Abstract
The effects of the visible light wavelengths on germination, mycelial radial growth, and conidial production of the plant pathogens Colletotrichum acutatum and Fusarium fujikuroi were studied. Both fungi were grown on potato dextrose agar medium (PDA) in the dark (control) or on PDA under continuous white, blue, green or red light. In addition, the conidia from each treatment were exposed to UV radiation. The germination and growth of both plant pathogenic fungi were not affected by any of the treatments. C. acutatum produced more conidia when the fungus grew under white and red light. F. fujikuroi produced more conidia in the dark. The tolerances to UV radiation of conidia produced on different light and dark treatments differed for both C. acutatum and F. fujikuroi. Conidia of C. acutatum were at least 30% more tolerant to UV radiation when they were produced under white light than under blue and green light and at least 20% more tolerant than conidia produced in the dark. Conidia of C. acutatum produced under red light were the least tolerant. Conidia of F. fujikuroi produced under white and blue light were at least 30% more UV tolerant than conidia produced in the dark, green, and red light. In conclusion, no differences were found for germination and growth for both fungi under different light regimes and dark; however, significant differences occurred both in production and UV radiation of conidia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most organisms on Earth sense visible light (380 to 740 nm) through the use of photoreceptive proteins specifically adapted to respond to it (Dasgupta et al. 2015). The response of fungi are reflected in different physiological responses such as conidial production (Yu et al. 2013; de Menezes et al. 2015), conidial stress tolerance (Idnurm and Heitman 2005; Rangel et al. 2011; Fuller et al. 2013; Aver'yanov et al. 2014; de Menezes et al. 2015; Rangel et al. 2015; Dias et al. 2020), conidial pigmentation (Fuller et al. 2013; Yu et al. 2013), virulence (Yu et al. 2013; Aver'yanov et al. 2014; Oliveira et al. 2018), germination speed (Fuller et al. 2013; Oliveira et al. 2018), and secondary metabolism (Tisch and Schmoll 2010; Fanelli et al. 2012). Fungal molecular biology studies have identified several genes that encode proteins, which are involved in the detection of visible light, and have investigated the mechanisms that activate physiological and morphological responses (Chen et al. 2009; Fuller et al. 2016). Most fungi perceive and respond to blue light, and some fungi can perceive and respond to wavelengths of light in the red, green, and ultraviolet regions of the spectrum (Idnurm et al. 2010; Yu and Fischer 2019). Blue light photoreceptors are White Collar 1 (WC-1 and homologous proteins), cryptochromes, and photolyases. Red and green wavelengths are sensed by phytochromes and opsins, respectively (Idnurm et al. 2010).
The insect-pathogenic fungi Metarhizium robertsii and M. acridum grown under visible light up-regulate many stress-related genes (Brancini et al. 2019; Dias et al. 2020). In turn, the corresponding changes in transcription and protein accumulation play a role in tolerance to stress by inducing higher conidial tolerance to osmotic stress, UV-radiation, and heat (Rangel et al. 2011; Rangel et al. 2015; Dias et al. 2020). In addition, M. robertsii conidia produced under white light germinate faster and are more virulent than conidia produced in the dark (Oliveira et al. 2018). In the fungus Botrytis cinerea, the White Collar Complex (WCC) is required for coping with excessive light and oxidative stress, as well as achieving full virulence (Canessa et al. 2013). This physiological outcome suggested the possibility that individual components of white light can also produce conidia with increased stress tolerance in other fungal species.
Conidial production is greatly regulated by visible light, but not all fungal species, and even different isolates from the same species, respond in the same way to visible light. Paecilomyces fumosoroseus (currently Isaria fumosorosea) produces the most conidia under white and blue light, less conidia under green light, and the least conidia under red light (Sanchez-Murillo et al. 2004). Aspergillus nidulans produces more conidia under the white light than in the dark (Atoui et al. 2010). Alternaria solani produces more conidia in the far-red, followed by red light and in the dark. Growth of A. alternata under blue, white and green light produces very few conidia (Igbalajobi et al. 2019). Beauveria bassiana produces more conidia under white and blue light and less conidia under green, purple, yellow, and red light and darkness (Zhang et al. 2009). Metarhizium robertsii produces more conidia under blue light than under red light and darkness (Oliveira et al. 2018). Neurospora crassa produces four-fold more conidia under the visible light than in the dark (Lauter et al. 1997).
Studying the effect of radiation on the development of plant pathogenic fungi is important to discover ways to control them in the environment. For example, Alternaria, Botrytis, and Stemphylium can be controlled by eliminating certain radiation wavelengths, and they sporulate only when they receive radiation in the ultraviolet (UV) range below 360 nm (Agrios 2005). Diseases of greenhouse vegetables caused by several species of plant pathogenic fungi can be controlled by covering or constructing the greenhouse with a special UV-absorbing vinyl film that blocks the transmission of wavelengths below 390 nm (Agrios 2005).
Therefore, our objective was to study the effects of different radiation wavelengths on the physiology of plant pathogens Colletotrichum acutatum, the causing agent of fruit rots as well as shoot, leaf, and flower blights (Agrios 2005), and Fusarium fujikuroi, the causal agent of the bakanae disease of rice (Hossain et al. 2016). The conidial production and germination as well as the mycelial radial growth of colonies and tolerance to UV radiation of these fungal species in different radiation wavelengths were studied.
Materials and methods
Fungal isolates
The Colletotrichum acutatum, isolate FDC 52, was provided by Gilberto U.L. Braga, Universidade de São Paulo. This fungus was isolated from orange plants by Fundecitrus in Taquarituba, SP, Brazil. This isolate is deposited in the Fundecitrus Collection.
The Fusarium fujikuroi, isolate FKMC 1995, was provided by Javier Avalos, Universidad de Sevilla, Spain. This fungus was isolated from rice in Taiwan and deposited in the Kansas State University Collection.
Stock cultures were maintained at 4 °C in test tubes on slants of potato dextrose agar (Difco Laboratories, Sparks, MD, USA) adjusted to pH 6.9.
Conidial production and harvesting
Conidia of C. acutatum and F. fujikuroi were produced on 23 ml of potato dextrose agar (Difco, Sparks, MD, USA) (PDAY) in 95 mm polystyrene Petri dishes in the dark. The pH was adjusted to 6.9 by using NaOH (1 M). A conidial suspension (100 μl of 107 conidia ml−1) was inoculated evenly with a glass spreader onto agar media. The cultures were incubated at 26 ± 1 °C and approximately 90% relative humidity (RH) for 14 days. Three different batches of conidia were produced, one for each replication of each type of stress experiment.
Light treatments
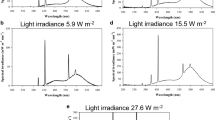
For the white light treatment, the Petri dishes with cultures on PDA medium with lids in place, in a single layer (not stacked) were maintained under continuous light provided by two 15 W cool white Philips (TL-D 15 W/75–650) broad-spectrum fluorescent light bulbs suspended at a distance of 25 cm above the samples. A sheet of 0.13 mm cellulose diacetate covered the plates to avoid medium dehydration. The integrated irradiance of the lamps that passed through the diacetate film and Petri dish lid was 5.0 W m−2 and 2230 lx (Fig. 1).
a Spectral irradiances of the lamp setups used. For white light treatment, the Petri dishes with cultures on PDA medium with lids in place, in a single layer (not stacked) were maintained under continuous light provided by two 15 W cool white Philips (TL-D 15 W/75–650) broad-spectrum fluorescent light bulbs suspended at a distance of 25 cm above the samples. A sheet of 0.13-mm cellulose diacetate covered the plates to avoid medium dehydration. The integrated irradiance of the lamps that passed through the diacetate film plus the Petri dish lid was 4.98 W m−2 and 2230 lx. For blue, green, or red light treatment, the Petri dishes with cultures on PDA medium with lids in place, in a single layer (not stacked) with LED light provided by four Color Led LLUM® E27 5 W (Jinli Lighting Co., China) bulbs each in three incubators. No heating effect by the LEDs was detected. b Incubators with white, red, green, and blue light irradiances
For blue, green, and red light treatments, the Petri dishes with cultures on PDA medium with lids in place, in a single layer (not stacked), were maintained under continuous blue, green or red light provided by three incubators that were adjusted to enable incubation of the cultures under different wavelengths of light. Incubator 1 contained four Color Led LLUM® E27 5 W light bulbs (Jinli Lighting Co., China), set in the blue wavelength providing a maximum output of integrated irradiance of 4.8 W m−2 and 645.5 lx. Incubator 2 contained four Color Led LLUM® E27 5 W light bulbs set in the green wavelength providing a maximum output of integrated irradiance of 2.2 W m−2 and 2602 lx. Incubator 3 contained four Color Led LLUM® E27 5 W light bulbs set in the red wavelength providing a maximum output of integrated irradiance of 2.8 W m−2 and 53 lx. The permitted distance of the incubator between the LEDs and the agar plates was 6 cm, the temperature of the incubators was adjusted to 26 °C, and no heating effect by the LEDs were detected (Fig. 1).
The spectral irradiances (in W m−2) of the incubators were measured with a spectroradiometer Ocean Optics (Dunedin, FL, USA) Model USB2000 + Rad connected to a laptop (Fig. 1) and the illuminance (in lux) of the incubators were measured with a Onset HOBO® data logger U12–012.
For dark treatments, all Petri dishes were maintained in the same incubator as the light treatment, but the Petri dishes were kept inside a perforated plastic box (to keep the cultures ventilated) and covered with a thick black cloth sleeve. Temperature and radiation inside the dark treatments were measured continuously using an Onset HOBO® data logger U12–012.
Conidial germination
Conidia from cultures grown for 14 days in PDA culture medium were collected with the aid of a microbiological loop and were suspended in 10 ml of sterile Tween 80 solution (0.01% v/v) in glass tubes. The suspensions were adjusted to a concentration of 105 conidia ml−1 and then stirred.
The conidial suspensions (40 μl) were inoculated in the center of the polyethylene Petri dishes (35 × 10 mm Greiner Bio-One) containing 6 ml of PDA. After inoculation, the plates were kept at 26 °C in the treatments: dark (control), white, blue, green or red light. Germination of C. acutatum and F. fujikuroi was evaluated for 2, 4, 6, 8, 10, 12, 14, and 16 h according to Rangel et al. (2004). After the incubation period, the conidia were stained with methylene blue solution (Braga et al. 2002), and germination was evaluated with 400 × magnification. Conidia were considered germinated when a projected germ tube from the conidia was visible (Milner et al. 1991). At least 300 conidia per plate were counted and the germination percentage was calculated. Conidia from both isolates and from all treatments were tested at the same time for each repetition. Three repetitions were done.
Mycelial radial growth
From the colonies of C. acutatum and F. fujikuroi grown on PDA medium in Petri dishes (90 × 15 mm), one disk of 5 mm diameter was removed with a cork borer and placed in the center of Petri dish containing 23 ml of PDA. The plates were kept at 26 °C for the treatments: dark (control), and white, blue, green or red light. The fungi C. acutatum and F. fujikuroi were grown for 5 days. Colony diameter of mycelial growth was measured on the fifth day horizontally and vertically (at a perpendicular axis). For each treatment, three Petri dish replicates were prepared, and three repetitions were performed on different days.
Measurement of conidial production
To measure conidial production under different treatment conditions, three agar plugs (per plate) were removed with a cork borer (5 mm diam) at different places on the medium surface with an even coverage of conidia, and the conidia were suspended in 1 ml sterile Tween 80 (0.1%) solution. After the conidial suspensions were vigorously shaken, the conidial concentrations were determined by hemocytometer counts. Each experiment was performed on three different dates, and each experiment used a new batch of cultures.
Conidial tolerance to UV radiation
Conidia from PDA medium from all four light treatments and the dark treatment were collected after 14 days of growth with a few passes of a microbiological loop (Decon Labs, Inc., PA, USA) and transferred to 10 ml of sterile Tween 80 (0.01% v/v). The amount of conidia collected were enough to produce a suspension of 105 conidia ml−1. The suspensions (1 × 105 conidia ml−1) were shaken vigorously using a vortex, and 40 μl were inoculated (dropped, but not spread) on the center of the medium (polyethylene plates 35 × 10 mm) containing 6 ml of PDA supplemented with benomyl 0.003% with 25% active ingredient (Hi-Yield Chemical Company, Bonham, TX, USA) (Milner et al. 1991). All suspensions were exposed to UV radiation (290–400 nm) immediately after preparation (Dias et al. 2018).
The plates were left open in laminar flow for 30 min to dry the conidial suspensions (Dias et al. 2018). After drying, the suspensions were exposed in a realistic test equipment for UV radiation using the Xenon Test Chamber QSUN XE-3-HC 340S (QLAB® Corporation, Westlake, OH, USA) with a Daylight-Q filter. The plates were covered with a diacetate film to avoid desiccation of the medium. Petri plates containing the dried suspension of all isolates were exposed for 160, 170, 180, 190, 200, and 210 min, equivalent to the irradiance of 5.7, 6.0, 6.4, 6.7, 7.1, and 7.5 kJ/m2, respectively. The exposure time and irradiances were used following previously published methods (Dias et al. 2018; Dias et al. 2020). The irradiance and temperature of the equipment was adjusted according to Dias et al. (2018).
The Quaite weighted irradiance inside the chamber was 1335 mW m−2. Spectral irradiance was measured as in Dias et al. (2018). The DNA-damage (cyclobutane pyrimidine dimer formation) action spectrum developed by Quaite et al. (1992) and normalized to unity at 300 nm was used to calculate weighted UV irradiances in mW m−2. Cellulose diacetate filters (JCS Industries, Le Miranda, CA, USA) are employed to exclude UV-C (230–280 nm) and short wavelength UV-B radiation (280–290 nm) (Rangel et al. 2006; Dias et al. 2018).
For the control, Petri dishes (one for each isolate and treatment) with the conidial suspension not exposed to UV radiation were placed in the chamber for 210 min covered with aluminum foil (Dias et al. 2018).
The germinations were observed, depending on the experiment, at 24 h (control plates) or at 48 h (UV-irradiated plates) after the conidial suspension was inoculated on the medium. The plates were kept at 26 °C in the dark for germination. The agar plate at the point of the inoculation was stained with 40 μl of methyl blue solution (Braga et al. 2002) and then covered with a circular glass coverslip (15 mm diameter) to avoid air between the medium and the coverslip. The germination was examined under light microscope at 400× magnification. Conidia were considered germinated when the germ tube showed a visible projection from the conidium. At least 300 conidia per plate were evaluated, and the percent of germination was calculated. All germinating or non-germinating conidia were counted on a single coverslip. The scanning pattern for counting was around the margin of the conidial suspension drop, which is an area commonly less populated by conidia. Each treatment was repeated at least four times with a fresh batch of conidia produced for each repetition.
Statistical analyses
The effect of white, blue, green or red wavelengths of radiation on germination, mycelial growth, conidial production, and conidial tolerance to UV irradiation was assessed with analysis of variance of a one-way factorial. Significance levels of pair-wise mean comparisons among treatments were controlled for experiment-wise type I error using the Tukey method with overall α = 0.05. All analyses were carried out with the statistical program Sisvar (Ferreira 1999; Ferreira 2011).
Results
Conidial germination
Continuous white, blue, green or red light did not affect the germination speed, compared to darkness for C. acutatum and F. fujikuroi conidia germinated on potato dextrose agar medium (Fig. 2).
Mycelial radial growth
No statistical differences in mycelial growth were found for C. acutatum and F. fujikuroi grown on potato dextrose agar medium in the dark or under continuous white, blue, green or red light (Fig. 3).
Radial growth of (a) Colletotrichum acutatum and (b) Fusarium fujikuroi colonies in the treatments: dark (control), white, blue, green, and red light at 26 °C. Conidia were point inoculated at the center of the plate containing PDA medium. The plates were kept at 26 °C for five days. Error bars represent the standard deviation of the average of three repetitions
Conidial production
C. acutatum produced more conidia when the fungus was grown under white and red light (Fig. 4a). However, growth under the blue and green light produced 76.2 and 64.7% fewer conidia than the white light treatment, respectively. Mycelial growth in the dark produced 42.1% fewer conidia than white light treatment (Fig. 4a).
Conidial production of (a) Colletotrichum acutatum and (b) Fusarium fujikuroi in the treatments: dark (control), white, blue, green, and red light. Error bars represent the standard deviation of the mean of three repetitions performed with independent experiments. Graph bars with the same letter are not significantly different (p < 0.01)
F. fujikuroi produced more conidia when grown in the dark than all light treatments. Growth under blue and green light produced 45.2 and 45.8%, respectively, fewer conidia than growth in the dark (Fig. 4b). Growth under red light produced 57.9% fewer conidia than growth in the dark. Growth under white light produced 77.7% fewer conidia than growth in the dark (Fig. 4b).
Conidial tolerance to UV radiation
For C. acutatum, conidia produced under white light were at least 30% more tolerant to UV radiation than conidia produced under blue, green, and red light and at least 20% more tolerant than conidia produced in the dark (Fig. 5a). Conidia produced under white light and in the dark had similar tolerance to UV radiation. However, at the higher exposure time of 220 min, conidia produced under white light were more tolerant than conidia produced in the dark. Conidia produced under red light displayed the least tolerance to UV radiation (Fig. 5a).
Germination percentage of (a) Colletotrichum acutatum and (b) Fusarium fujikuroi after exposure to UV radiation for 160, 170, 180, 190, 200, 210, and 220 min, equivalent to the irradiance of 5.7, 6.0, 6.4, 6.7, 7.1, 7.5, 7.8, and 8.2 kJ m−2, respectively. Error bars are the standard errors of at least three independent experiments conducted at different times. Graph bars with the same letter are not significantly different (p < 0.01)
F. fujikuroi conidia produced under blue light were most tolerant to UV radiation, and statistically similar to the tolerance of conidia produced under white light. On the other hand, growth under green and red light produced less tolerant conidia and similar tolerance as conidia produced in the dark (Fig. 5b).
Discussion
The plant-pathogenic fungi Colletotrichum acutatum and Fusarium fujikuroi control several aspects of their physiology in response to light. Light did not affect the germination and mycelial growth of these fungi. It has long been recognized, with few exceptions, e.g. in Botrytis cinerea that grows less under light (Canessa et al. 2013), that the effect of light on Fungal Kingdon is more important for reproduction than vegetative growth (Gottlieb 1950; Lilly and Barnett 1951; Cochrane 1958). Conversely, the conidial production as well as the conidial stress tolerance differs according to the different light treatments during mycelial growth.
C. acutatum produced more conidia under white and red light than in darkness, and blue and green light generated the least conidia (Fig. 4a), as also observed by de Menezes et al. (2015). Fusarium fujikuroi produced more conidia in the dark (Fig. 4b); however, F. verticillioides cultures grown under white, blue, yellow, green, and red wavelengths produce more conidia than the cultures grown in the dark (Fanelli et al. 2012). Conidiation is also stimulated by light in the wild type strain IMI58289 of F. fujikuroi (Avalos et al. 1985; Avalos and Estrada 2010), but in a different wild type strain of the same species mycelial growth in the dark produced more conidia than under light (Prado et al. 2004; Estrada and Avalos 2008; Estrada and Avalos 2009; Avalos and Estrada 2010). Estrada and Avalos (2008, 2009) found that growth in the dark of the same F. fujikuroi isolate which we used (FKMC 1995) generated more conidia than growth in the light. The results of our study agreed with Estrada and Avalos, in which this isolate also produced abundant conidia in the dark (Fig. 4b).
Growth under white light also improved conidial UV radiation tolerance of C. acutatum (Fig. 5a) and F. fujikuroi (Fig. 5b). Although C. acutatum conidia produced in the light were similarly tolerant to UV radiation as conidia produced in the dark in the lowest UV irradiances, at the highest UV irradiances, conidia produced under light were more tolerant than conidia produced in the dark. Mycelial growth under blue, green or red light generated conidia less tolerant than conidia produced in the dark (Fig. 5b). Similar to our results, growth of C. acutatum colonies under low irradiance of white light increased conidia and mucilage production, and conidia produced under the light were two-times more tolerant to UV radiation (de Menezes et al. 2015). C. acutatum is more pigmented under white and blue light, while the least pigmentation was observed in mycelia incubated in the dark (Yu et al. 2013). Blue light also enhances melanin production, enhancing virulence of C. acutatum (Yu et al. 2013), because appressorial wall melanin permits very high turgor pressure that the melanin deposition pattern directs into the penetration peg. Inhibition of melanin synthesis by tricyclazole is shown to prevent plant infection by fungal pathogens, and this process is usually attributed to inhibition of appressorial penetration (Butler et al. 2005). Moreover, the effect of green and red light stimulates less melanin production than blue light, leading to reduced disease severity (Yu et al. 2013). For F. fujikuroi, growth under white and blue light produces conidia two-fold more tolerant to UV radiation than conidia produced in the dark and under green and red light. Growth under illumination up-regulates many stress genes that are important for producing conidia with increased stress tolerance (Wu et al. 2014; Brancini et al. 2019; Dias et al. 2020). Light also promotes higher resistance of Aspergillus fumigatus against exogenous oxidative stress and enhances resistance to acute ultraviolet radiation (Fuller et al. 2013). M. robertsii conidia produced under white light exhibit higher tolerance to osmotic stress (Dias et al. 2020), heat (Rangel et al. 2011; Rangel et al. 2015), and UV radiation (Rangel et al. 2011; Rangel et al. 2015; Dias et al. 2020).
F. fujikuroi and C. acutatum grown under red light produced conidia very susceptible to UV radiation compared to conidia of these fungi produced under white and blue light. Similar results were found for M. robertsii, in which conidia produced under red light were less tolerant to osmotic stress caused by potassium chloride and UV radiation than conidia produced in the dark (Dias et al. 2020). In addition, exposure of fast-growing mycelia of M. acridum to white, blue or UV-A wavelengths induces tolerance to subsequent UV-B irradiation. However, red light induced lower mycelial tolerance to subsequent UV-B irradiation (Brancini et al. 2016). This observation may indicate that red-light represses genes for tolerance to stress. Therefore, pathogenic fungi use environmental cues to prepare their conidial offspring against challenges in the environment (Rangel et al. 2008; Rangel 2011; Rangel et al. 2012; Rangel et al. 2018; Rangel and Roberts 2018; Medina et al. 2020). Producing offspring more tolerant to the same or other stress conditions (Rangel et al. 2004; Rangel et al. 2011; Rangel et al. 2018) as well as enhancing their virulence (Oliveira et al. 2018; Oliveira and Rangel Oliveira and Rangel 2018) will support greater dispersal distances under daytime conditions, consequently, conidia which are more resistant to damage due to UV radiation exposure could also be an advantage in dispersal and infection, which could be a factor in the epidemiology of the diseases caused by these two pathogens.
Conclusion
In this study, the different wavelengths of blue, green, red and white light did not significantly affect the germination and mycelial radial growth of C. acutatum and F. fujikuroi, compared to germination or growth in the dark (control).
The conidial production of these fungi has different reactions to light. F. fujikuroi produced more conidia in the dark, while C. acutatum produced more conidia in white and red light.
White light induced higher tolerance to UV radiation in C. acutatum and F. fujikuroi. Blue light induced higher tolerance to UV radiation in F. fujikuroi only.
References
Agrios, G. N. (2005). Plant pathology (5th ed.). San Diego: Elsevier Academic Press.
Atoui, A., et al. (2010). Cross-talk between light and glucose regulation controls toxin production and morphogenesis in Aspergillus nidulans. Fungal Genet Biology, 47, 962–972. https://doi.org/10.1016/j.fgb.2010.08.007.
Avalos, J., Casadesús, J., & Cerdá-Olmedo, E. (1985). Gibberella fujikuroi mutants obtained with UV radiation and N-methyl-N'-nitro-N-nitrosoguanidine. Applied Environment Microbiology, 49, 187–191.
Avalos, J., & Estrada, A. F. (2010). Regulation by light in Fusarium. Fungal Genetic Biology, 47, 930–938. https://doi.org/10.1016/j.fgb.2010.05.001.
Aver'yanov, A. A., Lapikova, V. P., Pasechnik, T. D., Abramova, O. S., Gaivoronskaya, L. M., Kuznetsov, V. V., & Baker, C. J. (2014). Pre-illumination of rice blast conidia induces tolerance to subsequent oxidative stress. Fungal Biology, 118, 743–753. https://doi.org/10.1016/j.funbio.2014.06.003.
Braga, G. U. L., Rangel, D. E. N., Flint, S. D., Miller, C. D., Anderson, A. J., & Roberts, D. W. (2002). Damage and recovery from UV-B exposure in conidia of the entomopathogens Verticillium lecanii and Aphanocladium album. Mycologia, 94, 912–920 http://www.jstor.org/stable/3761859.
Brancini, G. T. P., Rangel, D. E. N., & Braga, G. U. L. (2016). Exposure of Metarhizium acridum mycelium to light induces tolerance to UV-B radiation. FEMS Microbiology Letters, 363(6), fnw036. https://doi.org/10.1093/femsle/fnw036.
Brancini, G. T. P., Ferreira, M. E. S., Rangel, D. E. N., & Braga, G. Ú. L. (2019). Combining transcriptomics and proteomics reveals potential post-transcriptional control of gene expression after light exposure in Metarhizium acridum. G3: Genes|Genomes|Genetics, 9, 2951–2961. https://doi.org/10.1534/g3.119.400430.
Butler, M. J., Gardiner, R. B., & Day, A. W. (2005). Fungal melanin detection by the use of copper sulfide-silver. Mycologia, 97, 312–319.
Canessa, P., Schumacher, J., Hevia, M. A., Tudzynski, P., & Larrondo, L. F. (2013). Assessing the effects of light on differentiation and virulence of the plant pathogen Botrytis cinerea: characterization of the white collar complex. PLoS One, 8(12), e84223. https://doi.org/10.1371/journal.pone.0084223.
Chen, C.-H., Ringelberg, C. S., Gross, R. H., Dunlap, J. C., & Loros, J. J. (2009). Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. The EMBO Journal, 28, 1029–1042. https://doi.org/10.1038/emboj.2009.54.
Cochrane, V. W. (1958). Physiology of Fungi. New York: John Wiley & Sons, Inc..
Dasgupta, A., Chen, C.-H., Lee, C., Gladfelter, A. S., Dunlap, J. C., & Loros, J. J. (2015). Biological significance of photoreceptor photocycle length: VIVID photocycle governs the dynamic VIVID-White Collar Complex pool mediating photo-adaptation and response to changes in light intensity. Plos Genetics, 11, e1005215. https://doi.org/10.1371/journal.pgen.1005215.
de Menezes, H. D., Massola, N. S., Flint, S. D., Silva, G. J., Bachmann, L., Rangel, D. E. N., & Braga, G. U. L. (2015). Growth under visible light increases conidia and mucilage production and tolerance to UV-B radiation in the plant-pathogenic fungus Colletotrichum acutatum Photochem Photobiol, 91, 397–402. https://doi.org/10.1111/php.12410.
Dias, L. P., Araújo, C. A. S., Pupin, B., Ferreira, P. C., Braga, G. Ú. L., & Rangel, D. E. N. (2018). The xenon test chamber Q-SUN® for testing realistic tolerances of fungi exposed to simulated full spectrum solar radiation. Fungal biol-Uk, 122, 592–601. https://doi.org/10.1016/j.funbio.2018.01.003.
Dias, L. P., et al. (2020). Outcome of blue, green, red, and white light on Metarhizium robertsii during mycelial growth on conidial stress tolerance and gene expression. Fungal biology, 124, 263–272. https://doi.org/10.1016/j.funbio.2019.04.007.
Estrada, A. F., & Avalos, J. (2008). The white collar protein WcoA of Fusarium fujikuroi is not essential for photocarotenogenesis, but is involved in the regulation of secondary metabolism and conidiation. Fungal Genet Biol, 45, 705–718. https://doi.org/10.1016/j.fgb.2007.12.003.
Estrada, A. F., & Avalos, J. (2009). Regulation and targeted mutation of opsA, coding for the NOP-1 opsin orthologue in Fusarium fujikuroi. J Mole Biol, 387, 59–73. https://doi.org/10.1016/j.jmb.2009.01.057.
Fanelli, F., Schmidt-Heydt, M., Haidukowski, M., Susca, A., Geisen, R., Logrieco, A., & Mule, G. (2012). Influence of light on growth, conidiation and fumonisin production by Fusarium verticillioides. Fungal Biol, 116, 241–248. https://doi.org/10.1016/j.funbio.2011.11.007.
Ferreira, D. F. (1999) SISVAR 4.3. Sistema de análises estatísticas, CD-ROM edn. Lavras: Universidade Federal de Lavras, UFLA.
Ferreira, D. F. (2011). Sisvar: A computer statistical analysis system. Ciência e Agrotecnologia, 35, 1039–1042.
Fuller, K. K., Ringelberg, C. S., Loros, J. J., & Dunlap, J. C. (2013). The fungal pathogen Aspergillus fumigatus regulates growth, metabolism, and stress resistance in response to light. MBio 4. https://doi.org/10.1128/mBio.00142-13.
Fuller, K. K., Cramer, R. A., Zegans, M. E., Dunlap, J. C., & Loros, J. J. (2016). Aspergillus fumigatus photobiology illuminates the marked heterogeneity between isolates. MBio 7(5):e01517–01516. https://doi.org/10.1128/mBio.01517-16.
Gottlieb, D. (1950). The Physiology of Spore Germination in Fungi Bot Rev, 16, 229–257.
Hossain, M. T., Khan, A., Chung, E. J., Rashid, M. H.-O., & Chung, Y. R. (2016). Biological control of rice bakanae by an endophytic Bacillus oryzicola YC7007. The Plant Pathology Journal, 32, 228–241. https://doi.org/10.5423/ppj.oa.10.2015.0218.
Idnurm, A., & Heitman, J. (2005). Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol, 3, 615–626. https://doi.org/10.1371/journal.pbio.0030095.
Idnurm, A., Verma, S., & Corrochano, L. M. (2010). A glimpse into the basis of vision in the kingdom. Mycota Fungal Genet Biol, 47, 881–892. https://doi.org/10.1016/j.fgb.2010.04.009.
Igbalajobi, O., Yu, Z., & Fischer, R. (2019). Red- and blue-light sensing in the plant pathogen Alternaria alternata depends on phytochrome and the white-collar protein. LreA mBio, 10, e00371–e00319. https://doi.org/10.1128/mBio.00371-19.
Lauter, F.-R., Yamashiro, C. T., & Yanofsky, C. (1997). Light stimulation of conidiation in Neurospora crassa: Studies with the wild-type strain and mutants wc-1, wc-2 and acon-2 journal of photochemistry and photobiology. B: Biology, 37, 203–211. https://doi.org/10.1016/S1011-1344(96)07405-2.
Lilly, V. G., & Barnett, H. L. (1951). Physiology of the Fungi. New York: McGraw-Hill Book Company.
Medina, E. Q. A., Oliveira, A. S., Medina, H. R., & Rangel, D. E. N. (2020). Serendipity in the wrestle between Trichoderma and Metarhizium Fungal Biol-Uk, 124, 418–426. https://doi.org/10.1016/j.funbio.2020.01.002.
Milner, R. J., Huppatz, R. J., & Swaris, S. C. (1991). A new method for assessment of germination of Metarhizium conidia. Journal Invertebrates Pathol, 57, 121–123.
Oliveira, A. S., Braga, G. U. L., & DEN, R. (2018). Metarhizium robertsii illuminated during mycelial growth produces conidia with increased germination speed and virulence. Fungal Biol-Uk, 122, 555–562. https://doi.org/10.1016/j.funbio.2017.12.009.
Oliveira, A. S., & Rangel, D. E. N. (2018). Transient anoxia during Metarhizium robertsii growth increases conidial virulence to Tenebrio molitor. Journal of Invertebrate Pathology, 153, 130–133. https://doi.org/10.1016/j.jip.2018.03.007.
Prado, M. M., Prado-Cabrero, A., Fernández-Martín, R., & Avalos, J. (2004). A gene of the opsin family in the carotenoid gene cluster of Fusarium fujikuroi. Current Genetics, 46, 47–58. https://doi.org/10.1007/s00294-004-0508-6.
Quaite, F. E., Sutherland, B. M., & Sutherland, J. C. (1992). Action spectrum for DNA damage in alfalfa lowers predicted impact of ozone depletion. Nature, 358, 576–578.
Rangel, D. E. N. (2011). Stress induced cross-protection against environmental challenges on prokaryotic and eukaryotic microbes world. Journal Microbiology Biotechnology, 27, 1281–1296. https://doi.org/10.1007/s11274-010-0584-3.
Rangel, D. E. N., Alston, D. G., & Roberts, D. W. (2008). Effects of physical and nutritional stress conditions during mycelial growth on conidial germination speed, adhesion to host cuticle, and virulence of Metarhizium anisopliae, an entomopathogenic fungus. Mycological Research, 112, 1355–1361.
Rangel, D. E. N., Braga, G. U. L., Fernandes, É. K. K., Keyser, C. A., Hallsworth, J. E., & Roberts, D. W. (2015). Stress tolerance and virulence of insect-pathogenic fungi are determined by environmental conditions during conidial formation. Current Gen, 61, 383–404. https://doi.org/10.1007/s00294-015-0477-y.
Rangel, D. E. N., Braga, G. U. L., Flint, S. D., Anderson, A. J., & Roberts, D. W. (2004). Variations in UV-B tolerance and germination speed of Metarhizium anisopliae conidia produced on artificial and natural substrates. Journal of Invertebrate Pathology, 87, 77–83. https://doi.org/10.1016/j.jip.2004.06.007.
Rangel, D. E. N., Butler, M. J., Torabinejad, J., Anderson, A. J., Braga, G. U. L., Day, A. W., & Roberts, D. W. (2006). Mutants and isolates of Metarhizium anisopliae are diverse in their relationships between conidial pigmentation and stress tolerance. Journal of Invertebrate Pathology, 93, 170–182. https://doi.org/10.1016/j.jip.2006.06.008.
Rangel, D. E. N., Fernandes, E. K. K., Anderson, A. J., & Roberts, D. W. (2012). Culture of Metarhizium robertsii on salicylic-acid supplemented medium induces increased conidial thermotolerance. Fungal Biol-Uk, 116, 438–442.
Rangel, D. E. N., Fernandes, E. K. K., Braga, G. U. L., & Roberts, D. W. (2011). Visible light during mycelial growth and conidiation of Metarhizium robertsii produces conidia with increased stress tolerance. FEMS Microbiology Letter, 315, 81–86. https://doi.org/10.1111/j.1574-6968.2010.02168.x.
Rangel, D. E. N., Finlay, R. D., Hallsworth, J. E., Dadachova, E., & Gadd, G. M. (2018). Fungal strategies for dealing with environmental and agricultural stress. Fungal Biology, 122, 602–612. https://doi.org/10.1016/j.funbio.2018.02.002.
Rangel, D. E. N., & Roberts, D. W. (2018). Possible source of the high UV-B and heat tolerance of Metarhizium acridum (isolate ARSEF 324). Journal Invertebrates Pathologica, 157, 32–35. https://doi.org/10.1016/j.jip.2018.07.011.
Sanchez-Murillo, R. I., de la Torre-Martinez, M., Aguirre-Linares, J., & Herrera-Estrella, A. (2004). Light-regulated asexual reproduction in Paecilomyces fumosoroseus. Microbiology, 150, 311–319.
Tisch, D., & Schmoll, M. (2010). Light regulation of metabolic pathways in fungi Appl Microbiol Biotechnol, 85, 1259–1277. https://doi.org/10.1007/s00253-009-2320-1.
Wu, C., et al. (2014). Genome-wide characterization of light-regulated genes in Neurospora crassa. G3-Genes Genomes Genetics, 4, 1731–1745. https://doi.org/10.1534/g3.114.012617.
Yu, S. M., Ramkumar, G., & Lee, Y. H. (2013). Light quality influences the virulence and physiological responses of Colletotrichum acutatum causing anthracnose in pepper plants. Journal of Applied Microbiology, 115, 509–516. https://doi.org/10.1111/jam.12252.
Yu, Z., & Fischer, R. (2019). Light sensing and responses in fungi. Nat Reviews Microbiology, 17, 25–36. https://doi.org/10.1038/s41579-018-0109-x.
Zhang, Y.-J., Li, Z.-H., Luo, Z.-B., Zhang, J.-Q., Fan, Y.-H., & Pei, Y. (2009). Light stimulates conidiation of the entomopathogenic fungus Beauveria bassiana. Biocontrol Science and Technology, 19, 91–101. https://doi.org/10.1080/09583150802588516.
Acknowledgments
The authors thank Alene Alder-Rangel (Alder’s English Services) and Luis M. Corrochano (Departamento de Genética, Facultad de Biología, Universidad de Sevilla) for their valuable review on the manuscript. The authors also thank Claudinéia A. S. Araújo for her assistance with the experiments. This research was supported by grants from National Council for Scientific and Technological Development (CNPq) of Brazil PQ1D 302100/2018-0 and to São Paulo Research Foundation (FAPESP) 2010/06374-1 and 2013/50518-6 for D.E.N.R. We sincerely thank FAPESP for the PhD fellowship for L.P.D 2013/25964-2, and for a technical fellowship to P.C.F. 2014/13573-1 and to C.A.S.A. 2014/02467-6.
Funding
This research was supported by grants from the National Council for Scientific and Technological Development (CNPq) of Brazil and São Paulo Research Foundation (FAPESP).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: DENR. Performed the experiments: LPD, DENR, PCF, and BP. Prepared all figures: DENR. Analyzed the data: BP and DENR. Wrote the paper: DENR, TPCC, and EMR.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
No ethical permissions were required for this work which involved no experimentation involving animals or human samples.
Consent for publication
All authors read and approved the final version of the manuscript, except PCF, who passed away in 2018.
Data availability
All datasets are available.
Additional information
Paulo C. Ferreira is Deceased
Rights and permissions
About this article
Cite this article
Costa, T.P.C., Rodrigues, E.M., Dias, L.P. et al. Different wavelengths of visible light influence the conidial production and tolerance to ultra-violet radiation of the plant pathogens Colletotrichum acutatum and Fusarium fujikuroi. Eur J Plant Pathol 159, 105–115 (2021). https://doi.org/10.1007/s10658-020-02146-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02146-y