Abstract
Stemphylium solani is an important foliar pathogen that infects many agricultural plants, especially solanaceous plants. The difficulty inducing sporulation in pure culture is a limiting factor for research on the different pathosystems involving S. solani. In this study, the influence of the culture medium, photoperiod with alternating temperatures, Petri dish cover materials (glass, polystyrene and PVC film) and stress factors of the colonies were investigated in the conidia production. Six sequential assays were performed with four isolates of S. solani, obtained from tomato plants. The inoculum produced was evaluated for infectivity on tomato plants under greenhouse conditions. The addition of fresh tomato juice to the agar medium and incubation temperatures of approximately 25 °C favoured mycelial growth. The ability to sporulate in vitro varied with the isolate, but in general the conidia production was significantly higher in V8 medium at 25 °C 6 h−1 light and 10 °C 18 h−1 of darkness. Sporulation was lower in glass Petri dishes but higher in transparent polystyrene dishes. The methodology allowed the production of viable and infective inoculum in sufficient quantity to inoculate plants under experimental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stemphylium solani Weber is an important foliar pathogen that infects cultivated solanaceous plants (tomato - Solanum lycopersicum, jilo - Solanum gilo, eggplant - Solanum melongena, sweet pepper and chili pepper - Capsicum spp.), wild solanaceous plants (Solanum lycocarpum, Solanum paniculatum, Nicandra physaloides, Solanum palinacanthum and Cyphomandra betacea), cotton (Gossypium hirsutum), basil (Ocimum basilicum) and garlic (Allium sativum) (Reis and Boiteux 2006; Zheng et al. 2009; Reis et al. 2011). Increasing losses in the Brazilian tomato crop are related to the cultivation of varieties susceptible to S. solani (Domingues et al. 2017a). Throughout the world, the other three species of Stemphylium are reported to be pathogenic on tomato plants. These species include Stemphylium lycopersici (Enjoji) W. Yamamoto (syn. Stemphylium floridanum Hannon and G. F. Weber), Stemphylium botryosum Wallr. and Stemphylium vesicarium (Wallr) Simmons. However, only the species S. solani and S. lycopersici have been reported in Brazil, and the first is prevalent, more polyphagous and better adapted to tropical conditions (Reis and Boiteux 2006; Domingues et al. 2017b).
The isolates of S. solani grow well in culture media but rarely sporulate under laboratory conditions (Domingues et al. 2017b), thus resulting in difficulty obtaining inoculum for research in controlled conditions. The growth rate and sporulation of the fungi in the culture media are most affected by the composition and concentration of nutrients, and the main factors for the incubation conditions are temperature, light and photoperiod (Dhingra and Sinclair 1995). Different methods have been studied to produce inoculum of Stemphylium as the culture in V8 juice agar medium at 25 °C and 12 h photoperiod (Bentes and Matsuoka 2005), and v8 agar medium at 21 °C (Mehta and Arias 2001). However, the results of these studies vary between laboratories and because of genetic variability in the pathogen population (Domingues et al. 2017b).
The culture media most cited for the cultivation of S. solani and Stemphylium spp. are widely used for Hyphomycetes and dematiaceous fungi: V8 juice agar (Diener 1952; Behare et al. 1991; Mehta and Arias 2001; Bentes and Matsuoka 2005) and potato-dextrose-agar (PDA) (Lima 1982; Bentes and Matsuoka 2005), although better results were obtained with V8 juice agar. Culture media with corn flour (Sandrock and Vanetten 1998) and malt peptone agar (Lima 1982) have also been reported to promote the sporulation of S. solani. The addition of fresh extracts from the host plant to the culture media is recommended to stimulate the production of the conidia of phytopathogenic necrotrophic fungi, although there have been no previous studies on the use of this procedure with S. solani (Dhingra and Sinclair 1995) or even involving isolates from tropical regions, or from Brazil.
Temperature and light are among the factors that most affect fungal development, including the formation of reproductive structures. Species of Stemphylium require higher temperatures to grow and form conidiophores, but low temperatures favour conidiogenesis (Leach 1967). Temperatures of 15 to 35 °C, with an optimum of 25 °C, are reported by Kim et al. (2004) to culture S. solani. The alternation of 12 h light/darkness is cited as being more favourable to the sporulation of Hyphomycetes as well as S. lycopersici (Bashi and Rotem 1975; Kim et al. 2004). The use of fluorescent lamps (380 to 775 nm) or near ultraviolet (NUV) light (320 to 420 nm) induces the in vitro production of spores of many foliar ascomycetes, as well as those of S. solani (Leach 1962; Pulz and Massola Jr. 2009).
Some practices that cause stress to the cultures or injuries to the mycelia can also stimulate the sporulation of different fungi, such as scraping the aerial mycelia, treating them with radiation (ultraviolet - UV and NUV), washing the colonies under tap water and dehydrating the cultures (Leach 1967; Wells et al. 1971; Rodrigues et al. 2010). However, little is known about the effect of these factors on the sporulation of S. solani.
The previous studies sought to establish the minimum conditions required stimulating the sporulation of S. solani in vitro. However, due the variation among isolates, pathogen populations and results from different laboratories, regional research is required to adapt the methodology, once variations are expected for fungus isolates originated from distinct phytogeographic regions.
In this study, sequential assays were carried out with four isolates of S. solani collected from tomato plants, originated from two distinct tomato producing regions of the Rio de Janeiro State, Brazil. The culture medium, temperature, light, photoperiod regimes with alternating temperatures, Petri dish cover materials (glass, polystyrene and polyvinyl chloride – PVC) and stress factors to the colonies were evaluated to determine the production of the conidia in vitro. The inoculum produced in vitro was tested for infectivity in tomato plants.
Materials and methods
Four isolates of S. solani isolated from tomato plants (S. lycopersicum) were used. The isolates SENA 106 and SENA 108 were collected in organic crop from Seropédica City, RJ, Brazil (22° 44' 29" S, 43° 42' 19" W, 26 m, climate tropical with dry season - Aw), and SENA 301 and SENA 304 were collected in conventional crop from Paty do Alferes City, RJ, Brazil (22° 25' 10" S, 43 ° 25' 21" W, 624 m, humid subtropical climate - Cfa).
All of the fungi were previously identified at the species level based on the size, colour and number of septa on the conidia (Ellis 1971) and characterized as being virulent to tomatoes. The pure cultures were preserved in PDA media and mineral oil for later use. The fungi were grown for 10 d in PDA at 25 °C and 12 h photoperiods to provide the inoculum required for further study. Six assays were carried out in sequence to evaluate the effect of culture media, temperature and photoperiod regimes, light source, application of stress factors in the colonies and the Petri dish cover materials (glass, polystyrene and PVC) on mycelial growth and conidia production. The completely randomized design was used in all of the assays, with five replications. Each replicate was comprised of a Petri dish. Additionally, the infectivity of the inoculum from the four isolates was evaluated in assays conducted under greenhouse conditions. Thus, a randomized block design was used with two plants per pot for each isolate and three repetitions.
Culture medium
The effects of different culture media on the growth and sporulation of the four isolates of S. solani were evaluated: 1) V8 juice agar (200 mL juice vegetables V8®, 20 g agar, 3 g CaCO3) (V8®, Campbell Soup Co., Camden, USA); (2) JT, juice tomato agar (300 g tomato boiled in 500 mL of water for 10 min, 3 g CaCO3, 20 g dextrose, 15 g agar); 3) PDA (42 g commercial product potato dextrose agar) (Kasvi, São José dos Pinhais, Brazil); 4) PDA + V8 (177 mL juice vegetable V8®, 12 g commercial PDA, 3.54 g CaCO3, 12 g agar brought to a volume of 650 mL with distilled water); 5) LT, tomato leaf agar (60 g extract cut tomato leaves cooked for 30 min, 18 g agar, 2.55 g CaCO3) (adapted from Brunelli et al. 2006); 6) MC, carrot agar (20 g extract of grated carrot that had been incubated in 400 mL water for 60 min, 20 g agar) (Alfenas and Maffia 2007); 7) MO, oats agar; and 8) FM, maize agar flour, prepared with 50 g oatmeal or cornmeal, both heated for 5 min in 1 L water, 20 g dextrose, 15 g agar (Dias Neto et al. 2010). The volume of all of the culture media was adjusted to 1 L with distilled water, and the pH was adjusted to 5.0 using lactic acid prior to autoclaving (121 °C for 20 h). Mycelial discs (5.0 mm diameter) from each of the four isolates were separately cut and transferred to the centre of glass Petri dishes containing the culture medium to initiate the assays. The isolates were incubated in a growth chamber at 25 ± 1 °C with a photoperiod of 12 h for 10 d followed by 15 ± 1 °C for an additional five days.
The colony diameter (mm) was measured daily in two orthogonal directions using a pachymeter. The amount of conidia produced was evaluated at 15 days by counting spores in triplicate (aliquots) in a Neubauer chamber (Fortuna, Wertheim, Germany). The conidia were removed from cultures, and 5 mL of distilled water was added to each plate, followed by careful scraping with a brush and the filtration of the suspension through gauze.
The number of conidia per mm2 of colony was calculated from the number of conidia and the colony diameter. The area under the curve of mycelial growth (AUCGM) was calculated based on the daily mycelial growth as described by Shaner and Finney (1977).
Temperature
Discs of the isolates were placed in the centre of Petri dishes containing V8 juice agar culture medium and incubated under different temperature regimes during a 12 h photoperiod: 1) 25 °C continuous; 2) 25 °C continuous for 10 d followed by 10 °C continuous for 5 d; 3) 25 °C for 10 d followed by 15 °C for 5 d; 4) 25 °C for 10 d followed by 20 °C for 5 d; 5) 25 °C and 10 °C alternating light/darkness; 6) 25 °C and 15 °C, alternating light/darkness, and 7) 25 °C and 20 °C, alternating light/darkness. After 15 days of incubation, the same evaluations were carried out using the procedures described in the previous assays.
Photoperiod
The isolates were placed in the centre of Petri dishes containing V8 juice agar medium and incubated in growth chambers at 25 °C (light) and 10 °C (darkness), in the following photoperiods: 1) 0/24 h; 2) 4/20 h; 3) 6/18 h; 4) 8/16 h and 5) 12/12 h, light/darkness. Four fluorescent lamps (20 watts) were used as a source of light and were positioned on the growth chamber door at a distance ranging from 10 to 30 cm from the dishes. The dishes were wrapped in aluminium foil for the dark continual conditions. The mycelial growth and sporulation were evaluated as described previously.
Light source
Mycelial discs from the four isolates were placed on the centre of Petri dishes containing V8 juice agar media. The dishes were incubated in a growth chamber for 6 h at 25 °C under light and for 18 h at 10 °C in the dark. Two light sources were utilized: 1) cool white fluorescent lamps (daylight) of 20 watts (380 to 775 nm) and 2) black fluorescent lamp NUV of 20 watts (310 to 440 nm). Four lamps for each treatment were placed in the doors of the respective growth chambers, and the dishes were positioned 10 to 30 cm away from the lamps. After 15 d of incubation, the number of conidia per mm2 was counted as previously described.
Stress factors for the colonies
Mycelial discs were placed on the centre of Petri dishes containing V8 juice agar media and incubated at preselected conditions of temperature, photoperiod and light source (25 °C/6 h light and 10 °C/18 h darkness using the cool white fluorescent lamps). After 10 d of growth, the different treatments were applied using three stress factors: 1) aerial mycelium scraping, 2) UV radiation, 3) scraping the aerial mycelium plus UV radiation and 4) control (intact cultures).
The aerial mycelia were scraped with a fine brush and washed with sterile distilled water. The UV radiation was applied in a laminar air flow chamber (UV lamp of 15 W for 5 h, positioned 40 cm from the uncovered dishes). After the treatments, the dishes were returned to the growth chambers for 5 d using the same incubation conditions. The number of conidia per mm2 was counted as described previously.
Types of Petri dish cover materials
The mycelial discs were placed on the centre of the Petri dishes containing V8 juice agar media and incubated under the preselected conditions of temperature, photoperiod and light source (25 °C/6 h light and 10 °C/18 h darkness, using the cool white fluorescent lamps). Two types of Petri dishes and three dish cover materials were used: 1) glass Petri dishes 9 cm in diameter, 2) glass Petri dishes, uncovered, but wrapped in transparent PVC films of 8 μm thickness (Banpack®, Bandeirante, Guarulhos, Brazil) and 3) Crystal polystyrene Petri dishes, 9 cm in diameter, disposable (J Prolab, São José dos Pinhais, Brazil). After 13 d of incubation, the production of conidia was evaluated as described previously.
Infectivity of isolates
The infectivity of the inoculum produced in vitro by the four isolates of S. solani was evaluated by inoculating tomato seedlings (‘Perinha Água Branca’ cultivar) susceptible to grey leaf spot (Domingues et al. 2017a). Seedlings cultivated in 150 mL pots were inoculated at the first leaf pair stage with a suspension of 104 conidia mL−1 until the liquid ran off both sides of the leaves. The plants were conditioned in a growth chamber model 1112FC (Eletrolab, São Paulo, Brazil) with alternating temperatures of 25 and 15 °C (light/darkness), a 12 h photoperiod and 100% relative humidity for 48 h. After this period, the humidity was adjusted to 50%. In addition, plants without inoculation which were sprayed with water (control) were kept under the same conditions). The disease severity was evaluated in the three terminal leaflets of two leaves per plant using the diagrammatic scale of Boff et al. (1991) from the 3rd to the 11th day after inoculation (DAI).
Statistical analysis
For all the tests, the data were submitted to analysis of variance, and the means were compared by the Tukey test (p < 0.05) using the SISVAR program (Ferreira 2011).
Results
Culture media
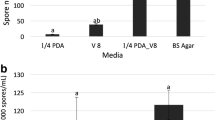
A significant interaction on the mycelial growth and sporulation was found between the culture medium and the isolates. Higher averages of colony diameters and amounts of spores were obtained with JT media and V8 media, respectively (Table 1). Generally, the four isolates grew well in the eight types of media tested, but they grew better in the treatments containing tomato juice as JT, followed by PDA + V8, V8 and MO. The isolates varied a great deal in the amount of spores they produced in the different media. The isolates SENA 106, SENA 304 and SENA 301 sporulated very well in the V8 media, while isolate SENA 108 sporulated more effectively in the JT medium. In general, the isolate SENA 108 produced the lowest number of spores. Only the SENA 301 isolate sporulated well in the four different media V8, JT, LT and PDA + V8, while isolates SENA 106 and SENA 304 sporulated well only in the V8 media. In general, the MC, MO and PDA media were unfavourable for the sporulation of the isolates tested (Table 1).
Temperature
There was a significant interaction on mycelial growth between the isolate and the temperature regime. However, in general, the mycelia grew at a higher rate when the temperature was 25 °C continuously for 15 d, followed by a continual 25 °C temperature during the first 10 days. There was also a significant effect of the interaction between the isolate and temperature on sporulation (Table 2). The SENA 106 isolate produced demonstrably higher amounts of conidia in all of the regimes tested, especially in the alternating light/darkness treatments in the temperature regimes of 25/10 °C, followed by 25/15 °C and 25/20 °C. Lower levels of sporulation were observed under a continual temperature regime of 25 °C. The alternation of light/darkness and temperature at 25/10 °C favoured sporulation the most effectively resulting in higher mean values for the four isolates, but there was variation among the isolates. The SENA 304 isolate sporulated more effectively at 25/20 °C and 25/15 °C, and the isolate 108 with the lowest number of conidia per mm2 of colony (mean) sporulated well at 25/10 °C and 25 + 10 °C (Table 2).
Influence of the photoperiod
A significant interaction on mycelial growth and sporulation was observed between the isolate and the photoperiod regime. In general, the isolates grew similarly. However, growth was higher during a 12 h photoperiod. A reduction in the amount of growth of all four isolates was observed as the period of darkness increased (Fig. 1a, c, e and g).
Mycelial growth expressed by the area under the curve of mycelial growth (AUCGM) and conidial production (conidia mm−2) of the four isolates of Stemphylium solani in vitro. SENA 106 (a and b), SENA 108 (c and d), SENA 301 (e and f) and SENA 304 (g and h) and the mean of the four isolates (i and j) as a function of the photoperiod, expressed by the number of darkness hours. Isolates were cultivated in V8 media at 25 °C and 10 °C light/darkness
Three of the four isolates sporulated more effectively during a photoperiod of 18 h darkness (Fig. 1b, d, f, h). Isolate SENA 304 sporulated less and had minimal variation as a function of photoperiod (Fig. 1h).
Effect of the light source, stress factors and type of Petri dishes
There was no effect on mycelial growth and sporulation from the type of the light source, cool white or NUV, with the exception of the SENA 304 and SENA 106 isolates, which growth and sporulated more effectively, respectively, under cool white fluorescent light bulbs. The application of stress such as UV light to the colonies, scraping and a combination of the two had a negative effect on the sporulation of the four S. solani isolates (Table 3).
The effect of the type of the Petri dish was not evaluated for the SENA 301 isolate because the fungus lost of sporulation capacity due maintenance in vitro. There was some variation among the isolates. However, in general, the disposable Crystal polystyrene Petri dishes resulted in higher levels of sporulation by the three isolates, followed by plates wrapped in transparent PVC film (Table 4).
Infectivity of the isolates
The conidia obtained by the most favourable method for the conidial sporulation of S. solani (V8 juice agar media, incubation under alternating conditions of temperatures 25/10 °C, photoperiod 6 h light/18 h darkness and cool white light) were considered viable and infective. After inoculation, disease symptoms were observed in the inoculated tomato seedlings. No symptoms were observed on leaves of no-inoculated plants (control). The first symptoms of grey leaf spot in the inoculated tomato seedlings appeared three days after inoculation with the isolates SENA 106 and SENA 301, and only at 11 days after inoculation with the isolates SENA 108 and SENA 304. The mean severity at 11 DAI was 3.4 and 9.7% for the first two isolates, respectively, and 1% for the last two isolates. The pathogen was re-isolated from the inoculated plants and the S. solani agent was confirmed.
Discussion
The greatest amount of S. solani mycelial growth occurred when fresh tomato juice was added to the media as has been reported for other fungi such as Alternaria cassiae (Ávila et al. 2000). Higher sporulation in the media to which commercial V8 juice had been added has also been observed with other species of Stemphylium (Diener 1952; Wells et al. 1971; Bentes and Matsuoka 2005). This result is probably due to the composition of V8 juice that has a more complex mix of nutrients, since it contains other vegetables such as beet, celery, carrot, lettuce, spinach, parsley and watercress. Another reason for this could be that the inclusion of susceptible plant tissue in culture medium is a technique that is recommended to stimulate the spore production of some fungi (Dhingra and Sinclair 1995). However, the culture medium to which tomato leaves (LT) had been added was not favourable to the mycelial growth or sporulation of S. solani.
The mycelial growth of S. solani was more vigorous at 25 °C, which was also observed by Kim et al. (2004). However, in this study, sporulation was stimulated more effectively at lower temperatures such as 10 °C, 15 °C, or even 20 °C, depending on the isolate. The photoperiod had little effect on the mycelial growth of S. solani, but it was a determinant factor in the induction of sporulation. Eighteen hours of darkness was optimal. These results indicate that the sporulation of S. solani is stimulated by alterations in temperature, photoperiod (light/darkness) and low temperatures at night. The alternation of light and darkness is necessary for some fungi to sporulate, because they require a period of light, even in short periods, to produce conidiophores, while they need darkness to induce conidial formation (Leach 1967). Our results were similar to these previously published. Fewer conidia were produced during continual darkness or light, and the conidiophores remained sterile. However, this study showed that the four S. solani isolates tested produced conidia when grown continually in the dark. The emission of conidia was significantly lower under this condition, although it indicates the ability of this species to produce conidiophores even in the absence of light.
The application of stress factors on the colonies is reported to induce the sporulation of other pleosporaceous fungi, such as Alternaria solani (Rodrigues et al. 2010). However, it did not encourage the sporulation of S. solani. Therefore, these procedures are not recommended for S. solani, since they negatively affect the sporulation of this species.
Some studies such, as Leach (1962) and Pulz and Massola Jr. (2009), report that the use of white fluorescent lamps or NUV stimulates conidiophore formation, and subsequent exposure to the darkness results in the formation of spores of Stemphylium spp. and other fungi. In general, both white and NUV light can be used to induce the sporulation of S. solani, since only one isolate responded differently to the light source. Rodrigues et al. (2010) reported higher sporulation of A. solani under black light and a 12 h photoperiod; the probable effect of certain wavelengths on photoreceptor pigments affected the results on the induction or inhibition of fungal sporulation. Thus, the lower sporulation of the fungus in glass Petri dishes may be associated with the lower transmittance of this radiation by the glass. If this is the case, the higher sporulation in the Crystal polystyrene Petri dishes and the glass Petri dishes covered with PVC film could be due to the stimulation of conidiophores by the radiation (Leach 1967) and in the polystyrene dishes, the greater aeration (oxygenation) of the cultures (Hawker 2016). The polystyrene dishes had grooves in the socket between the base and the cap that allowed gas exchange. The dishes wrapped in PVC film remained completely sealed. However, it is possible that some gases diffused through the PVC film. Further research should be conducted to evaluate the effect of aeration and atmospheric gas composition on the sporulation of S. solani.
As expected, the S. solani isolates varied in their ability to grow and sporulate in vitro. Isolates that sporulated with difficulty seemed to maintain this characteristic, even if the conditions favoured conidia formation (Namekata and Tokeshi 1967), due to the composition of the medium, regime of temperature or light. However, based on the results of this study, S. solani grew and sporulated most effectively in V8 agar media under incubation with alternating temperatures of 25 °C with 6 h light and 10 °C with 18 h darkness and light sources of cool white fluorescent lamps or NUV. Culture in the crystal polystyrene Petri dishes also favoured higher conidial production by S. solani. The conidia produced under these conditions could infect plants. Therefore, this methodology may be recommended for the production of S. solani inoculum. However, the need to pre-select the most prolific and virulent isolates remains. In a similar study, Namekata and Tokeshi (1967) observed a relationship between the sporulation capacity in pure culture and the virulence of S. solani isolates on tomatoes.
References
Alfenas, A. C., & Maffia, R. G. (2007). Métodos em Fitopatologia. Viçosa: Editora UFV.
Ávila, Z. D., Mello, S. D., Ribeiro, Z. D. A., & Fontes, E. M. G. (2000). Produção de inóculo de Alternaria cassiae. Pesquisa Agropecuária Brasileira. https://doi.org/10.1590/S0100-204X2000000300008.
Bashi, E., & Rotem, J. (1975). Effect of light on sporulation of Alternaria porri f. sp. solani and of Stemphylium botryosum f. sp. lycopersici in vivo. Phytoparasitica. https://doi.org/10.1007/BF02981223.
Behare, J., Laterrot, H., Sarfatti, M., & Zamir, D. (1991). Restriction fragment length polymorphism mapping of the Stemphylium resistance gene in tomato. Molecular Plant Microbe Interactions. https://doi.org/10.1094/MPMI-4-489.
Bentes, J. L. S., & Matsuoka, K. (2005). Localização de peróxido de hidrogênio durante a resposta de defesa de tomateiro contra Stemphylium solani. Fitopatologia Brasileira. https://doi.org/10.1590/S0100-41582005000600012.
Boff, P., Zambolim, L., & Vale, F. X. (1991). Escalas para avaliação de severidade da mancha-de-estenfílio (Stemphylium solani) e da pinta-preta (Alternaria solani) em tomateiro. Fitopatologia Brasileira, 16(4), 280–283.
Brunelli, K. R., Fazza, A. C., Athayde Sobrinho, C., & Camargo, L. E. A. (2006). Effect of culture media and light exposure on the sporulation of Cercospora zae-maydis. Summa Phytopatologica. https://doi.org/10.1590/S0100-54052006000100016.
Dhingra, O. D., & Sinclair, J. B. (1995). Basic Plant Pathology Methods. Florida: CRC Press.
Dias Neto, J. J., Santos, G. R., Castro Neto, M. D., Anjos, L. M., Cunha, C. F., & Ignácio, M. (2010). Influência do meio de cultura na esporulação de Magnaporthe grisea e da concentração de conídios na severidade da Brusone do Arroz. Bioscience Journal, 26(2), 173–179.
Diener, U. L. (1952). A method for inducing abundant sporulation of Stemphylium solani in pure culture. Phytopathology, 42(7), 141–145.
Domingues, D. P., Santos, C. A., Kowata-Dresch, L. S., & Carmo, M. G. F. (2017a). Quantification and progress of gray leaf spot in tomato cultivars under organic management. Caderno de Ciências Agrárias, 9(1), 09–18.
Domingues, D. P., Santos, C. A., Kowata-Dresch, L. S. K., Reis, C. A., Fernandes, M. C. A., & Carmo, M. G. F. (2017b). Sensitivity of Stemphylium solani to plant extracts and syrups and disease control in tomato at greenhouse. Revista de Ciências Agrárias. https://doi.org/10.19084/RCA15129.
Ellis, M. B. (1971). Dematiaceous Hyphomycetes. Kew, Commonwealth Mycological Institute.
Ferreira, D. F. (2011). Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia. https://doi.org/10.1590/S1413-70542011000600001.
Hawker, L. E. (2016). The physiology of reproduction in Fungi. Cambridge: Cambridge University Press.
Kim, B. S., Yu, S. H., Cho, H. J., & Hwang, H. S. (2004). Gray leaf spot in peppers caused by Stemphylium solani and S. lycopersici. Journal of Plant Pathology. https://doi.org/10.5423/PPJ.2004.20.2.085.
Leach, C. M. (1962). Sporulation of diverse species of fungi under near-ultraviolet radiation. Canadian Journal of Botany. https://doi.org/10.1139/b62-016.
Leach, C. M. (1967). Interaction of near-ultraviolet light and temperature on sporulation of the fungi Alternaria, Cercosporella, Fusarium, Helminthosporium and Stemphylium. Canadian Journal of Botany. https://doi.org/10.1139/b67-218.
Lima, D. M. M. (1982). Maltose-peptona-ágar, um meio de cultura para esporulação de Stemphylium solani. Pesquisa Agropecuária Brasileira, 17(1), 81–83.
Mehta, Y. R., & Arias, C. A. A. (2001). Herança da resistência a Stemphylium solani e insensibilidade a sua fitotoxina em cultivares de algodoeiro. Fitopatologia Brasileira. https://doi.org/10.1590/S0100-41582001000400013.
Namekata, T., & Tokeshi, H. (1967). Variabilidade de Stemphylium solani, Weber, agente causal de mancha foliar do tomateiro, no estado de São Paulo. Anais da Escola Superior de Agricultura Luiz de Queiroz. https://doi.org/10.1590/S0071-12761967000100026.
Pulz, P., & Massola Jr., N. S. (2009). Efeito de meios de cultura e fatores físicos no crescimento e esporulação de Alternaria dauci e A. solani. Summa Phytopathologica. https://doi.org/10.1590/S0100-54052009000200007.
Reis, A., & Boiteux, L. S. (2006). Círculo de hospedeiras de isolados de Stemphylium solani. Research report. Embrapa Hortaliças https://ainfo.cnptia.embrapa.br/digital/bitstream/CNPH-2009/32822/1/bpd_18.pdf Accessed 14 September 2016.
Reis, A., Boiteux, L. S., & Fonseca, M. N. (2011). Identification of solanaceous and non-solanaceous species as hosts of Stemphylium solani isolates in Brazil. Phytopathology. https://doi.org/10.5423/PPJ.OA.03.2016.0063.
Rodrigues, T. M. S., Maffia, L. A., Dhingra, O. D., & Mizubuti, E. S. (2010). In vitro production of conidia of Alternaria solani. Tropical Plant Pathology. https://doi.org/10.1590/S1982-56762010000400001.
Sandrock, R. W., & Vanetten, H. D. (1998). Fungal sensitivity to and enzymatic degradation of the phytoanticipin α-tomatine. Phytopathology. https://doi.org/10.1094/Phyto.1998.88.2.137.
Shaner, G., & Finney, R. E. (1977). The effect of nitrogen fertilization on the expression of slow-mildewing resistance in knox wheat. Phytopathology. https://doi.org/10.1094/Phyto-67-1051.
Wells, H. D., Forbes, I., & Markhan, C. R. (1971). A black, readily sporulating mutant of Stemphylium solani susceptible biotypes of blue lupine. Phytopathology. https://doi.org/10.1094/Phyto-61-575.
Zheng, L., Lv, R., Hsiang, T., & Huang, J. (2009). Host range and phytotoxicity of Stemphylium solani, causing leaf blight of garlic (Allium sativum) in China. European Journal of Plant Pathology. https://doi.org/10.1007/s10658-008-9387-x.
Acknowledgements
The authors are grateful to the postgraduate course “Fitotecnia” of the “Universidade Federal Rural do Rio de Janeiro,” the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES) by the Master’s scholarship for the first author and to the “Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro” (FAPERJ) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All authors are aware with the content of the manuscript and have agreed upon its submission to European Journal of Plant Pathology. 1) The manuscript has not been published in whole or in part elsewhere; 2) The manuscript is not currently being considered for publication in another journal; 3) The manuscript is not split up into several parts to increase the quantity of submissions;
Conflict of interest
No conflict of interest exits in the submission of this manuscript.
Rights and permissions
About this article
Cite this article
de Souza, F.C., da Silva, K.F., da Silveira, S.F. et al. Conidial sporulation of Stemphylium solani under laboratory conditions and infectivity of the inoculum produced in vitro. Eur J Plant Pathol 152, 691–700 (2018). https://doi.org/10.1007/s10658-018-1511-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1511-y