Abstract
Downy mildew in sweet basil (Ocimum basilicum L) was first recorded in Israel in late 2011. Within one year, the disease was reported all over the country, causing devastating economic damage to basil crops. The pathogen was reported to have similarly spread widely in Europe, the USA and Asia. Seed transmission and seedling trade were suggested as possible causes for rapid spread. Here, we report that some members of the Labiatae family beside O. basilicum serve as hosts of Peronospora belbahrii in Israel, thus possibly aiding its spread and survival. One hundred and two entries of Labiatae (Syn Lamiaceae), belonging to 22 genera and 53 species, were tested for sensitivity to P. belbahrii in artificial-inoculated plants in growth chambers and in field. Following artificial inoculation of potted plants in growth chambers and of field-grown plants, sporulation of P. belbahrii was observed in seven species: Salvia eigii, Salvia fruticosa, Salvia pinnata, Rosmarinus officinalis, Nepeta curviflora, Micromeria fruticosa, and Agastache spp. Six other entries (belonging to four other genera) showed small chlorotic spots but not sporulation. All other entries were symptom-free. This is the first report showing pathogenicity of P. belbahrii to Salvia, Rosmarinus, Nepeta and Micromeria spp. in Israel. Whether these species play a role in the epidemiology of basil downy mildew in Israel needs to be studied.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Basil downy mildew (BDM), caused by the oomycete Peronospora belbahrii (Thines), is a very destructive disease of sweet basil. It was first reported in Uganda in 1932 as Peronospora spp. and again in 1937 as P. lamii, seen as causing defoliation and death of sweet basil (Hansford 1933). The disease emerged in Switzerland in 2001 (Belbahri et al. 2005). First occurrence of the disease in Italy was next reported in 2003 (Garibaldi et al. 2004a), and in France and Belgium in 2004 (Coosemans 2004; Garibaldi et al. 2005). Since then, P.belbahrii has spread worldwide (Cohen et al. 2017).
Peronospora belbahrii is an obligate oomycete that attacks mainly sweet basil (Ocimum basilicum), producing chlorotic lesions on leaf blades and sporangia on the lower leaf surfaces. The lesions gradually turn necrotic and infected leaves abscised. Sporangia are purplish, oval, 30.4 ± 2.9 μm long × 21.4 ± 1.7 μm wide (Cohen et al. 2013a). Sporangiophores, which emerge from stomatal openings in saturated atmosphere, are hyaline, 400 to 600 μm long, dichotomously branched, with three to five branches per sporangiophore, and bear a single sporangium on each branchlet tip. Infected plants may sporulate during shipping and infected symptomless plants may turn dark upon marketing.
How P. belbahrii persists between growth seasons in Israel and elsewhere is still unknown. Possible routes of over-seasoning are green bridges, seed transmission, oospores in plant debris, and secondary hosts (Ben-Naim et al. 2017). Garibaldi et al. (2004b) and Farahani-Kofoet et al. (2012) both suggested transmission of the pathogen via seeds, although no direct evidence showing the pathogen inside the seed embryo of basil was presented. The fact that the pathogen is systemic suggests potential seed transmission (Falach-Block et al. 2018; Farahani-Kofoet et al. 2012). In Israel, seeds harvested from infected basil plants never developed downy mildew under confined conditions despite the presence of the pathogen’s DNA on the seeds (Cohen et al. 2017; Falach-Block et al. 2018).
We know little on oospore formation of P. belbahrii and on their role in the epidemiology of BDM. Cohen et al. (2013b) reported on the erratic occurrence of P. belbahrii oospores inside the mesophyll of BDM-infected basil leaves. Microscopic examination of infected leaves revealed brown, round oospores with a diameter of 46.2 ± 2.8 μm. Attempts to induce oospore formation in the laboratory by inoculating basil plants with sporangia of paired isolates from various years, various locations, or various hosts, provided no answer on whether the pathogen is homothallic or heterothallic, since oospore formation was erratic (Cohen et al. 2017). Our repeated attempts failed to germinate oospores in vitro or in vivo (unpublished data). Oospore production in other countries has not been reported to date.
P. belbahrii is reported as the causal agent of BDM in O. basilicum (sweet basil), attacking all known cultivars. It also affects other species members of the Ocimum genus (Ben-Naim et al. 2015; Farahani-Kofoet et al. 2014; Wyenandt et al. 2010). Only a few wild Ocimum species were found resistant to BDM (Ben-Naim et al. 2015, 2018; Farahani-Kofoet et al. 2014; Koroch et al. 2013). A resistant cultivar of sweet basil, Prospera, was recently released by Bar Ilan University.
In addition to Ocimum spp., three other species were reported as hosts of P. belbahrii: agastache (Agastache sp., Hyssops), coleus (Plectranthus scutellarioides Syn Solenostemon scutellarioides) and sage (mainly Salvia officinalis) (see below).
P. belbahrii was first reported on Agastache sp. in the U.K in 2009, and was reported the following year on O. basilicum (Henricot et al. 2010). Webb et al. (2012) confirmed P. belbahrii on both sweet basil and agastache based on reciprocal cross-contaminations and molecular analyses. In the USA, downy mildew in agastache was first recorded in Missouri in 2014 (Rivera et al. 2016a). Based on morphology, ITS (rDNA internal transcribed spacer) and cox 2, the authors concluded that the causal agent is closely related to P. belbahrii, but distinct.
P. belbahrii was first reported on coleus in the UK in 2014. ITS analyses of coleus isolates showed 99% homology to P. belbahrii taken from agastache (Denton et al. 2015). Similar findings were reported in the USA (Harlan et al. 2015). Rivera et al. (2016b) reported the first occurrence of P. belbahrii on coleus in Tennessee in 2015. In the United States, Peronospora spp. on coleus has been reported since 2006 in Florida, Louisiana, and New York, and P. belbahrii sensu lato has been reported in Michigan. Previous records indicated the presence of Peronospora sp. on coleus from various landscapes of Tennessee in 2006.
The prevailing assumption describes P. belbahrii as a complex of species that are defined by the host plant (Thines et al. 2009). Choi et al. (2009) identified three Peronospora species on sages: Peronospora swinglei, Peronospora salviae-plebeiae, and Peronospora salviae-officinalis. Each species was restricted to a different host: P. swinglei to S. reflexa, P. salviae-plebeiae to S. plebeia and P. salviae-officinalis to S. officinalis. They suggested that speciation might be allopatric, closely linked to the geographic distributions of the host plants. In the UK, sage is attacked by P. lamni, P. swinglei, and others, while mint is attacked by P. lamni only (Wright 2014).
Thines et al. (2009) confirmed that the occurrence of downy mildew on Solenostemon and Ocimum might be attributed to a single species or two different species, but both are unrelated to Peronospora lamii, which is a common pathogen of the weed Lamium purpureum.
Sage, rosemary and mint are hosts of P. lamni in the UK (Wright 2014). P. lamii persists in the woody plant material of sage between growth seasons, while in mint, systemic infection of this perennial plant allows persistence of the pathogen (Wright 2014).
The Labiatae (Syn Laminaceae) family has a cosmopolitan distribution. The Labiatae family includes 236 genera (Harley et al. 2004), with 6,900 (Heywood et al. 2007) to 7,200 (Harley et al. 2004) species. The largest genus is Salvia (900 species), followed by Clerodendrum (400 species), Scutellaria (360 species) Stachys (300 species), Plectranthus (300 species), Hyptis (280 species), Teucrium (250 species), Vitex (250 species), Thymus (220 species), and Nepeta (200 species) (Harley et al. 2004).
In the present study, we examined the response of various members of the family Labiatae to inoculation with an isolate of P. belbahrii collected from sweet basil in Israel. We identified new host species of this pathogen that may serve as sources of inoculum or as a bridge between production seasons to infect sweet basil.
Materials and methods
Germplasm
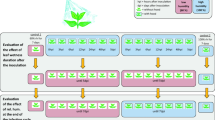
One hundred and two entries of Labiatae, belonging to 53 species and 22 genera, were tested for susceptibility to BDM (Table 1). Seeds were obtained from the following suppliers: USDA - Agriculture Research Service Plant Introduction Station, Iowa State University, Ames, Iowa; Hishtil Ltd. (Petach Tikva, Israel); Department of Archeological Botany, Bar-Ilan University (Ramat-Gan, Israel); Genesis Seeds Ltd. (Ashalim, Israel); and Danzinger Nurseries Ltd. (Mishmar Hashiva, Israel). Potted plants of ornamental species and hybrids were obtained from Hishtil Ltd. and from other local nurseries. The obtained entries were endemic, agricultural, or ornamental. A detailed description of the entries is provided in Table 1. Seeds were sown in multi-cell trays (cell size 2.5 × 2.5 cm) filled with a potting mixture (peat: vermiculite, 1:1, v/v), two or three seeds per cell. Trays were kept in in the greenhouse while plants grew. At the four-leaf stage, one set of plants was transplanted into 0.5 L pots and another set of plants was transplanted to the field.
Pathogen
Isolate Knafo 3 of P. belbahrii was used in all experiments. It was collected in 2013 at Ein-Tamar, Southern Jordan Valley, Israel and maintained by repeated inoculation of susceptible sweet basil plants at 25 °C.
Inoculation and disease assessment
Fresh sporangia of P. belbahrii were collected from sporulating plants into cold distilled water, adjusted to 5,000 spores/ml, and sprayed onto the upper leaf surfaces of the test plants, including the BDM-susceptible controls ‘Sweet Basil’ and ‘Peri’. Plants were placed in a dew chamber overnight at 18 °C to ensure infection and, thereafter, at 25 °C under continuous illumination (60 μmole m2 s−1) to ensure pathogen colonization. Plants were returned to the dew chamber at 9 days post inoculation (dpi) to enable sporulation of the pathogen on the inoculated plants. Disease symptoms and sporulation of the pathogen were visually estimated using the following ordered categorical scale (Ben-Naim et al. 2015):
-
No symptoms or sporulation.
-
(−) Hypersensitive response (HR) or minute chlorotic lesions with no sporulation.
-
(+) Chlorotic lesions with abundant sporulation.
In the field, plant entries, including the susceptible control plants, were spray-inoculated with P. belbahrii at 15 days after transplanting. Disease records were taken three times during a growing period of 52 days post-inoculation, using the scale described above.
Data analysis
Experiments in growth chambers were repeated twice with 5–30 plants per entry in each experiment. Percentage of plants showing sporulation of the pathogen was calculated in each experiment. Mean values and standard deviation (SD) of the mean are presented in Table 1.
The field experiment was conducted once.
Results
Growth chamber studies
Of the 22 genera tested, sporulation occurred in plants belonging to the following six genera: Ocimum, Salvia, Rosmarinus, Nepeta, Agastache, and Micromeria (Table 1). Restricted lesions with no sporulation occurred in plants belonging to four genera: Lamium, Mentha, Majorana, and Salvia (Table 1). Plants of all other genera showed no symptoms (Table 1, Fig. 1). Not all species of a genus reacted to inoculation in the same manner, indicating that susceptibility to BDM is determined at the species rather than the genus level.
Susceptibility of Ocimum
Sporulation of P. belbahrii on leaves of sweet basil (Ocimum basilicum) is shown in Fig. 2a. The following Ocimum species were highly susceptible to BDM: O. basilicum, O. basilicum var thyrsifolium, O. basilicum var minimum, O. basilicum var citrodorum, O. kilimandscharicum, O. tenuiflorum, O. americanom var americaum, and O. americanom var pilosum. Percentage of sporulating plants ranged between 92 and 100%. Two Ocimum species, O. gratissimum and O. campechianum were immune showing no symptoms or sporulation (Table 1).
The appearance of downy mildew caused by Peronospora belbahrii on some Labiatae species. a Sporulation on sweet basil leaves (Ocimum basilicum “Sweet basil”). b Sporulation on rosemary leaves (Rosemarinus officinalis “Barbecue”); c Sporulation on a sage leaf (Salvia eigii). d Symptoms of downy mildew on upper surface of a sage leaf (Salvia fruticosa). e Restricted chlorotic symptoms with no sporulation on sage leaf (Salvia officinalis “Maxiama”). f Sporulation on Nepeta leaf (Nepeta curviflora)
Susceptibility of Rosemarinus
Six out of fifteen cultivars belonging to Rosmarinus officinalis were susceptible to BDM (Fig. 2b). Percentage of sporulating plants among these susceptible cultivars ranged between 41.5–66.0% (Table 1).
Susceptibility of Salvia
Three species of Salvia, S. fruticose (Fig. 2c), S. pinnata (Fig. 2d), and S. eigii (not shown) were susceptible to BDM. Percent plants showing sporulation of P. belbahrii ranged between 46.0–61.6%. S. dominica (Fig. 2e) showed restricted chlorotic lesions with no sporulation. Other cultivars of S. officinalis showed HR symptoms without sporulation. All other Salvia species showed no symptoms or sporulation (Table 1).
Susceptibility of other species
Two endemic entries of Nepeta curviflora were susceptible to BDM (Fig. 2f). One Agastache entry (Agastache spp.) was susceptible and one Micromeria entry, M. fruticose, produced symptoms with sporulation. Disease symptoms with no sporulation were observed in several species. Mentha suaveolens, M. enthapulegium, and M. longifolia exhibited small necrotic HR lesions. Similar symptoms were seen in Lamium amplexicaule and Majorana syriaca.
Koch postulates
Sporangia were collected from the sporulating Labiatae plants that had been inoculated with P. belbahrii and re-inoculated onto sweet basil plants in growth chambers. All basil plants showed BDM symptoms within a week and sporulated abundantly upon one-night of incubation in a dew chamber. Sporangia were collected from these basil plants and re-inoculated onto all Labiatae species in growth chambers. Again, the same six susceptible entries mentioned above became infected and subsequently sporulated under moist conditions.
Field observations
The response of the Labiatae entries to BDM in the field was similar to their response in growth chambers. Sporulation of P. belbahrii in field-grown plants was observed in six genera: Ocimum, Salvia, Rosmarinus, Nepeta, Agastache, and Micromeria. Symptoms of downy mildew with no sporulation of the pathogen were observed in Mentha, Majorana, Lamium, and two entries of Salvia. Plants of all other genera were free of disease symptoms. These responses were maintained all through the growing season until seed set.
Coleus downy mildew
Sporangia of Peronospora spp. (unidentified species) (Cohen et al. 2017) were collected from coleus and inoculated in growth chambers onto coleus, Ocimum, Salvia, Rosmarinus, Nepeta, Agastache, and Micromeria. At 10 dpi, only coleus plants showed symptoms of downy mildew with sporulation, while the other species showed neither symptoms nor sporulation.
Discussion
The present study confirms that the oomycete pathogen Peronospora belbahrii has additional hosts within the Labiatae family beside its major host, Ocimum basilicum. It affects endemic, ornamental, and agricultural species belonging to Labiatae in Israel. Symptoms of downy mildew with sporulation were observed following artificial inoculation with a P. belbaharii isolate in growth chambers in Salvia eigii, Salvia fruticosa, Salvia pinnata, Rosmarinus officinalis, Nepeta curviflora, Micromeria fruticosa, and Agastache spp. These species were also attacked by P. belbahrii under field conditions when artificially inoculated with the same isolate. All members of Mentha, Lamium, Majorana, and some Salvia showed symptoms but no sporulation in both growth chambers and the field.
Previous studies conducted with sweet and wild basils revealed differences in virulence among isolates in different countries. For example, in field studies resistance against BDM in the USA was reported by Wyenandt et al. (2010) in ‘Spice’ (Ocimum spp.), ‘Blue Spice’ (Ocimum. americanum × Ocimum basilicum) and ‘Lime basil’ (O. americanum), whereas incomplete resistance was reported in these lines against Israeli isolates (Ben-Naim et al. 2015). Similarly, resistance was reported in the USA in O. basilicum var anisatum accessions PI 172996, PI 172997, PI 172998, and PI 174284 (Koroch et al. 2013; Pyne et al. 2014) while disease symptoms accompanied with sporulation were observed in these accessions in Israel (Ben-Naim et al. 2015; Pyne et al. 2014).
To the best of our knowledge, this is the first report on the susceptibility of Salvia eigii, S. fruticose, and S. pinnata to downy mildew caused by P. belbahrii in Israel. Entries of the most common Salvia species (S. officinalis) were all resistant to BDM. Salvia officinalis was reported to also be susceptible to another downy mildew Caused by Peronospora salviae-officinalis (Choi et al. 2009; Thines et al. 2009).
This is also the first report in Israel on the susceptibility of three other Labiatae to P. belbahrii: rosemary (Rosmarinus officinalis), white leaved savory (Micromeria fruticose), and Syrian catnip (Nepeta curviflora). Rosemary is the only Labiatae species besides basil that was seen naturally infected with P.belbahrii in Israel (unpublished data). Current attempts are aimed to acertain the infection other five species in nature.
P. belbahrii from basil was infectious to Agastache spp. and vice versa, as reported by Denton et al. (2015), but unlike the report of Henricot et al. (2010), it was incompatible with coleus (Plectranthus scutellarioides). Natural infection with downy mildew of ornamental coleus was first observed in Israel in 2015 (Cohen et al. 2017). The sporangia collected from infected coleus plants were noninfectious to sweet basil and vice versa, sporangia taken from sweet basil were noninfectious to coleus. Here, we show that coleus downy mildew pathogen was neither infectious to the Ocimum species nor to the Labiatae entries found susceptible to the P.belbahrii isolate used in this study. These observations corroborate with Thines et al. (2009) who stated that P.belbahhrii is not pathogenic to coleus.
References
Belbahri, L., Calmin, G., Lefort, F., & Pawlowski, J. (2005). Phylogenetic analysis and real time PCR detection of a new Peronospora species responsible for downy mildew disease of sweet basil and sage. Mycological Research, 109, 1276–1287.
Ben-Naim, Y., Falach, L., & Cohen, Y. (2015). Resistance against basil downy mildew in Ocimum species. Phytopathology, 105, 778–785.
Ben-Naim, Y., Falach, L., & Cohen, Y. (2017). Host range of Peronospora belbahrii, the causal agent of basil downy mildew. Phytoparasitica, 45, 260–261.
Ben-Naim, Y., Falach, L., & Cohen, Y. (2018). Transfer of downy mildew resistance from wild basil (Ocimum americanum) to sweet basil (O. basilicum). Phytopathology, 108, 114–123.
Choi, Y. J., Shin, H. D., & Thines, M. (2009). Two novel Peronospora species are associated with recent reports of downy mildew on sages. Mycological Research, 113, 1340–1350.
Cohen, Y., Vaknin, M., Ben-Naim, Y., & Rubin, A. E. (2013a). Light suppresses sporulation and epidemics of Peronospora belbahrii. PLoS One, 8, e81282.
Cohen, Y., Vaknin, M., Ben-Naim, Y., Rubin, A. E., Galperin, M., Silverman, D., Bitton, S., & Adler, U. (2013b). First report of the occurrence and resistance to mefenoxam of Peronospora belbahrii, causal agent of downy mildew of basil (Ocimum basilicum) in Israel. Plant Disease, 97, 692.
Cohen, Y., Ben Naim, Y., Falach, L., & Rubin, A. E. (2017). Epidemiology of basil downy mildew. Phytopathology, 107, 1149–1160.
Coosemans, J. (2004). First report of Peronospora lamii, downy mildew on basil (Ocimum basilicum) in Belgium. Parasitica, 60, 27.
Denton, G. J., Beal, E., Denton, J. O., & Clover, G. (2015). First record of downy mildew, caused by Peronospora belbahrii, on Solenostemon scutellarioides in the UK. New Dis. Rep., 31, 14.
Falach-Block, L., Ben-Naim, Y., & Cohen, Y. (2018). Systemicity of Peronospora belbahrii in basil plants. Phytoparasitica, 46, 313.
Farahani-Kofoet, R. D., Roemer, P., & Grosch, R. (2012). Systemic spread of downy mildew in basil plants and detection of the pathogen in seed and plant samples. Mycological Progress, 11, 961–966.
Farahani-Kofoet, R. D., Römer, P., & Grosch, R. (2014). Selecting basil genotypes with resistance against downy mildew. Scientia Horticulturae, 179, 248–255.
Garibaldi, A., Minuto, A., Minuto, G., & Gullino, M. L. (2004a). First report of downy mildew on basil (Ocimum basilicum) in Italy. Plant Disease, 88, 312.
Garibaldi, A., Minuto, G., Bertetti, D., & Gullino, M. L. (2004b). Seed transmission of Peronospora sp of basil. Journal Plant Dis Protection, 111, 465–469.
Garibaldi, A., Minuto, A., & Gullino, M. L. (2005). First report of downy mildew caused by Peronospora sp on basil (Ocimum basilicum) in France. Plant Disease, 89, 683.
Hansford, C. G. (1933). Annual report of the mycologist. Review Applied Mycologica, 12, 421–422.
Harlan, B., Linderman, S., Hyatt, L., & Hausbeck, M. (2015). Research gives clues for preventing coleus downy mildew. Greenhouse Grower, published online March, 11, 2015.
Harley, R. M., Atkins, S., Budantsev, A. L., Cantino, P. D., Conn, B. J., Grayer, R. J., Harley, M. M., de Kok, R. P. J., Krestovskaja, T. V., Morales, R., Paton, A. J., & Ryding, O. (2004). Labiatae. Pages 167–275 in: Flowering Plants - Dicotyledons. Springer, Berlin, Heidelberg, Labiatae.
Henricot, B., Denton, J., Scrace, J., Barnes, A. V., & Lane, C. R. (2010). Peronospora belbahrii causing downy mildew disease on Agastache in the UK: A new host and location for the pathogen. Plant Pathology, 59, 801.
Heywood, V. H., Brummitt, R. K., Culham, A., & Seberg, O. (2007). Flowering plant families of the world. Firefly Books, Ontario, Canada. ISBN 978-1-55407-206-4.
Koroch, A. R., Villani, T. S., Pyne, R. M., & Simon, J. E. (2013). Rapid staining method to detect and identify downy mildew (Peronospora belbahrii) in basil. Applications in Plant Sciences, 1, 1300032.
Pyne, R. M., Koroch, A. R., Wyenandt, C. A., & Simon, J. E. (2014). A rapid screening approach to identify resistance to basil downy mildew (Peronospora belbahrii). HortScience, 49, 1041–1045.
Rivera, Y., Salgado-Salazar, C., Creswell, T. C., Ruhl, G., & Crouch, J. A. (2016a). First report of downy mildew vaused by Peronospora sp on Agastache sp in the United States. Plant Disease, 100, 1249–1250.
Rivera, Y., Salgado-Salazar, C., Windham, A. S., & Crouch, J. A. (2016b). Downy mildew on Coleus (Plectranthus scutellarioides) caused by Peronospora belbahrii sensu lato in Tennessee. Plant Disease, 100, 655.
Thines, M., Telle, S., Ploch, S., & Runge, F. (2009). Identity of the downy mildew pathogens of basil, coleus, and sage with implications for quarantine measures. Mycological Research, 113, 532–540.
Webb, K., Sansford, C., MacLeod, A., & Matthews-Berry, S. (2012). Rapid assessment of the need for a detailed Pest risk analysis for Peronospora belbahrii. UK Risk Register Details for Peronospora belbahrii. UK Plant HealthRisk Register (DEFRA). https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=26813.
Wright, K. (2014). Outdoor herbs: Epidmiology and control of downy mildew in sage, parsley, mint and basil under protection. HDC Final Report of Project FV390.
Wyenandt, C. A., Simon, J. E., McGrath, M. T., & Ward, D. L. (2010). Susceptibility of basil cultivars and breeding lines to downy mildew (Peronospora belbahrii). HortScience, 45, 1416–1419.
Acknowledgments
We thank Eyal Klein of Hishtil Ltd. for supplying seeds and plant material.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was not submitted for publication to another journal. All authors listed have contributed to the work, have read the manuscript and declare that there are no potential conflicts of interest. This research was supported by Research Grant No. US-4947-16 R from BARD, the United States - Israel Binational Agricultural Research and Development Fund, and by a research grant from the Plant Council, Israel.
Rights and permissions
About this article
Cite this article
Naim, Y.B., Falach-Block, L., Ben-Daniel, BH. et al. Host range of Peronospora belbahrii, causal agent of basil downy mildew, in Israel. Eur J Plant Pathol 155, 789–799 (2019). https://doi.org/10.1007/s10658-019-01809-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01809-9