Abstract
The experiments were carried out under laboratory and greenhouse conditions to study the biocontrol potential of aqueous extracts of Pistacia lentiscus (L.) at different concentrations (C5: 100%, C4: 75%, C3: 50%, C2: 25%, C1:10% w/v) against Meloidogyne javanica and Fusarium oxysporum f.sp. lycopersici. In vitro assays, showed that the inhibition rate of F. oxysporum growth increased progressively by increasing the extract concentration, reaching about 83% at the highest concentration C5. However, the fungi growth rate (VC: mm/h) and the spore germination rate correlated negatively with the extract concentration. The results showed that M. javanica juvenile mortality increased significantly with all tested concentrations of plant extract after 72 h of exposure time. Furthermore, C3, C4 and C5 increased similarly the net mortality (C3: 96%, C4: 97%, C5: 96%) of M. javanica juveniles. Egg hatching decreased significantly with the increase of concentration of P. lentiscus aqueous extracts. The lowest level of egg hatching was recorded with C5 (38.2%). Furthermore, the P. lentiscus extract promoted the growth of tomato plant and reduced the incidence of wilt disease complex on tomato seedlings. The phytochemical analysis showed the high phenolic (quinic and gallic acids) and flavonoids (quercetin) content and antioxidant activity of the P. lentiscus extract. The considerable biocidal activities and valuable chemical composition suggested the future application of this plant in new bio-pesticides formulation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The root-knot nematodes (Meloidogyne spp. or RKN) are an important pest for many crops as a limiting factor for crop production on global agriculture (Moens et al. 2009). The Meloidogyne spp. are distributed globally (Sikora and Fernandez 2005). In the Mediterranean countries, the losses generated by root-knot nematodes can reach 30% (Talavera et al. 2012; Janati et al. 2018) and in Tunisia, Meloidogyne spp. alone can reduce the productivity of tomato crop in glasshouse cultivation by 12 up to 60% (Horrigue-Raouani 2003).
Fusarium wilt caused by the soil-borne pathogen Fusarium oxysporum f. sp. lycopersici (FOL), is one of the most economically important and widespread diseases of tomato crop (Borrero et al. 2004). The simultaneous occurrence of FOL with RKN, caused a wilt disease complex that results in greater damage to the host plant than either pathogen can cause alone (McGawely 2001; Hajji et al. 2016).
Often, management of plant diseases and pathogens is carried out by using chemical pesticides. Indiscriminate use of synthetic pesticides for pathogens control can lead to phytotoxicity, environmental pollution and pathogens resistance. Furthermore, the tomato monoculture enhanced the selection of virulent pathogens (Regaieg and Horrigue-Raouani 2008; Liu et al. 2013). The challenge of RKN and FOL management stimulated the research in biological control (Sikora 1992; Fravel et al. 2003).
Bio-pesticides from natural plant products could act as an alternative to chemical pesticides to control plant pathogens and will play a significant role in sustainable agriculture in the future. Several plants have been tested for their bio-control potential and different plant compounds have shown biocidal activities such as essential oils, terpenoids, lipids, sterols, alkaloids, flavonones and polyketides (Caboni and Ntalli 2014; Isman 2015).
Pistacia lentiscus L. belongs to the Anacardiaceae family and is commonly known as mastic tree, it has been used for treating various kinds of human and plant diseases. Its various plant parts contain a variety of chemical constituents that have medicinal importance such as resins, essential oils, gallic acid, anthocyanins, flavonol glycosides, nortriterpenoids, α-tocopherol and arabino-galactan proteins (Nahida et al. 2012). In previous works, the plant extract of P. lentiscus was already tested and confirmed to have antimicrobial (Bonsignore 1998) and antifungal (Kordali et al. 2003; Benhammou et al. 2008) activities, although no nematicidal effect was confirmed.
The present study aimed to characterize the phytochemical composition and the antioxidant potential of P. lentiscus dried leaves. Laboratory trials were carried out to test the nematicidal and fungicidal potential of extracts obtained from P. lentiscus. Finally, additional trials were performed to evaluate the influence of selected extract concentrations on the growth of tomato plants and the potential control of the wilt disease complex when compared to chemical pesticides.
Material and methods
Acquisition of nematode inoculum
Meloidogyne javanica (Treub) Chitwood egg masses, previously identified with perineal and isozyme patterns techniques were obtained from the roots of greenhouse grown tomato plants (Solanum lycorpersicum Mill.) ‘Riogrande’. Eggs masses were handpicked, collected from galled roots, and incubated at 27 ± 2 °C for three days to obtain second stage juveniles (J2) (Hussey and Barker 1973). Two stock suspensions were prepared with the final concentrations of 100 eggs and 20 J2 per ml, respectively.
Acquisition of Fungal inoculum
Fusarium oxysporum isolate “FOL14” was used in this study, it was originally isolated from severe wilted tomato plants in the centre of a Tunisian greenhouse and was previously tested to verify its pathogenicity in susceptible tomato cultivar ‘Riogrande’. The isolate was identified morphologically under the stereomicroscope according to Leslie and Summerell (2006) and by sequencing of the 18S ribosomal RNA (rRNA). A monoconidial culture was prepared by cultivation of a single conidia in PDA. Thus, after growth, it was stored in glycerol at −20 °C until usage in the experiments. The pot experiment inoculum was prepared by adding FOL mycelia discs into Erlenmeyer containing 200 ml of potato dextrose broth (PDB) amended with streptomycin sulphate (200 μg/ml) and incubated for 7–10 days at 25 ± 2 °C with a regular agitation to obtain uniform growth fungus. Spore concentration was measured by counting an aliquot in Hemocytometer chamber, being adjusted to 3 × 106 spores/ml. Ten ml of the spore suspension were poured into two holes to a 2–3 cm depth near the plant root system.
Preparation of P. lentiscus extract
Fresh and healthy leaves of P. lentiscus were collected in the mountains of the Kairouan region, Tunisia and were washed with tap water followed by sterile water. The samples were dried in the shade (35 °C) for seven days. A suspension was prepared by grinding 10 g of dried leaves in 100 ml distilled water using a pestle and mortar. The mixture was left standing for 48 h and the aqueous extract was filtered through a Whatmann®filter (N°1) and the filtrate was used immediately for in vitro and/or pot experiments. The obtained extract (10 g/10 ml) was considered the undiluted (or concentrated) dose (C5 = 100%). After this procedure, dilutions were prepared by adding distilled water to result in 75% (C4), 50% (C3), 25% (C2) and 10% (C1) extract. Distilled water was used as control (C0).
Phytochemical analysis
Sample preparation
The dried plant materials were grinded in a mortar. Thus, 1.5 g of leaves fine powder was mixed into 5 ml of absolute methanol by using magnetic stirrer for 30 min. This mixture was kept at 4 °C for 24 h in the dark. The methanolic extract was filtered through Whatmann paper N°1 and stored at 4 °C in a freezer until subsequent analysis (Dewanto et al. 2002).
Determination of total phenolic contents
The total phenolic content (TPC) was determined using Folin-Cicalteu reagent as described by Singleton and Rossi (1965). For this purpose, a 40 μl aliquot of methanolic extract was added 200 μl of the Folin reagent and 3.16 ml of bi-distilled water. The mixture was vortexed and incubated in the dark at room temperature for 3 min. Then, it was added to the mixture 600 μl of sodium carbonate (Na2CO3, 20%). After being incubated for 30 min at 40 °C, the absorbance was determined by spectrophotometer at 765 nm. The samples were analyzed in triplicate. TPC of dried leaves was expressed as milligram of gallic acid equivalents (GAE) per gram of dry weight (mg GAE/g DW) through the calibration curve with gallic acid (0–500 μg/ml range).
Determination of antioxidant activity
The antioxidant activity was determined by measuring the scavenging of the 1,1- Diphenyl-2-picryl-hydrazyl (DPPH) stable free radical. An aliquot of 210 μL of the plant extract was mixed into 1940 μL of DPPH solution (6 mg of DPPH in 25 ml of methanol). Then, the mixture were homogenized with a vortex for 45 min in darkness. The absorbance was determined by spectrophotometer at 517 nm and compared with methanol (control). The samples were analyzed in triplicate. The calibration curve with ascorbic acid (standard) was determined by preparing different concentrations (1 mg/ml) and diluting them using distilled water. The DPPH value of sample was compared with the scavenging effect of the control. The results were calculated according to this equation: DPPH scavenging effects = (AC-AS); where AC is the absorbance of control and AS is the absorbance of the sample. The values were expressed in mg of sample/ml (Brand-Williams et al. 1995).
Determination of total flavonoids
The total flavonoids content (TFC) was determined as described by Lamaison and Carnat (1990). An aliquot (1 ml) of the sample was added into 1 ml of freshly prepared AlCl3 solution (6H2O, 20%). After incubation for 40 min, the absorbance was determined by spectrophotometer at 430 nm. All samples were analyzed in triplicate. The result was expressed as mg quercetin equivalents (mg QE/g DW), through the calibration curve of quercetin (0–500 μg/mL range).
HPLC-DAD for phenolic profile determination
The analysis of phenolic compounds in the tested aqueous extracts obtained from dried leaves of P. lentiscus was performed by using a Shimadzu UFLC XR system (Kyoto, Japan), equipped with a SIL-20AXR auto-sampler, a CTO-20 AC column oven, a LC-20ADXR binary pump and a quadripole 2020 detector system. This instrument was equipped with a Inertsil ODS-4 C18 3 μm column (L150 × 3.0 mm i.d.). The column temperature was set at 40 °C and the injection volume was 5 μl with a flow rate of 0.5 ml/min. The plant were extracted by taking 5 g of dry powder and immersing into 100 ml distilled water for 48 h. After this, the suspension was filtrated was subjected to separation by HPLC with the following: (H2O (95%), Methanol (5%), acetic acid (0.15%) and (acetonitrile (50%), H20 (50%), folic acid (0,1%)) were used as mobile phases A and B, respectively. The phenolic content were identified comparing the retention time of the control. All samples were analyzed in triplicate and the results were presented as the mean values with standard deviation.
Laboratory test
Evaluation of nematicidal activity
In vitro bioassays were performed in sterilized 5 cm Petri dishes. The test of J2 mortality was performed by transferring 100 J2 (200μl) into 5 ml of aqueous extract C1, C2, C3, C4 and C5. As described for J2 mortality assays, the same volume of the extracts (C1 - C5) was used in the ovicidal assays, by adding the egg suspension (100 μl containing 100 eggs of M. javanica) into 5 ml of the extract. Distilled water was used as untreated control. For each treatment, five repetitions were used. Regardless of the experiment, the J2/eggs were incubated in darkness at 25 ± 3 °C.
In the J2 mortality assay, the J2 were counted daily for mortality and non-mortality under a stereoscopic microscope (X100) after 24, 48, and 72 h. Nematodes were considered dead if they were immobile even after mechanical stimulation with a needle. After 72 h, the nematodes were transferred to distilled water for 24 h and counted. The net J2 mortality rate (%) was calculated for each time, using the formula as described by Abbott’s (1925).
In the ovicidal assay, the number of hatched J2 were daily counted starting 24 h after treatment. Thus, the analyses were carried out through 7 days. Test solutions were discarded after counting the number of hatched juveniles and freshly made solutions replaced them, being added in the 50 mm Petri dishes. The number of hatched J2 was counted under the light microscope and the hatching percentage was calculated for 7 days post exposure. The relative egg hatching rate was determined in relation to the egg hatching percentage at the untreated controls.
Evaluation of antifungal activity
The antifungal activity was determined by following the poisoned food method (Nene and Thapliyal 1993). First, PDA was prepared and autoclaved during 1 h at 120 °C. Then, 20 ml of the cooled PDA (45 °C) were added into 1 ml of each aqueous leaf extracts (C1, C2, C3, C4 and C5) and placed on sterilized 9 cm Petri dish. When the media was solid, an FOL mycelia plug (5 mm in diameter) of 7 days old culture grown on PDA, was inoculated in the centre of each Petri dish.
Each treatment had five replicates. After incubating for 7 days at 25 ± 3 °C, radial mycelia growth of FOL was measured. The percent of growth inhibition over the control was determined according to Janssen et al. (1986): Inhibition growth (%) (I) = (1-Da/Db)*100.
Where: Da and Db are the diameter of the growth zone of FOL (mm) for the tested extract concentrations and control, respectively. The growth rate of mycelium (Vc) (7 days) (mm/h) was calculated according to (Mohammedi 2005).
For spore germination, 100-ml flasks containing 4.5 ml PDB were amended with 0.5 ml of several concentrations of the leaf extracts of P. lentiscus or distilled water, which was used as control. To each flask, a PDA disc of 5 mm diameter containing FOL mycelium was added to the medium. Each treatment was replicated five times and the flasks were incubated on an rotary shaker at 150 rpm and 25 ± 2 °C. After 24 h of incubation, a drop of spore suspension mixed with lacto phenol cotton blue was examined under the light microscope at 40x magnification to count the number of germinated and non-germinated spores. The percentage of spore germination (SG) was calculated.
Pot experiment
Pot experiments were conducted under greenhouse conditions at the Higher Agronomic Institute of Chott-Meriem, Sousse University, Tunisia. Tomato seeds ‘Riogrande’ were sterilized in hydrochloric acid (5%) for 5 min, washed 3 times with sterile distilled water and then sown in trays containing 100 individual wells filled with peat at one seed per well. The trays were then placed on benches in a glasshouse for germination until transplanting.
After four weeks plants were at the fourth true leaf stage and similar tomato seedlings were selected and transplanted to plastic pots (12 cm diameter) containing 1 l of sterilized substrate (sand, soil and peat, 1:1:1 (w/w/w)). Thus, one single plant was kept for each pot and used for the trial. One week after transplanting, the plants were inoculated with freshly hatched J2 of M. javanica by pipeting 2 ml containing 1500 J2 into two holes around the tomato plant (750 J2/hole). At the same time, the tomato plants were inoculated with FOL by pouring the spore suspension into two holes at 2–4 cm deep around root system. A chemical pesticide treatment for each pathogen was included for comparison with the efficacy of the extracts; For M. javanica, it was used the nematicide Mocap (active ingredient ethoprophos) at 10 kg/ha, corresponding to 0.5 g per pot. For FOL, it was used the fungicide Tachigazole 30% (active substance hymexazol) at 20 ml/10 l of water, corresponding to 0.15 ml per pot. Considering the results of in vitro experiment, the concentrations C3, C4 and C5 were chosen for pot experiment. Pots were divided into several treatments as described in Table 1. All treatments were arranged in a completely randomized design with six replicates. Plants were irrigated regularly and grown in the greenhouse under controlled conditions of temperature (27 ± 3 °C) and relative humidity (65 ± 5%) for 60 days after inoculation.
The tomato growth was recorded by measuring the shoot and root length and the fresh weight at the end of the experiment. The degree of FOL disease incidence and the RKN severity were determined. Roots were washed under running tap water and the gall index was estimated according to the 0–5 scale suggested Hussey and Janssen (2002); where 0 = no galling; 1 = trace infection with few small galls; 2 = ≤ 25% roots galled; 3 = 26 to 50%; 4 = 51 to 75%; and 5 = >75% roots galled. The final population density of M. javanica was assessed by extracting nematodes from the soil and root of each plant by decantation, sieving and double centrifugation flotation technique according to the modified method described by De Grisse (1969). The reproduction factor (RF) of M. javanica was calculated (final population/initial population) (Seinhorst 1967). The incidence of FOL was based in an index of Fusarium wilt (IF) on a scale of 0–4 and disease incidence (%) calculated per tomato plant according to Song et al. (2004) as described below:
Data analysis
All experiments were repeated twice with a two week interval and were performed with a completely randomized design. The presented data were the means of the two experiments. Data obtained from both experiments were subjected to one way analysis of variance using statistical package for social sciences (SPSS) version 20. Treatment means were separated and compared using the Tukey’s multiple range test at p ≤ 0.05. The bioassays data were analyzed by Probit regression to obtain the LD values.
Results
Phytochemical dried leaves composition
According to the results obtained for TPC, TFC and antioxidant activity, the dried leaves of P. lentiscus contained a notable levels of polyphenols, flavonoids and showed high antioxidant activities (Table 2). Total phenol content reached 406.52 μg GAE g of dried weight. Additionally, TFC reached 18,743.09 μg QE g of dried weight. Dried leaves of P. lentiscus exhibited 62.15% of DPPH radical scavenging activity (Table 2).
The overall chemical composition of P. lentiscus extract allowed the identification of a number of phenolic acids, mainly represented by quinic acid and gallic acid (Table 2). Additionally, the quercetin was the main flavonoid compound found. Nevertheless, other phenolic acids were present in the analyzed leaves of P. lentiscus such as protocatchuic, chlorogenic, 4–0-caffeoylquinic and p-coumaric acids with low rates.
Effect of P. lentiscus extract on juvenile mortality of M. javanica
In Table 3 is summarized the data obtained from the effect of aqueous leaf extracts of P. lentiscus on RKN juveniles mortality at different time points. All concentrations of the extracts had a significant positive effect on J2 mortality. Furthermore, the effect was dosage dependant with the higher concentration having the higher mortality. C3, C4 and C5 were more effective, and after 72 h of exposure, the J2 mortality rate were above 95%. In comparison to this, the control value for J2 mortality was 17.35%. After 24 h of exposure, juvenile mortality rate of 83% was obtained with C3 concentration. The calculated lethal doses LD50 and LD90 of P. lentiscus leaf aqueous extracts on M. javanica J2 were 7.727 μg/ml and 43.752 μg/ml respectively.
Effect of P. lentiscus extract on egg hatching of M. javanica
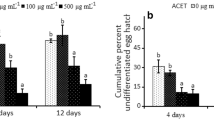
The data obtained regarding the efficacy of P. lentiscus leaf extracts on M. javanica egg hatching are shown in Table 4. The aqueous extracts inhibited significantly the egg hatching rate when compared with the control (C0), which reached 99.17% at 7 days after exposure. Egg hatching decreased significantly with the increasing concentrations of P. lentiscus aqueous extracts. Among the extracts, the lowest level obtained for the relative egg hatching rate compared with control was recorded with C5 (38.19%). On the other hand, the highest relative rate of M. javanica egg hatching was registered with C1 (85.50%). During the seven days of exposure to aqueous extract of P. lentiscus, the concentrations C3 and C4 inhibited the egg hatching.
Effect of P. lentiscus extract on growth and spore germination of Fusarium oxysporum f. sp. lycopersici
The inhibition rate of Fusarium wilt growth increased progressively with the increase of the extract concentrations (Table 5). The highest fungal growth inhibition obtained was observed in the higher concentration (C5) and reached about 83%. The fungal growth rate (VC) increased proportionally with decreasing aqueous extract concentrations ranging from 0.37 to 0.98 mm/h for C5 and C1, respectively. Additionally, results showed that all concentrations of aqueous leaf extracts inhibited germination of FOL spores and that inhibition increased with increasing concentration of the extracts. The concentrations C3, C4 and C5 presented negative effects, with the lowest value being obtained for C5 and the highest value obtained for C1. The control presented a 94.06% value.
Effect of aqueous extract of P. lentiscus on tomato growth
The data obtained from plant growth assessment presented on Table 6 showed that the Fusarium wilt and root-knot nematode complex disease decreased plant growth when compared to control tomato plants. Overall, M. javanica seemed to have the most considerable effect on tomato growth in case of individual inoculation or in combination with FOL when compared to single inoculation by FOL. The tomato plant height was reduced by the inoculation with M. javanica (34.63 cm) followed by M.j + FOL (37.93 cm) more than single infection by pathogenic fungus (41.62 cm) and control plants (43.60 cm).
The aqueous extracts concentrations and chemical treatments slightly enhanced the plant growth when comparing to the inoculated plants with both pathogens or separately by M. javanica and FOL. All concentrations of P. lentiscus extracts and chemical treatments improved the plant height and weight as compared to inoculated plant with pathogens. In case of single inoculation by M. javanica and combined with FOL, the root weight decreased with chemical and plant extract treatments when compared with galled tomato roots. However, the root length increased similarly by the chemical treatment and all concentrations as compared with separate or combined infected plants by pathogens (Table 6).
Effects on root galling and RF of Meloidogyne javanica
The gall index was significantly lower in all treatments when compared to the untreated plants inoculated with RKN (3.33) or co-infected with FOL (3.50). The three selected concentrations of aqueous leaf extracts reduced significantly the reproduction factor of root-knot-nematode (C5: 15.61, C4: 15.11, C3:17.44) followed by the chemical nematicide (27.49) in plants infested only with M. javanica. The co-infected tomato plants showed higher RF than those inoculated only with nematode. All tested extract concentrations and chemical nematicide showed a reduction on nematode reproduction when plants were co-infected by both pathogens (Table 7).
Effect on F. oxysporum f.sp. lycopersici development
Both, hymexazol and P. lentiscus aqueous extracts reduced significantly the disease incidence in the infected tomato seedlings with FOL 60 days after inoculation. The concentration C5 and the chemical fungicide reduced the wilt index and disease incidence of FOL when compared with inoculated seedlings with the fungus alone or combined with the nematode. The concentrations C4 and C3 of P. lentiscus aqueous extract also reduced the development of FOL, but at lower rates than the C5 concentration (Table 8).
Discussion
The results of the present research indicates that aqueous extracts of P. lentiscus leaves affected the growth and spore germination of the pathogenic fungus, FOL. The inhibitory effect of the plant extracts could be attributed to the presence of some antifungal toxicants. Furthermore, P. lentiscus aqueous extract showed an inhibitory effect against root-knot nematode which also could be explained by nemato-toxic properties of the tested plant. The classes of constituents identified previously in P. lentiscus which could explain the nematicidal and antifungal activities were the flavonoids (Romani et al. 2002), triterpenoids (Marner et al. 1991), phenolics mainly gallic acid and galloyl derivatives (Abdelwahed et al. 2007) and essential oils (De Pooter et al. 1991).
Considering the results of the chemical screening, the dried leaves of P. lentiscus contained phenolic compounds and flavonoids which exhibited high antioxidant ability. The suppressive effect of phytochemical compounds as flavonoids and phenols on plant parasitic nematodes and soil-borne fungi has been well reported in several pathosystems (Sukul 1992; Chitwood 2002; Edeoga et al. 2005).
The higher concentration of leaf extracts had an inhibitory action against nematode egg hatching. Thus, egg hatching was maximum after 72 h with the lowest concentration C1. Exposure time played an important role on nematode juvenile mortality and the fungal growth inhibition. According to Adegbite and Adesiyan (2005), the effect of exposure time on plant extracts and the concentrations affected the antagonistic activity. They reported that egg inhibition and juvenile mortality of root-knot nematodes decreased with an increase of extracts dilutions of all tested plants and increased with exposure time.
The results also indicated that P. lentiscus aqueous extracts treatments reduced the growth inhibition caused by F. oxysporum f.sp. lycopersici and / or Meloidogyne javanica on tomato plants. The promotional effect of P. lentiscus aqueous extract on tomato seedlings growth could be due to triterpene compounds. The plant growth promoting role of triterpene was previously reported by Srivastara (2000) who explained the effect of this plant secondary product by its role in primary processes such as photosynthesis, stability of cell membranes and as source compounds for several plant hormones mainly the steroids.
The significant reduction of wilt disease complex caused by M. javanica and FOL on tomato plants treated with P. lentiscus extract could be also due to the presence of chemical components, which have fungicidal and nematicidal proprieties. The potential of using plant extracts in controlling plant parasitic nematodes has been documented by several authors (Sosamma and Jayasree 2002; Caboni and Ntalli 2014). Additionally, several plant compounds are known to have antifungal properties such as phenolics, terpenoids, saponins, and alkaloids (Kordali et al. 2003; Copping and Duke 2007).
The dual effect of plants on pest control and plant growth promotion showed by P. lentiscus extracts was reported with previous study of Abbasi et al. (2008) who reported the plant growth and root-knot nematode control by Barleria acanthoides extracts on Okra and Brinjal. Other research reported that aerial plant extracts of several plants were efficient in controlling Meloidogyne incognita (Kofoid & White) Chitwood, and had growth promotion effects of cacao plants (Keniyi et al. 2010). Furthermore, recent studies showed that plant extracts boosted the plant growth and controlled the disease complex caused by root-knot nematode-Fusarium oxysporum (Hadian et al. 2011; El-Shennawy and Abo-Kora 2016).
Conclusion
In conclusion, the data presented in this paper showed the potential of biocidal activities of P. lentiscus for management of FOL and M. javanica in laboratory and pot experiment. These biological activities (nematicide and fungicide) could be related to high levels of flavonoîds, phenols and antioxidant activity. Considering these results, P. lentiscus could be a promoting component of new biopesticide on integrated approach which appears to be the most promising way of defeating disease complexes involving nematodes and soil-borne fungi.
References
Abbasi, W. M., Ahmed, N., Zaki, J. M., & Shaukat, S. S. (2008). Effect of Barleria acanthoides Vahl on root-knot nematode infection and growth of infected okra and brinjal plants. Pakistan Journal of Botany, 40(5), 2193–2198.
Abbott, W. S. (1925). A method of computing the effectiveness of an insecticide. Journal of Economic Entomology, 18, 265–267.
Abdelwahed, A., Bouhlel, I., Skandrani, I., Valenti, K., Kadri, M., & Guiraud, P. (2007). Study of antimutagenic and antioxidant activities of gallic acid and 1, 2, 3, 4, 6-pentagalloylglucose from Pistacia lentiscus: Confirmation by microarray expression profiling. Chemico-Biological Interaction, 165(1), 1–13.
Adegbite, A. A., & Adesiyan, S. O. (2005). Root extracts of plants to control root-knot nematode on edible soybean. World Journal Agricultural Sciences, 1(1), 18–21.
Benhammou, N., Atik Bekkara, F., & Panovska, T. K. (2008). Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. African Journal of Pharmacy and Pharmacology, 2, 22–28.
Bonsignore, L. (1998). Antibacterial activity of Pistacia lentiscus aerial parts. Fitoterapia, 69, 537.
Borrero, C., Trillas, M. I., Ordovás, J., Tello, J. C., & Avilés, M. (2004). Predictive factors for the suppression of Fusarium wilt of tomato in plant growth media. Phytopathology, 94(10), 94–101.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. Lebensmittel-Wissenschaft und -Technologie /Food Science and Technology, 28, 25–30.
Caboni, P., & Ntalli, N. G. (2014). Botanical Nematicides, recent findings. In C. et Al (Ed.), Biopesticides: State of the art and future opportunities (pp. 145–157). Washington DC: American Chemical Society.
Chitwood, D. J. (2002). Phytochemical based strategies for nematode control. Annual Review of Phytopathology, 40, 221–249.
Copping, L. G., & Duke, S. O. (2007). Natural products that have been used commercially as crop protection agents – A review. Pest Management Science, 63(6), 524–554.
De Grisse, A. T. (1969). Redescription ou modification de quelques techniques utilisée dans l’étude des nématodes phytoparasitaires. Mededelingen Rijksfaculteti der Landbouveten Gent, 351–369.
De Pooter, H. L., Schamp, N. M., Aboutabl, E. A., El Tohamy, S. F., & Doss, S. L. (1991). Essential oils from the leaves of three Pistacia species grown in Egypt. Flavour and Fragrance Journal, 6, 229–232.
Dewanto, V., Wu, X., Adom, K. K., & Liu, R. H. (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry, 50, 3010–3014.
Edeoga, H. O., Okwu, D. E., & Mbaebie, B. O. (2005). Phytochemical constituents of some Nigeria medicinal plants. African Journal of Biotehnology, 4(7), 685–688.
El-Shennawy, M. Z., & Abo-Kora, M. S. (2016). Management of wilt disease complex caused by Meloidogyne javanica and Fusarium oxysporum f.sp. lycopersici on tomato using some plant extracts. J. Plant Prot. and Path., 7(12), 797–802.
Fravel, D. R., Olivan, C., & Alabouvette, C. (2003). Fusarium oxysporum and its biocontrol. New Phytologist, 157(3), 493–502.
Hadian, S., Rahnama, K., Jamali, S., & Eskandari, A. (2011). Comparing neem extract with chemical control on Fusarium oxysporum and Meloidogyne incognita complex of tomato. Advances in Environmental Biology, 5(8), 2052–2057.
Hajji, L., Regaieg, H., M’Hamdi-Boughalleb, N., & Horrigue-Raouani, N. (2016). Studies on disease complex incidence of Meloidogyne javanica and Fusarium oxysporum f.sp. lycopersici on resistant and susceptible tomato cultivars. Journal of Agricultural Sciences and Food Technology, 2(4), 41–48.
Horrigue-Raouani N (2003) Variabilité de la relation hôte parasite dans le cas des Meloidogyne spp. (Nematoda: Meloidogynidae). Thèse de docteur d’état. Université Tunis-El Manar, Faculté des Sciences de Tunis, Tunisie, pp. 222.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula for Meloidogyne spp., including a new technique. Plant Disease Reporter, 57, 1025–1028.
Hussey, R. S., & Janssen, G. J. W. (2002). Root-knot nematode: Meloidogyne species. In J. L. Starr, R. Cook, & J. Bridge (Eds.), Plant Resistance to Parasitic Nematodes (pp. 43–70). Wallingford, UK: CAB International.
Isman, M. B. (2015). A renaissance for botanical insecticides? Pest Management Science, 71, 1587–1590.
Janati, S., Houari, A., Wifaya, A., Essarioui, A., Mimouni, A., Hormatallah, A., et al. (2018). Occurrence of the root-knot nematode species in vegetable crops in Souss region of Morocco. The Plant Pathology Journal, 34(4), 308–315.
Janssen, A. M., Scheffer, J. J. C., & Baerheim, S. A. (1986). Antimicrobial activity of essential oils: A 1976-86 literature review. Aspect of test methods. Planta Medica, 53, 395–398.
Keniyi, M. O., Fademi, O. A., Orisajo, S. B., Adio, S. O., Otunoye, H. A., & Adekunle, O. V. (2010). Effect of botanical extracts on root-knot nematode (Meloidogyne incognita) infection and growth of cacao seedlings. Journal of Applied Biosciences, 36, 2346–2352.
Kordali, S., Cakir, A., Zengin, H., & Duru, M. E. (2003). Antifungal activities of the leaves of three Pistacia species grown in Turkey. Fitoterapia, 74, 164–167.
Lamaison, J. L., & Carnat, A. (1990). Teneurs en principaux flavonoids des fleurs de Crataegeus monogyna Jacq et de Crataegeus laevigata (Poiret D.C) en fonction de la végétation. Pharmaceutica Acta Helvetiae, 65(11), 315–320.
Leslie, J. F., & Summerell, B. A. (2006). The Fusarium laboratory manual. Iowa: Blackwell Publishing Ltd.
Liu, S., Wu, F., & Wen, X. (2013). Allelopathic effects of root exudates of Chinese onion on tomato growth and the pathogen Fusarium oxysporum (Sch1) f.sp. lycopersici. Allelopathy Journal, 31(2), 387–403.
Marner, F. J., Freyer, A., & Lex, J. (1991). Triterpenoids from gum mastic, the resin of Pistacia lentiscus. Phytochemistry, 30, 3709–3712.
McGawely, E. C. (2001). Disease complex. In O. C. Maloy & T. D. Murray (Eds.), Encyclopedia of Plant Pathology (pp. 326–330). USA: John Wiley & Sons.
Moens M, Perry RN, Starr JL (2009) Meloidogyne species – A diverse Group of Novel and Important Plant Parasites In: Perry RN, Moens M, Starr JL (2009) Root-knot nematodes Wallingford Oxfordshire UK CAB International.
Mohammedi Z (2005) Etude de pouvoir antimicrobien et antioxydant des huiles essentielles et flavonoides de quelques plantes de la région de Tlemcen. Magistère. Université Abou Bakr Belkaid Tlemcen: pp 105.
Nahida, S., Ansari, H., & Siddiqui, A. N. (2012). Pistacia Lentiscus: A review on Phytochemistry and pharmacological properties. International Journal of Pharmacy and Pharmaceutical Sciences, 4, 16–20.
Nene YL, Thapliyal PN (1993) Fungicides in Plant Disease Control Oxford and IBH Publ. Co. (pp. 507). New Delhi.
Regaieg, H., & Horrigue-Raouani, N. (2008). Biological characteristics of two populations of Meloidogyne spp. virulent to the Mi resistance gene in tomato isolated from South Tunisia. The African Journal of Plant Science and Biotechnology, 2(1), 27–29.
Romani, A., Pinelli, P., Galardi, C., Mulinacci, N., & Tattini, M. (2002). Identification and quantification of Galloyl derivatives, flavonoid glycosides and anthocyanins in leaves of Pistacia Lentiscus L. Phytochemical Analysis, 13(2), 79–86.
Seinhorst, J. W. (1967). The relationships between population increase and population density in plant parasitic nematodes. II. Sedentary nematodes. Nematologica, 13, 157–171.
Sikora, R. A. (1992). Management of the antagonistic potential in agricultural ecosystems for the biological control of plant parasitic nematodes. Annual Review of Phytopathology, 30, 245–270.
Sikora RA, Fernandez E (2005) Nematode parasites of vegetables. In: Luc M, Sikora RA, Bridge J (Eds) Plant parasitic nematodes in subtropical and tropical agriculture. 2nd edition, CABI publishing: pp 319–392.
Singleton, V. L., & Rossi, J. A. (1965). Colorimetry of total phenolics with phosphor molybdic phosphotungstic acid reagents. American Journal of Enology and Viticulture, 16, 144–158.
Song, W., Zhou, L., Yang, C., Cao, X., Zhang, L., & Liu, X. (2004). Tomato Fusarium wilt and its chemical control strategies in a hydroponic system. Crop Protection, 23, 243–247.
Sosamma, V. K., & Jayasree, D. (2002). Effect of leaf extracts on the mortality of root-knot nematode, Meloidogyne incognita juveniles. Indian Journal of Nematology, 32, 183–233.
Srivastara, L. M. (2000). Plant growth and development hormones and environment. A.P. Elsevier Science, 173–174.
Sukul, N. C. (1992). Plants antagonistic to plant parasitic nematodes. Indian J L Sci, 12, 23–52.
Talavera, M., Sayadi, S., Chirosa-Ríos, M., Salmerón, T., Flor-Peregrín, E., & Verdejo-Lucas, S. (2012). Perception of the impact of root-knot nematode-induced diseases in horticultural protected crops of South-Eastern Spain. Nematology, 14(5), 517–527.
Acknowledgments
The authors are grateful to the review editor and the anonymous reviewers for their helpful comments and suggestions to improve the clarity of the research paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research does not contain any conflicts of interest, nor research involving humans or animals.
Rights and permissions
About this article
Cite this article
Hajji-Hedfi, L., Larayedh, A., Hammas, NC. et al. Biological activities and chemical composition of Pistacia lentiscus in controlling Fusarium wilt and root-knot nematode disease complex on tomato. Eur J Plant Pathol 155, 281–291 (2019). https://doi.org/10.1007/s10658-019-01770-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-019-01770-7