Abstract
A needle nematode of the genus Paralongidorus was isolated from Cuban royal palm (Roystonea regia) in China. Detailed morphological study confirmed that this species was the described needle nematode P. sacchari. Accordingly it was formally described and photographed. This nematode is characterised by a long body (4348–5825 μm), a board and anteriorly flat lip region slightly offset from body contour, bearing a stirrup-shaped amphidial fovea, with conspicuous slit-like aperture, a long and flexible odontostyle ca 101–110 μm long, stylet guiding ring located at 27.5–33.0 μm from anterior end, vulva near mid-body (48.0–50.0%), a short dorsally convex tail, with rounded terminus, and male absent. Molecular characterisation using near full-length 18S rRNA and D2-D3 expansion segments of 28S rRNA gene was also provided. Phylogenetic trees inferred from BI analysis of the two rRNA gene fragments revealed that P. sacchari could be distinguished from all described needle nematodes with molecular data, as well as the closely related species P. bikanerensis and P. sali. This nematode is a new record of Paralongidorus species from China. Cuban royal palm is a new host plant for P. sacchari.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Genus Paralongidorus was erected by Siddiqi et al. (1963) with two new species, P. sali Siddiqi et al., 1963 and P. sacchari Siddiqi et al., 1963. The genus Paralongidorus morphologically resembles Longidorus, but differs from the latter by shape of amphidial fovea (stirrup-shape, open goblet-shaped, open goblet-shaped, funnel-shaped, elongate stirrup-shaped or pouch-like vs pouch-like only) and amphidial opening (transverse slit-like vs pore-like) (Decraemer and Coomans 2007).
Like the genus Longidorus, Paralongidorus species are also migratory ectoparasites of plant roots (Decraemer and Robbins 2007). Paralongidorus species causes direct damage to a variety of host plant by feeding activity and one species, P. maximus, is also vectors of plant pathogenic viruses (Decraemer and Robbins 2007). Therefore, P. maximus is paid more attention to because its quarantine importance in many countries including China (Taylor and Brown 1997; Decraemer and Robbins 2007; Meador and Wu 2011).
Some 90 species of Paralongidorus have been recorded (Decraemer and Robbins 2007). As the number of nominal species increases, morphological identification of Paralongidorus species is a challenging task for the obvious interspecific overlapping and significant intraspecific variability of some diagnostic characters. Therefore, molecular techniques such as comprehensive analyses of fragments of rRNA genes are recommended to the identification of Paralongidorus spp. (Palomares-Rius et al. 2008, 2013; Pedram et al. 2012; Barsi and Luca 2017; Gutiérrez-Gutiérrez et al. 2018). In the past few years, several Paralongidorus species, i.e. P. litoralis Palomares-Rius et al., 2008, P. iranicus Pedram et al., 2012, P. plesioepimikis Palomares-Rius et al., 2013, P. francolambertii Barsi and Luca, 2017, P. lusitanicus Gutiérrez-Gutiérrez et al., 2018 were proposed as new species based on morphological characters and molecular approaches (Palomares-Rius et al. 2008, 2013; Pedram et al. 2012; Barsi and Luca 2017; Gutiérrez-Gutiérrez et al. 2018). However, molecular data of most Paralongidorus species are not currently available.
During 2015–2017, extensive surveys of plant nematodes on ornamental trees and shrubs were done in Guangdong Province. One population of Paralongidorus was found in a loamy soil in the rhizosphere of Cuban royal palm in China. Detailed morphological and molecular comparative study using previously reported data combined with molecular analyses showed that the population differed from all known Paralongidorus species except P. sacchari.
The objectives of this work were to: (i) characterize morphologically and molecularly P. sacchari from China; and (ii) study the phylogenetic relationships of this species with other Paralongidorus spp. and Longidorus spp. using sequences from near full-length 18S rRNA gene and D2-D3 expansion segments of 28S rRNA gene as inferred from Bayesian inference (BI) approaches.
Materials and methods
Nematode population and morphological studies
Twelve soil samples around the roots of Cuban royal palm were collected from different sites, Guangzhou, Guangdong province, China. For every sample, about 20 cm-depth topsoil was taken by stainless steel sampling tube. Needle nematodes of the genus Paralongidorus were isolated from soils by decanting and sieving method (Brown and Boag 1988). Needle nematodes were only detected in one sample from Tianhe district (isolate DWY: 23°9′ 47.1“ N, 113°21’ 21.7” E, 35 m a.s.l.). 34 nematodes of different stages were obtained from ca 200 mL soil.
Fresh nematodes were gentle heated, fixed in 4% formaldehyde and processed to pure glycerin (Seinhorst 1959). Nematodes from permanent slides were photographed and measured under a Nikon ECLIPSE Ni microscope (Nikon, Tokyo, Japan). A polytomous key from Escuer and Arias (1997) was used for species identification of the genus Paralongidorus.
DNA extraction, amplification and sequencing
DNA was extracted from individuals of female according to the method described by Mundo-Ocampo et al. (2008). Two rRNA gene fragments, i.e., near full-length 18S rRNA gene and D2-D3 expansion segments of 28S rRNA gene, were amplified from three specimens respectively. Primers for near full-length 18S rRNA gene amplification were 988F (5′-CTC AAA GAT TAA GCC ATG C-3′), 1912R (5′-TTT ACGGTC AGA ACT AGG G-30), 1813F (5′-CTG CGT GAG AGG TGA AAT-3′) and 2646R (50-GCT ACC TTG TTA CGA CTT TT-3′) (Holterman et al. 2006). Primers for D2-D3 expansion segments of 28S rRNA gene amplification were D2A (5’-ACA AGT ACC GTG GGG AAA GTT G-3′) and D3B (5’-TCG GAA GGA ACC AGC TAC TA-3′) (De Ley et al. 1999). Detailed protocols of PCR amplification were conducted as described by previous study (De Ley et al. 1999; Holterman et al. 2006). DNA fragments were sequenced as described in Zhuo et al. (2010). The newly obtained sequences were deposited in the GenBank database and the accession numbers are MH973643-MH973645 and MK920217-MK920220.
Phylogenetic analysis
The sequences of P. sacchari were compared with needle nematode sequences in GenBank using Standard Nucleotide BLAST (blastn) program. The close-related and published sequences of needle nematodes were selected for phylogenetic analyses. Outgroup taxa for each dataset were chosen according to previous phylogenetic study for needle nematodes (Pedram et al. 2012; Palomares-Rius et al. 2013). DNA sequences were aligned by ClustalW implemented in MEGA6.0 (Tamura et al. 2013) using default parameters. Nucleotide substitution models were evaluated using MODELTEST3.7 (Posada and Crandall 1998) combined with PAUP4.0 (Swofford 1998). The Akaike-supported model, the base frequencies, the proportion of invariable sites, the gamma distribution shape parameters and substitution rates were used in phylogenetic analyses. Bayesian analysis for both genes under the GTR + I + G model, was employed to confirm the tree topology using MrBayes 3.2 (Huelsenbeck and Ronquist 2001) running four chains for 1 × 106 generations and setting the ‘burn-in’ at 2500. The MCMC (Markov Chain Monte Carlo) method was performed within a Bayesian framework to estimate the posterior probabilities of the phylogenetic trees (Larget and Simon 1999) and generate a 50% majority rule consensus tree. TREEVIEW1.6 was used to display and edit the trees (Page 1996).
Results
Description of the needle nematode Paralongidorus sacchari
Measurements of females are listed in Table 1. Illustrations and photos are in Figs. 1, 2, and 3.
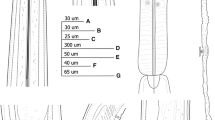
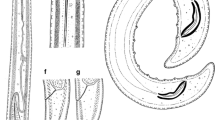
Females of Paralongidorus sacchari population in China under the light microscope. a entire body; (b) amphidial aperture; (c) pharyngeal region; (d) lip region and stylet; (e) amphidial fovea; (f) pharyngeal bulb; (g) vulval region and ovary; (h-i) tails (arrows show caudal pores). (Scale bars: a = 500 μm; b, e = 20 μm; c-d, f-k = 50 μm)
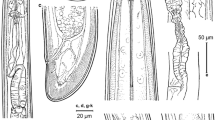
Juveniles of Paralongidorus sacchari population in China under the light microscope. (a-d) entire body of J1, J2, J3 and J4, respectively; (e) anterior region of J4; (f) amphidial fovea of J4; (g-i) anterior region of J3, J2 and J1, respectively; (j-m) tails of J4, J3, J2 and J1, respectively. (Scale bars: a-d = 200 μm; e-m = 20 μm)
Female
Body medium long, tapering gradually toward anterior end, open C-shaped when killed by gentle heat, more curved ventrally in posterior half (Fig. 2a). Cuticle appearing smooth under light microscope, 3.0–4.0 μm thick along the body, 7.5–9.2 μm thick at tail end. Lip region board, 14.4–15.6 μm wide, more than 2 times as long as high, anteriorly flat, continuous with body contour (Fig. 2: d-e). Amphidial fovea funnel-shaped or stirrup-shaped (Fig. 2e), with broad slit-like aperture, almost as wide as lip region (Fig. 2b). Odontostyle long and narrow, 1.5–1.9 times as long as odontophore, straight or slightly arcuate, odontophore weakly developed, with slightly swollen base (Fig. 2c). Stylet guiding ring single, 1.8–2.2 times head widths from anterior end. Nerve ring encircling narrower part of pharynx, slightly posterior to odontophore base. Anterior slender part of pharynx usually with looped region overlapping basal bulb, basal bulb cylindrical, 113–139 μm long, 21.0–29.5 μm diameter, 4.3–5.7 times as long as wide (Fig. 2f). Cardia conoid-rounded, 8–16 μm long. Dorsal pharyngeal gland nucleus in anterior part of bulb, 25.5–33.0 μm posterior to gland outlet, one ventrosublateral pair of nuclei near middle of bulb, 63.5–73.5 μm posterior to gland outlet (Fig. 2f). Vulva in form of a transverse slit, located at or slightly anterior to mid-body, vagina slopping backward, occupying 58.0–63.5% of corresponding body width, pars distalis vaginae and pars proximalis vaginae 16.5–18.5 μm and 15.5–18.5 μm long, respectively. Reproductive system with both genital branches almost equally developed. Anterior and posterior genital branches 321–424 μm and 253–302 μm long, respectively, with reflexed ovaries and single raw of oocytes (Fig. 2g). Well developed sphincter between oviduct and uterus. Prerectum 313–470 μm long and rectum 21–26.8 μm long. Tail short, 0.9–1.3 anal body diam. Long, dorsally convex, with rounded terminus (Fig. 2: h-j), bearing two caudal pores on each lateral side(Fig. 2k).
Male
Not found.
Juveniles
All four juvenile stages were found and distinguished by relative lengths of body and functional and replacement odontostyle (Table 1; Fig. 3: a-i), resembling adults in most respects except for size and development of reproductive system, more elongate and differently shaped tail (Fig. 3: j-m). First-stage juveniles (Jls) characterized by an elongate-conoid tail (Fig. 3m), odontostyle length 48–60 μm long (Fig. 3i), and shorter distance from anterior end to stylet guiding ring (Fig. 3i) than that in adult stages. However, morphology in all four juvenile stages (except for undeveloped genital structures) similar to that of female, including broadly rounded tail shape of fourth-stage juveniles, yet differed in shorter distance from anterior end to guiding ring (Fig. 3).
Hosts and localities
A population of P. sacchari extracted from rhizosphere of Cuban royal palm (Roystonea regia O.F.Cook) collected in Tianhe district, Guangzhou, Guangdong Province, China, in October, 2015. It has been recorded from the type locality in Australia from soils around the roots of sugarcane (Saccharunt officinarum) (Siddiqi et al. 1963). Other populations are discovered from sugarcane soil in India (Siddiqi et al. 1963) and soil around roots of Jubaea chilensis in Chile (Roca and Rios 2006).
Remarks
Females and Juveniles of population from China are morphologically coinciding with those from the type population from Australia, with minor difference in the reproductive system structures (genital branches asymmetrical in the Chinese population vs symmetrical in the type population). Females from China and Australia resemble those from India except shorter odontostyle length (101–110 μm and 105–114 μm vs 116–120 μm from India). Females from China and Australia are similar with those of population from Chile with an exception of some variations in three characters: wider lip region diameter [14.5–15.5 μm and ca 16 μm (inferred from illustration) vs 9.5–11.5 μm from Chile], more anterior position of the guide ring (27.5–33.0 μm and 30–33 μm vs 36–43 μm from Chile) and larger pharyngeal bulb size (113–139 × 21–29.5 μm and 127 × 22 μm vs 88–107 × 16–20 μm from Chile). First-stage juveniles (Jls) from China and Australia differ from those from Chile by shorter odontostyle (48–60 μm and 47–56 μm vs 62–74 μm from Chile), shorter replacement odontostyle (63–68 μm and 63–67 μm vs 73–94 μm from Chile), and more anterior position of the guide ring (16.5–18.0 μm and 16–18 μm vs 23–27 μm from Chile).
Molecular characterisation
A near full-length 18S rRNA gene of ca 1630 bp was obtained from P. sacchari population in China. Intra-population variations for P. sacchari China population were 0–0.1% (0-2 bp difference in compositon). A Blastn search of the 18S rRNA gene sequences showed 99.1%–99.3% similarities with P. bikanenrensis (JN032586) and 98.9%–99.3% similarities with P. sali (MG729696-MG729697). 18S rRNA gene sequences of P. sacchari differ from that of P. bikanerensis in 12 bp −14 bp, and from those of P. sali in 12 bp −19 bp. A phylogenetic tree (Fig. 4) based on near full-length 18S rRNA gene was from a multiple alignment of 1643 total characters with 1408 constant characters (85.7%). The average nucleotide composition was as follows: 26.71% A, 20.75% C, 26.51% G and 26.02% T. In the 18S rRNA gene trees, all Paralongidorus species with molecular data clustered into two separate groups. P. sacchari formed a clade with P. bikanerensis (JN032586) and P. sali (MG729696-MG729697) but with low support (PP = 51). Other species including P. litoralis (EU026158), P. lusitanicus (KY750569), P. plesioepimikis (JQ673405), P. paramaximus Heyns, 1965 (EU026157), P. maximus (Bütschli, 1874) Siddiqi 1964 (AJ875152), P. iranicus (JN032589) and P. rex Andrássy, 1986 (KJ427794) formed a major high support clade (PP = 100). The position of L. laevicapitatus Williams, 1959 (KX136873) was in a basal position of the BI tree.
Bayesian consensus tree inferred from near full-length 18S rRNA gene of Paralongidorus sacchari population in China under GTR + I + G model (lnL = 6014.6235; AIC = 12,049.2471; freqA = 0.2671; freqC = 0.2075; freqG = 0.2651; freqT = 0.2602; R(a) = 1.7119; R(b) = 3.9143; R(c) = 2.3649; R(d) = 0.5176; R(e) = 7.7103; R(f) = 1; Pinva = 0.7565; Shape = 0.5218). Posterior probability values exceeding 50% are given on appropriate clades. Newly obtained sequences are indicated in bold
Amplification of D2-D3 expansion segments of 28S rRNA gene from P. sacchari population in China yielded a single fragment of ca 850 bp. Intra-population variations in D2-D3 expansion segments of 28S rRNA gene sequences for P. sacchari China population were 0–0.1% (0-1 bp difference in length). A Blastn search of the 18S rDNA sequences showed 85.7% similarity with P. bikanenrensis (JN032584) and 84.8%–85.1% similarities with P. sali (MG729700-MG729701). D2-D3 expansion segments of 28S rRNA gene of P. sacchari differs from that of P. bikanerensis in 117 bp, and from those of P. sali in 122 bp −128 bp. A phylogenetic tree (Fig. 5) based on D2-D3 expansion segments of 28S rRNA gene was from a multiple alignment of 849 total characters with 331 constant characters (39.0%). The average nucleotide composition was as follows: 24.29% A, 21.55% C, 27.45% G and 26.71% T. Similar to the 18S rRNA gene trees, all sequences of Paralongidorus species also formed two separate groups in BI trees of D2-D3 expansion segments of 28S rRNA gene. P. sacchari, P. bikanerensis (JN032584) and P. sali (MG72970-MG729701) clustered together but with relative long genetic distance (PP = 100). The major clade contained P. rex (AY601582 and KJ427793), P. francolambertii (LT669805), P. iranicus (JN032587), P. maximus (AF480083), P. litoralis (EU026155), P. paramaximus (EU026156), P. plesioepimikis (JQ673403) and P. lusitanicus (KY750562), and received strong support (PP = 100). The position of L. laevicapitatus (KX136865) was in a basal position of the BI tree.
Bayesian consensus tree inferred from D2-D3 expansion segments of 28S rRNA gene of Paralongidorus sacchari population in China under GTR + I + G model (lnL = 15,454.2051; AIC = 30,928.4102; freqA = 0.2429; freqC = 0.2155; freqG = 0.2745; freqT = 0.2671; R(a) = 0.8533; R(b) = 2.4488; R(c) = 1.4415; R(d) = 0.6199; R(e) = 4.7947; R(f) = 1; Pinva = 0.3107; Shape = 0.8996). Newly obtained sequences are indicated in bold
Discussion
In this study, P. sacchari is closely related to P. bikanerensis molecularly and phylogenetically. However, P. sacchari can be distinguished morphologically from P. bikanerensis by more posterior vulva position (V = 48.0–50.0 vs V = 43–47), shorter odontostyle and odontophore (101–110 μm and 58–67 μm vs 121–132 μm and 66–76 μm, respectively), more anterior position of the guide ring (27.5–33.0 μm vs 32.5–37.4 μm), shorter tail hyaline region (7.5–9.2 μm vs 10.5–14.0 μm), larger c’ value (2.7–3.3 vs 2.5–2.6) of J1, and shorter J1 replacement odontostyle (63–68 μm vs 74.5–77 μm).
According to the original description, the amphidial fovea of P. sacchari was described as funnel-shaped. Female specimens of Paralongidorus with different orientations of the body might reveal a different shape of the fovea (Decraemer and Coomans 2007). Decraemer and Coomans (2007) examined some paratype females of P. sacchari and the amphidial fovea was considered to be stirrup-shaped. For the Chinese population of P. sacchari, one side of amphidial fovea wall is straight and the other side is curve. Therefore, the shape of the amphidial fovea for P. sacchari China population is between funnel-shaped and stirrup-shaped.
The phylogenetic relationship between Paralongidorus and Longidorus is still not clear. In both trees, two separate Paralongidorus groups are nested into Longidorus spp., and L. laevicapitatus was in a basal position. Both Paralongidorus and Longidorus are not monophyletic groups (Pedram et al. 2012; Palomares-Rius et al. 2013). Paralongidorus was even not accepted as a valid taxon (Gutiérrez-Gutiérrez et al. 2011).
Taxonomy status of the genus Longidoroides Khan et al., 1978 was controversial (Siddiqi et al. 1993; Coomans 1996; Escuer and Arias 1997). Siddiqi et al. (1993) synonymised Longidoroides with Paralongidorus, but this viewpoint was not admitted by Coomans (1996). P. bikanerensis, a member of former Longidoroides species, was the only species outside the major group of Paralongidorus in all phylogenetic trees reported in previous studies (Pedram et al. 2012; Palomares-Rius et al. 2013). P. sacchari showed close relationship with P. bikanerensis and P. sali in both trees, which further demonstrated the taxonomy status of Longidoroides as synomyn of Paralongidorus.
The Genus Longidorus, which is widely distributed in China, comprises about 18 species (Barsalote et al. 2018; Xu et al. 2018). However, the genus Paralongidorus was seldom discovered in China. P. sali collected from the rhizosphere of woody perennials, was the first Paralongidorus species recorded from China until 2018 (Cai et al. 2018). However, Paralongidorus species are very abundant in India (34 spp.), a neighboring country of China (Decraemer and Robbins 2007). The origin centre for Paralongidorus may be located in the region of South-East Africa to India (Coomans 1985; Palomares-Rius et al. 2008). Therefore, Paralongidorus diversity in China needs to be further investigated.
Cuban royal palm is susceptible to red ring nematode, Bursaphelenchus cocophilus (Cobb, 1919) Baujard 1989 and burrowing nematode, Radopholus similis (Cobb, 1893) Thorne 1949 (Goodey et al. 1965; Chuo and Wouts 1977). No other plant nematodes were discovered from Cuban royal palm before. In this study, P. sacchari was isolated from Cuban royal palm, further evaluation on its pathogenicity and economic damage is needed.
Palms are good hosts of needle nematodes (Paralongidorus spp.). There are 6 species of needle nematodes having the ability to parasitize the plant in Arecaceae. Date palm (Phoenix dactylifera) is the host of P. bikanerensis (Pedram et al. 2012) and P. georgiensis (Tulaganov, 1937) Siddiqi 1964 (FAO 2009). Coconut palm (Cocos nucifera) is the host of P. citris (Khan et al. 1989) and P. flexus Khan et al., 1971 (Khan et al. 1971). Chile cocopalm (Jubaea chilensis) is the host of P. sacchari (Roca and Rios 2006). Cuban royal palm (Roystonea regia) is anther Arecaceae host of P. sacchari as found in this study.
References
Andrássy, I. (1986). A new needle nematode species from Hungary, Paralongidorus rex sp. n. (Nematoda: Longidoridae) (Vol. 73, pp. 115–118). Állattani Kozlemények.
Barsalote, E. M., Tian, Z. L., Cai, R. H., Li, X. L., & Zheng, J. W. (2018). Description of two new records of genus Longidorus (Nematoda: Dorylaimida) in China. Journal of Zhejiang University (Agriculture & Life Science), 44(1), 31–40.
Barsi, L., & Luca, F. D. (2017). Morphological and molecular characterisation of Paralongidorus francolambertii sp. n. (Nematoda: Longidoridae) from Serbia. Nematology, 19(6), 681–695.
Baujard, P. (1989). Remarques sur les genres des sous-familles Bursaphelenchinae Paramonov, 1964 et Rhadinaphelenchinae Paramonov, 1964 (Nematoda: Aphelenchoididae). Revue de Nématologie, 12, 323–324.
Brown, D. J. F., & Boag, B. (1988). An examination of methods used to extract virus-vector nematodes (Nematoda: Longidoridae and Trichodoridae) from soil samples. Nematologia Mediterranea, 16, 93–99.
Bütschli, O. (1874). Zur Kenntnis der freilebenden Nematoden, insbesondere der des Kieler Hafens. Abhandl. d. Senckenb. naturf. Gesellsch, 9, 237–292.
Cai, R., Maria, M., Qu, N., Castillo, P., & Zheng, J. (2018). Morphological and molecular characterization of Paralongidorus sali Siddiqi, Hooper, and Khan, 1963 with a description of the first-stage juvenile and male of Longidorus jonesi Siddiqi, 1962 from China. Journal of Nematology, 50(3), 419–436.
Chuo, S. K., & Wouts, W. M. (1977). Royal Palm, Roystonea regia (HBK) cook, a new host of the burrowing nematode Radopholus similis (Cobb, 1893) Thorne, 1949. Singapore Journal of Primary Industries, 5, 91–95.
Cobb, N. A. (1893). Nematodes, mostly Australian and Fijian. In J. J. Fletcher (Ed.), Macleay memorial volume (pp. 252–308). Sydney: The Linnean Society of NSW.
Cobb, N. A. (1919). A newly discovered nematode (Aphelenchus cocophilus, sp. n.) connected with a serious disease of the coconut palm. West Indian Bulletin, 17(4), 203–210.
Coomans, A. (1985). A phylogenetic approach to the classification of the Longidoridae (Nematoda: Dorylaimida). Agriculture, Ecosystems and Environment, 12(4), 335–354.
Coomans, A. (1996). Phylogeny of the Longidoridae. Russian Journal of Nematology, (4), 51–60.
De Ley, P., Felix, M. A., Frisse, L. M., Nadler, S. A., Sternberg, P. W., & Thomas, W. K. (1999). Molecular and morphological characterisation of two reproductively isolated species with m irror-image anatomy (Nematoda: Cephalobidae). Nematology, 1(6), 591–612.
Decraemer, W., & Coomans, A. (2007). Revision of some species of the genus Paralongidorus sensu Siddiqi et al. (1993), with a discussion on the relationships within the family Longidoridae (Nematoda: Dorylaimida). Nematology, 9(5), 643–662.
Decraemer, W., & Robbins, R. T. (2007). The who, what and where of Longidoridae and Trichodoridae. Journal of Nematology, 39(4), 295.
Escuer, M., & Arias, M. (1997). Paralongidorus iberis sp. n. and P. monegrensis sp. n. from Spain with a polytomous key to the species of the genus Paralongidorus Siddiqi, Hooper & Khan, 1963 (Nematoda: Longidoridae). Fundamental and Applied Nematology, 20(2), 135–148.
FAO (2009). Study on the identification and population density of plant parasitic nematodes associated with date palm in the south of Iran [2006]. http://agris.fao.org/agris-search/search.do?recordID=IR2008001073. Accessed 23 October 2018.
Goodey, J., Franklin, M. T. H., & David, J. (1965). The nematode parasites of plants catalogued under their hosts (3rd ed.). Farnham Royal: Commonwealth Agricultural Bureaux.
Gutiérrez-Gutiérrez, C., Rius, J. E. P., Cantalapiedra-Navarrete, C., Landa, B. B., & Castillo, P. (2011). Prevalence, polyphasic identification, and molecular phylogeny of dagger and needle nematodes infesting vineyards in southern Spain. European Journal of Plant Pathology, 129(3), 427–453.
Gutiérrez-Gutiérrez, C., Mota, M., Castillo, P., Santos, M. T., & Palomares-Rius, J. E. (2018). Description and molecular phylogeny of one new and one known needle nematode of the genus Paralongidorus (Nematoda: Longidoridae) from grapevine in Portugal using integrative approach. European Journal of Plant Pathology, 151(1), 155–172.
Heyns, J. (1965). New species of the genera Paralongidorus and Longidorus (Nematoda: Dorylaimoidea) from South Africa. South African Journal of Agricultural Science, 8, 863–874.
Holterman, M., van der Wurff, A., van den Elsen, S., van Megen, H., Bongers, T., Holovachov, O., Bakker, J., & Helder, J. (2006). Phylum-wide analysis of SSU rDNA reveals deep phylogenetic relationships among nematodes and accelerated evolution toward crown clades. Molecular Biology and Evolution, 23(9), 1792–1800.
Huelsenbeck, J. P., & Ronquist, F. (2001). MR BAYES: Bayesian inference of phylogenetic trees. Bioinformatics, 17(8), 1754–1755.
Khan, E. (1981). Inagreius gloriosus gen. N., sp. n. and descriptions of three new species of Xiphinema Cobb, 1913 along with report on X. radicicola T. Goodey, 1936 and X. elongatum Sch. Stek. & Teun., 1938 (Nematoda: Longidoroidea) from India. Indian Journal of Nematology, 11(2), 189–204.
Khan, E., Seshadri, A. R., Weischer, B., & Mathen, K. (1971). Five new nematode species associated with coconut in Kerala, India. Indian Journal of Nematology, 1(2), 116–127.
Khan, E., Chawla, M. L., & Saha, M. (1978). Comments on the classification of the Longidoroidea (Nematoda) with description of three new species. Indian Journal of Nematology, 6(1), 47–62.
Khan, A., Saeed, M., Ali, T., & Khanaum, M. (1989). Efficacy of Tenekil against needle nematode Paralongidorus citri (Siddiqi, 1959) Siddiqi et al., 1963 associated with coconut palm (Cocos nucifera). Proceedings of Parasitology, (7/8), 202–205.
Larget, B., & Simon, D. L. (1999). Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution, 16, 750–759.
Meador, M., & Wu, X. (2011). People's Republic of China: Updated list of quarantine harmful bio-organisms. Global Agricultural Information Network Report Number CH11051. Washington, DC: USDA Foreign Agricultural Service.
Mundo-Ocampo, M., Troccoli, A., Subbotin, S. A., Cid, J., Baldwin, J. G., & Inserra, R. N. (2008). Synonymy of Afenestrata with Heterodera supported by phylogenetics with molecular and morphological characterisation of H. koreana comb. n. and H. orientalis comb. n. (Tylenchida: Heteroderidae). Nematology, 10(5), 611–632.
Page, R. D. M. (1996). TreeView: An application to display phylogenetic trees on personal computers. Computer Applications in the Biological Sciences, 12, 357–358.
Palomares-Rius, J. E., Subbotin, S. A., Landa, B. B., Vovlas, N., & Castillo, P. (2008). Description and molecular characterisation of Paralongidorus litoralis sp. n. and P. paramaximus Heyns, 1965 (Nematoda: Longidoridae) from Spain. Nematology, 10(1), 87–101.
Palomares-Rius, J. E., Cantalapiedra-Navarrete, C., GutiérrezGutiérrez, C., Liébanas, G., & Castillo, P. (2013). Morphological and molecular characterisation of Paralongidorus plesioepimikis n. sp. (Nematoda: Longidoridae) from southern Spain. Nematology, 15, 363–378.
Pedram, M., Pourjam, E., Namjou, S., Atighi, M. R., Cantalapiedra-Navarrete, C., Liébanas, G., PalomaresRius, J. M., & Castillo, P. (2012). Molecular and morphological characterisation of Paralongidorus iranicus n. sp. and P. bikanerensis (Lal & Mathur, 1987) Siddiqi, Baujard & Mounport, 1993 (Nematoda: Longidoridae) from Iran. Nematology, 14(4), 427–443.
Posada, D., & Crandall, K. A. (1998). Modeltest: Testing the model of DNA substitution. Bioinformatics, 14(9), 817–818.
Roca, F., & Rios, A. (2006). Paralongidorus sacchari Siddiqi, Hooper & Khan, 1963 and Paraxiphidorus michelluci Coomans & Chaves, 1995 (Nematoda: Longidoridae) from Chile. Nematology, 8(4), 619–625.
Seinhorst, J. W. (1959). A rapid method for the transfer of nematodes from fixative to anhydrous glycerin. Nematologica, 4, 67–69.
Siddiqi, M. R. (1959). Studies on Xiphinema spp. (Nematoda: Dorylaimoidea) from Aligarh (North India), with comments on the genus Longidorus Micoletzky, 1922. Proceedings of the Helminthological Society of Washington, 26(2), 151–163.
Siddiqi, M. R. (1964). Xiphinema conurum n. sp. and Paralongidorus microlaimus n. sp. with a key to the species of Paralongidorus (Nematoda: Longidoridae). Proceedings of the Helminthological Society of Washington, 31(2), 133–137.
Siddiqi, M. R., Hooper, D. J., & Khan, E. (1963). A new nematode genus Paralongidorus (Nematoda: Dorylaimoidea) with descriptions of two new species and observations on Paralongidorus citri (Siddiqi 1959) n. comb. Nematologica, 9(1), 7–14.
Siddiqi, M. R., Baujard, P., & Mounport, D. (1993). Descriptions of Paratylenchus pernoxius sp. n. and Paralongidorus duncani sp. n. from Senegal, and the synonymization of Longidoroides with Paralongidorus. Afro-Asian Journal of Nematology, 3(1), 81–89.
Swofford, D. L. (1998) PAUP*-phylogenetic analyses using parsimony (* and other methods). Version 4 b10. Sunderland: Sinauer Associates, 128 pp.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30(12), 2725–2729.
Taylor, C. E., & Brown, D. J. F. (1997). Nematode vectors of plant viruses (296 pp). Wallingford, UK: CABI.
Thorne, G. (1949). On the classification of the Tylenchida, new order (Nematoda, Phasmidia). Proceedings of the Helminthological Society of Washington, 16(2), 37–73.
Tulaganov, D. A. (1937). Nematoden der Tomate und des sie umgebenden Bodens. Zoologischer Anzeiger, 118, 283–285.
Xu, Y., Ye, W., Wang, J., & Zhao, Z. (2018). Morphological and molecular characterisation of Longidorus pinus sp. n. (Nematoda: Longidoridae) from China and a key to known species of Longidorus in China. Nematology, 20(7), 617–639.
Zhuo, K., Cui, R. Q., Ye, W. M., Luo, M., Wang, H. H., Hu, X. N., & Liao, J. L. (2010). Morphological and molecular characterization of Aphelenchoides fujianensis n. sp. (Nematoda: Aphelenchoididae) from Pinus massoniana in China. Zootaxa, 2509, 39–52.
Acknowledgements
This research was supported by the Forest Science and Technology Innovation Project of Guangdong Province (Grant number 2015KJCX045) and the Youth Innovation Talent Project founded by Department of Education of Guangdong Province (grant number 2017GkQNCX051).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
The article does not contain any studies with human or animal subjects performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Wang, H., Zhuo, K., Cai, R. et al. Morphological and molecular characterisation of Paralongidorus sacchari (Nematoda: Longidoridae), a new record of needle nematode in China. Eur J Plant Pathol 155, 615–625 (2019). https://doi.org/10.1007/s10658-019-01796-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01796-x