identificación y caracterización de Spiroplasma kunkelii Withcomb (Reino Eubacteria, Clase Mollicutes). In I. G. Laguna, V. Conci, P. Rodriguez Pardina, G. Truol, M. Fiorona, & L. Di Feo (Eds.), Procedimientos empleados en la identificación de organismos fitopatógenos (pp. 53–65). Ediciones INTA.
Gimenéz Pecci, M. P., García, A., Druetta, M., Cabral, J. B., Ramirez, E. H., Raspanti, J., Ferrer, M., Maurino, M. F., & Laguna, I. G. (2014). ePeriodicidad y distribución de dos enfermedades del maíz (Zea mays L.) en la década del 2000 en Argentina (pp. 46–47). Escuela de Matemática Aplicada a La Biología-BIOMAT.
Giménéz Pecci, M. P., Laguna, I. G., Avila, A. O., De Remes Lenicov, A. M. M., Virla, E. G., Borgogno, C., Nome, C. F., & Paradell, S. (2002). Difusión del Corn Stunt Spiroplasma del maíz (Spiroplasma kunkelii) y del vector (Dalbulus maidis) en la República Argentina. Revista de La Facultad de Agronomía de La Plata, 105(1), 1–8.
Gussie, J. S. (1995). Movement and multiplication of Spiroplasma kunkelii in Corn. Phytopathology, 85(10), 1093. https://doi.org/10.1094/Phyto-85-1093
Hidalgo, H., Jeffers, D., Castañon, G., & Rodriguez, F. (1998). Resistencia al achaparramiento del maíz mediante infestaciones de Dalbulus maidis en maíz. Agronomía MEsoamericana, 9(2), 119–124.
Hogenboom, N. G. (1993). Economic importance of breeding for disease resistance. In T. Jacobs & J. E. Parlevliet (Eds.), Durability of disease resistance. Current Plant Science and Biotechnology in Agriculture (pp. 5–9). Springer. https://doi.org/10.1007/978-94-011-2004-3_1
Lee, I. M. (1993). Universal amplification and analysis of pathogen 16S rDNA for classification and identification of mycoplasmalike organisms. Phytopathology, 83(8), 834. https://doi.org/10.1094/Phyto-83-834
Lenardon, S. L., Laguna, I. G., Gordon, D. L., Truol, G., Gómez, G., & Bratfute, O. E. (1993). Identification of Corn stunt spiroplasma in maize from Argentina. Plant Disease, 77(1), 100C. https://doi.org/10.1094/PD-77-0100C
Márquez Sánchez, F. (1982). The genetic improvement of resistance to the corn disease stunt and downy mildew in Nicaragua. Chapingo, 27, 26–27.
Massola, N. S., Bebendo, I. P., Amorin, L., & Lopes, J. R. S. (1999a). Effects of the inoculation time on corn with Spiroplasma kunkelii on yield components. Fitopatologia Brasileira, 24, 571–573.
Massola, N. S., Bebendo, I. P., Amorin, L., & Lopes, J. R. S. (1999b). Quantificação de danos causados pelo enfezamento vermelho e enfezamento pálido do milho em condições de campo. Fitopatologia brasileira, 24(2), 136–142 http://cat.inist.fr/?aModele=afficheN&cpsidt=10137411
Mendoza, M., López, A., Rodriguez, S. A., Oyervides García, A., De León, C., & Jeffers, D. (2002). Acción génica de la resistencia al Achaparramiento del maíz causado por espiroplasma, fitoplasmas y virus. Revista Mexicana de Fitopatología, 20, 13–17.
Nault, L. R. (1980). Maize Bushy Stunt and Corn Stunt: a comparison of disease symptoms, pathogen host ranges, and vectors. Phytopathology, 70(7), 659. https://doi.org/10.1094/Phyto-70-659
Oleszczuk, J. D., Catalano, M. I., Dalaisón, L., Di Rienzo, J. A., Giménez Pecci, M. P., & Carpane, P. (2020). Characterization of components of resistance to Corn Stunt disease. PLOS ONE, 15(10), e0234454. https://doi.org/10.1371/journal.pone.0234454
Pérez López, E., Olivier, C. Y., Luna Rodríguez, M., Rodríguez, Y., Iglesias, L. G., Castro Luna, A., Adame García, J., & Dumonceaux, T. J. (2016). Maize bushy stunt phytoplasma affects native corn at high elevations in Southeast Mexico. European Journal of Plant Pathology, 145(4), 963–971. https://doi.org/10.1007/s10658-016-0883-0
Ritchie, S. W., & Hanway, J. J. (1982). How a corn plant develops. Iowa State University of Science and Technology. Cooperative Extension Service Ames, Iowa. Special Report N° 48.
Sabato, E. O., Landau, E. C., Barros, B. A., & Oliveira, C. M. (2020). Differential transmission of phytoplasma and spiroplasma to maize caused by variation in the environmental temperature in Brazil. European Journal of Plant Pathology, 157(1), 163–171. https://doi.org/10.1007/s10658-020-01997-9
Scott, G. E., Rosenkranz, E. E., & Nelson, L. R. (1977). Yield loss of corn due to corn stunt disease complex 1. Agronomy Journal, 69(1), 92–94. https://doi.org/10.2134/agronj1977.00021962006900010024x
Sierra Macías, M., Becerra Leor, E., Palafox Caballero, A., Rodriguez Montalvo, F., Espinosa Calderón, A., & Valdivia Bernal, R. (2010). Tropical corn (Zea mays L.) genotypes with high yield and tolerance to corn stunt disease in the gulf of Mexico region. Tropical and Subtropical Agroecosystems, 12, 485–493.
Silva, R. G., Galvão, J. C., Miranda, G. V., & De Oliveira, E. (2003). Controle genético da resistência aos enfezamentos do milho. Pesquisa Agropecuária Brasileira, 38(8), 921–928. https://doi.org/10.1590/S0100-204X2003000800004
Sutula, C. L. (1986). Interpreting ELISA data and establishing the positive-negative threshold. Plant Disease, 70(8), 722. https://doi.org/10.1094/PD-70-722
Teixeira, F. F., Costa, F. M., Sabato, E. O., Leite, C. E., Meirelles, W., Guimarães, C., & Belicuas, S. (2013). Pré-melhoramento de milho quanto à resistência a enfezamentos. Pesquisa Agropecuária Brasileira, 48(1), 51–58. https://doi.org/10.1590/S0100-204X2013000100007
Virla, E. G., Díaz, C. G., Carpane, P., Laguna, I. G., Ramallo, J., Gerónimo Gómez, L., & Giménez Pecci, M. P. (2004). Evaluación preliminar de la disminución en la producción de maíz causada por el " Corn stunt spiroplasma " ( CSS ) en Tucumán. Argentina. Boletín de Sanidad Vegetal. Plagas, 30, 403–413.
Acknowledgments
We are grateful to Sergio Uhart (Corteva Agriscience Argentina) and Irma Graciela Laguna (Consejo Nacional de Investigaciones Científicas y Técnicas – CONICET - INTA) for critical reading of the manuscript.
Funding
This work was supported by a grant from PD-E4-I090-001 (INTA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that there are no competing economic interests or known personal relationships that may have interfered with the work reported in this document. All authors are informed and agree on the publication of the manuscript.
Research involving human participants and/or animals
Not applicable, the research does not involve humans or animals.
Informed consent
Not applicable, the research does not involve human participants.
Rights and permissions
About this article
Cite this article
Barontini, J.M., Malavera, A.P., Ferrer, M. et al. Infection with Spiroplasma kunkelii on temperate and tropical x temperate maize in Argentina and development of a tool to evaluate germplasm. Eur J Plant Pathol 162, 455–463 (2022). https://doi.org/10.1007/s10658-021-02415-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-021-02415-4