Abstract
Apple valsa canker (AVC), caused by Valsa mali (Vm), is a serious disease worldwide. In this study, a total of 193 actinomycete cultures were isolated from the rhizosphere of healthy wild apple trees in the Qinling Mountains in China. Six of these isolates had a strong antagonistic activity towards V. mali (inhibition rates >50%). The hyphal growth and conidial germination of V. mali were significantly inhibited by these isolates. The strain SL01 could induce the protoplasm leakage from V. mali hyphae. In a detached twig inoculation experiment, the fermentation broth of the six isolates showed significant inhibiting effects on disease development by V. mali. Among the six candidates, strain SL01 showed the best potential for biocontrol of AVC based on the inhibitory effects on hyphal growth, conidial germination and disease development by V. mali. Strain SL01 was identified as Streptomyces longissimus based on morphological, biochemical, physiological characteristics and 16S rDNA sequence analysis. In field studies, the SL01 strain showed an average of 90.3% inhibition on AVC recurrence in a two-year experiment. This study indicated that the actinomycete strain SL01 has the potential for biological control of AVC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apple, which belongs to the Rosaceae family and Pomoideae subfamily, is one of the most important fruit worldwide (Forsline et al. 2003). According to FAOSTAT (2016), the annual apple production reached nearly 89.3 million tons worldwide in 2016, and the apple production of China accounts for 49.7% of the world’s total production. Apple valsa canker (AVC), caused by the fungus Valsa mali Miyabe & G. Yamada (Wang et al. 2011), has seriously affected the development of the worldwide apple industry (Chen et al. 1987; Agrios 1997; Lee et al. 2006; Cao et al. 2009). AVC mainly occurs in eastern Asia, including China (Cao et al. 2009), Japan (Abe et al. 2007) and Korea (Lee et al. 2006). In China, according to a survey by the Apple Research & Development Center, China Agriculture Research System (Cao et al. 2009), the average disease incidence of AVC was 52.7% nationwide, and in some regions, it reached 85% in 2008. Currently, there are no effective disease-resistant apple varieties in production (Abe et al. 2007; Abe et al. 2011).
Valsa mali was usually considered to be a weakly parasitic fungus that mainly infected apple trees via pruning, wounding, sunburn, frostbite and other wounds (Willison 1936; Chen et al. 1981). The pathogen infects the host phloem and xylem of trunks, branches and twigs (Ke et al. 2013; Chen et al. 2016). Infections can take place throughout the year, and increase following winter pruning, which is a common practice used in apple production (Han 2011). The infected tissues are sagging and collapsed, eventually showing a swollen water-soaked and soft appearance.
To date, use of chemical fungicides is the most effective measure to control AVC. However, some chemical fungicides with the most effective control effect, such as asomate and other fungicides mixed with asomate, have been banned because, for example, of high toxicity and residue (Zhao et al. 2007). Application of environmentally-friendly and sustainable biocontrol agents to protect apple from AVC is a good choice. Previous studies showed a number of microorganisms screened were found to have antagonistic effects on V. mali in vitro (Deng et al. 2009; Xu et al. 2012; Wang et al. 2012; Zhang et al. 2013; Wang et al. 2014; Xin and Shang 2005; Zhang et al. 2015). However, application of the screened microorganisms for AVC control is in a preliminary stage (Li et al. 2015; Zhang et al. 2015).
The actinomycetes, a group of gram-positive bacteria, are widely distributed in soils. Actinomycetes produce abundant metabolites and antibiotics, and include strains with biocontrol activity towards plant pathogens (Abdelmohsen et al. 2014). Actinomycetes have been found that protect plants from pathogens by degrading the target fungal cell wall, producing antifungal compounds and stimulating plant induced systemic resistance. (Yuan & Crawford 1995; Doumbou et al. 2002; Getha & Vikineswary 2002; Bressan 2003; Eccleston et al. 2010; Abdelmohsen et al. 2014). They are therefore likely candidates for identifying new biocontrol agents from the environment to effectively control AVC.
The objectives of this study were to screen and identify new strains of actinomycetes from the rhizospheres of wild apple trees in the Qinling Mountains. The potential of newly isolated strains to control V. mali on apple trees was evaluated by determining the suppression of the hyphal growth, conidial germination and disease development on detached twigs. After screening, the strain showing the highest inhibitory activity to V. mali was characterized and identified, and its control efficacy against AVC was tested in the field.
Materials and methods
Isolation of actinomycete strains from soil and preliminary screening of antagonistic actinomycetes to Valsa mali.
Soil samples were collected from the rhizosphere of healthy wild apple trees in the Qinling Mountains, Shaanxi Province, China. Soil samples were air dried at room temperature, and then further dried in a drying oven at 70 °C for 2 h. One gram of each sample was added to 9 ml 0.8% NaCl solution and was cultivated in a shaker at 28 °C for 1 h. The soil solutions were serially diluted with sterile water. A volume of 50 μl 10−5, 10−6 and 10−7 soil solutions were spread onto Gause’s No. 1 agar and incubated at 28 °C for 5 days. The colonies were streaked onto new Gause’s No. 1 plates for purification. The purified colonies were coded and maintained on Gause’s No. 1 agar and stored at 4 °C.
The antagonistic ability of actinomycetes against V. mali was measured by a dual culture technique. The V. mali strain DB01 (Collection of Plant Pathology Laboratory of Northwest A & F University, Shaanxi, China) was grown on potato dextrose agar (PDA) at 25 °C for 5 days. A 5 mm agar disc from the edge of an actively growing colony was placed in the middle of a new 90-mm PDA plate. Afterwards, four discs (5 mm diameter) of 5-day-old cultures of actinomycete colonies were symmetrically placed at 2 cm away from edge of the DB01 colony. As a control, sterile Gause’s No. 1 agar discs were used at similar positions in all plates. Each treatment was repeated three times. All treatments were conducted at 25 °C. Five days later, the radial hyphal growth of V. mali towards the candidate actinomycete(Rt)and that on a control plate (Rc) were measured and the hyphal growth inhibition was calculated according to the formula: (Rc-Rt)/(Rc-0.25) × 100%. The actinomycete strains showing the strongest antifungal effect (inhibition rates >50.0%) were selected for further studies.
Suppression of hyphal growth of V. mali by fermentation broth of antagonistic actinomycetes
The effects of fermentation broth on hyphal growth of V. mali were determined using a filter paper method (Shan et al. 2010). The antagonistic strains were transferred to 100 ml of Gause’s No. 1 broth in 250 ml Erlenmeyer flasks and incubated on a rotary shaker at 180×g at 28 °C for 7 d to produce fermentation broth. A 5 mm agar disk from edge of the actively growing colony of V. mali strain DB01 was placed in the middle of a 90-mm PDA plate. Sterile filters (Φ = 8 mm) dipped with fermentation broth from antagonistic strains were symmetrically placed 2.5 cm away from the DB01 strain. All treatments were incubated at 25 °C for 5 d. Radial growth was then measured as described above. The assay was conducted with three replicates per treatment and repeated three times.
Effect of actinomycete fermentation filtrates on conidial germination in V. mali
To obtain the conidia, the V. mali colony on PDA plates were incubated for one month at 25 °C under continuous fluorescent light until golden pycnidia appeared. Conidia were harvested from pycnidia using a sterile inoculation needle. The needle point containing conidia was then stirred into 500 μl double distilled water to make a conidial suspension. The number of conidia was counted by a hemocytometer under an optical microscope (Olympus BX51), and then adjusted to the concentration of 106 conidia/ml. To prepare fermentation filtrates, the fermentation broth of the candidate antagonistic strains were transferred into 2-ml centrifuge tubes, and centrifuged at 6000×g for 10 min. The supernatants were decanted using sterilized syringes and then passed through 0.22 μm pore size filters to exclude the thallus of antagonistic strains. The cell-free fermentation filtrates were then used to test for inhibitory activity on conidial germination, using a slide method (Wang et al. 2012). The fermentation filtrates of antagonistic strains were mixed into the conidial suspension at concentrations of 50% (v/v), while the fermentation broth without antagonistic strains was added to conidial suspensions in the same proportion as the control. A 20-μl mixed solution was dropped onto 1 cm2 PDA on a glass slide using a micropipette. The glass slide was placed into a 9-cm petri dish containing 20 ml of water agar to maintain a 100% relative humidity, and the petri dish was sealed with paraffin film. All treatments were incubated at 25 °C. The glass slides were observed for conidial germination using an optical microscopy at 20 and 36 h post-inoculation (Olympus BX51). A conidium was considered germinated when the germ tube was longer than half of the length of the conidium. The number of germinated and total conidia was counted. Conidial germination rates were calculated based on examination of at least 400 conidia from a replicate. The assay was conducted with three replicates per treatment and repeated three times.
Effects of fermentation broth of antagonistic actinomycetes on disease development of detached twigs
Uniform two-year-old twigs were collected from a ‘Fuji’ apple orchard. The twigs were cut into 25 cm segments in length, immersed in 1% sodium hypochlorite solutions for 10 min for surface sterilization, and then washed with sterile water three times. The two ends of the twigs were dipped in heated liquid wax to seal the ends. V. mali inoculations were conducted by a scald - inoculation method (Zang et al. 2007). The twigs were scalded for a 6 mm - in - diameter round area at twig mid-points using a soldering iron. The concentration of prepared fermentation broth of each of six actinomycete strains was determined by dilution plating on Gause’s No. 1 agar, and then adjusted to the concentration of 106 colony forming units (CFU)/ml. For actinomycete treatments, a volume of 5-ml 106 CFU/ml fermentation broth of each strain was uniformly spread on whole twigs. For a blank treatment, twigs were applied with sterile water instead of antagonistic actinomycete fermentation broth. For a positive control treatment, a volume of 2-ml 400 μg/ml thiophanate methyl suspension (Xi’an Dingsheng biotechnology Co. Ltd., China) was spread on twigs. At 0.5 h post-inoculation, fungal disks of V. mali DB01 strain (Φ = 5 mm) were placed on the scalded area of the twigs previously treated with actinomycete fermentation broth, sterile water or thiophanate methyl suspension. The fungal disks were covered by degreasing cotton wetted with sterile water and wrapped with preservative film. The twigs were placed in an iron pallet. Sterile gauze moistened with sterile water was placed in the bottom of the iron pallet to maintain a high relative humidity (RH = 98 ± 1%). The iron pallet was wrapped with preservative film. The iron pallet with inoculated twigs was incubated at 25 ± 1 °C. At 2 days post-inoculation, the degreasing cotton and preservative film was removed. At 7 days post-inoculation, the twigs were observed for disease development. To measure the lesion areas, the epidermal layers of twigs around the inoculated sites were scraped off with a knife. The length and width of lesions was measured, and the lesion area was calculated according to the elliptical area formula:
The control effect was computed by the following equation:
Where, Sc is the lesion area of control (mm2) and St is the lesion area of treatment (mm2).
The assay was conducted with three replicates per treatment and repeated three times.
Characterization and identification of strain SL01
Actinomycete strain SL01, which showed the highest inhibitory effects against V. mali, was identified by morphological, physiological and molecular taxonomy.
Morphological and cultural characteristics of strain SL01
Strain SL01 was inoculated onto Gause’s No. 1 agar and incubated at 25 °C in darkness. After 7 days, the SL01 colonies were observed and photographed. For optical microscopy analysis, the coverslip insert method was used (Xiong et al. 2017). Sterile coverslips were inserted into Gause’s No. 1 agar at 45 degrees inclination. The actinomycete strain SL01 was inoculated at the interface between coverslips and medium, and incubated at 25 °C in darkness. At 7 d post-incubation, the coverslips containing aerial hyphae and spore chains were taken from the plates and observed by optical microscopy. For scanning electron microscopy (SEM) analysis, Gause’s No. 1 agar medium supporting SL01 growth were fixed in 3% glutaraldehyde solution for 24 h. The samples were washed three times with a 0.1 M phosphate buffer (pH = 7.0–7.2), then dehydrated in a series of aqueous-ethanol solutions (50%, 70%, 85%, 95 and 100%). Subsequently, samples were dried in a critical point dryer under CO2 and mounted with double-adhesive tape onto aluminum stubs and coated with gold. Samples were examined using a scanning electron microscope (Model S-4800, Hitachi, Japan).
Physiological characteristics and utilization of different carbon sources
Gram staining for strain SL01 was performed using aniline crystal violet and observed under an optical microscope (Burke 1922). Gelatin liquefaction, milk coagulation and peptonization, starch hydrolysis, cellulose decomposition, nitrate reduction, melanin formation and H2S production were determined according to Xu et al. (2007). Different carbon sources (2%) replaced soluble starch from Gause’s No.1 agar. Gause’s No.1 agar was used as the control and the plates were incubated in 28 °C for 5 d. The assay was conducted with three replicates per treatment and repeated three times.
Identification of strain SL01 by 16S rDNA gene sequence analysis
Molecular identification of strain SL01 was carried out using 16S rDNA sequences analysis. Genomic DNA was extracted from hyphae of strain SL01 by following the method of Hall et al. (1999). The 16S rDNA was amplified using two universal primers, 27f (5’-AGAGTTTGATCCTGGCTCAG-3′) and 1522r (5’-AAGGAGGTGATCCAGCCGCA-3′) (Hall et al. 2001). The polymerase chain reaction (PCR) mixture (25 μl) contained 2.5 μl of 10 × PCR buffer, 2.0 μl MgCl2 (25 mmol l−1), 2.0 μl dNTP mix (2.5 mmol l−1), 100 ng of template DNA, 1.0 μl each of reverse and forward primers (10 pmol μl−1), 1.0 μl Taq DNA polymerase (5 U μl−1) (Promega), and 14.0 μl of double distilled water. The PCR reaction conditions were as follows: initial denaturation was carried out for 5 min at 94 °C, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 54 °C for 1 min and extension at 72 °C for 2 min with a further 10 min extension at 72 °C. The amplified DNA was size-fractionated on a 1.5% agarose gel and then purified with TIAN gel Midi Purification Kit (TianGen Biotech Co., Ltd., Beijing, China) according to the manufacturer’s protocol and sequenced with the primers 27f (5’-AGAGTTTGATCCTGGCTCAG-3′) and 1522r (5’-AAGGAGGTGATCCAGCCGCA-3′) by Nanjing Genscript Biotech Co., Ltd., China. The 16S rDNA sequences of SL01 were compared with those available in GenBank using BLAST search at National Center of Biotechnology Information (NCBI) (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The nucleotide sequences of Streptomyces strains with more than 97% identity between SL01 and other Streptomyces sp. were used for the construction of the phylogenetic tree by the maximum likelihood method using Molecular Evolutionary Genetic Analysis (MEGA) software version 4.0 (Tamura & Nei 1993).
Field trials with Fuji apple trees
The field experiment was conducted at a Fuji apple orchard in Tongchuan City, Shaanxi Province. The orchard was under poor management, and fungicides had not been applied for 1 year before the experiment. The experimental trees were 15-years-old in 2012, and suffered seriously from AVC. Effect of SL01 on AVC recurrence was evaluated in 2012 and 2013. SL01 was prepared with 109 CFU/ml SL01 fermentation broth and 1% sodium alginate. SL01 fermentation broth was produced by the optimized SL01 fermentation method (Dai et al. 2016). To acquire optimized SL01 fermentation broth, a 50 ml optimum fermentation broth (2% millet powder, 1% yeast extract and 0.15% MgSO4·7H2O) in a 250 ml Erlenmeyer flask was inoculated with five 5-mm SL01 disks, and incubated in a constant temperature oscillator at 28 °C, 180×g for 7 days. Sodium alginate (1%) was added to SL01 fermentation broth for producing SL01 agent. The prepared SL01 agent was stored at 4 °C before use. For the field experiment, disease scabs of almost equivalent sizes (20 to 30 cm in length-diameter round or oval scabs) were selected for treatment. For SL01 treatment, a scab was scraped off with a knife to the xylem, leaving healthy tissues. A volume of 30 ml SL01 agent was uniformly spread on the scraped scab using a brush. For chemical control treatment, after shaving scabs, 30 ml of 400-μg/ml thiophanate methyl suspension (a common fungicide for control of AVC) was spread on a scab. For blank control, the shaved scabs were treated by applying 30 ml sterile water. The treatments were conducted in June 2012 and June 2013. For each treatment, five scabs with three replications from 15 trees were treated in 2012 and 15 scabs with three replications from 45 trees were treated in 2013. After 2 and 9 months post treatment, the treated scabs were observed for scab reoccurrence or healing, and the number of recurring scabs and healed scabs was recorded. Scab reoccurrence was defined as the tissues near the shaved scab became soft, collapsed and brown. A healed scab was defined as the treated tissues formed a complete callus around the shaved area. The width of the callus of healed scabs was measured at 9 months post treatment.
Statistical analyses
Data were subjected to a one-way analysis of variance (ANOVA), with a comparison of means by the Duncan’s multiple range test. This test was used to compare the significance of differences among data of hyphal inhibition, conidial germination inhibition, and disease inhibition on detached twigs and in the field. In all analyses, the level of significance was P < 0.05. Statistical data analysis was performed using the software SPSS 19.0 (Foster 2001).
Results
Isolation and antifungal activities of antagonistic actinomycetes
A total of 193 actinomycetes, isolated from soil samples, were screened for antagonistic potential against V. mali. Only 28 were found to have an antagonistic potential against V. mali, and six actinomycete strains (SL01, SF4, SF6, F46, SF3, SF2) showed a 50–69% inhibition on V. mali (Figs. 1A, B). Among these, the actinomycete strain SL01 had the strongest inhibiting effect against hyphal growth of V. mali (69.6%).
Effect of six actinomycete strains on hyphal growth of Valsa mali. A. The dual-culture assay. A colony radius is the colony length in the direction between the fungus agar disc and the actinomycete agar disc measured after 5 d at 28 °C. Means and standard deviations were calculated with data from three replicates. The same letters on each column are not significantly different (P > 0.05) according to the Duncan’s multiple comparison. B. Inhibition effects of five actinomycete strains on Valsa mali (middle) grown on the potato dextrose agar. Actinomycete strains were 2 cm away from V. mali
Effects of fermentation broth of antagonistic actinomycetes on hyphal growth of V. mali
Fermentation broth of six actinomycete strains were evaluated for effects on hyphal growth of V. mali. Compared with the V. mali hyphal growth with control broth treatment, the V. mali hyphal growth rates when treated by fermentation broth from strain SL01, SF6, SF4, F46, SF3 and SF2 were 34.1%, 39.0%, 41.5%, 46.5%, 51.2 and 54.9%, respectively (Fig. 2A). The hyphal growth of V. mali treated by fermentation broth from six actinomycete strains were significantly smaller than that treated by control broth (P < 0.05). Among them, the V. mali hyphal growth treated by fermentation broth from strain SL01 was the smallest. The V. mali hyphae grown in control broth were transparent, smooth, and tenuous (Fig. 2B). However, the hyphae of V. mali inhibited by SL01 were yellow, swollen, malformed, and ruptured (Fig. 2B). The hyphae grown in the presence of SL01 fermentation broth were twice the width of control hyphae (Fig. 2B).
A. Effect of fermentation broth from six actinomycete strains on colony growth of Valsa mali using the filter paper method on potato dextrose agar (PDA) plates. A colony radius was the colony length in the direction between the fungus agar disc and center of filter paper measured after 5 d at 28 °C. Means and standard deviations were calculated with data from three replicates. The same letters on each column are not significantly different (P > 0.05) according to the Duncan’s multiple comparison. B. The optical photomicrograph of hyphae of Valsa mali treated in the dual culture plate with strain SL01. The normal hyphae (CK) had the mean width of 2.5 μm on PDA. The hyphal malformation induced by SL01 and with mean width of 4.5 μm. White arrow indicates the malformation of hyphae. Bars = 20 μm
Effects of fermentation filtrates of antagonistic actinomycetes on conidial germination of V. mali
Fermentation filtrates of six actinomycete strains were evaluated for effects on conidial germination of V. mali. When conidial germination rates of V. mali using mock fermentation broth reached 90%, germination rates of V. mali by fermentation filtrates of six actinomycete strains were counted. The germination rates of V. mali conidia treated by fermentation filtrates from SL01, SF6, SF4, F46, SF3 and SF2 were 27.4%, 34.9%, 37.6%, 45.67%, 57.89%, and 64.33%, respectively (Fig. 3A). The germination rates of V. mali conidia treated by six actinomycete strains were significantly lower than that treated by mock filtrates (90.0%) (P < 0.05). Among them, germination rate treated by fermentation filtrates of strain SL01 was the lowest. The germination rate under the SL01 fermentation filtrate treatment was 30.4% (Fig. 3A). Conidial germination of V. mali underwent two phases, imbibition and germ tube formation. When conidia were treated with SL01 fermentation filtrates, at 20 h post treatment, 95.0% of conidia by mock treatment underwent imbibition, while only 16.7% of conidia by SL01 treatment became imbibed (Fig. 3B). At 36 h post treatment, more than 90% of conidia by mock treatment germinated, while only 27.4% of conidia with SL01 treatment germinated, and their germ tubes were much shorter than those by mock treatment (Fig. 3B).
Effect of fermentation filtrates from six actinomycete strains on conidial germination of Valsa mali. A. The germination rate of conidia on potato dextrose agar (PDA) after 36 h at 28 °C. Conidial germination is defined as the germ tube being longer than the short diameter of the conidium. Means and standard deviations were calculated with data from three replicates. The same letters on each column are not significantly different (P > 0.05) according to the Duncan’s multiple comparison. B. The optical photomicrograph of conidial germination of Valsa mali treated by the fermentation filtrate from strain SL01 on PDA agar at 28 °C. IC: imbibed conidium; C: conidium;GT: germ tube. Bars = 50 μm
Effect of fermentation broth of antagonistic actinomycetes on disease development in detached twigs
Fermentation broth of six actinomycete strains were applied on apple twigs to determine their effects on disease development caused by V. mali. The mean lesion areas in twigs treated by fermentation broth of SL01, SF6, SF4, F46, SF3 and SF2 were significantly smaller than that treated by control broth (Fig. 4A, B). The SL01 treatment reduced the mean lesion length the most (72.1%). But the SL01 treatment did not reduce the lesion size as much as the thiophanate-methyl treatment (inhibition rate = 90.4%) (Figs. 4A, B).
Effect of fermentation broth from six actinomycete strains on lesion extension of excised twigs caused by Valsa mali. The lesion area was calculated by the elliptical area formula. Means and standard deviations were calculated with data from three replicates. The same letters on each column are not significantly different (P > 0.05) according to the Duncan’s multiple comparison. Photographs were taken at 7 d after inoculation. CK was the extension of lesion size treated by sterile water. TM was the extension of lesion size treated by thiophanate-methyl
Identification of strain SL01
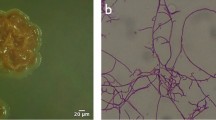
Strain SL01 produced short rod-shaped and smooth-surfaced spores, and these spore chains were long and straight (Fig. 5A, B). Substrate mycelia were bright orange and non-fragmented while aerial mycelia were white when grown on Gause’s No. 1 agar at 28 °C for 7 days, with no soluble diffusing pigments observed on the medium (Fig. 5C, D).
Morphology of strain SL01 (A-B) SEM and optical microscopy of strain SL01 grown on Gause’s No.1 agar at 28 °C for 7 d, showing long and straight spore chains and short rod and smooth-surfaced spores. (C) The bottom side of SL01 shows orange colonies grown on Gause’ s No.1 agar at 28 °C for 7 d. (D) The face side of SL01 shows dry and white colonies grown on Gause’s No.1 agar at 28 °C for 7 days
Strain SL01 was a gram-positive bacterium. Tests of gelatin liquefaction, coagulation and peptonization of milk, and hydrolysis of starch were all positive, and strain SL01 did not degrade cellulose or produce H2S. SL01 metabolized glucose, fructose, sorbose, mannitol, maltose, arabinose, galactose, and rhamnose as C sources, but not xylose and sucrose.
A phylogenetic tree was generated including 11 16S rDNA representative sequences and the SL01 sequence (GenBank accession numbers KM670435) by using the maximum likelihood method. The tree covers seven species of the genus Streptomyces according to the classification of the genus Streptomyces (Kämpfer et al. 1991). Strain SL01 sorted into the same branch with Streptomyces longissimus (AB890373.1) by 95% support rate, showing 98% sequence similarity (Fig. 6).
Phylogenetic analysis of Streptomyces generated from an alignment of 16S rDNA sequence using maximum likelihood, including strains of SL01 and other related strains from GenBank. The tree covers seven species of the Streptomyces genus according to the classification of the genus Streptomyces in 1991. Bootstrap values for each node are reported as percentages. To generate the tree, 16S rDNA sequences were aligned using Clustal W; Mega 6.0 was used to generate the phylogenetic tree with 1000 bootstraps for branch support. The scale bar at the bottom indicates genetic distance units based on Nei’s genetic distance
Based on morphological, biochemical, physiological characteristics and the 16S rDNA sequence analysis, Strain SL01 matched descriptions of S. longissimus (Shirling & Gottlieb 1968; Shirling & Gottlieb 1969; Shirling & Gottlieb 1972; Nonomura 1974). Therefore, SL01 was considered belonging to S. longissimus.
Performance of SL01 in field trials
The SL01 agent showed 100% inhibition on AVC recurrence in 2012 and 80.6% inhibition on AVC recurrence in 2013 (Table 1). Compared with the water treatment, the SL01 agents had significantly reduced the AVC reoccurrence (P < 0.05). The SL01 agent had a similar reoccurrence rate to the chemical treatments in 2012 and 2013 (Table 1). The average width (5.8 mm) of callus of non-recurrent scabs treated by SL01 treatment was significantly larger than the average width (2.1 mm) of that treated by water treatment.
Discussion
Apple valsa canker (AVC) causes enormous losses for apple growers (Wang et al. 2005; Cao et al. 2009). Many fungicides have been screened for AVC control (Wang et al. 2009; Ma et al. 2012). Using biocontrol agents to control AVC is being widely encouraged and adopted (Xin and Shang, 2005; Deng et al. 2009; Xu et al. 2012; Zhang et al. 2015). Actinomycetes are regarded as potential agents for biocontrol of plant diseases. Fermentation filtrates or fermentation broth of actinomycetes with antimicrobial activity are commonly applied to protect crops from pathogens (Prabavathy et al. 2006; Errakhi et al. 2009; El-Tarabily et al. 2009; Gopalakrishnan et al. 2011; Baz et al. 2012). In the current study, potential antibiotic-producing actinomycetes were isolated from the soils of the Qinling Natural Preserves in northwestern China. In a preliminary screening on Gause’s No. 1 plates, six actinomycete isolates with antimicrobial activity were selected from 193 actinomycete isolates for further study.
The overwintering and spreading of V. mali mainly depends on hyphae and conidia remaining in latent infected tissue (Bertrand 1976). In the current study, the fermentation broth of six actinomycete isolates had significant effects on the hyphal growth of V. mali, and the SL01 isolate had the best antagonistic effects. The fermentation broth of these six isolates may have included diffused antifungal substances which inhibited hyphal growth of V. mali insofar as there was no direct contact between the actinomycete isolates and the pathogens. The antifungal substances of SL01 could induce protoplasm leakage in V. mali hyphae. Fermentation filtrates of six isolates significantly reduced the conidial germination rate and the tube length compared with the control group. The lesion areas were greatly reduced after spreading fermentation broth of six isolates on detached twigs, and of the six isolates, strain SL01 again had the most inhibiting effect. Optimal fermentation conditions of strain SL01 have been studied to increase the antagonistic ability towards V. mali (Dai et al. 2016).
Strain SL01 was identified as Streptomyces longissimus using morphological characteristics, physicochemical properties, and carbon source utilization as well as phylogenetic analysis of 16S rDNA sequences. S. longissimus has been reported to produce hikizimycin and anthelmycin which have antifungal activity (Turner et al. 1977; Gonzalez et al. 1979; Ikemoto and Schreiber 1990). To our knowledge, the current study was the first applying S. longissimus to control plant diseases. The fermentation broth of SL01 inhibited hyphal growth of V. mali, and caused hyphal malformation and protoplasm leakage, and the fermentation filtrate of SL01 inhibited conidial germination of V. mali. The possible mechanism of SL01 involved in AVC reduction appears to be antibiosis by way of producing antifungal metabolites. Previous studies have shown that Streptomyces inhibited fungal pathogens by secreting cell wall degrading enzymes (Skujins et al. 1965; Prapagdee et al. 2008; Lee et al. 2012; Nagpure et al. 2013). Based on these studies, it is speculated that SL01 may produce fungal cell wall degrading enzymes, such as chitinase, protease and glucanase to inhibit V. mali hyphal growth.
The SL01 agent containing the SL01 optimum fermentation broth and sodium alginate was used for applying SL01 in the field. In a previous study, the antifungal activity of SL01 optimum fermentation broth was increased by 40% compared with Gause’s No.1 broth (Dai et al. 2016). In the current study, the SL01 optimum fermentation broth was used in producing the SL01 agent for field experiments. Oshiman (2000) indicated that sodium alginate was a common adjuvant of an antagonistic bacterium for prolonging the survival time of bio-control agents. SL01 fermentation broth contained living mycelia and spores in addition to antimicrobial metabolites. It is speculated that adding sodium alginate (1%) to SL01 fermentation broth may prolong the survival time of mycelia and spores. In addition, sodium alginate could increase SL01 fermentation broth viscosity so that SL01 agent adheres better to AVC lesions.
At the present, the main disease control measures for AVC are done by shaving infected tissues and applying with fungicides (Wang et al. 2009; Zhai et al. 2012). In field trails, fermentation broth of strain SL01 was spread onto shaved cankers to prevent recurrence of AVC. The sodium alginate (1%) had no effect on AVC (data not shown). The rates of reoccurrence by only shaving scabs were 73.3 and 68.7% in the two years of field trails (Table 1). Although the methods for shaving scabs could remove most hyphae in the infected tissue, many scabs reoccurred. One possible reason could be that the wounds caused by shaving the scabs were re-infected by conidia and hyphae. Moreover, the hyphae on the surface of the xylem were not removed. The method of shaving scabs combined with applying antagonistic strains because this method might be able to restrain the germination of new conidia and suppress hyphal infections.
References
Abdelmohsen, U. R., Bayer, B. K., & Hentschel, U. (2014). Diversity, abundance and natural products of marine sponge-associated actinomycetes. Natural Product Reports, 31, 381–399.
Abe, K., Kotoda, N., Kato, H., & Soejima, J. (2007). Resistance sources to Valsa canker (Valsa ceratosperma) in a germplasm collection of diverse Malus species. Plant Breeding, 126, 449–453.
Abe, K., Kotoda, N., Kato, H., & Soejima, J. (2011). Genetic studies on resistance to Valsa canker in apple: Genetic variance and breeding values estimated from intra- and inter-specific hybrid progeny populations. Tree Genetics & Genomes, 7, 363–372.
Agrios, G. N. (1997). Plant pathology. San Diego: Academic Press.
Baz, M., Lahbabi, D., Samri, S., Val, F., Hamelin, G., Madore, I., Bouarab, K., Beaulieu, C., Ennaji, M. M., & Barakate, M. (2012). Control of potato soft rot caused by Pectobacterium carotovorum and Pectobacterium atrosepticum by Moroccan actinobacteria isolates. World Journal of Microbiology and Biotechnology, 28, 303–311.
Bertrand, P. F. (1976). Release and dispersal of conidia and ascospores of Valsa leucostoma. Phytopathology, 66, 987–991.
Bressan, W. (2003). Biological control of maize seed pathogenic fungi by use of actinomycetes. BioControl, 48, 233–240.
Burke, V. (1922). Notes on the gram stain with description of a new method. Journal of Bacteriology, 7, 159–182.
Cao, K. Q., Guo, L., Li, B. H., Sun, G. Y., & Chen, H. J. (2009). Investigations on the occurrence and control of apple canker in China. Plant Protection, 35, 114–117.
Chen, C., Wang, J., Shi, X., & Li, M. (1981). Colonized position of Valsa mali on bark apple branch and infection entrance. Plant Protection, 7, 19–20.
Chen, C., Li, M. N., Shi, X. Q., & Wang, J. Y. (1987). Studies on the infection period of Valsa mali, the causal agent of apple tree canker. Acta Phytopathologica Sinica, 17, 65–68.
Chen, C., Dong, X. L., Li, B. H., Lian, S., Liang, W. X., & Wang, C. X. (2016). Effects of temperature, humidity, and wound age on Valsa mali infection of apple shoot pruning wounds. Plant Disease, 100, 2394–2401.
Dai, P. B., Lan, X. J., Zong, Z. F., & Wang, Y. (2016). Optimization of fermentation conditions for antifungal substance production of Streptomyces longissimus SL01 and its stability evaluation. Chinese Journal of Pesticide Science, 18, 729–737.
Deng, Z. S., Zhao, L. F., Zhang, W. W., Ji, Y. L., & Wei, G. H. (2009). Isolation of endophytic fungi from Ginkgo biloba L. and their antagonism on the Valsa mali Miyabe et Yamada. Acta Botanica Boreali Occidentalia Sinica, 29, 608–613.
Doumbou, C. L., Hamby Salove, M. K., Crawford, D. L., & Beaulieu, K. (2002). Actinomycetes: Promising tools to control plant diseases and to promote plant growth. Phytoprotection, 82, 85–102.
Eccleston, K. L., Brucks, P. R., & Kurtböke, D. I. (2010). Assessment of the role of local strawberry rhizosphere-associated Streptomycetes on the bacterially induced growth and Botrytis cinerea infection resistance of the fruit. Sustainability, 2, 3831–3845.
El-tarabily, K. A., Nassar, A. H., Hardy, G. E., & Sivasithamparam, K. (2009). Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. Journal of Applied Microbiology, 106, 13–26.
Errakhi, R., Lebrihi, A., & Barakate, M. (2009). In vitro and in vivo antagonism of actinomycetes isolated from Moroccan rhizospherical soils against Sclerotium rolfsii: A causal agent of root rot on sugar beet (Beta vulgaris L.). Journal of Applied Microbiology, 107, 672–681.
FAOSTAT (2016). http://www.fao.org/faostat/en/#data/QC.
Forsline, P.L., Aldwinckle, H.S., Dickson, E.E., Luby, J.J., & Hokanson, S. (2003). Collection, maintenance, characterization and utilization of wild apples of Central Asia. In: Janick, J., Forsline, P., Dickscon, E., Way, R., Thompson, M. (Eds.), Wild Apple and Fruit Trees of Central Asia, Hortic. Rev., 29, 1–61.
Foster, J. 2001. Data analysis using SPSS for windows versions 8–10: new edition.
Getha, K., & Vikineswary, S. (2002). Antagonistic effects of Streptomyces violaceusniger strain G10 on Fusarium oxysporum f. Sp. cubense race 4: Indirect evidence for the role of antibiosis in the antagonistic process. Journal of Industrial Microbiology & Biotechnology, 28, 303–310.
Gonzalez, A., Vazquez, D., & Jimenez, A. (1979). Inhibition of translation in bacterial and eukaryotic systems by the antibiotic anthelmycin (hikizimycin). Biochimica et Biophysica Acta (BBA)-Nucleic Acids and Protein Synthesis, 561, 403–409.
Gopalakrishnan, S., Kiran, B. K., Humayun, P., Vidya, M. S., Deepthi, K., Jacob, S., Vadlamudi, S., Alekhya, G., & Rupela, O. (2011). Biocontrol of charcoal-rot of sorghum by actinomycetes isolated from herbal vermicompost. African Journal of Biotechnology, 10, 18142–18152.
Hall, V., O’Neill, G. L., Magee, J. T., & Duerden, B. I. (1999). Development of amplified 16s ribosomal DNA restriction analysis for identification of Actinomyces species and comparison with pyrolysis-mass spectrometry and conventional biochemical tests. Journal of Clinical Microbiology, 37, 2255–2261.
Hall, V., Lewis-Evans, T., & Duerden, B. I. (2001). Identification of Actinomyces, Propionibacteria, lactobacilli and Bifidobacteria by amplified 16S rDNA restriction analysis. Anaerobe, 7, 55–57.
Han, Z. (2011). Dwarf-root-stock apple trees cultivation: Theory and practice. Beijing: Science Press.
Ikemoto, N., & Schreiber, S. L. (1990). Total synthesis of the anthelmintic agent hikizimycin. Journal of the American Chemical Society, 112, 9657–9659.
Kämpfer, P., Kroppenstedt, R. M., & Dott, W. (1991). A numerical classification of the genera Streptomyces and using miniaturized physiological tests. Journal of General Microbiology, 137, 1831–1891.
Ke, X. W., Huang, L. L., Han, Q. M., Gao, X. N., & Kang, Z. S. (2013). Histological and cytological investigations of the infection and colonization of apple bark by Valsa mali, var. Mali. Australas. Plant Path., 42, 85–93.
Lee, D. H., Lee, S. W., Choi, K. H., Kim, D. A., & Uhm, J. Y. (2006). Survey on the occurrence of apple disease in Korea from 1992 to 2000. Plant Pathol Journal, 22, 375–380.
Lee, S. Y., Tindwa, H., Lee, Y. S., Naing, K. W., Hong, S. H., Nam, Y., & Kim, K. Y. (2012). Biocontrol of anthracnose in pepper using chitinase, beta-1, 3 glucanase, and 2-furancarboxaldehyde produced by Streptomyces cavourensis SY224. Journal of Microbiology and Biotechnology, 22, 1359–1366.
Li, Z. P., Gao, X. N., Fan, D. Y., Yan, X., Kang, Z. S., & Huang, L. L. (2015). Saccharothrix yanglingensis strain Hhs.015 is a promising biocontrol agent on apple valsa canker. Plant Disease, 100, 510–514.
Ma, Y. Q., Li, J. P., Wang, L., Li, J. J., Hui, N. N., & Zhou, T. W. (2012). Inhibitory effects of five fungicides on apple tree valsa canker. Gansu Agricultural Science and Technology, 6, 20–21.
Nagpure, A., Choudhary, B., & Gupta, R. K. (2013). Mycolytic enzymes produced by Streptomyces violaceusniger and their role in antagonism towards wood-rotting fungi. Journal of Basic Microbiology, 54, 397–407.
Nonomura, H. (1974). Key for classification and identification of 458 species of the Streptomyces included in ISP. Journal of Fermentation Technology, 52, 78–92.
Oshiman, K. I. (2000). Sodium alginate as an adjuvant of an antagonistic bacterium, Pseudomonas fluorescens strain A11RN, to enhance biocontrol of turfgrass snow mold caused by Typhula ishikariensis. Journal of General Plant Pathology, 66, 258–263.
Prabavathy, V. R., Mathivanan, N., & Murugesan, K. (2006). Control of blast and sheath blight diseases of rice using antifungal metabolites produced by Streptomyces sp. PM5. Biological Control, 39, 313–319.
Prapagdee, B., Kuekulvong, C., & Mongkolsuk, S. (2008). Antifungal potential of extracellular metabolites produced by Streptomyces hygroscopicus against phytopathogenic fungi. International Journal of Biological Sciences, 4, 330.
Shan, W., Li, J., & Liu, H. (2010). Study on the inhibitory effects of different active materials screened by the filter paper on cotton Verticillium wilt. Chinese Agricultural Science Bulletin, 26, 285–289.
Shirling, E. B., & Gottlieb, D. (1968). Cooperative description of type cultures of Streptomyces III. Additional species descriptions from first and second studies. International Journal of Systematic Bacteriology, 18, 279–392.
Shirling, E. B., & Gottlieb, D. (1969). Cooperative description of type culture of Streptomyces. IV. Species descriptions from the second, third and fourth studies. International Journal of Systematic and Evolutionary Microbiology, 19, 391–512.
Shirling, E. B., & Gottlieb, D. (1972). Cooperative description of type culture of Streptomyces. V. Additional descriptions. International Journal of Systematic and Evolutionary Microbiology, 22, 265–394.
Skujins, J. J., Potgieter, H. J., & Alexander, M. (1965). Dissolution of fungal cell walls by a Streptomycete chitinase and β-(1, 3) glucanase. Archives of Biochemistry and Biophysics, 111, 358–364.
Tamura, K., & Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular Biology and Evolution, 10, 512–526.
Turner, J. R., Butler, T. F., Fuller, R. W., & Owen, N. V. (1977). 17th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.
Wang, L., Rui, Z., Huang, L. L., Xie, F. Q., & Gao, X. N. (2005). The investigation of apple tree Valsa canker in Guanzhong region of Shaanxi province. Journal of Northwest Sci-Tech University of Agriculture and Forestry, 33, 98–100.
Wang, L., Gao, Z. P., Huang, L. L., Wei, J. L., Zang, R., & Kang, Z. S. (2009). Screening fungicide for pathogen inhibition and disease control of apple tree valsa canker. Acta Phytophylacica Sinica, 39, 549–554.
Wang, X., Wei, J., Huang, L., & Kang, Z. (2011). Re-evaluation of pathogens causing valsa canker on apple in China. Mycologia, 103, 317–324.
Wang, C. X., Zhang, Q. M., Li, G. F., Dong, X. L., & Li, B. H. (2012). Identification of the antagonistic bacteria BJ1 and its antifungal activity against Valsa ceratosperma. Acta Phytophylacica Sinica, 39, 431–437.
Wang, W. X., Xu, B. L., Xue, Y. Y., Chen, Z., & Liang, X. D. (2014). Identification and antifungal activity of the antagonistic bacteria of Cytospora spp. Chinese Journal of Eco-Agriculture, 22, 1214–1221.
Willison, R. (1936). Peach canker investigations: II. Infection studies. Canadian Journal of Research, 14c, 27–44.
Xin, Y. F., & Shang, J. J. (2005). Bio-control trials of Chaetomium spirale ND35 against apple canker. Journal of Forestry Research, 16, 121–124.
Xiong, C. L., Wang, Q., Wang, S., Jiang, W. N., & Zhang, X. G. (2017). A method of double coverslips inserting on inducing Stemphylium eturmiunum conidia production. Journal of Fungal Research, 15, 130–132.
Xu, L. H., Li, W. J., Liu, Z. H., & Jiang, Q. L. (2007). Actinomycetes systematics: Principles, methods and practice (pp. 80–128). Beijing: Science Press.
Xu, T., Hu, T. L., Wang, Y. N., Wang, S. T., & Cao, K. Q. (2012). Isolation of endophytic fungi from apple bark and their potential for biological control of Valsa ceratosperma. Acta Phytophylacica Sinica, 39, 327–333.
Yuan, W. M., & Crawford, D. L. (1995). Characterization of Streptomyces lydicus WYEC108 as a potential biocontrol agent against fungal root and seed rots. Applied & Environmental Microbiology, 61, 3119–3128.
Zang, R., Huang, L. L., Kang, Z. S., & Wang, X. L. (2007). Biological characteristics and pathogenicity of different isolates of Cytospora spp. isolated from apple trees in Shaanxi province. Acta Phytopathologica Sinica., 37, 343–351.
Zhai, H. Z., Hu, T. L., Chen, Q., & Cao, K. Q. (2012). Control effect of 10 fungicides against apple valsa canker. Plant Protection, 38, 151–154.
Zhang, Q. M., Wang, C. X., Wang, H. Y., Li, B. H., Dong, X. L., & Li, G. F. (2013). Identification of antagonistic endophytic actinomycetes A-2 and evaluation of its activity against Valsa mali. Chinese Journal of Pesticide Science, 15, 286–292.
Zhang, J. X., Gu, Y. B., Chi, F. M., Ji, Z. R., Wu, J. Y., & Dong, Q. L. (2015). Bacillus amyloliquefaciens GB1 can effectively control apple valsa canker. Biology Control, 88, 1–7.
Zhao, Z. Y., Zhang, C. H., Liang, J., Liu, Z. L., & Gao, H. (2007). Studies on arsenic pollution in the apple orchards applied asomate. Acta Horticulturae Sinica, 34, 1117–1122.
Acknowledgements
This research was supported by National Natural Science Foundation of China (NO. 31371978) and by the Special Fund for Agroscientific Research in the Public Interest (NO. 201303025). We are grateful to Pro. John Richard Schrock (Emporia State University, USA) for proofreading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Dai, P., Zong, Z., Ma, Q. et al. Isolation, evaluation and identification of rhizosphere actinomycetes with potential application for biocontrol of Valsa mali. Eur J Plant Pathol 153, 119–130 (2019). https://doi.org/10.1007/s10658-018-1547-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1547-z