Abstract
Downy mildew, caused by the obligate oomycete Peronospora effusa (Pfs), is the most economically important disease of spinach. In recent years, numerous new races of the pathogen have overcome the resistance used in newly released cultivars. Resistance to Pfs in spinach is predominantly governed by dominant major resistance genes (RPFs) that are widely used in commercial spinach hybrid cultivars. Currently, a labor and resource-intensive inoculation test of whole plants, in a large tray format, in a temperature-controlled growth chamber and dew chamber, is employed to evaluate the resistance of spinach germplasm and to characterize races of the downy mildew pathogen. The objective of this work was to evaluate, standardize, and validate a more efficient detached leaf inoculation method to differentiate resistant and susceptible spinach genotypes and characterize Pfs races on a standardized set of spinach differential genotypes. Detached leaves and cotyledons of standard host differentials commonly used for race differentiation were placed on water agar in closed Petri dishes and inoculated by spraying the leaves with a spore suspension of Pfs inoculum. Disease incidence and severity on detached leaves and cotyledons were compared to the response of the corresponding cultivars in the standard whole-plant assay. There was a complete match between the disease reaction on whole plants and the disease reaction on detached leaves for all three races of Pfs examined. Furthermore, the obligate pathogen could infect, sporulate, and maintain pathogenicity by propagation solely on detached leaves. The detached leaf assay could facilitate advances in breeding for Pfs by evaluating resistance, pathogen race identification, and studies on epidemiology and genetics of the pathogen as the tests are less labor, resource-intensive than the whole-plant assay format, and environmental variables can be more accurately controlled.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Downy mildew (DM), caused by the obligate oomycete Peronospora effusa (formerly P. farinosa f. sp. spinaciae [Pfs]), is the most economically important disease of spinach in the US (Correll et al. 2011). Spinach production has increased dramatically in recent years in the US due to increased consumption as a result of the availability of high quality triple-washed packaged baby leaf spinach (Klosterman 2016; USDA-NASS 2019). Spinach is typically grown in high density plantings (5–10 million seed/ha) for 12 months of the year, and this plant density has favored the selection of new races of Pfs which overcome newly released resistant cultivars. Seventeen races of Pfs have been reported (Brandenberger et al. 1991; Feng et al. 2014, 2018; Irish et al. 2003, 2007; Satou et al. 2006), of which 14 new races have been described in the last three decades. A novel virulence pattern that is observed on the standardized differential spinach cultivars, as confirmed among multiple labs, is reported as a new race by the International Working Group on Peronospora (IWGP) (Dijkstra et al., 2011; https://spinach.uark.edu/amsterdam-october-2011/), and each new race represents a significant setback for breeders. The rate of emergence of races appears to be related to high density cultivation practices in California and Arizona. In addition, genetic variation resulting from asexual variation and sexual recombination of the pathogen can contribute to virulence variation in the pathogen.
Races of the downy mildew pathogen are defined based on the disease reactions of a set of standardized differential spinach cultivars in a whole plant bioassay (Brandenberger et al. 1991; Feng et al. 2014, 2018; Irish et al. 2007). Briefly, this bioassay involves growing the differentials until the plants are 2–3 weeks old. Conidia are washed off from infected spinach leaves, and the resulting spore suspension with a density of 105 spores per ml is used for inoculation. Inoculated plants are first placed in a temperature-controlled dew chamber (18 °C) for 24 h to provide free moisture, then transferred to temperature-controlled growth chamber (18 °C) for 5 days, and finally returned to the temperature-controlled dew chamber (18 °C) for 24 h to induce sporulation. On the seventh day, each plant of the differential set is rated for signs of the pathogen and symptoms of downy mildew. Based on the reaction pattern on the set of differentials, the race identity of the pathogen can be determined. Novel isolates exhibiting a unique reaction pattern on the standard set of spinach differentials go through a process whereby multiple labs that participate in the IWGP test the isolate. If the IWGP comes to a consensus on the disease reactions, they will denominate the novel isolates as a new race. Thus, the entire process typically takes 3–5 weeks, involves the maintenance and rental cost of a dew chamber and growth chamber, and labor to maintain all aspects of the bioassay. If adequate inoculum was not available in the original inoculation and the first round of inoculations did not produce conclusive disease reaction results on the differentials, the entire process needs to be repeated with the inoculum that was produced in the first cycle of the bioassay extending the effort to 6–8 weeks. Thus, the whole plant inoculation method has some major logistical disadvantages in terms of space, resources, and destructive phenotyping. The space requirements and risk of cross-contamination of different isolates also reduce the number of assays that can be performed at a given time.
Resistance against the downy mildew pathogen remains a primary focus of all major spinach breeding programs (Morelock and Correll 2008). Although quantitative resistance to the downy mildew pathogen has been identified (Irish et al. 2003), it is considerably more challenging to assess than qualitative resistance in the current whole plant bioassay. The development of a more efficient and reliable bioassay to screen for resistance against the pathogen and race-typing would be advantageous for any breeding program working on disease resistance, especially in the case of spinach, given the recent rise in the numbers of different pathotypes of the Pfs pathogen. A detached leaf inoculation assay is a non-destructive (for the whole plant) disease evaluation method, would require much less space and inoculum, and allow a given plant to be evaluated with multiple races. Although a detached leaf assay has been previously described for Pfs (Kubota et al. 2017; Yamauchi et al. 2011), the assays did not validate the disease reactions of known races nor compare the reactions to whole plant assays. Thus, the tests previously described could not authenticate the reactions and took considerably longer to perform than the assays described in the current study.
The objective of this study was to develop, standardize, and validate an efficient and reliable detached leaf inoculation assay for Pfs using three races/isolates with a known disease reaction on differentials. The described assays have the potential to considerably reduce the resources and labor needed to perform the standard whole plant assays. Further development and standardization of the detached leaf assay are anticipated to facilitate advances in breeding for Pfs resistance and in the analyses of pathogen epidemiology and genetics.
Materials and methods
Plant material
A standard set of differential cultivars were used for the distinction of denominated races of Pfs, as defined by diverse stakeholders in the International Working Group on Peronospora (IWGP) (Dijkstra et al., 2011; https://spinach.uark.edu/amsterdam-october-2011/). Three isolates of Pfs, UA0510C, UA201715, and UA2020-01E, representing races 13, 5, and a recently identified novel strain, respectively, were evaluated for disease incidence and severity in a standard whole plant bioassay and compared to disease reactions in a newly developed detached leaf bioassay (Table 1). Briefly, seeds of differential cultivars were sown in 25 × 50-cm plastic trays filled with potting soil (Sun Gro Horticulture, Canada), and a tray contained ten cultivars and 20 seed per cultivar. For Pfs 13, 13 differential genotypes were evaluated, while ten genotypes were evaluated for Pfs 5 and isolate UA2020-01E. The universally susceptible control cultivar Viroflay was also grown in a separate tray in a similar planting format to increase inoculum. Plants were grown in the greenhouse (at approximately 25 °C) for 2 weeks, watered daily, and fertilized weekly using Miracle-Gro® All Purpose Plant Food following a standard protocol (Feng et al. 2014, 2018).
Inoculum preparation for whole plant and detached leaf inoculations
The inoculum of each of the three isolates of Pfs was increased on the susceptible cultivar Viroflay and use to inoculate the standard set of differentials in a whole plant assay as previously described (Feng et al. 2014, 2018) and also in a detached leaf assay as described here. In brief, sporangia were washed off from the infected leaves of Viroflay in cold (4 °C) distilled water. Inoculum suspension was filtered using two layers of cheesecloth and diluted to 105 spores/ml and sprayed with a Badger basic spray gun (model 250) onto 2-week-old whole plants or detached leaves until the cotyledons and true leaves were wet.
After inoculation, plants were incubated following a standardized set of previously published conditions (Feng et al. 2014, 2018). Briefly, inoculated plants or detached leaves were incubated in a dew chamber (18 °C) for 24 h. Following the dew chamber incubation, the plants or detached leaves were moved to a growth chamber (18 °C, 12 h dark-light cycle). After 6 days, plants or detached leaves were returned to the dew chamber (18 °C) for 24 h to induce sporulation and then scored for the disease reaction.
Optimization of the detached leaf inoculation assay
Several preliminary detached leaf inoculation tests were conducted by placing detached leaves from the susceptible cultivar Viroflay in Petri dishes with moist filter paper, moist cheesecloth, or on the surface of 2% water agar. Detached leaves and whole plants of Viroflay were inoculated and incubated side by side with isolates UA0510C (race 13) following the routine inoculation method (Feng et al. 2014). The tests were conducted blind on a set of differential cultivars whereby the cultivar names were kept unknown until after phenotyping. Once the initial protocols were evaluated, the detached leaf tests were conducted by placing detached leaves on the surface of 2% water agar as this allowed for good pathogen development without the leaves desiccating.
Detached leaf inoculation experiment
For the detached leaf assays, one leaf and cotyledon from three to four plants of each genotype (Table 1) were excised and were placed in a Petri dish (100 × 15-mm) on the surface of 2% water agar medium with the abaxial surface facing upward (Fig. 1). Detached leaves in Petri dishes with the lids removed, along with the whole plants in the tray format, were inoculated following the standard procedures described above. The Petri dish lids were put back on the plates with detached leaves after inoculation, and the Petri dishes were incubated in a dew chamber (18 °C) for 24 h. Following the dew chamber incubation, the Petri dishes and plant trays were moved to a growth chamber (18 °C, 12 h dark-light cycle). Once placed in the growth chamber, the Petri dish lids were opened for 20 min to allow excess water on the detached leaves to evaporate. The detached leaves in the Petri dishes were examined daily to check moisture conditions, and water was sprinkled with a hand sprayer to the surface of the water agar if the leaves are desiccating. After 6 days, plant trays and Petri dishes were returned to the dew chamber (18 °C) for 24 h to induce sporulation, and disease reactions were recorded as previously described.
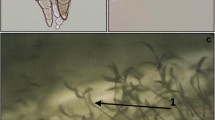
Downy mildew disease response of the spinach cultivars inoculated with Peronospora effusa isolate UA2020-01E following the detached leaf inoculation method. Disease signs and symptoms were examined at seven days post-inoculation. a. NIL3 is a resistant differential cultivar that does not show any sign of downy mildew. b. Viroflay is susceptible, and the leaves show chlorosis and sporulation
Maintenance of the downy mildew pathogen on detached leaves
To evaluate the effectiveness of propagating the obligate pathogen Pfs solely on detached leaves, an inoculum suspension of Pfs 13 isolate UA0510C was prepared exclusively from the infected detached leaves of the susceptible differentials and Viroflay to determine the potential of producing viable inoculum from infected detached leaves. A new set of detached leaves and cotyledons from the differentials were inoculated with an inoculum suspension obtained exclusively from the detached leaves.
Detached leaf assay on a tray format
In addition to the use of 2% water agar in Petri dishes to evaluate the disease reaction on detached leaves, an agarose gel tray format was used with 2% water agar. The cotyledons and true leaves of each cultivar were pinned with unique colored head needles (Fig. 2). The gel tray was placed on a rectangular plastic box with a 0.5-in. lid open during incubation periods in the dew chamber and growth chamber. Eight to 10 genotypes that fit on a tray were inoculated with either Pfs 5 or isolate UA2020-01E, and the downy mildew disease response was compared with the disease response on whole plants. The detached leaves in the gel tray format were sprinkled with water using a hand sprayer to avoid leaf desiccation during incubation.
Disease scoring
Disease reactions were evaluated for incidence as described by the IWGP (Dijkstra et al., 2011; https://spinach.uark.edu/amsterdam-october-2011/) by visually inspecting cotyledons and true leaves for sporulation using a magnifying lens, as previously described (Feng et al. 2014). Disease incidence was evaluated based on the development of chlorosis on cotyledons and true leaves and the presence (+) or absence (−) of sporulation on cotyledons and true leaves. A plant showing chlorosis and sporulation on cotyledons, true leaves, or both were classified as susceptible (+). A plant without chlorosis or sporulation was rated as resistant (−). A spinach cultivar was recorded as susceptible if more than 85% of the plants were diseased (+) or classified as resistant if less than 15% of plants were diseased (−) for each experimental unit of three plants or plant parts (cotyledons, true leaves).
Experimental design and analysis
The detached leaf and whole plant inoculation assay for isolates of Pfs UA201715 (race 5), UA0510C (race 13), and UA2020-01E were conducted in three independent tests. For the whole plant assay, three plants represented a replication, and three replications were scored for each cultivar. For the detached leaf assay, three detached leaves and three cotyledons of each cultivar placed on water agar in a Petri dish was a replicate, and each cultivar was inoculated in three replication. Disease incidence and severity scores of cultivars in each replication and inoculation method (detached leaf, whole plant) were calculated. The experiment was a multifactorial completely randomized design (CRD).
The assay to test the maintenance of the downy mildew pathogen on detached leaves, whereby inoculum of UA0510C (race 13) was prepared solely from detached leaves, was conducted in two independent tests. The assay to evaluate the disease reactions of Pfs 5 and UA2020-01E on whole plants and the detached leaves in the agarose tray format were conducted in two independent tests.
Results
The initial evaluations and optimization of the detached leaf assay indicated that good infection and symptom development on the detached leaves of susceptible cultivars occurred when they were placed in Petri dishes on the surface of 2% water agar following standard inoculation and incubation methods for whole plants as previously described (Feng et al. 2014, 2018). Once the initial detached leaf protocol was optimized and validated, replicated experiments were conducted on a set of differential cultivars to compare disease incidence and severity on the whole plants with those in the detached leaf inoculation assay. The disease response comparison between the detached leaf and whole plants were evaluated repeatedly with three different Pfs races: Pfs 5 (isolate UA201715), Pfs 13 (isolate UA0510C), and novel isolate UA2020-01E.
Initial detached leaf assays performed on the Petri dishes with Pfs 13 (isolate UA0510C), sporulation and chlorosis were observed on the true leaves and cotyledons of Viroflay, NIL2, NIL3, NIL4, NIL5, NIL6, Whale, and Califlay, but not on the true leaves and cotyledons of NIL1, Meerkat, Pigeon, Caladonia, and Hydrus (Fig. 1). The whole plant assays confirmed that Viroflay, NIL2, NIL3, NIL4, NIL5, NIL6, Whale, and Califlay were susceptible, while the cultivars NIL1, Meerkat, Pigeon, Caladonia, and Hydrus were resistant as expected for the Pfs race 13 (Table 1).
Similarly, to validate the effectiveness of the detached leaf inoculation assay with other Pfs races, the spinach differential lines were inoculated with isolate UA201715 (race 5) and UA2020-01E in the whole plant and detached leaf assay for three independent experiment run, and each differential lines were tested in three replications. Disease response on the detached leaf and the whole plants of the differential cultivars perfectly matched upon inoculation with the two Pfs races: Pfs 5 and isolate UA2020-01E (Table 1), which further indicates the effectiveness of detached leaf inoculation assay.
A resistant and susceptible downy mildew response was observed among the differential cultivar in the detached leaf inoculation test. Disease reactions of the spinach differentials inoculated with Pfs 5 (isolate UA201715), Pfs 13 (isolate UA0510C), and isolate UA2020-01E were the same in the whole plant inoculation assays as in the detached leaf assays in all three independent experiments, and the disease responses were averaged for each cultivar (Table 1). The susceptible and resistant disease reactions of each differential genotypes recorded in these experiments were consistent with previous reports (Feng et al. 2014, 2018). Similarly, the disease response between plant parts (cotyledons and true leaf) was not different in the detached leaf and the whole plant inoculation test in all tested cultivars (data not shown).
To determine if detached leaves could be used to propagate the downy mildew pathogen over multiple cycles, a series of inoculation tests were conducted on the detached leaves. Inoculum produced exclusively on the detached leaves of the susceptible cultivars was used to inoculate detached leaves of the differentials for two subsequent cycles. The data indicated that downy mildew response on the detached leaves of the differential cultivars inoculated with the inoculum produced exclusively on the detached leaves perfectly matched with the response recorded from all other inoculation methods (Table 1).
Finally, to make the detached leaf assay more user-friendly downy mildew disease response of several differential cultivars was evaluated in a large rectangular agarose gel tray that could accommodate 8–10 spinach genotypes instead of several Petri dishes. The disease response on the detached leaf and cotyledons of the differential lines on the agarose gel tray perfectly matched to the expected response for both Pfs 5 and isolate UA2020-01E on the whole plants (data not shown).
Discussion
A robust validated detached leaf inoculation assay was developed as an efficient method to screen for disease resistance to the spinach downy mildew pathogen and to characterize races of the pathogen. Disease incidence was evaluated on a set of spinach differential cultivars and compared between the detached leaf inoculation assay and a standard whole-plant inoculation assay using three different races of the downy mildew pathogen. The disease response of the differential cultivars showed a complete correspondence between the detached-leaf and whole-plant inoculation methods for all tested races across all repeats of each experiment with a complete congruence in the qualitative disease reactions observed. Thus, the detached leaf assay proved to be a reliable method to differentiate the resistant and susceptible spinach genotypes to a given race of the downy mildew pathogen. Similar results have been observed with other oomycete host-pathogen systems (Brooks 2008; Goth and Keane 1997; Nyassé et al. 1995), although some discrepancies in the disease response have been reported as in the Arabidopsis / Colletotrichum spp. host-pathogen system (Liu et al. 2007). Although a detached leaf assay had previously been used on spinach, neither the race of the pathogen was known nor was the expected resistance reaction among the spinach genotypes tested (Kubota et al. 2017; Yamauchi et al. 2011). As a result, it was not possible to authenticate the robustness of the tests. The results of the current effort offer a practical approach of using the detached leaf to identify the resistance response of the host. Indeed, the detached leaf assay, as reported here, can be reliably used to screen for a downy mildew disease response that allows distinguishing the resistance and susceptible spinach genotypes and characterizing the race identity of an isolate in a more resource-efficient approach.
As part of the study, the detached leaf assay also was used effectively to maintain the obligate pathogen through multiple infection cycles only on detached leaves. Disease response on detached leaves of the spinach differential cultivars inoculated using Pfs maintained solely on the detached leaves of a susceptible cultivar showed a complete match with the inoculum produced on whole plants. This indicates the potential use of detached leaves as a viable “in vitro” option to maintain, propagate, and store elite isolates of the downy mildew pathogen. Detached leaves on water agar have been used in a similar way to maintain and store the quinoa downy mildew pathogen Peronospora variabilis (Testen et al. 2014).
Although the detached leaf assay using Petri dishes with water agar was robust, a larger format detached leaf assay was also evaluated. A large electrophoresis gel tray format containing 2% water agar was sued. The larger scale format could accommodate the detached leaves of multiple genotypes. A complete correspondence in the disease response was observed between the detached leaf assay in the gel tray format and the whole plant. The gel tray format had some advantages over the Petri dish format in that large multiple leaves of genotypes could be evaluated under the same conditions in a single inoculation setting, further reducing the time and resources needed for the Petri dish assay.
The use of a robust detached leaf assay has some significant advantages over the whole-plant assay. The rapid emergence of new Pfs races has increased research focus on downy mildew disease studies and downy mildew resistance breeding efforts in spinach. A reliable, accurate, and efficient disease screening method for the downy mildew pathogen on spinach to characterize race-phenotypes could be an advantage for race typing of a larger sample of isolates from a field, from a given cultivar, or a region. Knowledge of what race or races are prevalent in a given area in real-time is critical for the development and deployment of effective resistance to the downy mildew pathogen. In addition to reducing the resources needed for screening, the detached leaf inoculation method also allows evaluating a single plant for resistance against multiple races of the spinach downy mildew pathogen, and it allows for a larger number of genotypes to be screened for resistance with reduced resources. This method is especially relevant to the spinach industry since the rate of appearance of new races of the pathogen has dramatically increased in the last fifteen years (Feng et al. 2018), likely as a result of asexual variation (Lyon et al. 2016) and sexual recombination (Kandel et al. 2019; Lyon et al. 2016) within the pathogen population.
Although not a direct part of this study, the detached leaf assay could also be used to provide a method to improve our understanding of the epidemiology of the spinach downy mildew pathogen by examining a wide range of variables, including sporangiospores viability, genetic diversity, oospore production, and population structure. Initial studies have shown that there is considerable diversity of the spinach downy mildew pathogen on a regional scale (Lyon et al. 2016). Recently, the detached leaf assay has been used to examine genetic crosses of Pfs arising from individual oospores of the pathogen and that this may, in turn, help to discern the population genetics and evolution of new pathotypes or races of the pathogen (Dhillon et al. 2020).
This is the first report to present a comparative evaluation of a downy mildew response on a set of spinach differential cultivars using a detached leaf assay and the standard whole plant assay. An efficient, reliable, and non-destructive (for mother plants) assay that can reduce labor and resource inputs will allow for the race characterization of a larger number of isolates from a given area and will also allow for the simultaneous screening of many spinach genotypes for resistance. This study presents that the detached leaf assay reflects the equivalent downy mildew disease response compared to the standard whole plant inoculation assay, and the detached leaf assay could be used to screen spinach cultivars for resistance to downy mildew, and the assay could be used to maintain and propagate downy mildew pathogen. Based on the resistant reactions of the differentials with multiple Pfs races, the data would indicate that the detached leaf disease reactions and the expected reactions from the genotypes with the various resistance genes in the field would completely coincide. Detached leaf inoculation assays have proven to be valuable in several other host-pathogen systems, including taro late blight (Brooks 2008), downy mildew in grapes (Boso and Kassemeyer 2008), and late blight in Solanum species (Vleeshouwers et al. 1999). Reduction in space requirements, ease of management, and lower inoculum requirements make the detached leaf inoculation technique a more attractive option in plant disease studies, including with the spinach downy mildew. In spite of some practical challenges of the detached leaf assay, this method shows the potential to increase our understanding of the epidemiology of the spinach downy mildew pathogen, the genetics of resistance, and accelerate the efforts to breed for resistance to this economically important pathogen.
Conclusion
In this study, the detached leaf assay was optimized, standardized, and validated in a set of spinach differential cultivars. Equivalent disease response on whole plants and detached leaves was obtained for multiple races of Pfs on 2% water agar media in the Petri dishes and the gel tray format. This report showed that the detached leaf assay offers a reliable and robust method to differentiate downy mildew resistance response of spinach differential cultivars. The Pfs pathogen could also be maintained solely on detached leaves and with no variation in disease responses compared to inoculum maintained on whole plants. For the obligate Pfs pathogen, the ability to handle and propagate pathogens on the detached leaves could help to generate pure cultures and to avoid cross-contamination. The detached leaf assay can be particularly helpful in an effort to characterize the resistance of a single plant to multiple races of the downy mildew pathogen.
Authors’s contributions
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Boso, S., & Kassemeyer, H. H. (2008). Different susceptibility of European grapevine cultivars for downy mildew. Vitis - Journal of Grapevine Research., 49, 1452–1462. https://doi.org/10.1016/j.cortex.2012.07.003.
Brandenberger, L. P., Correll, J. C., & Morelock, T. E. (1991). Identification of and cultivar reactions to a new race (race 4) of Peronospora farinosa f. sp. spinaciae on spinach in the United States. Plant Disease, 75, 630–634.
Brooks, F. E. (2008). Detached-leaf bioassay for evaluating taro resistance to Phytophthora colocasiae. Plant Disease, 92, 126–131. https://doi.org/10.1094/PDIS-92-1-0126.
Correll, J. C., Bluhm, B. H., Feng, C., Lamour, K., du Toit, L. J., & Koike, S. T. (2011). Spinach: Better management of downy mildew and white rust through genomics. European Journal of Plant Pathology, 129(2), 193–205. https://doi.org/10.1007/s10658-010-9713-y.
Dhillon, B., Feng, C., Villarroel-Zeballos, M. I., Castroagudin, V. L., Bhattarai, G., Klosterman, S. J., & Correll, J. (2020). Sporangiospore viability and oospore production in the spinach downy mildew pathogen, Peronospora effusa. Plant Disease. https://doi.org/10.1094/pdis-02-20-0334-re.
Dijkstra, J., Correll, J. C., Smilde, D., & Visser, J. de. (2011). The role of the International Working Group on Peronospora (IWGP). In International Spinach Conference 2011. https://spinach.uark.edu/amsterdam-october-2011/
Feng, C., Correll, J. C., Kammeijer, K. E., & Koike, S. T. (2014). Identification of new races and deviating strains of the spinach downy mildew pathogen Peronospora farinosa f. sp. spinaciae. Plant Disease, 98(1), 145–152. https://doi.org/10.1094/PDIS-04-13-0435-RE.
Feng, C., Saito, K., Liu, B., Manley, A., Kammeijer, K., Mauzey, S. J., Koike, S., & Correll, J. C. (2018). New races and novel strains of the spinach downy mildew pathogen Peronospora effusa. Plant Disease, 102(3), 613–618. https://doi.org/10.1094/PDIS-05-17-0781-RE.
Goth, R. W., & Keane, J. (1997). A detached-leaf method to evaluate late blight resistance in potato and tomato. American Potato Journal, 74, 347–352. https://doi.org/10.1007/BF02851579.
Irish, B. M., Correll, J. C., Koike, S. T., & Morelock, T. E. (2007). Three new races of the spinach downy mildew pathogen identified by a modified set of spinach differentials. Plant Disease, 91(11), 1392–1396.
Irish, B. M., Correll, J. C., Koike, S. T., Schafer, J., & Morelock, T. E. (2003). Identification and cultivar reaction to three new races of the spinach downy mildew pathogen from the United States and Europe. Plant Disease, 87(5), 567–572. https://doi.org/10.1094/PDIS.2003.87.5.567.
Kandel, S. L., Mou, B., Shishkoff, N., Shi, A., Subbarao, K. V., & Klosterman, S. J. (2019). Spinach downy mildew: Advances in our understanding of the disease cycle and prospects for disease management. Plant Disease, 103, 791–803. https://doi.org/10.1094/PDIS-10-18-1720-FE.
Klosterman, S. J. (2016). Spinach downy mildew: Threat, prevention & control. Progressive Crop Consultant, 1, 13–15.
Kubota, M., Tamura, O., Nomura, Y., Orihara, N., Yamauchi, N., Yonemoto, K., et al. (2017). Recent races of spinach downy mildew pathogen Peronospora farinosa f. sp. spinaciae in Japan. Journal of General Plant Pathology. https://doi.org/10.1007/s10327-017-0698-7.
Liu, G., Kennedy, R., Greenshields, D. L., Peng, G., Forseille, L., Selvaraj, G., & Wei, Y. (2007). Detached and attached Arabidopsis leaf assays reveal distinctive defense responses against Hemibiotrophic Colletotrichum spp. Molecular Plant-Microbe Interactions, 20, 1308–1319. https://doi.org/10.1094/MPMI-20-10-1308.
Lyon, R., Correll, J., Feng, C., Bluhm, B., Shrestha, S., Shi, A., & Lamour, K. (2016). Population structure of Peronospora effusa in the southwestern United States. PLoS One, 11, e0148385. https://doi.org/10.1371/journal.pone.0148385.
Morelock, T. E., & Correll, J. C. (2008). Spinach. In Vegetables I (pp. 189–218). Springer, New York, NY. https://doi.org/10.1007/978-0-387-30443-4_6.
Nyassé, S., Cilas, C., Herail, C., & Blaha, G. (1995). Leaf inoculation as an early screening test for cocoa (Theobroma cacao L.) resistance to Phytophthora black pod disease. Crop Protection. https://doi.org/10.1016/0261-2194(95)00054-2.
Satou, M., Nishi, K., Kubota, M., Fukami, M., Tsuji, H., & van Ettekoven, K. (2006). Appearance of race Pfs:5 of spinach downy mildew fungus, Peronospora farinosa f. sp. spinaciae, in Japan. Journal of General Plant Pathology, 72(3), 193–194. https://doi.org/10.1007/s10327-005-0266-4.
Testen, A. L., Jiménez-Gasco, M. D. M., Ochoa, J. B., & Backman, P. A. (2014). Molecular detection of Peronospora variabilis in quinoa seed and phylogeny of the quinoa downy mildew pathogen in South America and the United States. Phytopathology, 104, 379–386. https://doi.org/10.1094/PHYTO-07-13-0198-R.
USDA-NASS (2019). Agricultural statistics 2019. https://www.nass.usda.gov/.
Vleeshouwers, V. G. A. A., Van Dooijeweert, W., Keizer, L. C. P., Sijpkes, L., Govers, F., & Colon, L. T. (1999). A laboratory assay for Phytophthora infestans resistance in various Solanum species reflects the field situation. European Journal of Plant Pathology, 105(3), 241–250. https://doi.org/10.1023/A:1008710700363.
Yamauchi, N., Horinouchi, H., Sakai, K., Yonemoto, K., Satou, M., & Shirakawa, T. (2011). First report of spinach downy mildew caused by race Pfs:8 of Peronospora farinosa f. sp. spinaciae in Japan. Journal of General Plant Pathology. https://doi.org/10.1007/s10327-011-0313-2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All principles of ethical and professional conduct have been followed during the research and elaboration of this manuscript.
Ethical statement
All authors consent to the submission of this manuscript. The manuscript has been prepared following principles of ethical and professional conduct. The research did not involve human participants and did not involve animal subjects; therefore, neither submission of human consent nor statement on the welfare of animals is applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
All authors have reviewed the manuscript and approved its submission to the European Journal of Plant Pathology.
Rights and permissions
About this article
Cite this article
Bhattarai, G., Feng, C., Dhillon, B. et al. Detached leaf inoculation assay for evaluating resistance to the spinach downy mildew pathogen. Eur J Plant Pathol 158, 511–520 (2020). https://doi.org/10.1007/s10658-020-02096-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-020-02096-5