Abstract
Stigma/style somatic embryogenesis (SE) is proved to be effective in the complete elimination of the main Citrus virus and virus-like diseases in 100 % of analysed cases. In the present research SE was applied on 13 genotypes, belonging to the Algerian germplasm collection of two different Citrus species (Citrus limon and C. sinensis) infected by one or more graft-transmissible agents, to evaluate the genetic stability and the sanitation of regenerants. The infected genotypes were regenerated through stigma/style SE and the obtained plants were tested by serological, molecular and biological assays, 6 and 18 months after plantlet grafting, for assessing the elimination of the virus, viroid and virus-like agents present in the mother plants. No evidence of infections of virus and virus–like agents was present in the regenerated plants; whereas, after 18 months up to 24 % of tested plants were found to be infected by viroids, independently from the species and genotypes of the mother plants. Hop stunt viroid (HSVd) proved to be the most infectious viroid; conversely, Citrus exocortis viroid (CEVd) and Citrus viroid III (CVd-III) were present only in ‘Sécile’ lemon, as mixed infection with HSVd. SE from stigma/style explants was 100 % effective in the elimination of mixed viroid infections in two lemons (‘Lunario’ and ‘Sans pépins’) and one orange (‘Mitidja navel’). To evaluate genetic stability of regenerants, DNA analyses were performed. No somaclonal variability was observed in lemon regenerants. However, the amplification products of ‘Washington navel 251’ (C. sinensis) revealed genetic instability in some of the regenerated plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus virus, viroid and virus-like infections are widespread in the main citrus growing areas of the Mediterranean; they are primarily disseminated through the use of infected propagating material (Roistacher 1991; Bové 1995). Considering that graft-transmissible agents are widely distributed in the Mediterranean area, some of which may severely affect the citrus industry as for Citrus tristeza virus (Djelouah 2002; Karasev and Hilf 2010), and that the phytopatological status of citrus propagating material is still unknown in some countries, the use of ‘healthy’ plants is one of the most effective means for avoiding the entrance and spread of these infectious agents in new citrus growing areas and for improving citrus industry. Methods developed for sanitation of citrus plants from graft-transmissible pathogens include the use of nuclear embryos Citrus (Weathers and Calavan 1959; Juárez et al. 1976) and heat-treated buds (Calavan et al. 1972; Roistacher 1977), followed by in vitro shoot-tip-grafting (STG). The latter has been efficient in overcoming disadvantages of previous techniques, recovering pathogen-free and true-to-type citrus plants without a long juvenile phase (Murashige et al. 1972; Navarro et al. 1975). However, STG did not give satisfactory results in the elimination of some infectious agents (e.g. Citrus psorosis virus) and diseases, therefore requiring the combination of thermotherapy for effective virus elimination (Carvalho et al. 2002). Conversely, in vitro regeneration by somatic embryogenesis (SE) from stigmas and styles showed to be a very promising technique in recovering citrus plants, free from the main infectious agents, true-to-type and with a short juvenile phase (D’Onghia et al. 1997, 2001, 2002, 2003; Caruso et al. 2000). A wide range of organs and tissues has been used to produce new individuals through SE. SE produced using stigma/style explants have resulted in regenerating 100 % ‘healthy’ citrus plants (Carimi et al. 2013). Previous works on sanitation from graft transmissible agents by using stigma/style SE were conducted 6–12 months after grafting of regenerants using serological and molecular assays, but without assessing their genetic stability (D’Onghia et al. 1997; Meziane et al. 2012a,b; Caruso et al. 2000).
In the present work, regenerated plants by SE from infected Citrus limon and sinensis were analyzed for their genetic stability by using Inter-Simple Sequence Repeat (ISSR) markers and for the elimination of graft-transmissible agents by biological, serological and molecular assays.
Materials and methods
Explant preparation
After the assessment of the sanitary status of mother plants, flowers were collected before opening from 13 citrus genotypes belonging to two different citrus species at the Algerian Citrus Collection of Institut Technique d’Arboriculture Fruitière et de la Vigne (ITAFV): Citrus limon (L.) Burm. (cvs. ‘Béni Abbès’, ‘Bornéo’, ‘Dellys’, ‘Eureka 4’, ‘Eureka Maroc’, ‘Femminello’, ‘Lunario’, ‘Sans pépins’, ‘Villafranca’ and ‘Sécile’) and C. sinensis (L.) Osb. (cvs ‘Mitidja navel’, ‘Shamouti de station’ and ‘Washington navel 251’). The whole flowers (Fig. 1a) were surface-sterilized by immersion for 5 min in 70 % ethanol, 20 min in 2 % (w/v) sodium hypochlorite, followed by 5 min rinses in sterile distilled water for three times. Stigmas and styles were excised and plated vertically as single explants into medium-sized Petri dishes (100 × 15 mm) with the cut surface in contact with the medium (Fig. 1b).
Somatic embryogenesis procedure. a flowers used for somatic embryogenesis protocol; b stigma/style explants plated on MS medium; c, d creamy-white callus produced at the cut end of explants; e green somatic embryos produced from the surface of the callus; f germinated somatic embryo; g, h grafted plant
In vitro culture and in vivo transfer
Following the protocol of Carimi (2005), styles and stigmas were cultured on MS solidified medium (Murashige and Skoog 1962) with 8 g/l phyto agar, 500 mg/l malt extract and 146 mM sucrose as carbon source. The pH of the media was adjusted to 5.7 ± 0.1 with 0.5 M of KOH before autoclaving. Explants were cultured in the presence of 13 μM of 6-benzylaminopurine (BAP) added to the medium after autoclaving and filter sterilized through a 0.22 μm filter before use. The explants were plated in the same culture conditions described above and maintained in a growth chamber at 25 ± 1 °C under a 16 h day length, and a photosynthetic photon flux of 50 μmol m−2 s−1 provided by Osram cool-white 18 W fluorescent lamp. Explants and calluses were sub-cultured into fresh medium at 4–6 week intervals and maintained in a growth chamber under the same above-described culture conditions. Germinated embryos were isolated by using sterilized blades for dissection and transferred into test tubes (one embryo per 55 × 23 mm glass tube sealed with Parafilm M™) containing 20 ml of MS hormone-free solid medium; cultures were maintained into a growth chamber under the same above-described culture conditions. Within 2–3 months on MS medium, plantlets reached 5–6 cm in length and were grafted onto certified pathogen-free sour orange (C. aurantium L.) seedlings as described by De Pasquale et al. (1999). Grafted plants were covered with polyethylene bags and grown in the greenhouse at 24 °C. One month later, polyethylene bags were removed and plants were placed under greenhouse conditions.
Sanitary assays
In order to assess the presence of viruses, viroids and virus-like agents in the mother plants, the sources were tested by serological, molecular and biological assays. The same testing techniques were also performed on in vitro regenerated plants, to evaluate the elimination of the agents detected in the respective mother plants. The number of regenerated plants to be assayed by sanitary tests was based on the different embryogenic events and on the regeneration rate of each genotype; testing was repeated twice, 6 and 18 months after grafting during the acclimatization phase. It is important to highlight that the biological indexing was performed only 18 months after grafting when plant material from regenerants was suitable for grafting onto healthy indicator plants. The latter are produced from certified primary sources which are maintained under insect proof screenhouse at CIHEAM-MAIB.
Double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA)
DAS-ELISA (Clark and Adams 1977) was carried out to detect Citrus psorosis virus (CPsV) and Citrus tristeza virus (CTV), following the manufacturer protocols of the commercial kits (Agritest, Italy). Mature leaves were processed for CPsV, whereas leaf midribs for CTV.
Polystyrene plates were coated with specific polyclonal antisera diluted in the coating buffer. The plant tissues (0.5 g) were ground in the extraction buffer and incubated overnight at 4 °C. After three washings, 100 μl of diluted alkaline phosphatase conjugated specific antibodies were added to each well and the plates were incubated at 37 °C for 2 h. P-nitrophenyl phosphate (1 mg/ml) was then added to the substrate buffer and the plates were incubated at room temperature; photometric absorbance was read at 405 nm with an ELISA Thermo Multiskan EX reader.
Triple antibody sandwich ELISA (TAS-ELISA).
Young leaves were processed by TAS-ELISA (Cambra et al. 1994) for the detection of Citrus variegation virus (CVV), using the commercial kit (UCP Rabat, Morocco). After the first two steps (coating with Polyclonal antibodies and loading samples as detailed above), a third layer was added using diluted specific monoclonal antibodies; following the incubation at 37 °C and washing steps anti mouse enzyme-labelled conjugated antibodies were added; finally, as for DAS-ELISA, after adding the substrate, optical densities were measured at 405 nm with an ELISA Thermo Multiskan EX reader.
Molecular techniques for viroids detection
In order to assess the presence of the main citrus viroids (CEVd, HSVd and CVd-III), molecular techniques were performed on mother plants and on regenerants (6 months after acclimatization). Moreover, the same assay was carried out on inoculated ‘Etrog’ citron (C. medica L.) plants which were maintained at 35 °C for viroid replication. Samples were tested by RT-PCR, using the primers set as reported by Wang et al. (2009).
Total nucleic acid (TNA) extraction and complementary DNA synthesis
Bulked small pieces of leaves were collected from different parts of ‘Etrog’ citron plants maintained at high temperature. Leaves were immersed in liquid nitrogen, then ground with a sterile plastic pestle and about 10 mg were collected and homogenised with 60 μl TES buffer [100 mM Tris–HCl, 2 mM EDTA and 2 % (w/v) SDS] and 60 μl of water-saturated phenol/chloroform/isoamyl alcohol (25:24:1 v/v/v). The homogenised samples were incubated in a water bath at 70 °C for 10 min, followed by centrifugation at 12,000 g for 5 min; the TNAs were obtained through the use of mini columns by following a slightly modified protocol from Zhou et al. (2001). Successively, an aliquot of 5 μl of the obtained TNA extract was mixed with 1 μl/μg random primer and 7 μl sterile distilled water, denatured at 95 °C for 3 min, and immediately cooled in ice for 5 min. Then, 4 μl of M-MLV buffer 5X (50 mM tris-HCl pH 8.3, 75 mM KCl, 3 mM MgCl2), 1.2 μl DTT (10 mM), 1 μl dNTPs (10 mM) and 0.5 μl M-MLV (Moloney Murine Leukaemia virus) (Invitrogen, USA) were added to the mix, reaching 20 μl as final volume; finally, the mix was incubated at 37 °C for 90 min followed by 10 min at 72 °C.
Reverse transcriptase polymerase chain reaction (RT-PCR)
An aliquot of 3 μl of cDNA was used as template for PCR amplifications in 5 μl Taq polymerase buffer (5×) (Promega, USA), 1.5 μl MgCl2 (50 mM), 0.4 μl dNTPs (10 mM), 0.4 μl of 10 mM corresponding reverse and forward primers (Wang et al. 2009), 0.2 μl Go Taq DNA polymerase (5 units/μl) in a final volume of 25 μl. The cDNA amplification was done in Perkin Elmer Cetus Thermal Cycler apparatus. After initial denaturation at 94 °C for 5 min, the 35 cycles consisted of: denaturation at 94 °C for 30 s, primer annealing at 58 °C for 30 s and extension at 72 °C for 45 s. The final extension step was at 72 °C for 10 min. PCR products were analysed by electrophoresis in 1.2 % agarose gel in 1X TAE buffer, then stained with ethidium bromide and visualized under UV illumination.
Biological indexing on woody indicators
Biological indexing onto ‘healthy’ woody indicators was performed for assessing mainly the presence of virus-like diseases of unknown aetiology, as the diseases inducing the oak-leaf patterns (OLP): Citrus concave gums, Cristacortis and Impietratura (Roistacher 1991). However, this technique reveals also the presence of viruses and viroids infections, thus confirming results obtained by serological and molecular assays. For regenerants biological indexing was carried out 18 months after grafting when plant material was suitable for grafting onto indicator plants. Graft inoculations were made onto four citrus indicators: ‘Madame vinous’ orange [C. sinensis (L.) Osb.], ‘Dweet’ tangor (C. reticulata x C. sinensis), ‘Volkameriana’ lemon [C. limon (L.) Burm.], ‘Mexican’ lime [C. aurantifolia (Christm.) Swing.] and ‘Etrog’ citron. The latter was also used for viroid replication before performing molecular assays. Four plants/each indicator were grafted with two bud/bark patches (10 × 5 mm) of tissue. Two un-inoculated indicator plants were used as negative controls and two inoculated plants as positive controls. Indicator plants were grown in greenhouse at 18–24 °C for the observation of virus and virus-like symptoms. Only ‘Etrog’ citron plants were maintained at 32–35 °C for viroid symptoms and replication (Roistacher 1991).
Assessment of genetic stability
DNA extraction
Total DNA was isolated from young, fresh leaves of plants growing in the field (for the maternal plant) and in vitro (for the regenerated clones). The mother plants and their SE regenerants, coming from different embryogenic events, were selected from each genotype for the analysis of genetic stability. All samples were frozen in liquid nitrogen and stored at −80 °C until used. They were ground in a mortar with liquid nitrogen and genomic DNA extraction was carried out by the Doyle and Doyle (1987) CTAB method. The DNA concentration of each sample was quantified by measuring the absorbance at 260 nm as described by Sambrook et al. (1989) and also visualized on a 1 % agarose gel to confirm relative concentrations among different samples.
ISSR fingerprinting
A total of twelve ISSR primers were used to amplify the DNA: ENEA34, ENEA36, ISSR1–6, ISSR2 + 2b, ISSR3 + 3b, ISSR4 + 4b, ISSR5 + 5b, ISSR7–9, ISSR8 + 8b, ISSR10 + 10b, ISSR11 + 11b and ISSR12 + 12b (Siragusa et al. 2007).
PCR amplifications were performed in 25 μl reaction mixture as described in Polat et al. 2014. Amplification was performed in a 96-well GeneAmp PCR System 9700 thermocycler (Applied Biosystems) equipped with a Hot Bonnet under the following cycle program: initial denaturation for 5 min at 94 °C; followed by 36 cycles at 94 °C for 30 s (denaturation), 49, 53 and 56 °C (depending on primers used) for 45 s (annealing), and 72 °C for 120 s (extension), followed by a final extension step at 72 °C for 10 min. In both cases, the amplification products were separated by 1.5 % agarose gel electrophoresis and stained with ethidium bromide. About 25 μl of reaction product (with an adequate amount of loading buffer) was loaded and the gel was run for 5 h at 110 V. The gel was then visualized under ultraviolet light. The reproducibility of the DNA profiles was tested by repeating the PCR amplifications three times.
Data analysis
For in vitro trials, a total of six replicates (Petri dishes) per genotype and five explants per replicate were prepared. Each treatment comprised 30 explants. Percentages of successful embryogenic explants were counted after 4 months of incubation and percentages of acclimatised plants were evaluated after 2 months from grafting. Embryogenic response of explants was expressed as percentages on a Petri dish basis. The effect of genotypes on embryogenic response and surviving plants was tested by Analysis of Variance and differences among means were tested by Tuckey’s test.
To investigate genetic fidelity, for each ISSR markers only those bands showing consistent amplification in the range from 200 to 2600 bp were considered; smeared and weak bands were excluded. Polymorphic ISSR markers were scored for the presence (1) or absence (0) of bands for all samples (both mother and regenerated plants) analysed. All the PCR-amplified samples coming from plantlets regenerated in the same culture conditions were run in the same gel compared with the PCR-product of the mother plant. The percentage of polymorphism was given as number of polymorphic loci/number of total loci, regardless of allele frequencies. To confirm the obtained polymorphisms, the analysis was repeated performing separate PCRs. DNA of plantlets showing polymorphisms was re-extracted and tested again.
Results
In vitro culture
Most explants of different genotypes produced a friable creamy-white callus at the cut end of the styles 4–9 days after culture initiation (Fig. 1c, d) and successively embryos differentiated on the surface of the callus thereafter. Somatic embryos appeared 38–150 days after culture initiation according to the genotype. Somatic embryos were formed more rapidly from ‘Eureka Maroc’ lemon explants than from other genotypes. Green and easy to detach somatic embryos were observed simultaneously at different developmental stages (Fig. 1e).
A strong effect of genotype on SE was observed, in facts the percentage of responsive styles varied from 1.9 % (‘Mitidja navel’ orange) to 36.6 % (‘Femminello’ lemon) (Table 1). The lemon genotypes tested showed a higher embryogenic potential with percentages ranging from 5.2 % (‘Sans pépins’) to 36.6 % (‘Femminello’). Although it was possible to obtain embryos from all the sweet orange genotypes tested, the embryogenic potential varied greatly, with percentages ranging from 1.9 % for ‘Mitidja navel’ to 17.3 % for ‘Washington navel 251’ (Table 1). Somatic embryos were transferred to germinate in Petri dishes containing hormone-free solid medium (Fig. 1f). The rate of germinated embryos also varied according to genotypes being higher in lemon than in sweet orange (80 % and 58 % respectively); their conversion to plantlets was 73 % and 84 % for sweet orange and lemon, respectively. Generated plantlets were grown in tube for 2 months and reached 5–6 cm in length; these plantlets were grafted onto sour orange before the in vivo transfer phase (Fig. 1g, h). Percentages of acclimatized plants after grafting varied according to genotypes and successful percentages vary from 13.3 % (‘Femminello’ lemon) to 100 % (‘Mitidja navel’ sweet orange and ‘Sans pépins’ lemon) (Table 1).
Phytosanitary assays
Results of serological, molecular and biological assays showed that most of the citrus mother trees were infected by more than one graft-transmissible pathogen, primarily viroid agents (Table 2). The latter were detected by biological and molecular means in all citrus mother trees, with the exception of the ‘Washington navel 251’ sweet orange. The most prevalent viroids were HSVd and CEVd, followed by CVd-III. However, all viroids were present as mixed infections excluding CVd-III, which was found in ‘Eureka Maroc’ lemon as single infection (Table 2).
Concerning the viruses tested by serological and biological assays, (CTV, CPsV and CVV), the total infection rate was 35.8 % and ‘Washington navel 251’ orange was infected by all of them. CTV, the most severe virus of citrus, was detected in two lemon cvs. (‘Villafranca’ and ‘Dellys’) and in all tested sweet oranges. The latter were also infected by CPsV that was also found in two lemon mother trees (‘Sans pépins’ and ‘Béni Abbès’). As for CVV, three lemons (‘Bornèo’, ‘Eureka 4’ and ‘Sans pépins’) and the ‘Washington navel 251’ orange were found infected. Moreover, OLP symptoms were observed on the new flushes of ‘Madame vinous’ orange and ‘Dweet tangor’ plant indicators, inoculated with material from three lemons (‘Béni Abbès’, ‘Eureka 4’ and ‘Sécile’) and two sweet oranges (‘Mitidja navel’ and ‘Washington navel 251’). Among the tested mother trees, ‘Mitidja navel’ orange was the most infected (only CVV-negative), whereas ‘Eureka Maroc’ lemon was the least infected (positive only to CVd-III).
Sanitary assays carried out on the plants regenerated by stigma and style culture from the infected mother trees showed negative results for virus infections and OLP diseases, both at 6 and 18 months after grafting. On the contrary, viroids were not detected by molecular assay in regenerated plants 6 months after grafting but were present in 12 % of the regenerants 18 months after grafting using the inoculated ‘Etrog’ for molecular assay. This result was obtained independently of species and genotypes (Table 3). HSVd proved to be the most prevalent viroid (24.2 %), which was found in 16 out of 66 plants regenerated. Conversely, CEVd and CVd-III were detected, as mixed infection with HSVd, only in regenerated plants from ‘Sécile’ lemon. However, SE from stigma/style explants was 100 % effective in the elimination of mixed viroid infections in ‘Lunario’ and ‘Sans pépins’ lemons and ‘Mitidja navel’ orange.
Genetic fidelity of somatic embryo-derived plants
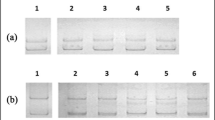
Regenerated plantlets of C. limon (‘Dellys’, ‘Bornéo’ and ‘Béni Abbès’) and C. sinensis (‘Washington navel 251’) were analysed (Table 4) to evaluate the genetic stability. Twelve ISSR primers previously used to characterize citrus (Siragusa et al. 2007) gave rise to a total of 179 well-resolved and reproducible bands (average ca. 15 bands/primer, ranging from 8 to 25). The amplified fragments ranged in size from 230 bp (primers ISSR11 + 11b and ISSR12 + 12b) to 2.5 kb (primers ENEA34, ISSR1–6, ISSR3 + 3b and ISSR10 + 10b). In C. limon, the ISSR primers gave rise to a mean of 111 amplification products (Table 5), and all amplicons were monomorphic among the analysed regenerants. These in turn were similar to the mother plant (Fig. 2a), demonstrating that regenerants were true-to-type plants. On the contrary, in ‘Washington navel 251’ the markers generated 112 bands of which 18 (15.9 %) were observed to be polymorphic in the regenerated clones compared to their mother plants (Table 5). In C. sinensis the number of polymorphic bands generated per primer varied between 3 (primer ENEA36) and 8 (primer ISSR12 + 12b). Fifty percent of regenerated clones from ‘Washington navel 251’ genotype showed genetic polymorphic profile compared to the mother plant (Fig. 2b).
Genetic fidelity assessment of C. limon and C. sinensis with ISSR marker, profiles were obtained with primer ISSR1–6. The amplification products in C. limon (panel a) were monomorphic across all the regenerated plants compared to the mother; while in C. sinensis (panel b) the amplification patterns revealed genetic instability. The black arrows indicate clones with polymorphic bands
Discussion
Results of this investigation indicated that SE starting from stigma and style culture has been successfully applied to regenerate different Citrus genotypes of important Citrus species. Percentages of explants producing embryos varied according to genotypes and, within species, a different regeneration potential was observed as reported in previous papers (De Pasquale et al. 1994; Carimi et al. 1999; Carimi and De Pasquale 2003; Carimi 2005; Meziane et al. 2012a,b).
All regenerated plants from stigma and style SE were free from tested viruses and virus-like diseases, both at 6 and 18 months after grafting, thus confirming the previous reported results (D’Onghia et al. 1997, 2001, 2002, 2003; Meziane et al. 2012a). Differently from previously reported in literature (Caruso et al. 2000; Meziane et al. 2012a), regenerants were not all viroid-free when molecular assays were performed 18 months after grafting using the inoculated ‘Etrog’ citron for viroid replication. Probably 6–12 months, generally considered as a proper time for a conclusive sanitary assessment in regenerants, is not enough for viroid replication and their concentration is too low to detect. On the contrary, the serological and molecular analysis on material obtained from inoculated woody indicators maintained in a thermo-conditioned screenhouse, is more sensitive and accurate for the detection of these agents. As ‘Etrog’ citron is the preferred host for viroids and their replication increases at warmer temperatures, the sanitary assessment of viroids in regenerants should be carried out on ‘Etrog’ citron maintained at 35 °C, when plant material is suitable for graft-inoculation. Interestingly, SE from stigma/style explants was 100 % effective in the elimination of mixed viroid infections in three lemons (‘Eureka Maroc’, ‘Lunario’ and ‘Sans pépins’) and one orange (‘Mitidja navel’); moreover, despite the mixed viroid infections present in the flower donor plants, more than 73 % of regenerants were found free from these agents. Accordingly, SE is also very effective in viroid elimination even if the sanitation rate is not 100 % as for viruses and virus-like agents.
The reason why virus and virus-like agents are completely eliminated through SE differently from viroids is not clear. Citrus viroids are considered highly infective and readily transmitted mechanically from citron to citron by slash inoculation through knife blade under greenhouse and field conditions (Barbosa et al. 2005). It is known from literature that grapevine embryos regenerated in vitro are viroid-free while the callus from which embryos regenerated is infected (Gambino et al. 2011). Despite this, we consider it unlikely that viroids could have been mechanically transmitted in vitro by infected tools during citrus embryos dissection from the infected callus (Navas-Castillo et al. 1995) but it could be possible that a low percentage of embryos may be accidentally damaged and, as a result, infected by these agents. Probably this is the reason why we observed a low percentage of infected regenerants. In light of this hypothesis, it is suggested that tools need to be continuously disinfected in order to avoid viroid transmission from callus to regenerated somatic embryos.
It is important to ensure that regenerants are genetically true to their mother plants. Although under our culture conditions a callus phase was detected during culture initiation, the DNA analysis indicates no differences between lemon regenerants and mother plants. A different result was achieved with sweet orange; in fact the DNA analysis of regenerants as compared to the mother plants showed induction of somaclonal variation. To our knowledge, this is the first report of genetic instability of regenerants during a sanitary procedure. However this result is consistent with those reported in other studies on the incidence of somaclonal variation at morphological, cytological, biochemical and molecular levels in C. sinensis (Fang and Roose 1997). In particular it was reported that somaclonal variation is a genetically stable phenomenon in C. sinensis and several new improved clones have been selected from somaclones (Grosser et al. 1997, 2003). The high number of ‘healthy’ plants, genetically identical to the mother trees, regenerated in this work from stigma and style culture will allow obtaining primary sources of several Citrus genotypes for the production of certified true-to-type and pathogen-free nursery plants. For the routine use of this technique in citrus clonal and sanitary improvement programmes, particular attention should be given to the presence of viroids in regenerated plants, primarily HSVd, and for the genetic fidelity, primarily of sweet oranges, which showed high levels of somaclonal variation.
References
Barbosa, C. J., Pina, J. A., Pérez-Panadés, J., Bernad, L., Serra, P., Navarro, L., & Duran-Vila, N. (2005). Mechanical transmission of citrus viroids. Plant Disease, 89, 749–754.
Bové, J. M. (1995). Virus and virus-like diseases of Citrus in the near east region (p. 518). FAO Rome eds.
Calavan, E. C., Roistacher, C. N., & Nauer, E. M. (1972). Thermotherapy of Citrus for inactivation of certain viruses. Plant Disease Report, 56, 976–980.
Cambra, M., Asensio, M., Gorris, M. T., Perez, E., Camarasa, E., Garcia, J. A., Moya, J. J., Lopez-Abella, D., Vela, C., & Sanz, A. (1994). Detection plum pox potyvirus using monoclonal antibodies to structural and non-structural proteins. EPPO Bulletin, 24, 569–577.
Carimi, F., De Pasquale, F. (2003). Micropropagation of Citrus. In: Kluwer Academic Publishers (eds) Micropropagation of Woody trees and fruits, pp 590–619.
Carimi, F. (2005). Somatic Embryogenesis Protocol: Citrus. In: Springer (eds) Protocol for Somatic Embryogenesis in Woody Plants, pp 321–343.
Carimi, F., De Pasquale, F., & Crescimanno, F. G. (1999). Somatic embryogenesis and plant regeneration from pistil thin cell layers of Citrus. Plant Cell Reports, 8, 935–940.
Carimi, F., D’Onghia, A. M., Carra, A., & Djelouah, K. (2013). Somatic embryogenesis, genetic fidelity of somatic, embryo derived plantlets and virus elimination in Citrus. In P. S. Srivastava & M. P. Sharma (Eds.), Junaid Aslam (pp. 124–145). New Delhi Somatic Embryogenesis and Gene Expression: Narosa Publishing House.
Caruso, A., Guardo, M., Reforgiato-Recupero, G., Russo, G., Terranova, G., Pietro Paolo, D. (2000). Viroid detection in Citrus flowers and use of style culture as an alternative method in sanitation programs. In: Proceedings of the 9th International Citrus Congress (Orlando Florida, 2000), 110–111.
Carvalho, A., Santos, F. A., & Machado, M. A. (2002). Psorosis virus complex elimination from Citrus by shoot-tip-grafting associated to thermotherapy. Fitopatologia Brasileira, 27, 306–308.
Clark, M. F., & Adams, A. N. (1977). Characteristics of the microplate method for enzyme-linked immunosorbent assay for the detection of plant viruses. Journal of General Virology, 34, 475–483.
D’Onghia, A.M., Carimi, F., De Pasquale, F., Djelouah, K., Martelli, G.P. (2003). Somatic embryogenesis from style, a new technique for sanitation, conservation and safe exchange of Citrus germplasm. In: Proceedings of the 9thInternational Citrus Congress (Orlando Florida, 2000), 1:147–149.
D’Onghia, A. M., Carimi, F., De Pasquale, F., Fiore, S., Djelouah, K., & Martelli, G. P. (2002). Somatic embryogenesis from style and stigma cultures eliminates Citrus tristeza virus (CTV) and Citrus variegation virus (CVV). In: proceedings of the. In 15th conference of the International Organization of Citrus Virologists (Paphos Cyprus, 2001) (pp. 413–416).
D’Onghia, A. M., Carimi, F., De Pasquale, F., Djelouah, K., & Martelli, G. P. (2001). Elimination of Citrus psorosis virus by somatic embryogenesis from stigma and style cultures. Plant Pathology, 50, 266–269.
D’Onghia, A. M., De Pasquale, F., Carimi, F., Savino, V., & Crescimanno, F. G. (1997). Somatic embryogenesis from style culture as possible means for virus elimination in Citrus. Journal of Phytopathology, 145, 77–79.
De Pasquale, F., Carimi, F., & Crescimanno, F. G. (1994). Somatic embryogenesis from styles of different cultivars of Citrus limon (L). Brum. Australian Journal of Botany, 42, 587–594.
De Pasquale, F., Giuffrida, S., & Carimi, F. (1999). Minigrafting of shoots, roots, inverted roots and somatic embryos for rescue of in vitro regenerants of Citrus. Journal of the American Society for Horticultural Science, 124, 152–157.
Djelouah, K., Frasheri, D., & D’Onghia, A. M. (2002). Serological diagnosis of Citrus psorosis virus (CPsV) and Citrus tristeza virus (CTV) using flower parts. In: proceedings of the. In 15th conference of the International Organization of Citrus Virologists (Paphos Cyprus, 2001) (pp. 363–365).
Doyle, J. J., & Doyle, J. L. (1987). A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Physics Bulletin, 19, 11–15.
Fang, D. Q., & Roose, M. L. (1997). Identification of closely related Citrus cultivars with inter-simple sequence repeat markers. Theoretical and Applied Genetics, 95, 408–417.
Gambino, G., Navarro, B., Vallania, R., Gribaudo, I., & Di Serio, F. (2011). Somatic embryogenesis efficiently eliminates viroid infections from grapevines. European Journal of Plant Pathology, 130, 511–519.
Grosser, J. W., Gmitter, F. G., & Chandler, J. L. (1997). Development of improved sweet orange cultivars using tissue culture methods. P Fl St Hortic Soc, 110, 13–16.
Grosser, J.W., Chandler, J.L., Gmitter, F.G. (2003). Development of improved sweet oranges via somaclonal variation. Proceedings of the International Society of Citriculture, 1:42–45.
Juárez, J., Navarro, L., & Guardiola, L. (1976). Obtention de plants nucellaires de divers cultivars de clémentiniers au moyen de la culture de nucelle in vitro. Fruits, 31, 751–762.
Karasev, A. V., & Hilf, M. E. (2010). Citrus tristeza virus complex and tristeza diseases. The American Phytopathological Society, St. Paul, MN: APS Press.
Meziane, M., Boudjeniba, M., Frasheri, D., D’Onghia, A.M., Carra, A., Carimi, F. (2012a). A first attempt on sanitation of Algerian Citrus genotypes by stigma/style somatic embryogenesis. Proceedings of the International Symposium on the challenge for a sustainable production, protection and consumption of Mediterranean fruits and nuts, IHC 2010, Lisbon, pp 713–717.
Meziane, M., Boudjeniba, M., Frasheri, D., D’Onghia, A. M., Carra, A., Carimi, F., Haddad, N., Boukhalfa, S., & Braneci, S. (2012b). Regeneration of Algerian Citrus germplasm by stigma/style somatic embryogenesis. African Journal of Biotechnology, 11, 6666–6672.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–497.
Murashige, T., Bitters, W. S., Rangan, T. S., Nauer, E. M., Roistacher, C. N., & Holliday, B. P. (1972). A technique of shoot apex grafting and its utilization towards recovering virus-free Citrus clones. Hortscience, 7, 118–119.
Navarro, L., Roistacher, C. N., & Murashige, T. (1975). Improvement of shoot tip grafting in vitro for virus free Citrus. Journal of the American Society for Horticultural Science, 100, 471–479.
Navas-Castillo, J., Moreno, P., & Duran-Vila, N. (1995). Citrus psorosis, ringspot, cristacortis and concave gum pathogens are maintained in callus culture. Plant Cell, Tissue and Organ Culture, 40, 133–137.
Polat, İ., Baysal, Ö., Mercati, F., Kitner, M., Cohen, Y., Lebeda, A., & Carimi, F. (2014). Characterization of Pseudoperonospora cubensis isolates from Europe and Asia using ISSR and SRAP molecular markers. European Journal of Plant Pathology, 139, 641–653.
Roistacher, C.N. (1977). Elimination of Citrus pathogens in propagative budwood. Budwood selection, indexing and thermotherapy. In: Proceedings International Society of Citriculture, 3:965–972.
Roistacher, C.N. (1991). Graft-transmissible diseases of Citrus in handbook for detection and diagnosis. FAO Rome eds, pp 286.
Sambrook, J., Fritsch, E.F., Maniatis, T. (1989). Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, NY, 2nd Ed.
Siragusa, M., Carra, A., Salvia, L., Puglia, A. M., De Pasquale, F., & Carimi, F. (2007). Genetic instability in calamondin (Citrus madurensis Lour.) plants derived from somatic embryogenesis induced by diphenylurea derivatives. Plant Cell Reports, 26, 1289–1296.
Wang, X., Zhou, C., Tang, K., Zhou, Y., & Li, Z. (2009). A rapid one-step multiplex RT-PCR assay for the simultaneous detection of five Citrus viroids in China. European Journal of Plant Pathology, 124, 175–180.
Weathers, L. G., & Calavan, E. C. (1959). Nucellar embryony - a means of freeing Citrus clones of viruses. In Citrus Virus Diseases (pp. 197–202). Berkeley: University of California Division Agricultural Science.
Zhou, C.Y., Hailstones, D., Connor, R., Barkley, P., Bowyer, J. (2001). A method for micro and rapid extraction of Citrus tristeza virus (CTV) nucleic acid applied to RT-PCR amplification. Journal of Fujian Agricultural University, 30: 200.
Author contribution statement
AC, AMD, FM, KD and FC conceived and designed the study; MM, DF, MB and FM developed the methodology and performed the experiments; AC, AMD, FM KD and FC analysed data; MM, AC, AMD, FM, KD and FC prepared the manuscript. All authors discussed the results and commented on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meziane, M., Frasheri, D., Carra, A. et al. Attempts to eradicate graft-transmissible infections through somatic embryogenesis in Citrus ssp. and analysis of genetic stability of regenerated plants. Eur J Plant Pathol 148, 85–95 (2017). https://doi.org/10.1007/s10658-016-1072-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-016-1072-x