Abstract
Spinach anthracnose caused by Colletotrichum spinaciae is a newly emergent disease that causes serious losses in the spinach fields of the Mediterranean Region of Turkey. The most popular spinach cv. Matador is very susceptible to the anthracnose. Currently, anthracnose disease is not effectively controlled in Turkey due to the lack of understanding of its epidemiology. In order to understand the effect of environmental parameters on anthracnose, the influences of inoculum concentration, temperature, leaf wetness duration, plant and leaf age on lesion development of spinach anthracnose was studied under controlled conditions. Seedlings of spinach cv. Matador at 4-leaf stage were inoculated with four inoculum concentrations, 102, 104, 106, 2 × 106 conidia/ml, and subjected to seven temperatures of 15, 18, 22, 25, 28, 30, and 33 °C, and nine leaf wetness durations of 3, 5, 10, 20, 24, 30, 44, 48, and 53 h. The mean number of lesions per plant increased at the rate of 50.7% with increasing inoculum density from 104 to 106 conidia per millilitre. Inoculum concentration with 106 conidia per ml of C. spinaciae was appropriate for infection and lesion development. Disease was observed at all temperatures and increased with wetness duration. The development of lesions per plant was observed more gradually when incubation temperature increased from 15 °C to 22 °C. The mean lesion number of anthracnose increased on spinach seedlings as wetness duration increased to 24 h and temperatures to 22 °C and 25 °C. Regression analysis revealed that there was a linear relationship between the increase in number of lesions per plant and wetness duration at 18, 25, and 30 °C. Optimum disease development was recorded at 22 °C with 24 h leaf wetness period. The highest number of lesions was observed at 45 d-old plants. In the same time, older leaves were more severely infected than younger leaves. This kind of information can be used to target key stages of an epidemic and may allow tailoring of spray programs or disease forecasts to cultivar with different levels of anthracnose resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinach (Spinacia oleracea L.) is an economically important green leafy vegetable crop in many countries (Correll et al. 1994). World production of spinach is estimated at 23.2 million tonnes (MT) from an estimated 911.000 ha with an average yield of 250 Kg/ha (FAOSTAT 2015). Based on FAO statistics, the five spinach producing countries are China (21 MT), USA (0.336 MT), Japan (0.258 MT), Turkey (0.220 MT), and Indonesia (0.131 MT). Spinach plant is cultivated mainly for fresh consumption in fall, winter, or spring seasons annually with an approximately 16.600 ha throughout the Turkey (TUIK 2015). Diseases cause significant problems affecting both yield and quality during growing season of spinach in Turkey and worldwide (Kurt et al. 2016; Correll et al. 1994). Spinach is susceptible to several fungal and viral diseases, including anthracnose that is generally recognized as economically important (Correll et al. 1994). Numerous pathogens have been implicated as causal agents with this diseases and yield decline problem. The fungi, Albugo occidentalis, Peronospora farinosa, Colletotrichum spinaciae, Cladosporium variabile, Stemphylium botryosum, Rhizoctonia solani, Fusarium oxysporum f.sp. spinaciae, and Verticillium dahlia, were reported as the most important pathogens (Larsson and Gerhardson 1992). The genus Colletotrichum includes a number of plant pathogens of major importance, causing a wide variety of diseases in woody and herbaceous plants. It has a primarily tropical and subtropical distribution, although there are some high-profile species affecting temperate crops. As plant pathogens, Colletotrichum species are primarily described as causing anthracnose diseases (Cannon et al. 2012).
Anthracnose caused by the filamentous fungal plant pathogen Colletotrichum spinaciae Ellis and Halst. [Colletotrichum dematium (Pers.) Grove f.sp. spinaciae (Ellis and Halst.) Arx], can cause serious symptoms on spinach leaves and food losses of about 50% of total production under some advantageous environmental conditions (Koike and Correll 1993; Correll et al. 1994; Cannon et al. 2012). In earlier studies, the disease has been reported as Colletotrichum dematium from Canada (Cerkauskas et al. 1991), Australia (Washington et al. 2006), and as C. spinaciae from Italy, Netherlands and UK (Damm et al. 2009). Anthracnose was described for the first time as a spinach pathogen in Hatay, Adana, and Mersin provinces in the Mediterranean Region of Turkey (Kurt et al. 2016). Anthracnose lesions were also observed on some host in and around the spinach fields. Therefore, some plants such as lamb’s-quarters, purslane, parsley, and clover were tested as hosts for C. spinaciae by inoculation under controlled conditions. After all, infection was detected on tested plants (Kurt et al. 2014). The plant pathogen C. spinaciae occupies a well-defined sub clade distinct from a separate sub clade made up of the putatively saprobes species such as C. dematium, C. lineola, C. fructi and C. anthrisci (Damm et al. 2009; Cannon et al. 2012). Ellis and Halsted (1890) first described the spinach anthracnose fungus from New Jersey as C. spinaciae. Sherf and MacNab (1986) described two species capable of causing spinach anthracnose C. spinaciae and C. spinacicola. C. spinacicola had small straight conidia and was more virulent than C. spinaciae. Currently, neither species designation is considered valid. Von Arx (1970) described the spinach anthracnose pathogen as C. dematium but, recognizing host specialization, designated it as C. dematium f.sp. spinaciae. It has been widely reported as a pathogen of spinach and beet and research indicates that individually strains can be highly host specific (Damm et al. 2009). However, after all we have often continued to use the name C. spinaciae when referring to a pathogen that is associated with spinach anthracnose.
Anthracnose symptoms on both young and old leaves at first include small, circular, water-soaked, and tan coloured lesions. These often enlarge and become chlorotic, blight and necrotic as well as stunting of infected spinach plants. Under wet or humid conditions, anthracnose lesions produce dark acervuli, an asexual reproductive structure, with setae which are useful diagnostic characteristics in the middle of some leaf spots (Correll et al. 1994; Washington et al. 2006; Kurt et al. 2016). In this stage, conidia produced in acervuli, appressorial development and secondary conidiation of the pathogen have an important role for epidemiology of the disease. Ultimately, spinach leaves become blighted as lesions coalesced.
Factors such as inoculum concentration, temperature, and moisture can affect the progress of anthracnose infections. Extensive studies have been conducted to examine effects of inoculum density, wetness duration, plant age, inoculation method, and cultivar resistance on the development of pepper anthracnose caused by Colletotrichum coccodes. In result, as inoculum density of C. coccodes increased from 103 to 106 conidia per ml, symptoms of anthracnose developed. Disease severity also increased with increasing time of wetness duration from 0 to 60 h. However, wetness duration above 48 h and a high inoculum density at 106 conidia/ml caused severe defoliation and blight symptoms in pepper seedlings (Hong and Hwang 1998). In a previous study, it was indicated that anthracnose severity on tomato fruit increased as inoculum density increased from 103 to 106 conidia/ml (Dillard 1989).
Wetness duration and temperature are generally the main microclimatic parameters determining the development of fungal plant diseases (Diéguez-Uribeondo et al. 2011). For most fungal pathogens in most terrestrial environments, infection is usually limited by the duration of surface wetness or high humidity and temperature (Campbell and Madden 1990). Therefore, temperatures and wetness duration are generally known to be the main microclimatic parameters determining the development of fungal plant diseases (Monroe et al. 1997; Byrne et al. 1998). Thus, models to predict anthracnose and other foliar diseases in different host systems have been based on climatic parameters and relationships between temperature and leaf wetness duration derived from experiment under controlled conditions (Monroe et al. 1997). The incidence and severity of anthracnose can change from season to season and according to regions, demonstrating the influence of environment on disease development (Sosnewski et al. 2005). C. spinaciae infections observed in Turkey revealed that environmental conditions such as high moisture, temperature and rainfall, along with inoculum density of the pathogen and plant age, affected incidence and development of spinach anthracnose (Kurt et al. 2014). Such conditions with well-defined environmental parameters provide a basis for understanding the effects of temperature and leaf wetness duration on disease development. Damicone et al. (2013) briefly reported that maximum severity of spinach anthracnose was between 18.3 °C and 26.7 °C, and about 50% at 96 h of wetness duration in desiccator. In controlled environment experiments conducted to determine the relationship between temperature (Monroe et al. 1997), leaf wetness duration, and infection of watermelon by Colletotrichum orbiculare, watermelon seedlings were inoculated and exposed to various combinations of temperature (12, 15, 18, 21, 24, 27, and 30 °C) and leaf wetness duration (2, 4, 8, 12, 16, and 24 h). Anthracnose incidence, defined as the percentage of symptomatic seedlings in each flat 10 days after inoculation, increased with increasing leaf wetness duration at all levels of temperature. The optimum temperature for infection ranged from 21 to 24 °C. In this context, leaf wetness is not only important factor for disease development, but its duration will also affect the minimum temperature required for disease to develop. Furthermore, there is no information available on the effects of plant and leaf ages and different inoculum densities on development of spinach anthracnose. Much research has been performed on epidemiology of anthracnose diseases caused by Colletotrichum spp. infecting different plants (Byrne et al. 1998; Chongo and Bernier 2000; Guyot et al. 2005; Dieguez-Uribeondo et al. 2011; Moral et al. 2012; Lima et al. 2015), but little information exists on the effects of environmental factors on epidemics of spinach anthracnose. It is currently not understood what factors have triggered the higher occurrence of anthracnose on spinach in Turkey. Specific effects of environmental factors on spinach anthracnose in the Mediterranean Region of Turkey are not known. More detailed studies on the effects of environmental and other factors on spinach anthracnose could provide a better understanding of the disease and lead to appropriate disease control strategies under natural conditions. Such understanding would contribute toward developing genetic improvement programs for this crop. However, because of the lack of epidemiological information, these results will be used as a benchmark to represent the minimum temperature and leaf wetness conditions necessary for fungal infection of spinach leaves under the specific environmental conditions.
An integrated disease management approach including the use of disease-resistant cultivars, crop rotation, careful irrigation, fertility management, weed control, and fungicides is often necessary to produce a high-quality product (Correll et al. 1994; Kurt et al.2014). There are no fungicides currently labelled for anthracnose caused by C. spinaciae on spinach in Turkey. It is recommended preventative control for anthracnose (Colletotrichum spp.) on various crops with multisite protectant, quinone inhibitors or strobilurins, and demethylation inhibitor fungicides such as azoxystrobin, famoxadone, trifloxystrobin, pyraclostrobin, copper hydroxide, mancozeb, chlorothalonil, fludioxonil plus cyprodinil, difenoconazole, famoxadone plus cyprodinil (Harp et al. 2014; Rodriguez-Salamanca et al. 2015). Also, disease management using programs containing fungicides, biological control agents, and systemic acquired resistant activators have been incorporated into disease control programs. Quantitative knowledge of the favourable factors for disease development could help for both timing and reducing the number of fungicide applications, and developing efficient breeding strategies in growing resistant cultivars. Also, measures for controlling anthracnose disease could be improved if the interactions between the above-mentioned factors and disease development were known.
Recent evidence of the increasing incidence of this study highlights the need for epidemiological studies of the disease. Thus, the present experiment was undertaken to understand the effects of inoculum concentration, temperature, leaf wetness duration, plant and leaf age on lesion development of anthracnose on spinach under controlled conditions. Outcomes of this study are believed to provide practical solutions for spinach growers.
Materials and methods
Plant production
Seeds of spinach were sown into 0.9-l plastic pots filled with sterilized mixture of peat and perlite (4:1, v/v) for the experiments consisting of inoculum concentration, leaf-wetness duration, temperatures and plant age. The spinach cultivar ‘Matador’ used in this trial is commercially cultivated in Hatay province and other spinach growing areas of Turkey. Plants were grown in a controlled growth chamber (Percival Scientific, Inc., USA) adjusted to 20–27 °C / 12 h fluorescent light and 18–22 °C/12 h dark. Two weeks after seeding, spinach seedlings were watered with deionized water and fertilized with water-soluble herbal organic fertilizer (1 ml/l of water) once a week to field capacity of the growing medium. Fertilization proceeded weekly during the course of the study.
Inoculum preparation
Infected tissues showing typical symptoms of anthracnose were gathered from commercially spinach fields of Hatay province in the Mediterranean region of Turkey during 2011 to 2013. The fungus C. spinaciae was isolated from symptomatic leaf tissues on spinach dextrose agar (SDA, juice of 250 g spinach, 20 g of dextrose, 20 g agar in 1 l distilled water) amended with 50 mg/l of antibiotic streptomycin sulphate to inhibit bacterial growth in petri dishes. Molecular identification of single conidial isolate of C. spinaciae was also confirmed by actin and β-tubulin (TUB 2) gene sequencing (Kurt et al. 2016). SCs1 isolate of C. spinaciae was used in trials. Petri plates were incubated at 25 °C for 7–10 days in darkness. A sterile filter paper discs were aseptically placed on the surface of SDA ¼ strength media in Petri plates. Mycelial plugs (3 mm diameter) of C. spinaciae were then placed on the sterile filter paper and allowed to grow for 7–10 days in the plates until the mycelial growth completely covered the filter paper dishes. The filter paper containing fungal growth was then raised from the agar, allowed to air-dry for one day under sterile conditions, and maintained at 24 °C on SDA in the dark until used.
Prior to inoculation of C. spinaciae, the filter paper discs were removed from storage, placed on potato dextrose agar (PDA) plates amended with streptomycin sulphate at the same concentration, and incubated at 24 °C for 7 days under florescent light at 12 h day/night cycles. Agar blocks (approximately 2 × 2 mm) containing actively growing mycelia of C. spinaciae were then subcultured to PDA plates, the plates were wrapped with parafilm, and incubated at the same conditions. For inoculum, conidial suspension was made by flooding the surface of the colony with sterile distilled water by scraping the surface of the plates using sterilized needle and by splitting acervuli with pestle. The obtained suspension was then filtered through four layers of sterile cheesecloth. Tween 20 (0.1%) was added to conidial suspension as wetting agent. An aqueous suspension was adjusted to 106 conidia per ml with the aid of a haemocytometer. Later, 5-week-old plants of spinach cv. Matador, highly susceptible against spinach anthracnose (Kurt et al. 2014), were inoculated with 106 conidia ml−1 suspension by using a handheld pump sprayer until incipient run-off. Conidial suspension of C. spinaciae isolate SCs1 (106 conidia/ml) was used in all other experiments. The seedlings were incubated at 20 °C for 48 h by covering the plastic pots with polyethylene bags in a moist chamber. After removing the bags, plants were maintained for 24 h at 80–90% relative humidity (RH). Alternatively, agar disc inoculation method was used to prepare the fungal inoculum. For this purpose, fresh spinach leaves with 70% ethanol were placed on PDA medium in Petri dishes. Then, fungal mycelial discs of C. spinaciae were placed on fresh leaves of spinach, and incubated at 25 °C, 70% relative humidity (RH), and 24 h dark for 7 days.
Effects of inoculum concentration on lesions development
The effect of inoculum density on incidence of leaf lesions was examined by spraying on both sides of leaves of 4- to 6- week-old seedlings of spinach cv. Matador with desired conidial concentration using a handheld pump sprayer. The concentration of spore suspensions 102, 104,106 and 2 × 106 conidia/ml of C. spinaciae were prepared with a series of dilutions in sterile distilled water containing Tween 20 as wetting agent. Fifteen plants treated with sterile distilled water only were used as control. Inoculated and uninoculated plants of spinach were sealed with a moist polyethylene bags to maintain a saturated atmosphere and placed in a growth chamber at 20 °C for 48 h to allow disease development. After incubation, the plants were removed, and maintained in the natural growing environment (approximately night at 5–6 °C, daytime at 16–18 °C, winter season) for 30 days. Under these conditions, maximum humidity (100%) occurs in plants through the winter rains. Plots were arranged in a completely randomized design with three replications per treatment. Each plot consisted of 15 plants. Forty-five plants were scored for disease evaluation at each inoculum concentration. Spinach seedlings were checked daily until the first lesion appeared. Each plant was individually evaluated for the anthracnose incidences. The experiment was conducted twice. After incubation is complete, lesions on the 4 leaves of each spinach plant were counted one by one. Disease incidence was calculated as the average lesion number per leaf on spinach plants inoculated with isolate SCs1 of C. spinaciae.
Effects of log inoculum concentration on lesion development of anthracnose disease were analysed using the IBM-SPSS Statistics 22 software (SPSS, an IBM Company, and Chicago, IL, USA). Process means were separated using Duncan’s Multiple Range Test (p = 0.05) (Kurt and Tok 2006). Regression procedures were performed to assess the relationship between inoculum concentration (independent variable x) and number of anthracnose lesions (dependent variable y). Regression equations were developed for the 45 treatment means using the IBM-SPSS Statistical version 22.
Effect of temperature and leaf wetness duration on lesion development
A factorial design of seven different temperature levels, ranging from 15 to 33 °C (15, 18, 22, 25, 28, 30, and 33 °C) and nine leaf wetness durations (3, 5, 10, 20, 24, 30, 44, 48, and 53 h) for lesion development were evaluated as stated below. For each temperature regime, plants were exposed to nine leaf wetness periods. Seedlings at 4-leaf stage were inoculated by spraying conidial suspensions (106 conidia/ml of C. spinaciae) as described above. Based on a preliminary experiment testing the effect of inoculum concentration on lesion development, 106 conidia/ml were an effective concentration to use for inoculation. These seedlings were covered with plastic bags immediately after inoculation. Then, they were removed 3, 5, 10, 20, 24, 30, 44, 48, 53 h later, while the control left uncovered (0 h). At each temperature, inoculated and un-inoculated plants were placed in the dew chamber. Seedlings dried with fan were then moved to natural growing environment. The pots were watered with tap water every few days during wetness period to keep the potting mixture moist. The experimental design was a split-plot with all units arranged with three replications in a randomized complete block. A total of 15 plants were selected for each wetness duration-temperature combination. Fifteen plants treated with sterile distilled water only were used as control. Each treatment of temperature/duration of leaf wetness trial was repeated two times. Individual plants were assessed in detail at 3- day intervals after inoculation by counting the total number of leaf lesions.
Effect of temperature and leaf wetness duration on lesion development of anthracnose were analysed by using the IBM-SPSS Statistics 22 software (SPSS, an IBM Company, and Chicago, IL, USA). The mean number of plants with anthracnose infection was compared using Duncan’s Multiple Range Test (p = 0.05). The relationship between the mean number of leaf lesions and wetness periods was analysed by linear regression separately for each temperature regime. Regression procedures were performed to assess the relationship between epidemiological parameters temperature and leaf wetness duration (independent variable x) and number of anthracnose lesions (dependent variable y). Regression equations were developed for the 15 treatment means using SPSS software.
Effect of temperature on C. spinaciae sporulation
In the present study, effects of low (15 °C) and high (33 °C) temperatures on sporulation of the C. spinaciae were investigated. Spinach plants at the 4–6 leaf stages were sprayed with conidial suspension at 106 conidia/ml of C. spinaciae. Each treatment with three replications consisted of 15 plants. Inoculated and uninoculated plants were placed in the moist chamber and incubated separately in both 15 °C and 33 °C temperatures during 48 h. Later, spinach seedlings were moved to natural growing conditions for infection and lesion development. Twenty days after inoculation, the number of lesion per cm2 of spinach leaf surface and the number of spores per lesion were calculated on 15 plants. Lesion number (cm−2 leaf) and the number of spores per lesion were evaluated using a pair multiple comparison based on t-test at p = 0.05.
Effect of plant and leaf age on lesion development
In order to determine effects of plant and leaf age on lesion development, 20, 45, and 60-day-old spinach plants were spray- inoculated with a conidial suspension at 106 conidia/ml. Inoculated plants were placed in the moist chamber and incubated during 48 h. Then, they were moved to natural growing ambiance for lesion development. After 30 day of incubation, the number of lesions was counted in 20, 45, and 60-day-old spinach plants and the youngest and the oldest leaves of each plant. Lowest two leaves of the spinach plants were determined as the oldest leaves and upper two leaves as the youngest leaves. The experiments were arranged in a randomized complete block design with four replications (fifteen plants per replication) per treatment. Fifteen plants treated with sterile distilled water only were used as control.
Effects of plant and leaf age on lesion development of anthracnose disease were assessed by analysis of variance using SPSS statistical software. Means of treatment were separated using Duncan’s multiple range test (p = 0.05). Regression procedures were performed to assess the relationship between epidemiological parameters plant and leaf age (independent variable x) and number of anthracnose lesions (dependent variable y). Regression equations were developed for the 15 treatment means using SPSS software.
Results
Effect of inoculum density on lesions development
The fungal developments on surface-sterilized plant tissues plated onto SDA demonstrated that the causal agent produced a cottony white to pale-grey mycelium with hyaline, one-celled, falcate conidia borne on conidiophores in acervuli.
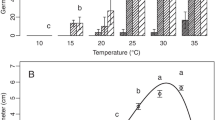
Conidia produced on leaves of infected spinach plants are undoubtedly important in the epidemiology of spinach anthracnose caused by C. spinaciae. When evaluated at different log conidial concentrations of C. spinaciae isolate SCs1, thirty days after inoculation, the lesion development of anthracnose was significantly (P ≤ 0.05) affected by leaf age (Fig. 1). The mean number of lesions per plant increased at the rate of 50.7% with increasing inoculum concentration from 104 to 106 conidia per ml. Ten days after inoculation, anthracnose symptoms were first appeared on leaves of spinach plants. Disease development occurred in all trials of the experiment. Inoculum density with 106 conidia per ml of C. spinaciae was appropriate for infection and lesion development. Subsequently, the highest number of lesion were recorded at concentration of 1 × 106 conidia per ml with 15.04% on first leaves, followed by concentration at 2 × 106 conidia per ml with 13.33% on same leaves (Fig. 1). The lowest lesion number was found in all concentrations on fourth leaves. To our knowledge, this is the first study on epidemiological factors influencing the development of spinach anthracnose.
Effect of log conidial concentration on lesion area per leaf on spinach plants inoculated with isolate SCs1 of Colletotrichum spinaciae in a controlled environment chamber. Values represent the mean lesion number of 45 plants inoculated with each of four inoculum concentrations (102, 104, 1 × 106, 2 × 106 conidia/ml). In each inoculum concentration, values followed by the same letter on lines are not significantly (P = 0.05) different according to Duncan Multiple Range Test. Bar represent standard error of the mean
Regression analysis indicated that there is a linear positive relationship between inoculum concentration and lesion development at both young and old leaf stages. Nevertheless, the coefficient of determination (r2) was 0.938 for all log inoculum concentrations. Also, control spinach plants inoculated with sterile distilled water and tween 20 showed no symptoms.
Effect of temperature and leaf wetness duration on lesion development
Leaf wetness duration (P ≤ 0.05) and temperatures (P ≥ 0.1) had a significant effect on anthracnose development. C. spinaciae isolate SCs1 was able to infect leaves and cause symptoms in all combinations of temperatures and leaf wetness duration tested. Un-inoculated plants without a wetness period were not infected. The findings indicated that the development of lesions per plant was observed more gradually when incubation temperature increased from 15 °C to 22 °C. The lowest lesion number was recorded at 33 °C with mean value of 0.61 in all wetness duration. The mean lesion number of anthracnose increased on spinach seedlings as wetness duration increased to 24 h and temperatures to 22 °C and 25 °C. But, a slight reduction was observed in lesion development when the wetness period increased from 30 to 53 h. The lowest number of lesions was recorded at 3 h wetness duration. Additionally, optimum disease development was recorded at 22 °C with 24 h leaf wetness period (Fig. 2). Knowledge on optimal disease development is an important factor in assessing epidemiology of spinach anthracnose. Data on temperature and wetness period mentioned above confirm the natural infection conditions of the pathogen on spinach plant in which the disease is widespread in November and April.
The linear responses derived from the effect of temperature and wetness duration on anthracnose symptoms required for disease severity and equations describing them are shown in Fig. 3. Regression analysis revealed that there was a linear relationship between the increase in number of lesions per plant and wetness duration (P ≤ 0.05) at 18, 25 and 30 °C. But, it was not observed a significant relationship between lesion development and leaf wetness durations at temperatures 15, 22, 28 and 33 °C (P ≤ 0.05) (Fig. 3).
Effect of temperature on sporulation of the C. spinaciae
In this trial, effects of temperature on lesion development per plant and number of spore in each lesion were investigated. Consequently, the number of lesions per cm−2 of each leaf was 0.493 and 0.450 at 15 °C and 33 °C, respectively. Furthermore, the mean spore number of lesions cm−2 of inoculated leaf surface was significantly (P ≤ 0.05) higher (3.86) on plants that were incubated at 15 °C, when compared (0.0) with plants that were incubated at 33 °C (Table 1).
Effect of plant and leaf age on lesion development
In the present study, the first anthracnose symptoms appeared on leaves of spinach plants 20 days after inoculation. Disease development occurred in all trials of the experiment. The fungus C. spinaciae produced and released conidia from old lesions on leaves, when wetted for several days, but no acervuli were detected. The highest number of lesions was observed at 45 d- old plants (Fig. 4). In the same time, older leaves were more severely infected than younger leaves. At 20 days, the number of lesion on young leaves was higher than old leaves. At 45- to 60- d- old plants, on the contrary, lesion number on old leaf was significantly higher than young leaf. Fifteen days later, lesion numbers on both plant ages decreased significantly to approximately 50% level. ANOVA exhibited that plant age and leaf age had statistically significant (P ≤ 0.05) effects on lesion development on spinach leaves. Regression analysis on spinach plants with different ages showed that there was no a linear relationship between younger and older leaves (Fig. 5).
Discussion
The present study has revealed the importance of inoculum concentration, temperature, the duration of wetness period, and plant age in the infection development of spinach with conidia of C. spinaciae. As expected, this epidemiological study demonstrated that all these factors had significant effects on anthracnose. Likewise, in southern Turkey, it provides important information for the development of strategies such as programs containing fungicides, biological control agents, and systemic acquired resistant activators to manage spinach anthracnose. Also, the present study will help testing conditions required to differentiate resistant from susceptible genotypes in greenhouse seedling experiments.
Inoculum density of C. spinaciae significantly influenced the development of anthracnose in spinach seedlings, which confirmed the findings on various crops of Hong and Hwang (1998), Chongo and Bernier (2000), and Sosnowski et al. (2005). Foliar application of a conidial suspension produced anthracnose lesions on spinach leaves at all development stages of the plants. Consistent with the findings for C. spinaciae, in this study, it was indicated that anthracnose severity on tomato fruit increased as inoculum density increased from 103 to106 conidia/ml in a previous study (Dillard 1989). In a low level of inoculum density (102 conidia/ml), a few symptoms appeared on leaves of spinach seedlings after 10 days of incubation. These findings were similar to the results of an experiment conducted on pepper by Hong and Hwang (1998) who demonstrated that primary inoculum density (103 conidia/ml) of C. coccodes seemed to be important for producing typical anthracnose lesions on pepper plants. The knowledge on the effect of inoculum concentration on disease development is of great importance in screening some genotypes and cultivars of spinach for resistance to anthracnose. From these results we reported that the inoculum concentration used in this study (106 conidia per ml) was adequate for producing the disease reaction under favourable conditions. Also, it was used in all other experiments.
The present epidemiological study proposed that infection of spinach by C. spinaciae was influenced by temperature and duration of leaf wetness similar to what has been reported in other Colletotrichum spp. infecting various hosts (Wilson et al. 1990; Byrne et al. 1998; Sullivan et al. 2002; Diéguez-Uribeondo et al. 2011; Moral et al. 2012). The findings indicated that when the temperature was increased from 15 to 22 °C, the development of anthracnose lesions per leaf in spinach plants increased gradually. Results from our studies demonstrated that the optimal temperature for anthracnose development on spinach was between 15 and 22 °C. In Turkey conditions, spinach seedlings in the field are subjected to rainy days and the overhead irrigation implemented by growers, which creates periods of leaf wetness and relative humidity (RH) conducive to infection and symptom development. Also, modern spinach producers are growing plants in high planting density. These increased plant densities may favor the development of epidemics of airborne pathogens by increasing the duration of wetness on the leaves. Therefore, disease development could peak in the autumn and spring in the field when temperatures became between 13 and 25 °C. With a physiological point of view, this could result from the stomata being open more frequently when the temperature was higher than 20 °C. In this stage, conidial germination, appressorial development and secondary conidiation of the pathogen may be increased with increasing periods of leaf wetness. Thus, a previous study (Leandro et al. 2003) with Colletotrichum acutatum exhibited that the largest number of appressoria was observed at 17.6 to 20.8 °C 132 h after inoculation of C. acutatum to strawberry leaves. Noteworthy, only one study (Damicone et al. 2013) reported that the optimum temperature for anthracnose lesions on spinach was between 18.3 and 26.7 °C. Also, experiments were performed in winter season conditions (approximately 16–18 °C during the day and 5–6 °C at night) in Hatay province in the Mediterranean Region of Turkey. The differences in the optimal temperatures observed for spinach anthracnose in both studies may be related to the incubation conditions and cultivars used for the development of C. spinaciae conidia. Similar relationships between temperature and wetness duration, which also effects infection in other Colletotrichum pathosystems, have been well documented for a variety of plants and diseases for a long time all over the world (Wilson et al. 1990; Leandro et al. 2003; Diéguez-Uribeondo et al. 2011; Moral et al. 2012).
From these results we assume that the optimum disease development was recorded at the temperature 22 °C with a wetness period of 24 h. As expected, anthracnose incidence increased with the length of the leaf wetness period in temperatures between 18 and 30 °C. The range of temperature and leaf wetness duration at which infection occurs is in accordance with the values obtained by Damicone et al. (2013). They reported the effects of wetness duration on disease severity increased from 2% at 3 h to a maximum of approximately 50% at 96 h. Thompson and Jenkins (1985) observed similar results with cucurbit anthracnose (Colletotrichum lagenarium), where maximum disease development occurred with ≥16 h of leaf wetness. Additionally, our results were similar to the findings on pepper of Hong and Hwang (1998) who reported that 24–36 h of leaf wetness seemed to be necessary for inducing naturally occurring symptoms of anthracnose under the greenhouse conditions. In this case, infection of C. spinaciae is the result of a prolonged period of dew, and it is strongly influenced by fluctuations in temperatures in semiarid countries such as Turkey (Kurt and Tok 2006). For this purpose, further experiment would be necessary to determine if there is any interaction between cultivars of spinach and epidemiological parameters such as temperature and wetness period. Thus, this kind of knowledge can be used to target key stages of an epidemic and may allow tailoring of spray programs. However, a range of isolates must be tested to explore if isolate response to temperature and wetness periods exists.
The current study firstly documented the effects of temperature on sporulation and ages of plant and leaf on the development of spinach anthracnose under field conditions. Field conditions is winter season conditions (approximately night at 5–6 °C, daytime at 16–18 °C, winter season) in Hatay province in the Mediterranean Region of Turkey. We observed that the mean spore number of lesions cm−2 of inoculated leaf surface was higher on plants that were incubated at 15 °C, when compared with plants that were incubated at 33 °C. Likewise, the influence of temperature on sporulation of C. coccodes was determined tomato fruit (Dillard 1989). This study has revealed that there was no significant difference in the number of conidia produced from lesion incubated at 19 or 22 °C. Furthermore, conidial production was greatest at 28 °C and decreased at 31 °C. These results highlight the importance of temperature in the development of anthracnose infection. This study demonstrated that older leaves were more severely infected than younger leaves. From these results we recommend that leaf age was one of the most effective factors affecting infection of spinach leaves by C. spinaciae under an optimum range of temperature and leaf wetness conditions (Fig. 5). At 20 days, lesion number in young leaves was higher than old ones. At 45 days, conversely, lesion number in old leaf was significantly (P = 0.05) higher than young leaf (Fig. 5). One possible reason for the greater disease severity on old leaves of spinach is that spinach cv. Matador used is the highly susceptible (Kurt et al. 2014) against spinach anthracnose in Turkey. Thus, these facts are consistent with findings reported on anthracnose (Colletotrichum truncatum) of lentil by Chongo and Bernier (2000), who found that symptoms were more severe on 4- to 6- weeks-old plants. Also, our results were very similar to the results on strawberry fruit of Wilson et al. (1990) who demonstrated that disease incidence for mature fruit generally was higher than that of immature fruit at the same temperature and wetness duration, indicating that mature fruit appear more susceptible. However, our findings were inconsistent with those reported in pepper plants by some researches (Kim et al. 1989; Hong and Hwang 1998) who demonstrated that as pepper plants grew older, they became more resistant to C. coccodes. It could be explained by the age-related biochemical or structural alterations in the tissue. In this context, it would be of interest to investigate whether or not different spinach cultivars and plant growth stages are encountered in optimum conditions.
In conclusion, this work represents the first cooperative epidemiological investigation of C. spinaciae prevalent in spinach fields in the Mediterranean Region of Turkey. The data generated on the temperature, wetness duration, and leaf ages trials should be taken into account in the development of a fundamental forecaster for anthracnose incidence on spinach and other Colletotrichum spinaciae hosts (Damm et al. 2009) such as weeds Chenopodium album, Medicago sativa, and Portulaca oleracea selected. In Turkey, C. spinaciae infection was detected on some plants such as Chenopodium album, Portulaca oleracea, Petroselinum crispum and Trifolium pratense tested as hosts for C. spinaciae and present in and around the spinach field. Thus, it was suggested that alternative hosts such as Chenopodium album, Medicago sativa, Portulaca oleracea, Trifolium pratense, and Petroselinum crispum may serve as potential inoculum source (Damm et al. 2009; Kurt et al. 2014; Lima et al. 2015). Additionally, the determination of the precise environmental requirements for C. spinaciae infection would be of great use in timing fungicide applications.
References
Byrne, J. M., Hausbeck, M. K., Meloche, C., & Jarosz, A. M. (1998). Influence of dew period and temperature on foliar infection of greenhouse-grown tomato by Colletotrichum coccodes. Plant Disease, 82, 639–641.
Campbell, C. L., & Madden, L. V. (1990). Introduction to plant disease epidemiology. New York: John Wiley & Sons.
Cannon, P. F., Damm, U., Johnston, P. R., & Weir, B. S. (2012). Colletotrichum-current status and future directions. Studies in Mycology, 73, 181–213.
Cerkauskas, R. F., Nauta, R., & McDonald, M. R. (1991). First report of spinach anthracnose in Ontario. Plant Disease, 75, 101.
Chongo, G., & Bernier, C. C. (2000). Effects of host, inoculum concentration, wetness duration, growth stage, and temperature on anthracnose of lentil. Plant Disease, 84, 544–548.
Correll, J. C., Morelock, T. E., Black, M. C., Koike, S. T., Brandenberger, L. P., & Dainello, F. J. (1994). Economically important diseases of spinach. Plant Disease, 78, 653–660.
Damicone, J., Pierson, T., & Stoner, A. (2013). Biology and management of spinach anthracnose. Phytopathology, 103(Suppl.1), S1.3.
Damm, U., Woudenberg, J. H. C., Cannon, P. F., & Crous, P. W. (2009). Colletotrichum species with curved conidia from herbaceous hosts. Fungal Diversity, 39, 45–87.
Diéguez-Uribeondo, J., Förster, H., & Adaskaveg, J. E. (2011). Effect of wetness duration and temperature on the development of anthracnose on selected almond tissues and comparison of cultivar susceptibility. Phytopathology, 101, 1013–1020.
Dillard, H. R. (1989). Effects of temperature, wetness duration, and inoculum density on infection and lesion development of Colletotrichum coccodes on tomato fruit. Phytopathology, 79, 1063–1066.
Ellis, J. B., & Halsted, B. D. (1890). New fungi. Journal of Mycology, 6, 34–35.
FAOSTAT (2015). FAOSTAT online database. http://www.faostat3.fao.org/browse/Q/QC/E.
Guyot, J., Omanda, E. N., & Pinard, F. (2005). Some epidemiological investigations on Colletotrichum leaf disease on rubber tree. Crop Protection, 24, 65–77.
Harp, T., Kuhn, P., Roberts, P. D., & Pernezny, K. L. (2014). Management and cross-infectivity potential of Colletotrichum acutatum causing anthracnose on bell pepper in Florida. Phytoparasitica, 42, 31–39.
Hong, J. K., & Hwang, B. K. (1998). Influence of inoculum density, wetness duration, plant age, inoculation method, and cultivar resistance on infection of pepper plants by Colletotrichum coccodes. Plant Disease, 82, 1079–1083.
Kim, Y. J., Hwang, B. K., & Park, K. W. (1989). Expression of age-related resistance in pepper plants infected with Phytophthora capsici. Plant Disease, 73, 745–747.
Koike, S. T., & Correll, J. C. (1993). First report of spinach anthracnose caused by Colletotrichum dematium in California. Plant Disease, 77, 318.
Kurt, Ş., & Tok, F. M. (2006). Influence of inoculum concentration, leaf age, temperature, and duration of leaf wetness on Septoria blight of parsley. Crop Protection, 25, 56–561.
Kurt, Ş., Uysal, A., & Soylu, E. M. (2014). Reactions of differential host plant species and some cultivars against anthracnose disease (Colletotrichum spinaciae) in spinach. 7th symposium of National Vegetable Agriculture, 2-4 September, Tekirdağ, Turkey, p.134.
Kurt, Ş., Uysal, A., & Akgül, D. S. (2016). First report of anthracnose caused by Colletotrichum spinaciae on spinach in the Mediterranean region of Turkey. Plant Disease, 100(1), 219.
Larsson, M., & Gerhardson, B. (1992). Disease progression and yield losses from root diseases caused by soilborne pathogens of spinach. Phytopathology, 82, 403–406.
Leandro, L. F. S., Gleason, M. L., Nutter Jr., F. W., Wegulo, S. N., & Dixon, P. M. (2003). Influence of temperature and wetness duration on conidia and appressoria of Colletotrichum acutatum on symptomless strawberry leaves. Phytopathology, 93, 513–520.
Lima, N. B., Lima, W. G., Towar-Pedreza, J. M., Michereff, S. J., & Camara, M. P. S. (2015). Comparative epidemiology of Colletotrichum species from mango in northeastern Brazil. European Journal of Plant Pathology, 141, 679–688.
Monroe, J. S., Santini, J. B., & Latin, R. (1997). A model defining the relationship between temperature and leaf wetness duration, and infection of watermelon by Colletotrichum orbiculare. Plant Disease, 81, 739–742.
Moral, J., Jurado-Bello, J., Sánchez, M. I., Oliveira, R., & Trapero, A. (2012). Effect of temperature, wetness duration, and planting density on olive anthracnose caused by Colletotrichum spp. Phytopathology, 102, 974–981.
Rodriguez-Salamanca, L. M., Quesada-Ocampo, L. M., Naegele, R. P., & Hausbeck, M. K. (2015). Characterization, virulence, epidemiology, and management of leaf curling and petiole anthracnose in celery. Plant Disease, 99, 1832–1840.
Sherf, A. F., & MacNab, A. A. (1986). Vegetable diseases and their control (Second ed.p. 728). New York: John Wiley and Sons.
Sosnowski, M. R., Scott, E. S., & Ramsey, M. D. (2005). Temperature, wetness period and inoculum concentration influence infection of canola (Brassica napus) by conidia of Leptosphaeria maculans. Australasian Plant Pathology, 34, 339–344.
Sullivan, M. J., Damicone, J. P., & Payton, M. E. (2002). The effects of temperature and leaf wetness period on the development of spinach white rust. Plant Disease, 86, 753–758.
Thompson, D. C., & Jenkins, S. F. (1985). Effect of temperature, moisture and cucumber resistance on lesion size and increase and conidial production by Colletotrichum lagenarium. Phytopathology, 75, 828–832.
TUIK (2015). Crop production statistics. Turkish Statistical Institute, Ankara. http://www.turkstat.gov.tr/.
Von Arx, J. A. (1970). A revision of the fungi classified as Gloeosporium. Bibliography Mycology, 24, 1–203.
Washington, W. S., Irvine, G., Aldaoud, R., De Alwis, S., Edwards, J., & Pascoe, I. G. (2006). First record of anthracnose of spinach caused by Colletotrichum dematium in Australia. Australasian Plant Pathology, 35, 89–91.
Wilson, L. L., Madden, L. V., & Ellis, M. A. (1990). Influence of temperature and wetness duration on infection of immature and mature strawberry fruit by Colletotrichum acutatum. Phytopathology, 80, 111–116.
Acknowledgements
Authors would like to thank Professor Emine Mine Soylu for her scientific and technical support. This investigation was supported by a Grant (No. 10720) from the Coordinatorship of the Scientific Research Projects of Mustafa Kemal University, Hatay, Turkey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure of potential conflicts of interests
The authors declare that they have no conflict of interest.
Research involving human participants and/ or animals
For this type of study formal consent is not required. This study does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent is not required.
Rights and permissions
About this article
Cite this article
Uysal, A., Kurt, Ş. Influence of inoculum density, temperature, wetness duration, and leaf age on infection and development of spinach anthracnose caused by the fungal pathogen Colletotrichum spinaciae . Eur J Plant Pathol 149, 1041–1052 (2017). https://doi.org/10.1007/s10658-017-1249-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1249-y