Abstract
Streptomyces mycarofaciens SS-2-243 has been found to produce non-volatile compounds possessing antifungal activity against the growth of plant pathogens, but the effect of its volatile compounds remain unknown. Therefore, the efficacy of volatile compounds from S. mycarofaciens SS-2-243 (volatiles SS-2-243) grown on wheat seeds for 12 days was evaluated against four pathogenic fungi. In vitro studies using an antifungal bioassay tests on PDA dishes indicated that the volatiles SS-2-243 could totally suppress the growth of all four strains with most pronounced activity (100% inhibition) against Aspergillus parasiticus TISTR 3276 and A. flavus TISTR 3041. Identification of the volatiles SS-2-243 using gas chromatography–mass spectrometry (GC–MS) revealed 33 compounds, with the most abundant being 2-methylisoborneol. Effect of the inoculum size and spore concentration of S. mycarofaciens SS-2-243 prepared as a wheat seed inoculum on the suppression of the two aflatoxin producing fungi was studied. Complete growth inhibition (100%) was achieved at the optimum wheat seed inoculum size of at least 30 g L−1 and 107 spore mL−1. Use of 30 g L−1 wheat seed culture of S. mycarofaciens SS-2-243 could completely kill the conidia of A. parasiticus TISTR 3276 and A. flavus TISTR 3041 with 1 h and 3 h exposures, respectively. Based on the promising antifungal activity of the volatiles SS-2-243, fumigation with 30 g L−1 wheat seed culture of S. mycarofaciens SS-2-243 for 24 h completely controlled the growth of the two aflatoxin producing fungi infecting maize seeds, without adverse effects on maize seed germination. The main effect of the volatiles SS-2-243 was damage and complete inhibition of conidia germination as evident by scanning electron microscope (SEM) images. Therefore, this biofumigant has good potential to control the two aflatoxin producing fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maize (Zea mays L.) is among the most important crops around the world as human food and animal feed (Valdez-Ortiz et al. 2007; Shaista et al. 2011), and is now also used for bioethanol production (Eckert et al. 2018). Fungal contamination of stored seeds, including those of maize, is a common problem for the warehouses in Thailand with humid and warm weather. Aspergillus parasiticus and A. flavus are the most common spoilage fungi in food and feed, and are known to produce aflatoxins (Ng'ang'a et al. 2016; Picot et al. 2017). Aflatoxins are well-known for being hazardous to animals and humans (Zain 2011). Infections by toxigenic fungi reduce the economic value and quality of stored seeds (Razzaghi-Abyaneh et al. 2013; Ng'ang'a et al. 2016).
Chemical fungicides are usually used to control the spoilage fungi. However, these agents have negative effects via toxic residues in food, environmental and human health effects, as well as selection effects favoring fungicide resistant microorganisms (Chen et al. 2016; Tamreihao et al. 2016). The development of efficient antifungal microbial agents could be an alternative method to control food spoilage pathogens. Among the various antagonistic microorganisms, Streptomyces spp. is most promising for controlling pathogenic fungi, because of producing both volatile (Wan et al. 2008; Li et al. 2010, 2012; Boukaew et al. 2013) and non-volatile antimicrobial compounds (Boukaew et al. 2011, Boukaew & Prasertsan, 2014; Elango et al. 2015; Li et al. 2015; Chen et al. 2016; Shakeel et al. 2016; Singh and Gaur 2017) with diverse biological activities.
Streptomyces species have been widely evaluated as biocontrol agents against plant pathogens, because of their ability to produce a large number of antibiotics and other secondary metabolites (Demain 2000; Chen et al. 2016). The volatile antifungal compounds from Streptomyces spp. could help control plant pathogenic fungi such as Colletotrichum spp. (Shimizu et al. 2009; Boukaew et al. 2018), R. solani (Boukaew et al. 2013), Sclerotinia sclerotiorum (Wan et al. 2008), Penicillium italicum (Li et al. 2010) and B. cinerea (Li et al. 2012; Boukaew et al. 2017a). As well as Streptomyces, the endophytic fungus Muscodor albus (Strobel 2011; Suwannarach et al. 2013) and yeasts such as Candida intermedia (Huang et al. 2011), and bacteria such as Bacillus pumilus and B. thuringiensis (Zheng et al. 2013) also produce volatile antifungal compounds that are applied to control food spoilage microorganisms worldwide (Arrebola et al. 2010; Huang et al. 2011; Li et al. 2012; Braun et al. 2012; Suwannarach et al. 2013, 2015).
Streptomyces mycarofaciens SS-2-243 was previously found to produce non-volatile antimicrobial compounds that inhibited the growth of S. rolfsii and Ralstonia solanacearum (Boukaew et al. 2011) but it has never been studied for its volatile antimicrobial compounds against aflatoxin-producing fungi. Therefore, it was the aim of this work to investigate the efficacy of the volatiles from S. mycarofaciens SS-2-243 (volatiles SS-2-243) as a biofumigant to control the growth of aflatoxin producing fungi. The specific objectives were: (i) to test the antifungal activity of the volatiles SS-2-243 against four pathogenic fungi and to determine the effects of the volatiles SS-2-243 at different wheat seed culture ages against the selected fungi; (ii) to identify the chemical nature of the volatiles SS-2-243; (iii) to assess the effects of wheat seed inoculum size and spore concentration of S. mycarofaciens SS-2-243 on the selected fungi grown on PDA dishes and maize seeds; (iv) to study fungicidal period of the volatiles SS-2-243 against the selected fungi; (v) to evaluate the efficacy of the volatiles SS-2-243 on protection of maize seed; (vi) to study the effect of the fumigation period of the volatiles SS-2-243 for controlling the selected fungi and its effect on seed germination; and (vii) to investigate the mode of action of the volatiles SS-2-243 by studying the ultrastructure of the selected fungi.
Materials and methods
Microorganisms
Streptomyces mycarofaciens SS-2-243 was isolated by Boukaew et al. (2011) and grown on GYM agar dishes at 28 ± 2 °C for 10 days before use. A. parasiticus TISTR 3276, A. flavus TISTR 3041, A. niger ATCC 6275, and Penicillium sp. were maintained on potato dextrose agar (PDA 39 g L−1) medium at 4 °C. The four pathogenic fungi were provided by Microbiology Laboratory of the Department of Industrial Biotechnology, Faculty of Agro-Industry, Prince of Songkla University, Songkhla Province, Thailand. A. parasiticus TISTR 3276 and A. flavus TISTR 3041 reportedly produce aflatoxins (Rammanee and Hongpattarakere 2011; Sangmanee and Hongpattarakere 2014).
Preparation of wheat seed culture of S. mycarofaciens SS-2-243 and spore inoculum of four pathogenic fungi
Wheat seed culture of of Streptomyces mycarofaciens SS-2-243 was prepared by inoculating a spore suspension (107 spores mL−1) on sterile wheat seeds (autoclaved at 121 °C / 15 min) in conical flasks (250 mL) at 1 mL per 100 g of wheat seeds and incubated at 28 ± 2 °C on a rotary shaker (150 rpm) for 12 days.
Spore inoculum of the four pathogenic fungi were prepared by inoculating 5 mm diameter mycelial plug cutting from the periphery of a growing cultures (at 28 ± 2 °C for 10 days) from each strain and mixed with 5 mL sterile distilled water. The spore counts was done using a hematocytometer. Inoculums were prepared by dilution with sterile distilled water to the required spore concentration of each experiment.
Antifungal activity of the volatiles SS-2-243 against four pathogenic fungi and the effect of wheat seed culture age
The influence of the volatiles from 12 days old wheat seed culture of Streptomyces mycarofaciens SS-2-243 on the mycelial growth of the four pathogenic fungi was investigated using an antifungal bioassay (Li et al. 2012). The bioassay was performed following the procedure as previously described (Boukaew et al. 2013). The inhibition of mycelial growth was calculated using the equation:
Effects of the volatiles SS-2-243 from different wheat seed culture ages (0, 3, 6, 9, 12 and 15 days) against the selected pathogenic fungi (based on the above results) was studied. In this experiment, the wheat seed culture of S. mycarofaciens SS-2-243 was prepared as described above except that the amount of spore suspension added was the autoclaved wheat seeds 1 mL per 30 g of wheat seeds and incubated (at 28 ± 2 °C) on a rotary shaker (at 150 rpm) for 15 days. Wheat seed culture samples were taken every 3 days for use in antifungal assays (Li et al. 2012) as described above. The colony size in each treatment was recorded and inhibition of mycelial growth was calculated as above.
Identification of the volatiles SS-2-243 by gas chromatography–mass spectrometry
Identification of the volatilesSS-2-243 was carried out following the procedure as described by Wan et al. (2008) with minor modifications. Briefly, volatile compounds from the head space of a 100 ml sealed Duran bottle containing 30 g autoclaved wheat seeds or wheat seed cultures of Streptomyces mycarofaciens SS-2-243 were collected by means of solid-phase micro-extraction (SPME) and analyzed with a gas chromatograph mass spectrometer (GC-MS) (Trace GC Ultra/ISQMS, Thermo Scientific Inc., USA) equipped with a TR-5MS column (30 m × 0.25 mm, film thickness 0.25 μm, Agilent, Santa Clara, CA, USA). Mass spectra were obtained using the scan modus (total ion count, 35–500 m/z). Confirmation of the compound identities was achieved by comparison of the mass spectra and retention times with those of available standards from the Library of the National Institute of Standards and Technology (NIST). In addition, any baseline volatile compounds from autoclaved wheat seeds were measured and subtracted. The experiments were conducted three times.
Effects of wheat seed inoculum size and spore concentration of S. mycarofaciens SS-2-243 on mycelial growth, conidia germination and sporulation of the selected pathogenic fungi growing on PDA dishes or on maize seeds
Effects of wheat seed inoculum size tested on agar dishes
The effect of inoculum size (1, 5, 10, 15, 30, 45 and 60 g) of wheat seed culture of Streptomyces mycarofaciens SS-2-243 per L of airspace in the flask) on the mycelial growth, conidia germination and sporulation of the selected pathogenic fungi were studied on PDA dishes, using an antifungal bioassay (as described above). Autoclaved wheat seeds (not inoculated with S. mycarofaciens SS-2-243) in similar amounts were used as a control treatments. The diameters of the colonies in the smaller dishes were recorded after incubating (at 28 ± 2 °C) for two days. The inhibition of mycelial growth was calculated as above.
For the conidia germination, 50 μL of spore inoculum (104 spore mL−1) of the selected pathogenic fungi were spread on three of the smaller dishes containing 5 mL PDA and the fourth dish had each concentration of the seven inoculum sizes tested. After 24 h incubation at 28 ± 2 °C, the conidia germination in the smaller dishes was recorded. The inhibition of conidia germination was calculated using the equation:
For the sporulation of the selected pathogenic fungion PDA dishes, the experiment was as described above (for conidia germination). After incubation at 28 ± 2 °C for 5 days, the total number of conidia per plate was assessed by diluting with sterile water before counting the number density of conidia under a compound microscope (Omano OM88, USA).
Effects of wheat seed inoculum size tested on maize seeds
Maize seeds were prepared by soaking in distilled water (100 mL) for 5 h, and then autoclaving at 121 °C for 15 min (Yang and Chang 2010). Five maize seeds were transferred on each of the three smaller dishes, a 50 μL spore inoculum (104 spore mL−1) of the selected pathogenic fungi was dropped on each maize seed. Wheat seed inoculum sizes of S. mycarofaciens SS-2-243 at various concentrations (1, 5, 10, 15, 30, 45 and 60 g L−1) were added to the fourth Petri dishes. The same concentration of wheat seeds (not inoculated with S. mycarofaciens SS-2-243) was used in the control cases. After incubation at 28 ± 2 °C for five days, the total number of conidia per maize seed was determined by flushing the infected maize seeds with sterile water, then counting the number of conidia in the flush water under a compound microscope.
Effects of spore concentration
The effect of spore concentration of Streptomyces mycarofaciens SS-2-243 on the mycelial growth, conidia germination and sporulation of the selected pathogenic fungi were investigated on PDA, using the antifungal bioassay (as stated above) using spore concentrations of 104, 105 106, 107 and 108 spores mL−1 at 1 mL per 30 g wheat seeds inoculated. For the conidia germination, 50 μL of spore inoculum (104 spores mL−1) of the selected pathogenic fungi were spread on three of the smaller dishes containing 5 mL PDA, and the fourth dish had 30 g of wheat seed inoculum at five spore concentrations of S. mycarofaciens SS-2-243. The incubation and calculation of inhibition were as described above. The sporulation of the selected pathogenic fungi was tested on PDA dishes or on maize seeds. After incubation at 28 ± 2 °C for five days, the total number of the conidia per PDA plate was determined as described above. In the experiment on maize seeds, five maize seeds were placed on each of the three smaller dishes, then a 50 μL of spore inoculum (at 104 spore mL−1) of the selected pathogenic fungi were dropped on each maize seed, and the fourth dish contained wheat seed inoculum of S. mycarofaciens SS-2-243 with five spore concentrations tested. The same concentrations of wheat seed (without inoculation of S. mycarofaciens SS-2-243) were used in the control. After incubation at 28 ± 2 °C for five days, the total number of conidia per maize seed was determined by flushing the infected maize seeds with sterile distilled water, then counting the number of conidia in the flush water with the use of a compound microscope.
Fungicidal period of the volatiles SS-2-243 against the selected pathogenic fungi
To assess the fungicide period of the volatiles SS-2-243, fifty μL of spore inoculum (104 spore mL−1) of the selected pathogenic fungi were spread on PDA. The cultures were fumigated with 30 g L−1 wheat seed inoculum of Streptomyces mycarofaciens SS-2-243 for 0, 1, 3, 6, 9, 12, or 15 h. Equivalent amounts of autoclaved wheat seeds not inoculated with S. mycarofaciens SS-2-243 were used as the control. The spore fumigant was removed after each fumigation period and the cultures were incubated at 28 ± 2 °C for 48 h. For each treatment, there were three replicates. The total number of conidia alive on PDA dishes were determined (Li et al. 2013).
The fungicidal period was defined as the minimum time required for fumigation to effect total inhibition of a pathogen.
Efficacy of the volatiles SS-2-243 at various inoculum sizes and spore concentrations of S. mycarofaciens SS-2-243 to protect maize seeds against the selected aflatoxin producing fungi
Evaluation of the volatiles SS-2-243 at different inoculum sizes and spore concentrations of Streptomyces mycarofaciens SS-2-243 to protect maize seed against contamination by the selected aflatoxin producing fungi was done using the antifungal bioassay. Five maize seeds were transferred on each of the three smaller dishes, then 50 μL of spore inoculum (104 spore mL−1) of the selected aflatoxin producing fungi was dropped on each maize seed, and the fourth dish had some amount (1, 5, 10, 15, 30, 45 and 60 g L−1) of wheat seed culture from varied spore concentrations (inoculated at 1 mL of 104, 105 106, 107 or 108 spores mL−1 on 30 g wheat seeds) of S. mycarofaciens SS-2-243. Equivalent amounts of autoclaved wheat seeds not inoculated with S. mycarofaciens SS-2-243 were used as the control. The dishes were incubated at 28 ± 2 °C for 5 days. Then, the maize seeds in the dishes were individually analyzed for infection under a stereo-binocular microscope (Sumalan et al. 2013) and the protection against infection of seeds was calculated: Percentage of protection = [{(Control-treatment)/ Control} × 100]. The experiment was repeated twice with three replicates in each.
Effects of the fumigation period with the volatiles SS-2-243 for controlling the selected aflatoxin producing fungi on maize seeds, and its effect on seed germination
The fumigation period with the volatiles SS-2-243 for controlling the aflatoxin producing fungi on maize seeds using the antifungal bioassay was investigated. The minimum dose (30 g L−1 wheat seed culture) of Streptomyces mycarofaciens SS-2-243 that completely controlled the infection of maize seeds was chosen for all treatments. The four treatments all had aflatoxin producing fungal infection, followed by 0, 6, 12 or 24 h of S. mycarofaciens SS-2-243 fumigation. The fungal fumigant was removed after each fumigation period and the Petri dishes were restored to incubation at 28 ± 2 °C. The contamination of maize seeds was observed every 2 days for 12 days. The growth of fungi on maize seeds was visually assessed using a stereo-binocular microscope. The seed contamination index (SCI) was obtained using the formula: % SCI = [(Number of contaminated seeds / Total number seeds) × 100] (Doolotkeldieva 2010).
Streptomyces mycarofaciens SS-2-243 with strong antifungal activity against the selected aflatoxin producing fungi was tested on the effects on maize seed germination. One hundred seeds were fumigated with 30 g L−1 of S. mycarofaciens SS-2-243 for 24 h and placed on moist paper on the bottom of a plastic box (120 mm width × 170 mm length × 68 mm height with 1 L inner volume). Equivalent amounts of autoclaved wheat seeds not inoculated with S. mycarofaciens SS-2-243 were used in the control. The effects of the volatiles SS-2-243 on maize seed germination were assessed after 10 days of incubation at 28 ± 2 °C.
Mode of action of the volatiles SS-2-243 by studying the ultrastructure of the selected aflatoxin producing fungi
Fungal biomass obtained from a 5-day-old culture grown on PDA with or without fumigation exposure to the volatiles SS-2-243 was subjected to scanning electron microscopy (SEM) to assess possible effects of the volatile compounds on the ultrastructure of the fungi. The mycelium affected by the volatiles SS-2-243 was transferred to a glass cover slip, then fixed with 1.5% glutaraldehyde and dehydrated with a graded series of ethanol washes followed by drying in a desiccator (Walter and Crawford 1995). Samples were affixed to SEM stubs using carbon tape followed by a thin coating with gold and examined using a SEM (FEI Quanta 400, SEM-Quanta).
Statistical analysis
All experiments were repeated twice with three replicates. The data were analyzed using Statistical Package for the Social Sciences (SPSS) software version 15 for Windows. Statistical significance was evaluated using Duncan’s Multiple Range and accepted at a p = 0.05.
Results
Antifungal activity of the volatiles SS-2-243 against four pathogenic fungi and the effect of wheat seed culture age
The volatiles generated by the 12 days old wheat seed culture of Streptomyces mycarofaciens SS-2-243 could inhibit the mycelial growth of the four pathogenic fungi. The growth inhibitions on A. parasiticus TISTR 3276, A. flavus TISTR 3041, Penicillium sp. and A. niger ATCC 6275 (on PDA) were 100%, 100%, 49%, and 22%, respectively. The least inhibited was A. niger ATCC 6275 while the most pronounced inhibition (100% inhibition) was against the two aflatoxin producing fungi A. parasiticus TISTR 3276 and A. flavus TISTR 3041. Therefore, the two aflatoxin producing fungi were selected for further studies as they were completely inhibited by the volatiles SS-2-243.
The effect of the volatiles SS-2-243 at different wheat seed culture ages against the two aflatoxin producing fungi was investigated. The results indicated that the growth inhibition increased with the wheat seed culture age from 0 to 15 days (Fig. S1; Supplementary data). Growth of both pathogens was inhibited >60% after 6 days incubation and complete inhibition (100%) occurred at 9 days of cultivation for A. flavus TISTR 3041 and at 12 days for A. parasiticus TISTR 3276. Therefore, A. parasiticus TISTR 3276 was more resistant to the volatiles SS-2-243 than A. flavus TISTR 3041.
Identification of the volatiles SS-2-243
Identification of the chemical composition by GC–MS revealed 33 volatile compounds from the 12 days old wheat seed culture of S. mycarofaciens SS-2-243 (Table 1). Streptomyces mycarofaciens SS-2-243 excreted multiple antimicrobial compounds, and the two major constituents were 2-methylisoborneol (29.7%) followed by 3,7-dimethylocta-2,6-dien-1-ol (geosmin) (10.4%). The identified volatile compounds fell into several categories, including alcohols, alkenes, aromatic hydrocarbons, ketones, alkanes and carboxylic acids.
Effect of wheat seed inoculum size and spore concentration of S. mycarofaciens SS-2-243 on mycelial growth, conidia germination and sporulation of the selected pathogenic fungi growing on PDA dishes or on maize seeds
Effect of wheat seed inoculum size
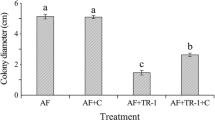
The effects of inoculum size (1, 5, 10, 15, 30, 45 and 60 g L−1) of the wheat seed culture of Streptomyces mycarofaciens SS-2-243 was tested against the two selected aflatoxin producing fungi. The reduction on mycelial growth, conidia germination and sporulation were dependent on the wheat seed inoculum size (Fig. 1). The optimum wheat seed inoculum size was 10 g L−1 for total growth inhibition (100%) of A. parasiticus TISTR 3276 and 30 g L−1 for A. flavus TISTR 3041 (Fig. 1a). In addition, the optimum inoculum size for conidia germination was 15 g L−1 for both aflatoxin producing fungal strains (Fig. 1b). The sporulation of the two strains was tested on PDA and on maize seeds using various inoculum sizes of S. mycarofaciens SS-2-243. The wheat seed inoculum with 15 g L−1 of S. mycarofaciens SS-2-243 completely inhibited sporulation (100%) of both A. parasiticus TISTR 3276 and A. flavus TISTR 3041 on PDA plate (Fig. 1c) and on maize seeds (Fig. 1d). The inoculum size 30 g L−1 was chosen for further study as it could completely inhibit growth, conidia germination and sporulation of both alfatoxigenic strains.
Effects of wheat seed inoculum size on suppression of mycelial growth (a), conidial germination (b), sporulation on PDA plate (c), and sporulation on maize seeds (d) of A. parasiticus TISTR 3276 and A. flavus TISTR 3041, when fumigated with the volatiles SS-2-243. Different letters above bars indicate significant differences (p < 0.05) using ANOVA after the Duncan’s Multiple Range Test in each group. Each bar represented mean value with standard error of three replicates
Effects of spore concentration
Effects of the volatiles SS-2-243 from different spore concentrations (104, 105, 106, 107 and 108 spore mL−1) of Streptomyces mycarofaciens SS-2-243 in the selected wheat seed inoculum size (30 g L−1) on mycelial growth, conidia germination and sporulation of A. parasiticus TISTR 3276 and A. flavus TISTR 3041 were studied (Fig. 2). Increasing the concentration from 104 to 107 spore mL−1 increased the growth inhibition from 55.8% to 100% for A. parasiticus TISTR 3276 and from 34.5% to 100% for A. flavus TISTR 3041, respectively (Fig. 2a). Therefore, the optimum spore concentrations of S. mycarofaciens SS-2-243 were 107 and 106 spore mL−1 to completely inhibit the growth of A. parasiticus TISTR 3276 and A. flavus TISTR 3041, respectively. In addition, conidia germination was completely inhibited (100%) when the spores of these pathogenic fungi were treated with 104 spore mL−1 of S. mycarofaciens SS-2-243 (Fig. 2b). The spore concentration of 107 spore mL−1 of S. mycarofaciens SS-2-243 completely inhibited sporulation of A. parasiticus TISTR 3276 (Fig. 2c) and A. flavus TISTR 3041 (Fig. 2d). Therefore, the spore concentration 107 spore mL−1 was chosen for further study, as it could completely inhibit growth, conidia germination and sporulation of both A. parasiticus TISTR 3276 and A. flavus TISTR 3041.
Effects of spore concentration on suppression of mycelial growth (a), conidial germination (b), sporulation on PDA plate (c), and sporulation on maize seeds (d) of A. parasiticus TISTR 3276 and A. flavus TISTR 3041, when fumigated with the volatiles SS-2-243. Different letters above bars indicate significant differences (p < 0.05) using ANOVA after the Duncan’s Multiple Range Test in each group. Each bar represented mean value with standard error of three replicates
Fungicidal period of the volatiles SS-2-243 against the two aflatoxin-producing fungi
The growth inhibition kinetics of A. parasiticus TISTR 3276 and A. flavus TISTR 3041 by the volatiles SS-2-243 is presented in Fig. S2 (Supplementary data). Exposure of the two aflatoxin producing fungal spores to the volatiles SS-2-243 for a period of 0–15 h decreased conidia germination with exposure time. The volatiles SS-2-243 totally inhibited (100%) spore germination of A. parasiticus TISTR 3276 at 1 h of exposure, while it look 3 h for A. flavus TISTR 3041.
Efficacy of the volatiles SS-2-243 at various inoculum sizes and spore concentrations of S. mycarofaciens SS-2-243 to protect maize seeds against the selected aflatoxin producing fungi
Various concentrations of the wheat seed inoculum (1, 5, 10, 15, 30, 45 and 60 g L−1) and spore concentrations (104, 105, 106, 107 and 108 spore mL−1) of Streptomyces mycarofaciens SS-2-243 in the fumigation of A. parasiticus TISTR 3276 and A. flavus TISTR 3041 on maize seeds were investigated for effects on fungal growth (Fig. 3). The percentages of protection on maize seeds significantly (p < 0.05) increased with the concentration of the wheat seed inoculum and with the spore concentration of S. mycarofaciens SS-2-243. The most effective wheat seed inoculum dose and spore concentration were 30 g L−1 (Fig. 3a) and 104 spore mL−1 (Fig. 3b), respectively, as the fumigation could completely protect (100%) maize seeds against infection by either alfatoxigenic fungus after 5 days of incubation at 28 ± 2 °C. Therefore, the optimum wheat seed inoculum size and spore concentration of S. mycarofaciens SS-2-243 were 30 g L−1 and 104 spore mL−1, respectively, providing complete growth inhibition of A. parasiticus TISTR 3276 and A. flavus TISTR 3041 on maize seeds.
Effects of inoculum size (a) and spore concentration (b) on the protection of maize seeds inoculated with A. parasiticus TISTR 3276 and A. flavus TISTR 3041 when fumigated with the volatiles SS-2-243 after cultivation at 28 ± 2 °C for five days. Different letters above bars indicate significant differences (p < 0.05) using ANOVA after the Duncan’s Multiple Range Test in each group. Each bar represented mean value with standard error of three replicates
Effects of the fumigation period of the volatiles SS-2-243 for controlling the selected aflatoxin producing fungi on maize seeds, and its effect on maize seed germination
The fumigation period (0, 6, 12 or 24 h) had an influence on the level of infection by either pathogen (Table 2). Treated and non–fumigated maize seeds showed initial wheat seed contamination symptoms after 2 days, with contamination levels 46.6% for A. parasiticus TISTR 3276 and 33.3% for A. flavus TISTR 3041. With 6 h of fumigation, the initial wheat seed contamination (< 30%) of both pathogens occurred on the 8th day. Interestingly, the 24 h fumigation treatment completely controlled (100%) the two aflatoxin producing fungi on maize seeds. Therefore, 24 h fumigation was sufficient to control the two aflatoxin producing fungi on maize seeds.
The maize seeds were fumigated with 30 g L−1 wheat seed culture of Streptomyces mycarofaciens SS-2-243, incubated for 24 h, and placed in moist plastic boxes for 10 days. The volatiles SS-2-243 did not significantly (p < 0.05) affect maize seed germination relative to the control (data not shown). Therefore, the fumigation with S. mycarofaciens SS-2-243 can be applied to control aflatoxin producing fungi during maize seed storage.
Mode of action of the volatiles SS-2-243 by studying the ultrastructure of the selected aflatoxin producing fungi
Ultrastructure alterations of the two aflatoxin producing fungi after five days of treatment with the volatiles SS-2-243 were imaged by SEM (Fig. 4). In the control, there were no morphological changes in A. flavus TISTR 3041 (Fig. 4a) and A. parasiticus TISTR 3276 (Fig. 4d). The individual ordered mycelia could be clearly distinguished in the control samples, and a large quantity of well formed conidiophores grew at the fungal tips. On the other hand, images of the two aflatoxin producing fungi samples exposed to the volatiles SS-2-243 showed evident damage and completely inhibited conidia germination of A. flavus TISTR 3041 (Fig. 4b, c) and A. parasiticus TISTR 3276 (Fig. 4e, f). The two aflatoxin producing fungi were markedly shriveled, with crinkled cell-walls and flattened hyphae in the SEM images (Fig. 4c and f).
Scanning electron micrographs (SEM) of normal hypha, conidiophores, and spores of and A. parasiticus TISTR 3276 (a) and A. flavus TISTR 3041 (d) without exposure to the volatiles SS-2-243 (the control) after incubation at 28 ± 2 °C for five days. Mycelia treated with the volatiles SS-2-243 of A. parasiticus TISTR 3276 (b and c) and of A. flavus TISTR 3041 (E and F) after incubation at 28 ± 2 °C for five days
Discussion
Streptomyces mycarofaciens SS-2-243 was previously reported to produce non-volatile compounds with antimicrobial activity in a culture broth (Boukaew et al. 2011, 2017a), but production of volatile compounds by this strain SS-2-243 has never been assessed. In this study, S. mycarofaciens SS-2-243 was found to produce volatile compounds while growing on autoclaved wheat seeds, and the volatiles significantly (p < 0.05) affected the mycelial growth of four phytopathogenic fungi on PDA agar dishes. The volatile compounds were most active against two aflatoxin producing fungal strains (A. parasiticus TISTR 3276 and A. flavus TISTR 3041). Streptomyces spp. is known to produce volatile compounds that inhibit and kill plant pathogenic fungi (Wan et al. 2008; Li et al. 2010, 2012; Boukaew et al. 2013, 2018; Wang et al. 2013). Among the 33 volatile compounds from S. mycarofaciens SS-2-243 grown on autoclaved wheat seeds and identified by GC-MS analysis, 2-methylisoborneol was found as the largest component (29.7%) followed by 3,7-dimethylocta-2,6-dien-1-ol (geosmin) (10.4%). They could be chemically grouped into alcohols, alkenes, aromatic hydrocarbons, ketones, ester and alkanes, some of which were known antimicrobial agents (Wan et al. 2008). The 2-methylisoborneol was also the most abundant compound among 27 volatile compounds from S. alboflavus TD-1 (Wang et al. 2013). In contrast, trans-1,10-dimethyl-trans-9-decalol (geosmin) was the major component among 16 volatile substances released by cultures of S. platensis F-1 grown on autoclaved wheat seeds (Wan et al. 2008). Geosmin was also the most abundant compound among 41 volatile organic compounds from S. globisporus JK-1 growing on wheat seeds (Li et al. 2010). Unlike these cases, 3,7-dimethylocta-1,6-dien-3-ol (L-linalool) was the major compound among 36 volatile compounds from S. philanthi RM-1-138 growing on autoclaved wheat seed (Boukaew et al. 2013). Some of the volatiles SS-2-243, such as s-methyl −3-methyl butanethioate, 4-isopropenyl-1-methyl-1-cyclohexen, 4,8-dimethyl-nona-1,3,7-triene, octane,6-ethyl-2-methyl, penta-decanoic acid, verticellol and hexadecanoic acid have not been previously reported from Streptomyces spp. (Wan et al. 2008; Li et al. 2010; Boukaew et al. 2013; Wang et al. 2013). Thus, different Streptomyces species may produce similar or different major components of volatile compounds, with various effects. Three components of the volatiles SS-2-243 have been reported to possess antimicrobial activities: phenylethyl alcohol in S. globisporus JK-1 (Li et al. 2010) and Muscodor albus (Grimme et al. 2007; Strobel et al. 2007), acetophenone in S. globisporus JK-1 (Li et al. 2010) and hexadecanoic acid in Holarrhena antidysenterica (Preethi et al. 2010). In addition to their antimicrobial function, the volatile compounds from Bacillus amyloliquefaciens IN937 and B. subtilis GB03 were reported to promote the growth of Arabidopsis thaliana (Ryu et al. 2003; Vespermann et al. 2007) and induce systemic resistance in A. thaliana against Erwinia carotovora subsp. carotovora (Ryu et al. 2004). In addition, volatile compounds from bacteria and yeast inhibited pigment production by sapstain fungi (Bruce et al. 2003). Finally, volatile compounds can regulate aflatoxin biosynthesis in Aspergillus parasiticus (Roze et al. 2007).
The wheat seed inoculum size and the spore concentration of S. mycarofaciens SS-2-243 had significant (p < 0.05) effects on growth, conidia germination, and sporulation of both aflatoxin producing fungal strains growing on PDA or on maize seeds. The efficacy of the volatile compounds of this strain increased with concentration and size of the inoculum, and the effective dose was found to be at least 30 g L−1 inoculum size with spore concentration of 107 spore mL−1. Spores are important for the survival and spreading of aflatoxin producing fungi (Rabea et al. 2003). A significant (p < 0.05) decrease in their spore germination was observed for A. parasiticus TISTR 3276 and A. flavus TISTR 3041 with time of exposure to the volatiles. The kinetic study revealed that 1 h exposure to volatiles from 30 g L−1 wheat seed culture of S. mycarofaciens SS-2-243 could completely kill (100%) the conidia of A. parasiticus TISTR 3276, while the same effect occurred at 3 h for A. flavus TISTR 3041.
Although in vitro testing of the volatiles SS-2-243 was an important first step in checking their antifungal potential, in vivo testing is needed to confirm these results. In a maize seed assay, the volatile compounds exhibited 100% protection of maize seeds against contamination by the two aflatoxin producing fungi, with the effective wheat seed inoculum size (30 g L−1) and spore concentration (107 spore mL−1). This demonstrated the biofumigation effect. In this study, the wheat seed inoculum size differed from other biocontrol reports that have studied volatiles from Streptomyces spp. For example, fumigation with 15 g L−1 of wheat seed culture of S. philanthi RM-1-138 completely inhibited Rhizoctonia solani in in vitro and in vivo experiments (Boukaew et al. 2013). The effective inoculum size against Botrytis cinerea was a wheat seed culture with 120 g L−1 of S. globisporus JK-1 (Li et al. 2012), while biofumigation with 240 g L−1 of this culture completely inhibited Penicillium italicum (Li et al. 2010). Rhizoctonia solani, Sclerotinia sclerotiorum, and Botrytis cinerea could also be effectively inhibited by fumigation with 300 and 75 g L−1 of the wheat seed culture of S. platensis F-1 (Wan et al. 2008).
Furthermore, increasing the fumigation period, or exposure to the volatile compounds, from 6 to 24 h significantly (p < 0.05) reduced the fraction of infected maize seeds. Therefore, fumigation with 30 g L−1 wheat seed inoculum for at least 24 h provided complete control of maize seed infection by both A. parasiticus TISTR 3276 and A. flavus TISTR during the 12 days tested. Based on this result, maize seeds must be fumigated with the volatiles SS-2-243 to protect against infection by the aflatoxin producing fungi before seed storage. In general, some compounds produced by microorganisms (Qiming et al. 2006; Boukaew et al. 2011) or plant essential oils (Kordali et al. 2009; Young and Bush 2009; Boukaew et al. 2017b) have been reported to inhibit seed germination. In this study, S. mycarofaciens SS-2-243 volatile compounds had no significant (p < 0.05) effect on maize seed germination when compared to the control.
Results from both in vitro and in vivo observations showed that the volatiles SS-2-243 completely inhibited (by 100%) mycelial growth, conidia germination, and sporulation of A. flavus TISTR 3041 and A. parasiticus TISTR 3276 at 30 g L−1 of wheat seed inoculum. The SEM results confirmed that the suppression activity of the volatiles SS-2-243 on the two aflatoxin producing fungi incurred alterations in mycelial morphology, led to a gradual destruction of mycelia, and completely inhibited conidia germination. This confirms the potent inhibitory potential of these pathogenic fungi. Similar damage is induced by the volatile substances of S. globisporus JK-1 against P. italicum (Li et al. 2010) and B. cinerea (Li et al. 2012). Therefore, the main action of the volatiles SS-2-243 was on the conidia germination of A. flavus TISTR 3041 and A. parasiticus TISTR 3276.
Conclusion
Use of volatile compounds has been emerging as a possible alternative for the control of aflatoxin producing fungi. Streptomyces mycarofaciens SS-2-243 could be used as a biofumigant to protect maize seeds against the two aflatoxin producing fungi. The strain produced volatile compounds that have good potential as a biofumigant for controlling aflatoxin producing fungi, without any adverse effect on maize germination.
References
Arrebola, E., Sivakumar, D., & Korsten, L. (2010). Effect of volatile compounds produced by Bacillus strains on postharvest decay in citrus. Biological Control, 53, 122–128.
Boukaew, S., & Prasertsan, P. (2014). Suppression of rice sheath blight disease using heat stable culture filtrate of Streptomyces philanthi RM-1-138. Crop Protection, 61, 1–10.
Boukaew, S., Chuenchit, S., & Petcharat, V. (2011). Evaluation of Streptomyces spp. for biological control of Sclerotium root and stem rot and Ralstonia wilt of chili pepper. BioControl, 56, 365–374.
Boukaew, S., Plubrukarn, A., & Prasertsan, P. (2013). Effect of volatile substances from Streptomyces philanthi RM-1-138 on growth of Rhizoctonia solanion rice leaf. BioControl, 58, 471–482.
Boukaew, S., Prasertsan, P., Troulet, C., & Bardin, M. (2017a). Biological control of tomato gray mold caused by Botrytis cinerea by using Streptomyces spp. BioControl, 62, 793–803.
Boukaew, S., Prasertsan, P., & Sattayasamitsathit, S. (2017b). Evaluation of antifungal activity of essential oils against aflatoxigenic Aspergillus flavus and their allelopathic activity from fumigation to protect maize seeds during storage. Industrial Crops and Products, 97, 558–566.
Boukaew, S., Petlamul, W., Bunkrongcheap, R., Chookaew, T., Kabbua, T., Thippated, A., & Prasertsan, P. (2018). Fumigant activity of volatile compounds of Streptomyces philanthi RM-1-138 and pure chemicals (acetophenone and phenylethyl alcohol) against anthracnose pathogen in postharvest chili fruit. Crop Protection, 103, 1–8.
Braun, G., Vailati, M., Prange, R., & Bevis, E. (2012). Muscodor albus volatiles control toxigenic fungi under controlled atmosphere (CA) storage conditions. International Journal of Molecular Science, 13, 15848–15858.
Bruce, A., Stewart, D., Verrall, S., & Wheatley, R. E. (2003). Effect of volatiles from bacteria and yeast on the growth and pigmentation of sapstain fungi. International Biodeterioration & Biodegradation, 51, 101–108.
Chen, Y. Y., Chen, P. C., & Tsay, T. T. (2016). The biocontrol efficacy and antibiotic activity of Streptomyces plicatus on the oomycete Phytophthora capsici. Biological Control, 98, 34–42.
Demain, A. L. (2000). Small bugs, big business: The economic power of the microbe. Biotechnology Advances, 18, 499–514.
Doolotkeldieva, T. D. (2010). Microbiological control of flour-manufacture: Dissemination of mycotoxins producing fungi in cereal products. Microbiology Insights, 3, 1–15.
Eckert, C. T., Frigo, E. P., Albrecht, L. P., Albrecht, A. J. P., Christ, D., Santos, W. G., Berkembrock, E., & Egewarth, V. A. (2018). Maize ethanol production in Brazil: Characteristics and perspectives. Renewable and Sustainable Energy Reviews, 82, 3907–3912.
Elango, V., Manjukarunambika, K., Ponmurugan, P., & Marimuthu, S. (2015). Evaluation of Streptomyces spp. for effective management of Poria hypolateritia causing red root-rot disease in tea plants. Biological Control, 89, 75–83.
Grimme, E., Zidack, N. K., Sikora, R. A., Strobel, G. A., & Jacobsen, B. J. (2007). Comparison of Muscodor albus volatiles with a biorational mixture for control of seedling diseases of sugar beet and root-knot nematode on tomato. Plant Disease, 91, 220–225.
Huang, R., Li, G. Q., Zhang, J., Yang, L., Che, H. J., Jiang, D. H., & Huang, H. C. (2011). Control of postharvest Botrytis fruit rot of strawberry by volatile organic compounds of Candida intermedia. Phytopathology, 101, 859–869.
Kordali, S., Cakir, A., Akcin, T. A., Mete, E., Akcin, A., Aydin, T., & Kilic, H. (2009). Antifungal and herbicidal properties of essential oils and n-hexane extracts of Achillea gypsicola hub-Mor and Achillea biebersteinii Afan. Industrial Crops and Products, 29, 562–570.
Li, Q., Ning, P., Zheng, L., Huang, J., Li, G., & Hsiang, T. (2010). Fumigant activity of volatiles of Streptomyces globisporus JK-1 against Penicillium italicum on Citrus microcarpa. Postharvest Biology and Technology, 58, 157–165.
Li, Q., Ning, P., Zheng, L., Huang, J., Li, G., & Hsiang, T. (2012). Effects of volatile substances of Streptomyces globisporus JK-1 on control of Botrytis cinerea on tomato fruit. Biological Control, 61, 113–120.
Li, W. R., Shi, Q. S., Ouyang, Y. S., Chen, Y. B., & Duan, S. S. (2013). Antifungal effects of citronella oil against Aspergillus niger ATCC 16404. Applied Microbiology and Biotechnology, 97, 7483–7492.
Li, J., Liu, W., Luo, L., Dong, D., Liu, T., Zhang, T., Lu, C., Liu, D., Zhang, D., & Wu, H. (2015). Expression of Paenibacillus polymyxa β-1,3-1,4-glucanase in Streptomyces lydicus A01 improves its biocontrol effect against Botrytis cinerea. Biological Control, 90, 141–147.
Ng'ang'a, J., Mutungi, C., Imathiu, S., & Affognon, H. (2016). Effect of triple-layer hermetic bagging on mould infection and aflatoxin contamination of maize during multi-month on-farm storage in Kenya. Journal of Stored Products Research, 69, 119–128.
Picot, A., Ortega-Beltran, A., Puckett, R. D., Siegel, J. P., & Michailides, T. J. (2017). Period of susceptibility of almonds to aflatoxin contamination during development in the orchard. European Journal of Plant Pathology, 148, 521–531.
Preethi, R., Vimal Devanathan, V., & Loganathan, M. (2010). Antimicrobial and antioxidant efficacy of some medicinal plants against food borne pathogens. Advances in Biological Research, 4, 122–125.
Qiming, X., Haidong, C., Huixian, Z., & Daqiang, Y. (2006). Allelopathic activity of volatile substance from submerged macrophytes on Microcystin aeruginosa. Acta Ecologica Sinica, 26, 3549–3554.
Rabea, E. I., Badawy, M. E. T., Stevens, C. V., Smagghe, G., & Steubaurt, W. (2003). Chitosan as antimicrobial agent:Applications and mode of action. Biomacromolecules, 4, 1457–1465.
Rammanee, K., & Hongpattarakere, T. (2011). Effects of tropical citrus essential oils on growth, aflatoxin production, and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Bioprocess Technology, 4, 1050–1059.
Razzaghi-Abyaneh, M., Saberi, R., Sharifan, A., Rezaee, M. B., Seifili, R., Hosseini, S. I., Shams-Ghahfarokhi, M., Nikkhah, M., Saberi, I., & Amani, A. (2013). Effects of Heracleum persicum ethyl acetate extract on the growth,mycelialultrastructure and aflatoxin biosynthesis in Aspergillus parasiticus. Mycotoxin Research, 29, 261–269.
Roze, L. V., Beaudry, R. M., Arthur, A. E., Calvo, A. E., & Linz, A. E. (2007). Aspergillus volatiles regulate aflatoxin synthesis and asexual sporulation in Aspergillus parasiticus. Applied and Environmental Microbiology, 73, 7268–7276.
Ryu, C. M., Farag, M. A., Hu, C. H., Reddy, M. S., Wei, H. X., Pare, P. W., & Kloepper, J. W. (2003). Bacterial volatiles promote growth in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America, 100, 4927–4932.
Ryu, C. M., Farag, M. A., Hu, C. H., Reddy, M. S., Kloepper, J. W., & Pare, P. W. (2004). Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiology, 134, 1017–1026.
Sangmanee, P., & Hongpattarakere, T. (2014). Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control, 40, 224–233.
Shaista, K., Memon, A. N., Ghanghro, A. B., & Ibtessam, T. (2011). Fractionation and characterization of seed storage proteins from different wheat varieties cultivated in Sindh on SDS-PAGE electrophoresis. Pakistan Journal of Nutrition, 10, 139–142.
Shakeel, Q., Lyu, A., Zhang, J., Wu, M., Chen, S., Chen, W., Li, G., & Yang, L. (2016). Optimization of the cultural medium and conditions for production of antifungal substances by Streptomyces platensis 3-10 and evaluation of its efficacy in suppression of clubroot disease (Plasmodiophora brassicae) of oilseed rape. Biological Control, 101, 59–68.
Shimizu, M., Yazawa, S., & Ushijima, Y. (2009). A promising strain of endophytic Streptomyces sp. for biological control of cucumber anthracnose. Journal of General Plant Pathology, 75, 27–36.
Singh, S. P., & Gaur, R. (2017). Endophytic Streptomyces spp. underscore induction of defense regulatory genes and confers resistance against Sclerotium rolfsii in chickpea. Biological Control, 104, 44–56.
Strobel, G. (2011). Muscodor species-endophytes with biological promise. Phytochemistry Reviews, 10, 165–172.
Strobel, G. A., Kluck, K., Hess, W. M., Sears, J., Ezra, D., & Vargas, P. N. (2007). Muscodor albus E-6, an endophyte of Guazuma ulmifolia making volatile antibiotics: Isolation, characterization and experimental establishment in the host plant. Microbiology, 153, 2613–2620.
Sumalan, R. M., Alexa, E., & Poiana, A. M. (2013). Assessment of inhibitory potential of essential oils on natural mycoflora and Fusarium mycotoxins production in wheat. Chemistry Central Journal, 7, 1–12.
Suwannarach, N., Kumla, J., Bussaban, B., McKenzie, E. H. C., Hyde, K. D., Mutsui, K., & Lumyong, S. (2013). Molecular and morphological evidence support four new species in the genus Muscodor from northern Thailand. Annals of Microbiology, 63, 1341–1351.
Suwannarach, N., Bussaban, B., Nuangmek, W., Pithakpol, W., Jirawattanakul, B., Matsuif, K., & Lumyonga, S. (2015). Evaluation of Muscodor suthepensis strain CMU-Cib462 as a postharvest biofumigant for tangerine fruit rot caused by Penicillium digitatum. Journal of the Science of Food and Agriculture, 96, 339–345.
Tamreihao, K., Ningthoujam, D. S., Nimaichand, S., Singh, E. S., Reena, P., Singh, S. H., & Nongthomba, U. (2016). Biocontrol and plant growth promoting activities of a Streptomyces corchorusii strain UCR3-16 and preparation of powder formulation for application as biofertilizer agents for rice plant. Microbiological Research, 192, 260–270.
Valdez-Ortiz, A., Medina-Godoy, S., Valverde, M. E., & Paredes-Lopez, O. (2007). A transgenic tropical maize line generated by the direct transformation of the embryo-scutellum by A. tumefaciens. Plant Cell Tiss Organ Cult, 91, 201–214.
Vespermann, A., Kai, M., & Piechulla, B. (2007). Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Applied and Environmental Microbiology, 73, 5639–5641.
Walter, M. Y., & Crawford, D. L. (1995). Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Applied and Environmental Microbiology, 61, 3119–3128.
Wan, M., Li, G., Zhang, J., Jiang, D., & Huang, H. C. (2008). Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biological Control, 46, 552–559.
Wang, C., Wang, Z., Qiao, X., Li, Z., Li, F., Chen, M., Wang, Y., Huang, Y., & Cui, H. (2013). Antifungal activity of volatile organic compounds from Streptomyces alboflavus TD-1. FEMS Microbiology Letter, 341, 45–51.
Yang, E. J., & Chang, H. C. (2010). Purification of a new antifungal compound produced by Lactobacillus plantarum AF1 isolated from kimchi. International Journal of Food Microbiology, 139, 56–63.
Young, G. P., & Bush, J. K. (2009). Assessment of the allelopathic potential of Juniperus ashei on germination and growth of Bouteloua curtipendula. Journal of Chemical Ecology, 35, 74–80.
Zain, M. E. (2011). Impact of mycotoxins on humans and animals. Journal of Saudi Chemical Society, 15, 129–144.
Zheng, M., Shi, J., Shi, J., Wang, Q., & Yanhua, Y. (2013). Antimicrobial effects of volatiles produced by two antagonistic Bacillus strains on the anthracnose pathogen in postharvest mangos. Biological Control, 65, 200–206.
Acknowledgements
This research study was financially supported by the Agricultural Research Development Agency (Public Organization) grant number PRP5905021490 and the Thailand Research Fund (grant no. RTA6080010). The copyediting service of RDO/PSU and proof-reading by Dr. Seppo Karrila are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare having no conflict of interest.
Human and animal studies
This research did not involve human and/or animal participants.
Electronic supplementary material
ESM 1
(DOCX 62 kb)
Rights and permissions
About this article
Cite this article
Boukaew, S., Petlamul, W., Phitthayaphinant, P. et al. Potential use of Streptomyces mycarofaciens SS-2-243 as a biofumigant to protect maize seeds against two aflatoxin producing fungi. Eur J Plant Pathol 155, 489–503 (2019). https://doi.org/10.1007/s10658-019-01782-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01782-3