Abstract
The ability to control soil-borne pathogens in agriculture is highly conditioned by the restricted use of synthetic pesticides. Allelopathy, the antimicrobial activity of plant extracts, is a promising option against crop pathogens. Extracts from Lycium spp. such as L. barbarum, L. chinense and L. intricatum possess biological and therapeutic properties. Individual methanolic extracts from leaves and stems of the Mediterranean medicinal species L. europaeum collected in two locations of Tunisia were each evaluated in vitro against Verticillium dahliae (Vd), Sclerotinia sclerotiorum (Ss) and Harpophora maydis (Hm). The mycelial growth of the three fungi was significantly reduced by all the extracts at doses of 10 and 30 μl mL−1 (equivalent to 1 and 3 mg plant tissue mL−1). The sporulation of Hm was almost completely inhibited in all the amendments, but that of Vd was stimulated by one of the leaf extracts when 1 and 3 mg dried plant tissue mL−1 were used. Sclerotia of Ss were formed in a smaller number, their total weight increasing at extract doses equivalent to 1 mg plant tissue mL−1 and higher. In greenhouse, the pathogenicity of Hm was confirmed as early as 6 weeks after inoculation, since it caused significant decreases of weights in both roots and aboveground parts of maize. The detrimental effect of Hm on maize root weight in greenhouse was significantly counteracted by one of the leaf extracts added by watering. In total, 11 phenolic compounds were separated in the four extracts. The hydroxycinnamic acid family, including chlorogenic acid as a major compound, represented more than 50% of the total content in all the samples. Rutin was the most abundant flavonoid. The results of this work show the detrimental effect of L. europaeum extracts against the soil-borne pathogens Hm, Ss and Vd, and highlight their potential in crop protection if adequately developed into final products and used in combination with other tools.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the major current challenges of European agriculture is that for many crop – pest (jointly referring to phytophagous insects and diseases) situations, no effective or economically feasible alternatives to synthetic pesticides are available, or are still under development (Geiger et al. 2010; Hillocks 2012). Regarding crop diseases, a large diversity of soil-borne plant pathogens, including fungi such as Verticillium spp. (causing Verticillium wilts), Sclerotinia spp. (causing Sclerotinia rots), and Fusarium oxysporum ff. spp. (causing Fusarium wilts), oomycetes such as systemic Downy mildews (Peronospora spp., Plasmopara spp.) and Phytophthora root rots (Phytophthora spp.), plant parasitic nematodes (e.g., Meloidogyne spp., Globodera spp., etc.) and parasitic plants (e.g., Orobanche spp., Striga spp.) are major concerns raised in agricultural food production worldwide (Agrios 1970; Fernández-Aparicio et al. 2016; Molinero-Ruiz et al. 2015; Pegg and Brady 2002; Thines and Kamoun 2010). Among those, Verticillium dahliae and Sclerotinia sclerotiorum are soil-borne pathogens of major importance, since they have a wide range of host crops and cause important losses by wilt, and mold and rot diseases respectively (Pegg and Brady 2002; Heffer Link and Johnson 2007; Martin-Sanz et al. 2018). Others, like Harpophora maydis occur in a particular crop (i.e. maize) endemically in some countries (Egypt and India), and are considered a threat to production in others like the USA (Bergstrom et al. 2008). The ability to control soil-borne pathogens is conditioned by factors such as the restrictions on the use of synthetic pesticides and the difficult access to active infection courts, which are mostly located beneath the soil surface (Chellemi et al. 2016). Genetic resistance is increasingly in use to control soil-borne pathogens, particularly those of herbaceous crops, but is hindered by the emergence of pathogen populations with new races (García-Ruiz et al. 2014; Molinero-Ruiz et al. 2002, 2008, 2015) or by a partial expression of resistance as determined by environmental conditions (Molinero-Ruiz et al. 2009; Ortiz-Bustos et al. 2016). Thus, enlarging the set of tools for efficient disease management, while maintaining crop yield and profitability, is crucial.
Late wilt of maize, caused by the soil-borne fungus Harpophora maydis, is a disease of increasing concern in the Iberian Peninsula, where incidences of 60% have been associated with 40% yield losses (Molinero-Ruiz et al. 2010; Ortiz-Bustos et al. 2016). Although plant infection occurs during the first month after sowing, symptoms appear suddenly at or shortly after crop flowering. Genetic resistance is the most effective control method (Bergstrom et al. 2008; Degani and Cernica 2014; Ortiz-Bustos et al. 2015, 2016). The pathogenic ability of H. maydis on the host or, conversely, the reaction of maize genotypes to the fungus, has mostly been assessed at the moment of the appearance of its symptoms (Abd El-Rahim et al. 1998; Ortiz-Bustos et al. 2015, 2016; Soliman and Sadek 1998). However, efficient phenotyping of genetic material in maize breeding programmes relies on the fast screening of a large number of entries, and that is mostly achieved under controlled conditions.
The allelopathic potential of plant extracts has been known and used in agriculture since ancient times. Allelochemicals can stimulate or inhibit plant germination and growth (Cheng and Cheng 2015; Chou 2010) and even plant-derived products can be effective tools that, adequately formulated, reduce the reliance on synthetic pesticides (Gahukar 2012). Currently, allelopathy offers an attractive environmentally friendly alternative to pesticides in agricultural pest management, and by extension, to those targeting crop pathogens (Farooq et al. 2011). Evidence for the antimicrobial activity of plant extracts and plant-derived compounds has accumulated in the literature over the last decade. In vitro experiments have shown the antifungal activity of Flourensia spp. extracts on Alternaria sp., Rhizoctonia solani and Fusarium oxysporum (Jasso de Rodríguez et al. 2007), or that of Rhus muelleri Standl. & F.A. Barkley against Fusarium oxysporum f. sp. lycopersici (Jasso de Rodríguez et al. 2015). Some extracts have even effectively reduced disease symptoms in the plants upon pathogen infection. Extracts of Vitex agnus-castus showed strong antifungal activity against Pythium ultimum under in vitro conditions and also reduced Pythium root rot and wilt in tomato (Švecová et al. 2013). Similarly, the extract of Boerhavia diffusa L. has recently reduced symptoms of Phytophthora capsici in pepper and P. infestans in tomato (Švecová et al. 2017).
The genus Lycium (Solanaceae family) is widely distributed in the world, and Lycium spp. are well known as a traditional herbal medicine and functional food (Amagase and Farnsworth 2011). Extracts from some Lycium species possess a range of biological and therapeutic properties (Potterat 2010), including antioxidant activities such as those from Lycium barbarum L., L. chinense Mill. and L. intricatum Boiss (Abdennacer et al. 2015; Mocan et al. 2014). Lycium barbarum and L. chinense have also been reported for the in vitro antimicrobial activities of their extracts (Mocan et al. 2014). Lycium europaeum L. is a frequent species in dry and sandy soils of the Mediterranean Basin, and a widespread representative of the genus Lycium in Tunisia. The antiproliferative capacity, protective properties, and antioxidant activity of its extracts in cancer treatments have been reported (Ghali et al. 2015). In addition, extracts of Lycium spp., including L. europaeum, are rich sources of phenolic compounds (Abdennacer et al. 2015; Ghali et al. 2015). Phenolic compounds are secondary metabolites which accumulate in plant tissues and, because of their antimicrobial effect (Cowan 1999), they have been proposed to serve as useful alternatives for the control of fungal pathogens such as Fusarium oxysporum in agricultural crops (Langcake et al. 1981).
The objectives of the current work were to: a) conduct the in vitro evaluation of the effect of extracts from L. europaeum against the soil-borne fungi Sclerotinia sclerotiorum, Verticillium dahliae and H. maydis, b) analyse the phenolic composition of the extracts, c) characterize the reaction of maize to the pathogen H. maydis under greenhouse conditions and at initial growth stages, and d) to assess the effect of extracts from L. europaeum on the maize – H. maydis pathosystem at early stages of the disease.
Material and methods
Fungal isolates
The extracts were tested by their effect against single-spore cultures of S. sclerotiorum (Ss) and V. dahliae (Vd), pathogenic to sunflower, and H. maydis (Hm), pathogenic to maize. All isolates used in this work are from the culture collection of the Laboratory of Diseases of Field Crops at the Institute for Sustainable Agriculture (IAS-CSIC) in Córdoba, Spain, and their complete information is presented in Table 1. Active cultures of the isolates were obtained on fresh potato dextrose agar (PDA) by incubation at 25 ± 1 °C in the dark for 5–7 days.
Plant extracts
Aboveground parts (leaves and stems) of L. europaeum were randomly sampled during the vegetative stage of the plants in February 2015. Collection sites were located in Tunisia: Gafsa (362 km south of Tunis; longitude 8° 46′ 60″ E, latitude 34° 25′ 0″ N, altitude 297 m; superior arid bioclimatic stage) and Jdaida (20 km north of Tunis; longitude 9° 55′ 26.97″ E, latitude 36°54′ 10.68″ N, altitude 17 m; superior semi-arid bioclimatic stage). Between 10 and 18 plants were sampled at each location. The samples were deposited in plastic bags and transported to the laboratory. They were rinsed with deionised water and air dried. The leaves were carefully separated from the stems and both leaves and stems were lyophilised separately prior to extraction.
In order to obtain the plant extracts of L. europaeum, approximately 7 g tissue samples were powdered and then extracted with 70 mL of a methanol aqueous solution (MeOH/H2O, 80:20, vol:vol) under stirring at 150 rpm for 30 min. The methanolic extracts were kept at −4 °C for 24 h in the dark, filtered (Whatman filter paper No.1) through a vacuum filtration system and stored at −20 °C until needed.
Analysis and quantification of phenolic compounds by HPLC–DAD–MS
Separation and characterization of phenolics
Separation and characterization of phenolics were carried out using a Jasco-LC-Net II ADC liquid chromatography system equipped with a diode array detector (DAD). Phenolic compounds were separated by using a Mediterranea Sea C18 reverse-phase analytical column (25 cm length × 4.6 mm i.d., 5 μm particle size; Teknokroma, Barcelona, Spain). The gradient profile for the separation was formed using solvent A (water with 1% formic acid) and solvent B (acetonitrile with 1% formic acid) with the following procedure: the proportion of B was increased from 0% B to 20% B for the first 20 min, then to 21% B over the next 8 min, maintained at 21% B for 2 min, then to 30% B over the next 10 min, and to 100% over the next 5 min, maintained at 100% B for 5 min and finally returned to the initial conditions over the next 5 min. The flow rate was 1 mL min−1 and the column temperature was 30 °C. Spectra from all peaks were recorded in the 200–600 nm range and the chromatograms were acquired at 280 nm for hydroxycinnamic acids and 360 nm for flavonoids.
Identification of Phenolics by HPLC-DAD-MS
Phenolic compounds detected in Lycium extracts were separated by HPLC as described above and identified by their electron impact mass data collected on a quadrupole mass analyzer (ZMD4, Micromass, Waters Inc., Manchester, U.K.). Electrospray ionisation (ESI) mass spectra were obtained at ionisation energies of 50 and 100 eV (negative mode) and 50 eV (positive mode), with MS scans from m/z 100 to 1000. Capillary voltage was 3 kV, desolvation temperature was 200 °C, source temperature was 100 °C, and extractor voltage was 12 V. The flow was maintained at 1 mL min−1.
Quantitative evaluation of phenolics
This was carried out as described by Fuentes-Alventosa et al. (2007). Individual phenolics were identified by using their retention times and both spectroscopic and mass spectrometric data. Chlorogenic acid and flavonoid glycosides (rutin, quercetin-3-O-glucoside and nicotiflorin) were quantified by HPLC–DAD using an eight-point regression curve in the range of 0–250 μg on the basis of standards. When standards were not available, as in the case of the different hydroxycinnamoylquinic acids described in the present work, quantification was based on chlorogenic acid (5-O-caffeoylquinic acid), because responses were essentially similar within classes. Results were calculated from the mean of three replicates.
In vitro effect of the extracts against soil-borne fungi
The effect of the extracts against the fungal vegetative growth (Hm, Vd and Ss), sporulation (Hm and Vd) and formation of sclerotia (Ss) was analysed in independent experiments for each pathogen using the amended plate technique with PDA as culture medium. The experiments were conducted twice and similar results were obtained. The methanolic extract concentrations tested were 0.01, 0.1, 1, 10, 30, 50 μl mL−1. As L. europaeum samples were extracted with methanol at a sample to solvent ratio of 1:10 (w/v), the doses assayed corresponded to 0.001, 0.01, 0.1, 1, 3, 5 mg mL−1 of dried plant tissue. For each treatment, and in order to exclude any antifungal effect due to the MeOH itself, PDA plates amended with the methanol aqueous solution at the same concentrations were included. Extracts were added, at the corresponding dose, after autoclaving and when the temperature of the medium reached about 50 °C. Unamended PDA plates served as controls. All treatments are presented in Table 2. Five-mm-diameter PDA discs were obtained from the edge of the actively growing 5–7 day-old colonies of each of the three fungi and then placed onto the centre of the amended plates. The cultures were incubated in laboratory lighting conditions at 24 °C for 3 days (Ss), 25 °C for 31 days (Vd) or 28 °C for 5 days (Hm), the growth conditions and time needed for the control of each fungus to reach the edge of the plate.
The colony radial growth was measured in each plate as the average of two diameters taken in 90°, with the measurements being taken three times from plate inoculations until the control treatment reached the edge of the plates. Four replicates (plates) were used per treatment and concentration. Sequential values of colony radial growth were used to calculate the area under the growth progress curve (AUGPC) over time by the trapezoidal integration method (Campbell and Madden 1990). The percent inhibition of AUGPC for each treatment, concentration and replication was determined relative to the AUGPC on the control and calculated according to the formula:
Where AUGPCc is the AUGPC of the control and AUGPCt is the AUGPC of the different treatments tested.
The effect of the extracts on the sporulation of Vd and/or Hm was assessed at the end of the experiments. In each plate, four 5-mm- diameter disks were cut at 2 cm from the centre and towards the edge of the colony, added to a glass tube containing 5 mL of sterile deionised water (SDW), then sonicated (90 U) for 10 min and filtered through four layers of cheesecloth to remove mycelial structures. Serial suspensions of conidia (from 1 to 10−5) were prepared. Conidia were counted using a Neubauer haemocytometer and converted into the concentration in the initial suspension. Sclerotia of Ss started to form on the media 30 days after plate inoculation. The effect of the extracts on the sclerotia formation of Ss was assessed 60 days after plate inoculation (Gulya et al. 1997). Sclerotia formed in each plate were counted under a stereomicroscope and weighed using a precision balance.
Pathogenicity of H. maydis at initial stages after infection
The reaction of maize to Hm (isolates HmLT809-S and Hm01–15) (Table 1) at early growth stages was studied in an experiment conducted twice in the greenhouse. Each isolate was cultured on PDA and incubated at 28 °C with a 12-h photoperiod per day for 10 days. Plates were flooded with SDW and scraped to loosen the spores. The spore suspension was filtered through four layers of sterile cheesecloth, and its concentration determined using a Neubauer hemacytometer and diluted with SDW to obtain a final concentration of 106 conidia mL−1. The inoculum consisted of a soil mixture (sand:cornflour:deionised water, 9:1:1, vol:vol:vol) (SCFW) to which fungal conidial suspensions were added at a final concentration of 15 × 103 conidia g−1 SCFW prior to incubation at room temperature of 22–24 °C in the dark for 3 weeks. After incubation, the infested SCFW was thoroughly mixed with sterile (autoclaved twice, 120 °C for 30 min) vermiculite at a rate of 50% (vol:vol) and added to 0.3-L pots. The inoculum density in the vermiculite-SCFW mixture, determined by dilution-plating assay on water agar medium (Borrego-Benjumea et al. 2013), was 1.3 × 106 and 1.4 × 106 colony forming units g−1 soil for HmLT809-S and Hm01–15, respectively.
The maize varieties MO1501 and MO1504, genetically susceptible and resistant, respectively, to the disease, were provided by Monsanto (Madrid, Spain). Eight seeds (replications) of each variety were surface-disinfested by immersing them in 10% sodium hypochlorite for 5–10 min, then thoroughly rinsed in deionised water and incubated in Petri plates in the dark at saturation humidity in a germinator at 24–28 °C until radicles were 5–10 mm long. Individual seedlings were inoculated by transplanting them into the infested pots 24 h after preparation of the vermiculite-SCFW mixture. Vermiculite with non-infested SCFW was used as the control treatment. Six plants of each variety were additionally included for each treatment in order to conduct weekly destructive assessments of their growth and root development. The experiment was carried out in a completely randomised factorial design.
The plants were grown for 6 weeks in the greenhouse at 25 ± 2 °C and a 12-h photoperiod per day. They were fertilized three times a week with 30 mL of Hoagland’s nutrient solution per pot, and watered as required. Every week, one plant from each treatment was uprooted and its roots carefully cleaned to remove substrate, washed and air-dried. The roots were observed for the presence of lesions caused by the pathogen and compared to those of the control plants. Tissue samples were taken in order to re-isolate the fungus from the inoculated plants. At the end of the experiment all the replications of each treatment were uprooted and their roots cleaned and washed. Roots and aboveground parts of each plant were independently weighed after drying at 65 °C for 62 h.
In vivo effect of the extracts against H. maydis
The effect of the extracts LG and LJ on the pathosystem maize – Hm was assessed in the greenhouse. The susceptible variety MO1501 was inoculated with isolate Hm01–15 following the methodology previously detailed. The extracts were applied twice by watering, 3 and 5 days after sowing, 2 mL (equivalent to 200 mg dried plant tissue) each to inoculated plants, as well as to non-inoculated plants. In order to exclude the antifungal effect due to the MeOH itself, plants watered with MeOH at the same dose as the one in the extracts were included. Control and only inoculated plants were watered with deionised water. Treatments are summarized in Table 3. Four replications (plants) were established for each of the treatments. Plants were grown in greenhouse for 6 weeks under the same conditions previously described. During the experiment, inoculated plants were observed for development of wilting symptoms caused by the infection by Hm or for any lesion associated with the treatments with the extracts, but no visible differences were detected as compared to the respective controls. At the end of the experiment all the plants were uprooted and their roots cleaned and washed. Roots and aboveground parts of each plant were air-dried and independently weighed. The experiment was conducted twice and similar results were obtained.
Statistical analyses
When assessing the in vitro effect of the extracts against soil-borne fungi, values of AUGPC, sporulation (Vd and Hm) and number and weight of sclerotia (Ss) were analysed, for each concentration, using analysis of variance (ANOVA) and following complete randomized statistical designs. Fisher’s unprotected LSD tests (P = 0.05) were used for comparisons of means. Values of inhibitions of: growth of the three fungi, sporulation of Hm and number of sclerotia of Ss were determined with respect to the variable values for the corresponding control treatment and used for representing data in figures and tables.
Dry weights of aboveground parts (DWAP) and roots (DWR) (pathogenicity of Hm at initial stages) and fresh weights of aboveground parts (FWAP) and roots (FWR) (in vivo effect of the extracts against Hm) were analysed by ANOVA. When significant effects were obtained, Fisher’s unprotected LSD tests (P = 0.05) were used for comparisons of means. Statistical analyses of data were performed using STATISTIX 10.0 software (Analytical software, Tallahassee, FL, USA).
Results
In vitro effect of the extracts against soil-borne fungi
The extracts inhibited growth of the three fungi, although their effect depended on the species. Doses of 1 μl mL−1 (0.1 mg dried plant tissue mL−1) and higher ones significantly (P ≤ 0.029) reduced the mycelial growth of Hm, with the leaf extract from L. europaeum from Gafsa (LG) exerting the highest effect (Fig. 1a). The inhibition of Ss growth was observed at doses of 10 μl mL−1 (1 mg dried plant tissue mL−1) and higher (P ≤ 0.0004). The reduction in mycelial growth was significantly higher than that of the methanol treatments for all the extracts at doses of 10 and 30 μl mL−1 (1 and 3 mg dried plant tissue mL−1 respectively), particularly when plates were amended with 30 μl mL−1 (3 mg dried plant tissue mL−1) of leaf extract from Jdaida (LJ) (Fig. 1b). Finally, the strongest inhibitory effect was that on Vd, whose growth was affected at all the doses. Significant reductions in mycelial growth of Vd occurred in extract amendments at doses of between 0.01 and 30 μl mL−1 (0.001 and 3 mg dried plant tissue mL−1 respectively) (P ≤ 0.0368). In general, LG and LJ had the greatest inhibitory effect on the growth of Vd as compared with other amendments (Fig. 1c). In experiments with Ss and Vd, complete inhibition of mycelial growth occurred at the highest dose of 50 μl mL−1 (5 mg dried plant tissue mL−1), methanol providing by itself a control of the growth of both fungal species that could not be distinguished from that of the extracts.
Effect of different concentrations of four extracts from Lycium europaeum and methanol on the vegetative growth of Harpophora maydis (a), Sclerotinia sclerotiorum (b) and Verticillium dahliae (c) by amendment of potato dextrose agar growth medium and expressed as the Inhibition of the Area under the growth progress curve compared with the unamended control. Vertical upper bars represent the standard error of the mean of four replications. When existing, significance is indicated as: * = 0.01 < P < 0.05; ** = 0.001 < P < 0.01; and *** = 0.0001 < P < 0.001
On the other hand, all the extracts, as well as methanol, almost completely inhibited the sporulation of Hm, with values that varied between 99 and 100% at different doses (Table 4). Contrary to Hm, sporulation of Vd was clearly stimulated by SJ at the highest doses (30 and 50 μl mL−1, equivalent to 3 and 5 mg dried plant tissue mL−1; P = 0.0074 and P = 0.0219, respectively), or in the amendments with either methanol at 50 μl mL−1 (P = 0.0219) (Fig. 2a). Significant decreases in Vd sporulation were only obtained at doses of 50 μl mL−1 and in amendments with leaf extracts (LG and LJ) (0.0675 × 106 conidia mL−1, averaged across both extracts) in comparison with methanol (0.242 × 106 con mL−1) (Fig. 2a). As for sclerotia of Ss, they were formed in a smaller number upon extract application, particularly at doses of 10 and 30 μl mL−1 (P = 0.0068 and P = 0.0470, respectively) but their total weight increased at doses of 1 μl mL−1 or higher (P ≤ 0.0026). The effect of the extracts by themselves on the number and weight of sclerotia was undistinguishable from that of methanol at 50 μl mL−1 (Fig. 2b and c).
Effect of different concentrations of four extracts from Lycium europaeum and methanol on the sporulation of Verticillium dahliae (a), and on the number (b) and the weight (c) of sclerotia of Sclerotinia sclerotiorum by amendment of potato dextrose agar growth medium. In b, the inhibition of the number of sclerotia is presented in comparison with the unamended control. Vertical upper bars represent the standard error of the mean of four replications
Phenolic composition of the extracts
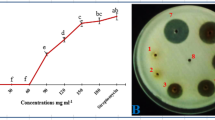
The identification and quantification of the individual phenolic compounds present in the methanolic extracts from leaves and stems of L. europaeum from Gafsa and Jdaida were made by HPLC-DAD and HPLC-MS. As can be observed in Fig. 3, the analytical method allowed the separation of 11 phenolic compounds that included hydroxycinnamic acids and flavonoids, which were identified as: 1: 3-O-caffeoyl, 4-O-feruloylquinic acid; 2: 4-O-caffeoyl, 5-O-feruloylquinic acid; 3: 5-O-caffeoylquinic acid (Chlorogenic acid); 4: 3-O-caffeoylquinic acid; 5: 4-O-caffeoylquinic acid; 6: -O-p-coumaroylquinic acid; 7: 3-O-feruloylquinic acid; 8: 5-O-feruloylquinic acid; 9: Quercetin-3-O-Rhamnoglucoside (Rutin); 10: Q-3-O-glucoside and 11: Kaempherol-3-O-Rhamnoglucoside (Nicotiflorin). Rutin, Q-3-O-glucoside and nicotiflorin, as well as chlorogenic acid, were identified on the basis of their spectral characteristics and comparison to standards. The seven other hydroxycinnamic acids were tentatively identified by means of a combination of the retention times (tR), UV, and mass spectra obtained by HPLC-DAD-MS. As the UV spectra from all the hydroxycinnamic acids are very similar, they were not very useful for identification. MS fragmentation patterns of these compounds were used to obtain more information on their molecular masses and structural characteristics. These analyses revealed the respective presence of two isomers of chlorogenic acid (peaks 3 and 4), the isomer 5-O- being the main hydroxycinnamic acid in all the samples investigated, with the exception of leaves from Gafsa, in which the isomer 3-O- was the most abundant. Regarding the flavonoids, the major content corresponded to rutin (quercetin-3-O-rutinoside) in the four samples analysed.
As can be observed in Table 5, total content of phenolic compounds from the four samples varied from 0.33 to 1.84 mg g−1 dried plant tissue. There were significant differences between leaves and stems from both Gafsa and Jdaida samples. Leaves from those two locations contained a larger amount and greater variability of phenolics than the stems (1.15 and 1.84 mg g−1 versus 0.34 and 0.33 mg g−1). Among the high diversity of phenolic acids obtained in the extracts, hydroxycinnamic acids and derivatives, including chlorogenic acid as a major compound, represented more than 50% of the total content in all the samples. Leaves from Jdaida contained 1.10 mg g−1 whereas the content of leaves from Gafsa was slightly lower (0.98 mg g−1). Smaller amounts of flavonoid compounds were also quantified in leaves, rutin being the most abundant one in samples from both origins, Jdaida (0.66 mg g−1) and Gafsa (0.14 mg g−1). It is worth noting that, although rutin was clearly the most abundant flavonoid, also present were significant amounts of two other flavonoid compounds, one of them (quercetin-3-O-glucoside) derived from the same aglycone as rutin, but the other, nicotiflorin (kaempherol-3-O-rutinoside), was derived from the aglycone kaempherol. The SJ extracts only contained chlorogenic acid in their two isomeric forms in a total quantity of 0.33 mg g−1, and the SG extracts contained two other chlorogenic derivatives and a total amount of 0.34 mg g−1. Flavonoids were only detected in trace amounts in SJ extracts and they were not detected in SG extracts.
Pathogenicity of H. maydis at initial stages after infection
Small necrotic lesions (2–4 mm long) were observed on the roots of inoculated susceptible plants (MO1501) as early as 3 weeks after inoculation. Their size increased over time with lengths of 10–14 mm at the end of the experiment. Lesions caused by isolate Hm01–15 in roots of a four-week-old susceptible plant compared to healthy roots of the control plant are shown in Online Resource 1. Necrotic lesions on the roots of the inoculated resistant plants (MO1504) were very scant and, when they occurred, were no longer than 4 mm. The same time was necessary for the plants to sequentially reach phenological stages irrespective of their reaction to the pathogen (resistant or susceptible) or of the treatment (inoculated or controls). In fact, the phenology of the plants was that expected according to the Growing Degree Days and, at the final time (6 weeks), all the plants were in V6 and close to V7 (Abendroth et al. 2011).
At the end of the experiment, decreases in dry weights of aboveground parts of the plants inoculated with Hm were obtained as compared with control inoculations. The DWAP was significantly dependent on the genotype and on the treatment (P ≤ 0.0401), but not on their interaction, showing that both genotypes reacted similarly to the pathogen. Additionally, no statistical differences in DWAP were found between the two isolates. The DWAP of the susceptible genotype MO1501 was reduced from 1.10 g for the control to 0.59 g (averaged across HmLT809-S and Hm01–15) (Fig. 4a). In the resistant genotype MO1504, the fungus caused a decrease in DWAP from 1.36 g for the control to 0.89 g averaged across isolates (Fig. 4b).
Dry weight of aboveground parts and roots of the susceptible (a) and the resistant (b) genotypes MO1501 and MO1504 respectively, upon inoculation with one of two isolates of Harpophora maydis and growth in the greenhouse for 6 weeks. Vertical upper bars represent the standard error of the mean of eight replications. Bars with the same letter are not significantly different according to the least significant difference test (P = 0.05)
Harpophora maydis also affected the maize roots as early as 6 weeks after inoculation. In fact, DWR was statistically dependent on genotype and on the treatment, as well as on their interaction (P ≤ 0.0006). In the susceptible MO1501, the DWR of inoculated plants was 0.63 g (averaged across HmLT809-S and Hm01–15) compared with 1.09 g of the control plants (Fig. 4a). The significance of the interaction between genotype and treatment showed that reductions in DWR in the resistant MO1504 were much higher: 0.95 g (averaged across isolates) as compared to 2.66 g of the non- inoculated plants (Fig. 4b).
In vivo effect of the extracts against H. maydis
As observed in the pathogenicity experiment (subheading above), the effect of Hm alone was evidenced by significantly decreased weights in both roots and aboveground parts of the plants. Decreased weights were also associated with the treatment with MeOH. The FWAP of the plants depended significantly (P = 0.0002) on the treatment, all of them resulting in significantly lower values of FWAP as compared to those of the controls. No significant effect of the extracts was obtained with respect to the FWAP decreases associated with MeOH or Hm (Fig. 5). In relation to the FWR of the plants, a significant (P = 0.0074) effect from treatments was also obtained. Lower values of FWR were recorded for the Control-MeOH treatment as well as for treatments with LJ and the one with only Hm. However, the detrimental effect of either MeOH or Hm on the plants was counteracted by the LG extract: upon inoculation and watering with it, the FWR of the Hm-LG treatment (18.56 g) did not statistically differ from that of either the controls (21.38 g) or the LG-treated plants (19.85) (Fig. 5).
Fresh weight of aboveground parts and roots of maize upon inoculation with isolate Hm01–15 of Harpophora maydis, watering with methanol or with extracts LG or LJ from Lycium europaeum and growth in the greenhouse for 6 weeks. Vertical upper bars represent the standard error of the mean of four replications. Bars with the same upper (aboveground parts) or lower (roots) case letters are not significantly different according to the least significant difference test (P = 0.05)
Discussion
Sclerotinia sclerotiorum and V. dahliae are among the most important crop pathogens worldwide, and H. maydis causes serious economic losses in maize grown in Mediterranean countries such as Egypt, Israel or Spain (El-Shafey and Claflin 1999; Molinero-Ruiz et al. 2010). Since the limitations of fungicide treatments exert increasing pressure in many regions, current control of these fungi relies heavily on host resistance and cultural practices. Varied alternatives that can be considered should be identified for the management of crop diseases and to ensure the profitability of crops. Under the framework of sustainable agriculture, the use of plant extracts in crop protection is increasing in importance.
The antioxidant activity of extracts from Lycium spp. is widely known (Mocan et al. 2014; Abdennacer et al. 2015; Ghali et al. 2015) and their antimicrobial activity in human health has even been reported (Mocan et al. 2014). To the best of our knowledge, this research describes for the first time the efficacy of Lycium extracts against the growth and sporulation of soil-borne plant pathogens. Mycelial growth of the three fungi and sporulation of H. maydis were clearly reduced and, in some cases, completely inhibited depending on the extract and dose. The inhibition of vegetative growth of phytopathogenic fungi by plant extracts has been previously reported (Abdel-Monaim et al. 2011; Hernández-Castillo et al. 2010; Latha et al. 2009). Regarding increased sporulation (Vd) and enhancement of sclerotia formation (Ss), both have been observed as being a consequence of mycelial stress due to adverse conditions (Carvalho et al. 2008) even upon treatment with plant extracts (Alizadeh et al. 2011; Ochoa-Fuentes et al. 2012). The antifungal properties of some plant extracts against crop pathogens are widely documented (Jasso de Rodríguez et al. 2007; Pane et al. 2017; Švecová et al. 2017).
In agreement with other authors (Jasso de Rodríguez et al. 2015; Wu et al. 2014), the current research found that antifungal activity can be related to contents of total phenolics. The bioactive properties of many plant extracts have been attributed to particular phytochemical constituents, mainly phenolic compounds (Singh et al. 2016; Treutter 2006; Kumar and Pandey 2013). Among them, hydroxycinnamic acids and their derivatives, and flavonoids, possess a wide range of biological activities, including antifungal activity against soil-borne pathogens (Grayer and Harborne 1994; Lattanzio et al. 2006). Previous research has reported the antifungal effect of asparagus by-product extracts enriched in flavonoids against F. oxysporum ff. spp. pathogenic to horticultural crops (Rosado-Alvarez et al. 2014). In the current work, the most abundant phenolics in extracts from L. europaeum were hydroxycinnamic acids and their derivatives (particularly chlorogenic acid), accompanied by minor quantities of flavonoid compounds. Chlorogenic acid is hydroxycinnamic acid ester conjugated to quinic acid, which is widely known for its nutraceutical and antibiotic activities (Santana-Gálvez et al. 2017). The finding that chlorogenic acid is an abundant phenolic derivative in L. europaeum was evidenced by the high contents detected in extracts from leaves as well as from stems of plants collected in both locations (Gafsa or Jdaida). The bioactivity of extracts in this research is likely associated with their high chlorogenic acid content, in agreement with Martínez et al. (2017), who recently reported that this compound inhibits spore germination and reduces mycelial growth of several soil-borne phytopathogenic fungi. As far as flavonoids are concerned, the identification of small amounts of nicotiflorin in addition to rutin is of interest from a functional point of view as it has been well established that the synergistic action among different flavonoids may contribute to enhancing the biological activities attributed to these compounds (Cushnie and Lamb 2005). Further research should address the fungicide effect particularly associated with chlorogenic acid, as well as the role of all compounds detected as conferring antifungal properties to extracts from L. europaeum.
In the current research, the disease of maize caused by H. maydis was successfully reproduced in maize at early stages and under controlled greenhouse conditions. The methodology developed in this work could have applications such as the in vivo assessment not only of plant extracts, but of other types of novel treatments for controlling maize late wilt at the seedling stage.
When the effect of the extracts from L. europaeum on the maize – H. maydis pathosystem was assessed, it was found that the weight of roots from the inoculated plants treated with LG extract, as well as that of the plants only treated with LG, did not significantly differ from that of the non-inoculated controls. Besides a direct effect on H. maydis, a beneficial effect of LG on maize was suggested by these results. Although disease reduction was only observed in treatments with LG, in this research potential use of L. europaeum extracts in crop protection was indicated. In any case, the protective effect of the phenolic compounds studied in this work against late wilt of maize should be further confirmed by means of experiments that address their effect at later growth stage. Bioactive principles present in plant products may act directly on plant pathogens or induce systemic resistance in host plants, resulting in a reduction in disease development. Nevertheless, the allelopathic effects of L. europaeum on soil-borne fungal plant pathogens, on the physiology of the plant, and even on soil microbiota, constitute an underexplored and intriguing research field. In summary, bioactive L. europaeum extracts, if adequately developed into final products and used in combination with other strategies, could play a significant role in crop protection.
References
Abd El-Rahim, M. F., Fahmy, G. M., & Fahmy, Z. M. (1998). Alterations in transpiration and stem vascular tissues of two maize cultivars under conditions of water stress and late wilt disease. Plant Pathology, 47, 216–223.
Abdel-Monaim, M. F., Abo-Elyousr, K. A. M., & Morsy, K. M. (2011). Effectiveness of plant extracts on suppression of damping-off and wilt diseases of lupine (Lupinus termis Forsik). Crop Protection, 30, 185–191.
Abdennacer, B., Karim, M., Yassine, M., Nesrine, R., Mouna, D., & Mohamed, B. (2015). Determination of phytochemicals and antioxidant activity of methanol extracts obtained from the fruit and leaves of Tunisian Lycium intricatum Boiss. Food Chemistry, 174, 577–584.
Abendroth, L. J., Elmore, R. W., Boyer, M. J., & Marlay, S. K. (2011). Corn growth and development, PMR 1009. Ames: Iowa State University Extension.
Agrios, G. N. (1970). Plant pathology. New York and London: Academic Press.
Alizadeh, H., Leung, D. W. M., & Cole, A. L. J. (2011). Conidiogenic effects of mannose-binding lectins isolated from cotyledons of red kidney bean (Phaseolus vulgaris) on Alternaria alternata. Phytochemistry, 72, 94–99.
Amagase, H., & Farnsworth, N. R. (2011). A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (goji). Food Research International, 44, 1702–1717.
Bergstrom, G., Leslie, J., Huber, D., Lipps, P., Warren, H., Esker, P., et al. (2008). Recovery plan for late wilt of corn caused by Harpophora maydis syn. Cephalosporium maydis. Washington, DC: National Plant Disease Recovery System.
Borrego-Benjumea, A., Basallote-Ureba, M. J., Melero-Vara, J. M., & Abbasi, P. A. (2013). Characterization of fusarium isolates from Asparagus fields in southwestern Ontario and influence of soil organic amendments on fusarium crown and root rot. Phytopathology, 104, 403–415.
Campbell, C. L., & Madden, L. V. (1990). Introduction to plant disease epidemiology. New York: John Wiley & Sons.
Carvalho, D. D. C., Alves, E., Batista, T. R. S., Camargos, R. B., & Lopes, E. A. G. L. (2008). Comparison of methodologies for conidia production by Alternaria alternata from citrus. Brazilian Journal of Microbiology, 39, 792–798.
Chellemi, D. O., Gamliel, A., Katan, J., & Subbarao, K. V. (2016). Development and deployment of systems-based approaches for the Management of Soilborne Plant Pathogens. Phytopathology, 106, 216–225.
Cheng, F., & Cheng, Z. (2015). Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Frontiers in Plant Science, 6, 1020. https://doi.org/10.3389/fpls.2015.01020.
Chou, C. H. (2010). Roles of allelopathy in plant biodiversity and sustainable agriculture. Critical Reviews in Plant Sciences, 18, 609–636.
Cowan, M. M. (1999). Plant products as antimicrobial agents. Clinical Microbiology Reviews, 12, 564–582.
Cushnie, T. P., & Lamb, A. J. (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26, 343–356.
Degani, O., & Cernica, G. (2014). Diagnosis and control of Harpophora maydis, the cause of late wilt in maize. Advances in Microbiology, 4, 94–105.
El-Shafey, H. A., & Claflin, L. E. (1999). Late wilt. In D. G. White (Ed.), Compendium of corn diseases (3rd ed., pp. 43–44). St. Paul: American Phytopathological Society (APS Press).
Farooq, M., Jabran, K., Cheema, Z. A., Wahid, A., & Siddique, K. H. M. (2011). The role of allelopathy in agricultural pest management. Pest Management Science, 67, 493–506. https://doi.org/10.1002/ps.2091.
Fernández-Aparicio, M., Reboud, X., & Gibot-Leclerc, S. (2016). Broomrape weeds. Underground mechanisms of parasitism and associated strategies for their control: A review. Frontiers in Plant Science, 7, 135. https://doi.org/10.3389/fpls.2016.00135.
Fuentes-Alventosa, J. M., Rodríguez, G., Cermeño-Sacristán, P., Jiménez, A., Guillén, R., Fernández-Bolaños, J., et al. (2007). Identification of flavonoid diglycosides in several genotypes of asparagus from the Huétor Tájar population variety. Journal of Agricultural and Food Chemistry, 55, 10028–10035.
Gahukar, R. T. (2012). Evaluation of plant-derived products against pests and diseases of medicinal plants: A review. Crop Protection, 42, 202–209.
García-Ruiz, R., García-Carneros, A. B., & Molinero-Ruiz, L. (2014). A new race of Verticillium dahliae causing leaf mottle of sunflower in Europe. Plant Disease, 98, 1435. https://doi.org/10.1094/PDIS-04-14-0360-PDN.
Geiger, F., Bengtsson, J., Berendse, F., Weisser, W. W., Emmerson, M., Morales, M. B., Ceryngier, P., Liira, J., Tscharntke, T., Winqvist, C., Eggers, S., Bommarco, R., Pärt, T., Bretagnolle, V., Plantegenest, M., Clement, L. W., Dennis, C., Palmer, C., Oñate, J. J., Guerrero, I., Hawro, V., Aavik, T., Thies, C., Flohre, A., Hänke, S., Fischer, C., Goedhart, P. W., & Inchausti, P. (2010). Persistent negative effects of pesticides on biodiversity and biological control potential of European farmland. Basic and Applied Ecology, 11, 97–105.
Ghali, W., Vaudry, D., Jouenne, T., & Marzouki, M. N. (2015). Lycium europaeum fruit extract: Antiproliferative activity on A549 human lung carcinoma cells and PC12 rat adrenal medulla Cancer cells and assessment of its cytotoxicity on cerebellum granule cells. Nutrition and Cancer, 67, 637–646. https://doi.org/10.1080/01635581.2015.1017054.
Grayer, R. J., & Harborne, J. B. (1994). A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry, 37, 19–42.
Gulya, T., Rashid, K., & Masirevic, S. (1997). Sunflower diseases. In A. A. Scheneiter (Ed.), Sunflower technology and production (pp. 263–379). Madison: Am. Soc. Agr., Crop Sci. Soc. Am. and Soil Sci. Soc. Am.
Heffer Link, V., & Johnson, K. B. (2007). White mold. The Plant Health Instructor. American Phytopathological Society. https://doi.org/10.1094/PHI-I-2007-0809-01.
Hernández-Castillo, F. D., Castillo-Reyes, F., Gallegos-Morales, G., Rodríguez-Herrera, R., & Aguilar-González, C. N. (2010). Lippia graveolens and Carya illinoensis organic extracts and their in vitro effect against Rhizoctonia solani Kühn. American Journal of Agricultural and Biological Sciences, 5, 380–384.
Hillocks, R. J. (2012). Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Protection, 31, 85–93.
Jasso de Rodríguez, D., Hernández-Castillo, D., Angulo-Sánchez, J. L., Rodríguez-García, R., Villarreal Quintanilla, J. A., & Lira-Saldivar, R. H. (2007). Antifungal activity in vitro of Flourensia spp. extracts on Alternaria sp., Rhizoctonia solani, and Fusarium oxysporum. Industrial Crops and Products, 25, 111–116.
Jasso de Rodríguez, D., Trejo-González, F. A., Rodríguez-García, R., Díaz-Jimenez, M. L. V., Sáenz-Galindo, A., Hernández-Castillo, F. D., Villarreal-Quintanilla, J. A., & Peña-Ramos, F. M. (2015). Antifungal activity in vitro of Rhus muelleri against Fusarium oxysporum f. Sp. lycopersici. Industrial Crops and Products, 75, 150–158.
Kumar, S., & Pandey, A. (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal, 2013, 1–16. https://doi.org/10.1155/2013/162750.
Langcake, P., Irvine, J. A., & Jeger, M. J. (1981). Alternative chemical agents for controlling plant disease. Philosophical Transactions of the Royal Society B, 295, 83–101.
Latha, P., Anand, T., Ragupathi, N., Prakasam, V., & Samiyappan, R. (2009). Antimicrobial activity of plant extracts and induction of systemic resistance in tomato plants by mixtures of PGPR strains and Zimmu leaf extract against Alternaria solani. Biological Control, 50, 85–93.
Lattanzio, V., Lattanzio, V. M. T., & Cardinali, A. (2006). Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. In F. Imperato (Ed.), Phytochemistry: Advances in research (pp. 23–67). Kerala: Research Signpost.
Martínez, G., Regente, M., Jacobi, S., Del Rio, M., Pinedo, M., & de la Canal, L. (2017). Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pesticide Biochemistry and Physiology, 140, 30–35. https://doi.org/10.1016/j.pestbp.2017.05.012.
Martin-Sanz, A., Rueda, S., Garcia-Carneros, A. B., Gonzalez-Fernandez, S., Miranda-Fuentes, P., Castuera-Santacruz, S., et al. (2018). Genetics, host range, and molecular and pathogenic characterization of Verticillium dahliae from sunflower reveal two differentiated groups in Europe. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2018.00288.
Mocan, A., Vlase, L., Vodnar, D. C., Bischin, C., Hanganu, D., Gheldiu, A. M., et al. (2014). Polyphenolic content, antioxidant and antimicrobial activities of Lycium barbarum L. and Lycium chinense mill. leaves. Molecules, 19, 10056–10073. https://doi.org/10.3390/molecules190710056.
Molinero-Ruiz, M. L., Dominguez, J., & Melero-Vara, J. M. (2002). Races of isolates of Plasmopara halstedii from Spain and studies on their virulence. Plant Disease, 86, 736–740.
Molinero-Ruiz, M. L., Pérez-Vich, B., Pineda-Martos, R., & Melero-Vara, J. M. (2008). Indigenous highly virulent accessions of the sunflower root parasitic weed Orobanche cumana. Weed Research, 48, 169–178. https://doi.org/10.1111/j.1365-3180.2007.00611.x.
Molinero-Ruiz, M. L., García-Ruiz, R., Melero-Vara, J. M., & Dominguez, J. (2009). Orobanche cumana race F: Performance of resistant sunflower hybrids and aggressiveness of populations of the parasitic weed. Weed Research, 49, 469–478. https://doi.org/10.1111/j.1365-3180.2009.00708.x.
Molinero-Ruiz, M. L., Melero-Vara, J. M., & Mateos, A. (2010). Cephalosporium maydis, the cause of late wilt in maize, a pathogen new to Portugal and Spain. Plant Disease, 94, 379.
Molinero-Ruiz, L., Delavault, P., Pérez-Vich, B., Pacureanu-Joita, M., Bulos, M., Altieri, E., & Domínguez, J. (2015). History of the race structure of Orobanche cumana and the breeding of sunflower for resistance to the parasitic weed: A review. Spanish Journal of Agricultural Research, 13, e10R01. https://doi.org/10.5424/sjar/2015134-8080.
Ochoa-Fuentes, Y. M., Cerna-Chávez, E., Landeros-Flores, J., Hernández-Camacho, S., & Delgado-Ortiz, J. C. (2012). Evaluation in vitro of the anti-fungal activity of four methanol plant extracts for the control of three species of Fusarium spp. Φyton, 81, 69–73.
Ortiz-Bustos, C. M., García-Carneros, A. B., & Molinero-Ruiz, L. (2015). La marchitez tardía del maíz (Zea mays L.) causada por Cephalosporium maydis en la Península Ibérica, y otros hongos asociados. Summa Phytopathologica, 41, 107–114. https://doi.org/10.1590/0100-5405/1998.
Ortiz-Bustos, C. M., Testi, L., García-Carneros, A. B., & Molinero-Ruiz, L. (2016). Geographic distribution and aggressiveness of Harpophora maydis in the Iberian peninsula, and thermal detection of maize late wilt. European Journal of Plant Pathology, 144, 383–397. https://doi.org/10.1007/s10658-015-0775-8.
Pane, C., Francese, G., Raimo, F., Mennella, G., & Zaccardelli, M. (2017). Activity of foliar extracts of cultivated eggplants against sclerotinia lettuce drop disease and their phytochemical profiles. European Journal of Plant Pathology. https://doi.org/10.1007/s10658-016-1126-0.
Pegg, G. F., & Brady, B. L. (2002). Verticillium wilts. New York: CABI Publishing ISBN 0851995292.
Potterat, O. (2010). Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Medica, 76, 7–19.
Rosado-Alvarez, C., Molinero-Ruiz, L., Rodríguez-Arcos, R., & Basallote-Ureba, M. J. (2014). Antifungal activity of asparagus extracts against phytopathogenic Fusarium oxysporum. Scientia Horticulturae, 171, 51–57.
Santana-Gálvez, J., Cisneros-Zevallos, L., & Jacobo-Velázquez, D. A. (2017). Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules, 22, E358. https://doi.org/10.3390/molecules22030358.
Singh, G., Passsari, A. K., Leo, V. V., Mishra, V. K., Subbarayan, S., Singh, B. P., et al. (2016). Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Frontiers in Plant Science, 7, 407. https://doi.org/10.3389/fpls.2016.00407.
Soliman, F. H. S., & Sadek, S. E. (1998). Combining ability of new maize inbred lines and its utilization in the Egyptian hybrid program. Bulletin of the Faculty of Agriculture, Cairo University, 50, 1–20.
Švecová, E., Proietti, S., Caruso, C., Colla, G., & Crinò, P. (2013). Antifungal activity of Vitex agnus-castus extract against Pythium ultimum in tomato. Crop Protection, 43, 223–230.
Švecová, E., Colla, G., & Crinò, P. (2017). Antifungal activity of Boerhavia diffusa L. extract against Phytophthora spp. in tomato and pepper. European Journal of Plant Pathology, 148, 27–34. https://doi.org/10.1007/s10658-016-1065-9.
Thines, M., & Kamoun, S. (2010). Oomycete–plant coevolution: Recent advances and future prospects. Current Opinion in Plant Biology, 13, 427–433.
Treutter, D. (2006). Significance of flavonoids in plant resistance: A review. Environmental Chemistry Letters, 4, 147–157. https://doi.org/10.1007/s10311-006-0068-8.
Wu, H. S., Zhou, X. D., Shi, X., Liu, Y. D., Wang, M. Y., Shang, X. X., Gu, D. L., Wang, W. Z., & Wu, C. W. (2014). In vitro responses of Fusarium oxysporum f.Sp. niveum to phenolic acids in decaying watermelon tissues. Phytochemistry Letters, 8, 171–178.
Acknowledgements
The authors thank A.B. García-Carneros for excellent technical assistance.
Funding
This research was partially supported by grants AGL2010–17909 (Ministerio de Economía y Competitividad, Spain) and P12-AGR1281 (Andalusian Government, Spain) and the European Regional Development Fund (ERDF). The stay of R. Tej was granted by the Ministry of Higher Education and Scientific Research in Tunisia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Each of the authors declare that the/she has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
We hereby state that our manuscript complies to the Ethical Rules applicable for the European Journal of Plant Pathology.
Electronic supplementary material
ESM 1
(PPTX 539 kb)
Rights and permissions
About this article
Cite this article
Tej, R., Rodríguez-Mallol, C., Rodríguez-Arcos, R. et al. Inhibitory effect of Lycium europaeum extracts on phytopathogenic soil-borne fungi and the reduction of late wilt in maize. Eur J Plant Pathol 152, 249–265 (2018). https://doi.org/10.1007/s10658-018-1469-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-018-1469-9