Abstract
The plant defense activator, acibenzolar-S-methyl (ASM) was tested for its ability to protect tomato seedlings against verticillium wilt caused by Verticillium dahliae. ASM exhibited in vitro an antifungal activity in dose-dependent manner against three strains of the pathogen with the less virulent strain SH being the most inhibited. This inhibition of mycelial growth reached 75 % when ASM was applied at concentrations higher than 50 μg ml−1. ASM also induced an elevated protection against the strain SH in the greenhouse when it was applied twice as foliar sprays at 100 μg ml−1 before root inoculation. It reduced leaf alteration index by 67 %, vessel browning index and growth alteration by 80 %. The plant defense activator markedly enhanced accumulation of H2O2. Furthermore, ASM primed tomato seedlings for enhanced activity of peroxidase and polyphenol oxidase. These results suggest that ASM protects tomato from V. dahlia directly by inhibiting the growth of pathogen and indirectly by activating plant defenses responses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Verticillium wilt of tomato (Verticillium dahliae Kleb.) is a severe disease which can cause heavy yield losses both in greenhouse and in the field. V. dahliae persists in soil as microsclerotia and penetrates plants through root tips or wounds on roots before reaching the xylem (Klosterman et al. 2009). Once inside the xylem vessels systemic infections and spread to the aerial parts of the infected plants occur because the fungus rapidly proliferates and conidia are passively carried with the upward transpiration flow. Consequently, leaves will show marginal chlorosis and necrosis as well as vascular discoloration and severe defoliation as well as stunting leading to death of the plant.
Control methods are mainly preventive and are intended to limit the spread of the fungus by tool sterilization and reducing nitrogen supply (Wilhel and Taylor 1965). Crop rotation and chemical treatment of soil are also commonly used to control verticillium wilt; however, soil fumigation with methyl bromide will be no longer used because of its harmful impact on the environment (Rekanovic et al. 2007). The most commonly used fungicides to control verticillium wilt include, benzimidazoles-benomyl, carbendazim and thiophanate-methyl (Talboys 1984; Tian et al. 1998). However, these fungicides showed limited efficacy because of the pathogen’s ability to survive in the environment for a long time and to spread readily, in addition to their high cost and their adverse effect to the environment (Rekanovic et al. 2007).
The use of induced resistance in plants is a promising environment-friendly strategy for controlling plant diseases. The best characterized is systemic acquired resistance (SAR), which is induced by necrotizing pathogens. It is long-lasting and effective against a broad spectrum of pathogens and is correlated with the activation of several antimicrobial defense responses of the plant including genes encoding for pathogenesis-related (PR) proteins (Durrant and Dong 2004). In addition to this direct activation SAR is associated with a primed state, a physiological condition in which plants are able to better or more rapidly mount defense responses to stress. It provides long-lasting resistance based in host defense reaction upon pathogen infection and can be maintained long after the initial stimulus (Conrath et al. 2006). SAR can be triggered by some chemicals, including salicylic acid (SA) and its synthetic analogues, such as acibenzolar-S-methyl (ASM), a derivative of the benzo [1,2,3] thiadiazole-7-carbothioic acid-S-methyl ester (BTH). It is commercially available as Actigard in the United States and as Bion in Europe and Africa. In itself, ASM has no anti-microbial effect, but has been reported to protect several plant species against broad spectrum of pathogens including viral, bacterial and fungal diseases (Friedrich et al. 1996; Benhamou and Belanger 1998; Cole 1999; Ishii et al. 1999; Pappu et al. 2000; Louws et al. 2001; Buonaurio et al. 2002; Faize et al. 2004; Mandal et al. 2008; Tripathi and Pappu 2015). Protection afforded by ASM is either due to the direct activation of plant defenses or to a priming state (Katz et al. 1998; Cools and Ishii 2002; Faize et al. 2004; Faize et al. 2009).
To our best knowledge ASM have not been tested in the tomato/V. dahliae pathosystem. The objective of this work was to evaluate the possibility of usingASM to control tomato against verticillium wilt disease. We first examined whether or not ASM exhibited a direct antimicrobial activity against V. dahliae; then we tested its ability to provide in vivo protection and to activate some plant defense responses. Our results showed that ASM has a dual effect; it was able to inhibit the growth of V. dahliae in vitro and to protect tomato plants in the greenhouse. In addition, it was able to enhance H2O2 production and to prime tomato peroxidase and polyphenol oxidase after pathogen attack.
Material and methods
Fungal material, chemical and plant material
Two virulent strains (SJ and SE) and the less virulent strain (SH) of V. dahliae isolated from single spore in different regions of Morocco were used (Cherrab et al. 2002). They were maintained by regular subculture in petri dishes containing potato dextrose agar (PDA) culture medium incubated at 25 °C.
The chemical used in this study is the Bion (Syngenta), the active material is acibenzolar-S-methyl (ASM) provided as water soluble granules with a formulation of 50 % of active ingredient.
Solanum lycopersicum variety ‘Pomodoro’ was used in this study. Tomato seeds were disinfected with 5 % sodium hypochlorite for 3 min and rinsed three times with sterile distilled water and germinated in pots containing a sterile mixture of peat and sand (3:1) in a depth of 2 cm. Seedlings were grown in greenhouse with a 12 h photoperiod at 26 °C and 70 % relative humidity.
In vitro inhibition mycelial growth test
Sterile distilled water or ASM solutions were added aseptically to PDA medium before solidification to get the final concentrations of 0, 25, 50, 75 and 100 μg ml−1. Three implants of 5 mm diameter taken with a punch on the outskirts of a pure growing strain were placed on solid medium and Petri dishes were then incubated in the dark at 26 °C.
Monitoring mycelial growth was performed after 2, 3, 4 and 5 days of incubation. Mycelial growth was estimated by measuring the mean diameter of the colony. The percentage of inhibition was expressed relative to the control grown in the same conditions. The regression line between colony diameter of V. dahliae and ASM concentration was calculated. Then, ASM sensitivity was measured by calculating effective MIC50 values.
Elicitation, inoculation of tomato plants and determination of disease incidence and disease severity
Plants at 15 days after planting were sprayed with distilled water (DW) or ASM (10 and 100 μg ml−1) until runoff. Four days after the first treatment plants were sprayed again with DW or ASM (10 and 100 μg ml−1). Three days later they were challenged by the strain SH of V. dahlia (107 conidia ml−1) or with DW (Mock inoculation). Treated plants were dug up and their roots were washed with water then dipped in spore suspension for 10 min and replanted. For controls roots were dipped in sterile distilled water. All plants were replanted in separate plastic pots and maintained in a greenhouse as described above. They were arranged in a randomized block design with three blocks, and one experimental unit consisted of eight plants. The experiment was repeated two times and data of typical experiment is shown.
The quantitative and qualitative assessment of the disease was determined from 24 plants based on external and internal symptoms. Several parameters were considered to assess the degree of expression of the disease. Foliar alteration index (FAI) was measured periodically during 3 weeks. It reflects the expression of foliar damage on each plant. A score (N) was attributed to each cotyledon and leaf according to the following scale: (0) absence of foliar symptoms; (1) yellowing or partial necrosis of cotyledon; (2) cotyledon scar; (3) yellowing of leaf; (4) wilting or necrosis of leaf; and (5) leaf scar (Daayf et al. 1995). FAI was then calculated for each inoculated plant as FAI = (Σ(N)/(4 + 5n)), where 4 is the maximum score for cotyledons, 5 is the maximum score for each leaf and n is the number of leaves of each plant. Disease incidence was calculated as percent of infection = (Ip/Tp) × 100, where, Ip represents the number of plants showing external symptoms and Tp is the total number of plants.
The stunting index (SI) was calculated by measuring the elongation of the epicotyl 3 weeks after inoculation according to the following formula: SI = ((Eplc-Eplx)/Eplc) × 100, where Eplc and Eplx represent the mean of epicotyl lengths for control plants and inoculated plants, respectively.
Browning index of browning (BI) was also used as the expression of internal symptoms for each internode and calculated according to the method of Erwin et al. (1976). Discoloration was scored (ΣN) for each internode based on the following scale: (0) absence of discoloration; (1) some localized brown regions within the vascular tissues of the same internode; (2) browning of long stretches of the vascular tissue; (3) browning of all the vessels but not the adjacent tissues; and (4) browning of both vessels and adjacent tissues. BI was then calculated for each plant as ΣN/4n, where: N represents the scores assigned to the internodes, 4 is the maximum score for an internode; and n is the total number of internodes.
Enzymes extractions, activities and H2O2 determination
Fifteen days-old tomato plants were sprayed with ASM (100 μg ml−1) or DW until runoff. A second treatment with ASM or DW was added 4 days later. Three days after, plants were challenged by root-inoculation of the strain SH of V. dahlia (107 conidia ml−1) or with DW (Mock inoculation). Treated and inoculated plants were arranged in randomized three blocks design and one experimental unit consisted of three plants (three plants for enzymatic activities and other three plants for H2O2 determination). Each sample was a pool of apical leaves from three different plants harvested at 0, 0.25, 1, 2, 4, 7, 9, 11 and 15 days after the first treatment. Samples were frozen in liquid nitrogen then stored at −80 °C until use.
Enzymes were extracted at 4 °C by grinding 400 mg of fresh young leaves in an ice bath with 3 ml of 50 mM phosphate buffer, pH 7.5, containing 0.01 % (v/v) Triton X-100, 1 mM polyethylene glycoland 8 % polyvinylpyrrolidone phosphate (PVPP). The homogenate was centrifuged at 16,000 × g for 20 min at 4 °C, and the supernatant was assayed immediately for enzymatic activities. The total protein concentration was determined using bovine serum albumin (BSA) as a standard, according to Bradford (1976).
Peroxidase (POX, EC 1.11.1.7) activity was determined at 470 nm as described by Moerschbacher et al. (1986). The reaction mixture consisted of 25 mM guaiacol (2-methoxyphenol) prepared in 50 mM phosphate buffer pH 7.5, 100 mM H2O2, and 25 μl of extract. Changes in absorbance at 470 nm were recorded for 2 min, and enzyme activity was expressed as μmol per minute per milligram of protein (ε 470 = 26.6 mM−1 cm−1).
Polyphenol oxidase (PPO, EC 1.10.3.1) activity was measured as described by Masia et al. (1998). The reaction mixture contained 25 mM pyrogallol prepared in 100 mM phosphate buffer, pH 7.5, and 100 μl of extract. Changes in absorbance were followed for 3 min at 410 nm. The activity of PPO was expressed as ΔDO per minute per milligram of protein.
H2O2 was measured according to the method of Alexieva et al. (2001). 500 mg of leaf tissues were homogenized in ice bath with 5 ml 0.1 % trichloroacetic acid. The homogenate was centrifuged at 16,000 × g for 20 min and the supernatant was assayed immediately for H2O2 measurement. The reaction mixture consisted of 250 μl of supernatant, 500 μl of 100 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M KI. The reaction was carried out for 1 h in darkness and absorbance was measured at 390 nm. The H2O2 content was determined by using an extinction coefficient of 0.28 μM cm−1 and expressed as mmol g−1 FW.
Statistical analysis
For determination of parameters of disease severity and enzymatic activities as well as H2O2 the statistical design was the completely randomized blocks. The analysis of variance was carried for each variable and means compared by Tukey’s test.
Results
In vitro antimicrobial activity of ASM
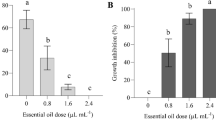
ASM was added to solid PDA medium at 4 different concentrations (25 μg ml−1, 50 μg ml−1, 75 μg ml−1 and 100 μg ml−1) and tested for its possible direct antimicrobial activity against three strains of V. dahliae with different levels of virulence (Fig. 1).
The less virulent strain SH was inhibited even with the lowest concentrations (Fig. 1a). At 25 μg ml−1, about 44 % of mycelial growth was inhibited after 5 days of incubation. When the concentration was increased up to 50 and 75 μg ml−1 approximately 60 % of the inhibition of mycelial growth was observed at the fifth day after incubation. With the highest concentration (100 μg ml−1) more than 75 % of inhibition was observed from the 2nd day until the fifth day. The regression line determined at 5 days after incubation indicated the equation y = 0.344 x + 36 (R2 = 0.936). The MIC50 value was 38.7 μg ml−1.
The highly virulent strain SJ was only slightly inhibited at the lowest concentration used (25 μg ml−1) (Fig. 1b). In fact, at this concentration less than 5 % of growth inhibition was obtained. When this concentration increased to 50 μg ml−1, about 40 % inhibition was observed after 4 and 5 days of incubation. At 75 μg ml−1 and 100 μg ml−1 inhibition increased from the 2nd day and reached levels above 75 % in the 4th and the 5th day of the experiment. The regression line determined at 5 days after incubation revealed the equation y = 0.9468 x – 12.9 (R2 = 0.903) and MIC50 value of 66.2 μg ml−1.
For the highly virulent strain SE and with the lowest concentration about 30 % of mycelial growth inhibition was observed after 4–5 days of incubation (Fig. 1c). This increased to 40 % with a concentration of 50 μg ml−1 on the same dates. At higher concentrations (75 and 100 μg ml−1) these inhibitions hardly exceeded 60 %. The regression line showed the equation y = 0.436 x + 25.3 (R2 = 0.966) and MIC50 value of 56.7 μg ml−1.
In planta protection induced by ASM
Two concentrations of ASM were compared to induce protection of the tomato in planta: 10 μg ml−1 and 100 μg ml−1. Plants were treated twice with ASM at 7 days and 3 days before Verticillium inoculation. To highlight the phenomenon of protection we evaluated several parameters such as the disease incidence, foliar alteration index, stunting and browning index of the vessels.
The disease incidence was followed over 21 days (Fig. 2a). No differences were observed between plants treated with 10 μg ml−1 of ASM and the control during the whole duration of the experiment, whereas significant differences were detected when the concentration of ASM increased to 100 μg ml−1. At this concentration the percentage of protection averaged 52 % at the end of the experiment.
Effect of ASM treatment on the disease incidence and severity caused by Vertivcillium dahliae after treatment with ASM at 10 or 100 μg. ml−1 or distilled water (control). Seedlings were treated twice at 0 days and 4 days. At the 7th day plants were root inoculated with the strain SH of V. dahlia at 107 spore ml−1. a Disease incidence, b Foliar alteration index, c stunting index and d Browning index were calculated from 24 plants. Values are means ± standard errors (5 %). Letters indicate significant differences within each treatment group according to post-hoc analysis (Tukey’s HSD; P < 0.05) after one-way ANOVA. **P < 0.01, *P < 0.05 between treatment groups according to t-test. Experiment repeated twice with similar results and data from one representative experiment are given
The index of foliar alteration gradually increased in the control from the first day after inoculation and it reached 0.35 at 21 days after inoculation (Fig. 2b). Plants treated with 10 μg ml−1 of ASM showed leaf alteration index slightly lower than that observed with the control and differences were not significant at only at 12 days after inoculation. In plants treated with 100 μg ml−1 of ASM symptoms started only at 8 days after inoculation and leaf alteration index was three times lower than in the control at 21 days after inoculation giving rise to a percentage of protection of about 67 %.
The stunting index was compared at the end of the experiment (Fig. 2c). V. dahlia induced growth reduction that averaged 17 % in plants treated with DW. This was significantly reduced in tomato plants treated with 10 μg ml−1 of ASM (7 %) or 100 μg ml−1 of ASM (2 %).
The browning index of vessels caused by V. dahliae was also compared at the end of the experiment (Fig. 2d). In plants treated with 10 μg ml−1 of ASM browning index was significantly lower than in plants treated with DW, giving rise to a percentage of protection of 40 %. This jumped to 80 % when 100 μg ml−1 of ASM was used.
Activation of plant defense and H2O2 by ASM
The activities of POX (Fig. 3a) and PPO (Fig. 3b) as well as H2O2 accumulation (Fig. 4) were studied in seedlings pretreated with 100 μg ml−1 of ASM or with DW and challenged 7 days afterward with V. dahlia.
Effect of the ASM treatment on a POX and b PPO activity in tomato leaves. Plants treated with ASM at 100 μg. ml−1 or distilled water (DW), twice at 0 days and 4 days. At the 7th day plants were root inoculated with the strain SH of V. dahlia at 107 spore ml−1. For control, plants were sprayed with water or ASM but inoculated with DW (Mock inoculation). Arrow indicates the beginning of root inoculation. Data are means and standard errors from three biological replicates. Letters indicate significant differences within each treatment group according to post-hoc analysis (Tukey’s HSD; P < 0.05) after one-way ANOVA
Effect of the ASM treatment on H2O2 production in tomato leaves. Plants treated with ASM at 100 μg. ml−1 or distilled water (DW), twice at 0 days and 4 days. At the 7th day plants were root inoculated with the strain SH of V. dahlia at 107 spore ml−1. For control, plants were sprayed with water or ASM but inoculated with DW (Mock inoculation). Arrow indicates the beginning of root inoculation. Data are means and standard errors from three replicates. Letters indicate significant differences within each treatment group according to post-hoc analysis (Tukey’s HSD; P < 0.05) after one-way ANOVA
In non-inoculated plants no significant differences were observed between those pretreated with ASM or with DW. In inoculated plants pretreated with DW a significantly elevated POD activity was observed when compared to the non-inoculated plants at 9 days post treatment (or 2 dpi). This increase remained significant at 11 after treatment and decreased at 15 days. However, seedlings pretreated with ASM and challenged with V. dahlia had considerably higher levels of POD activity than other treatments. This increase remained stable and was two times higher than that observed in inoculated plants pretreated with DW and three times higher than that observed in non-inoculated plants pretreated with DW.
In seedlings treated with ASM but not challenged with V. dahliae, PPO activity increased significantly only at 1 and 2 days after treatment.
Treatment of seedlings with ASM followed by inoculation with V. dahliae also resulted in a marked increase in PPO activity (Fig. 3b). The activity of PPO in these plants was significantly higher than that observed in plants after any of the other treatments at 9, 11 and 15 days after treatment. In inoculated plants pretreated with DW a significantly elevated PPO activity was observed when compared to the non-inoculated plants at 11 days post treatment (or 4 dpi) and slightly decreased at 15 days after treatment (or 8 dpi). These results suggest that the expression of induced resistance in tomato was associated with enhanced POD and PPO activities.
ASM induced an early and a significant accumulation of H2O2 in non-inoculated plants (Fig. 4). Concentrations measured from leaves treated with ASM were 2 times higher than in the control at 6 h after treatment. These significant differences lasted for the whole duration of the experiment, although a decrease was observed after 2 days. At 9 days after treatment (2 days after inoculation), H2O2 dropped in the control but remained significantly higher in ASM treated and in inoculated plants; it started to increase again at 11 and 15 days after treatment where the highest concentrations were recorded from inoculated plants pretreated with ASM.
Discussion
ASM is known as an elicitor of plant defense without having direct antimicrobial activity against the pathogen. However, in this study we showed that it exhibited a direct activity against three strains of V. dahlia. To our knowledge, only one study has shown that the ASM has a direct antimicrobial activity. Indeed, Muñoz and Moret (2010) reported that ASM was able to reduce the growth of Botrytis cinerea, the causal agent of gray mold, in vitro. However, the concentration required to inhibit 50 % of the mycelium growth was very high and exceeded 3 g l−1. Concentrations used in our study were 45 to 78 times lower than their concentrations and are even slightly lower than those used in the greenhouse or in the field to induce resistance (Benhamou and Belanger 1998; Buonaurio et al. 2002; Faize et al. 2004; Louws et al. 2001; Pappu et al. 2000). Also in this study we have identified a differential ability of ASM to inhibit the growth of V. dahliae. Indeed, the less virulent strain SH was the most sensitive as only 38.7 μg ml−1 were needed to inhibit 50 % of mycelial growth whereas with the most virulent strains SE and SJ concentrations higher than 56.7 μg ml−1 were necessary.
Despite this direct action, we examined if ASM was able to protect tomato plant against the strain SH of V. dahliae in the greenhouse. At the lowest concentration used (10 μg ml−1) ASM was able to induce some level of protection. However, the best protection was achieved when 100 μg ml−1 were applied. Indeed, foliar alteration was 3 times lower than that observed with the control, giving rise to a percentage of protection of 67 % protection. This reached 80 % when the parameter considered was the vessel discoloration or stunting index. All of these data indicated that twice application of 100 μg ml−1 of ASM is effective in controlling tomato Verticillium. Several reports indicated that similar concentrations were needed to achieve elevated levels of protection. Soylu et al. (2003)eported that the application of 200 μg ml−1 of ASM on tomatoes reduces the severity due to Clavibacter michigenensis by 75 %. Generally, the number, frequency and application time play an important role in the efficiency of ASM in different pathosystems. Faize et al. (2004) showed that a single application of ASM at 100 μg ml−1 was neither sufficient to induce plant defenses of Japanese pear nor to protect against Venturia nashicola, whereas two applications were necessary to induce protection and to activate plant defenses. Similarly, Matheron and Porchas (2002) showed that four applications of ASM at 75 μg ml−1 were more effective against Phytophthora capsici than a single application.
In this work ASM was also able to early activate H2O2 production in tomato leaves. ASM is known as a potent inhibitor of catalase and ascorbate peroxidase allowing H2O2 accumulation (Wendehenne et al. 1998). Increased H2O2 production confers enhanced disease resistance through the regulation of the expression of genes associated with plant defense mechanisms that are involved in lignification, cell wall crosslinking and direct killing of the pathogen (Foyer and Noctor 2005). ASM-induced resistance is often associated with plant defense responses priming, characterized by the enhancement of the basal level of plant resistance, resulting in stronger and faster durable resistance upon pathogen attack (Conrath et al. 2006). In the present study, POX and PPO activities were primed by ASM after inoculation of tomato seedlings with V. dahliae. Enhancement of POX and PPO activities results in the reinforcement of cell walls by lignification, which acts as a physical barrier that prevents or reduces pathogen colonization (Anterola and Lewis 2002; Nicholoson and Hammerschmidt 1992). In addition, PPO is capable of catalyzing the oxidation of phenolic compounds to quinones, which are highly toxic compounds against pathogens and its overexpression in tomato plants resulted in increased disease resistance against Pseudomonas syringae pv. syringae (Li and Steffens 2002).
In conclusion, our results clearly show the duality of ASM in the pathosystem tomato/V. dahlia; namely a direct antifungal activity and activation of plant defenses.
References
Alexieva, V., Sergiev, I., Mapelli, S., & Karanov, E. (2001). The effect of drought and ultraviolet radiation growth and stress markers in pea and wheat. Plant, Cell & Environment, 24(12), 1337–1344.
Anterola, A. M., & Lewis, N. G. (2002). Trends in lignin modification: a comprehensive analysis of the effects of genetic manipulations/mutations on lignification and vascular integrity. Phytochemistry, 61(3), 221–294.
Benhamou, N., & and Belanger, R. (1998). Benzothiadiazole-mediated induced resistance to Fusarium oxysporum f. sp. radicis-lycopersici in tomato. Plant Physiology 118 (4), 1203–1212.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of proteins utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1–2), 248–254.
Buonaurio, R., Scarponi, L., Ferrara, M., Sidoti, P., & Bertona, A. (2002). Induction of systemic acquired resistance in pepper plants by acibenzolar-S-methyl against bacterial spot disease. European Journal of Plant Pathology, 108(1), 41–49.
Cherrab, M., Bennani, A., Charest, P. M., & Serrhini, M. N. (2002). Pathogenecity and vegetative compatibility of verticillium dahlia kleb. Isolates from olives in morocco. Journal of Phytopathology, 150(11–12), 703–709.
Cole, D. L. (1999). The efficacy of acibenzolar-S-methyl, an inducer of systemic acquired resistance, against bacterial and fungal diseases of tobacco. Crop Protection, 18(4), 267–273.
Conrath, U., Beckers, G. J. M., Flors, V., García-Agustín, P., Jakab, G., Mauch, F., Newman, M. A., Pieterse, C. M. J., Poinssot, B., Pozo, M. J., Pugin, A., Schaffrath, U., Jurriaan, T., Wendehenne, D., Zimmerli, L., & Mauch-Mani, B. (2006). Priming: getting ready for battle. Molecular Plant-Microbe Interactions, 19(10), 1062–1071.
Cools, H. J., & Ishii, H. (2002). Pre-treatment of cucumber plants with acibenzolar-S-methyl systemically primes a phenyl alanine ammonia lysae gene (PAL1) for enhanced expression upon attack with a pathogenic fungus. Physiological and Molecular Plant Pathology, 61, 273–280.
Daayf, F., Nicole, M., & Geiger, J. P. (1995). Differentiation of verticillium dahlia on the basis of vegetative compatibility and pathogenicity on cotton. European Journal of Plant Pathology, 101(1), 69–79.
Durrant, W. E., & Dong, X. (2004). Systemic acquired resistance. Annual Review of Phytopathology, 42, 185–209.
Erwin, D. C., Tsai, S. D., & Khan, R. A. (1976). Reduction of the severity of verticillium wilt of cotton by the growth retardant, tributyl (5 chloro-2-thienyl methyl) phosphonium chloride. Phytopathology, 66, 106–110.
Faize, M., Faize, L., Koike, N., Ishizaka, M., & Ishii, H. (2004). Acibenzolar-S-methyl-induced resistance to Japanese pear scab is associated with potentiation of multiple defense responses. Phytopathology, 94(6), 604–612.
Faize, M., Faize, L., & Ishii, H. (2009). Gene expression during acibenzolar-S-methyl-induced priming for potentiated responses to venturia nashicola in Japanese pear. Journal of Phytopathology, 157(3), 137–144.
Foyer, C. H., & Noctor, G. (2005). Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell, 17(7), 1866–1875.
Friedrich, L., Lawton, K., Ruess, W., Masner, P., Specker, N., Rella, M. G., Meier, B., Dincher, S., Staub. T., Uknes S., Metraux J. P., Kessmann, H., & Ryals, J. (1996). A benzothiadiazole derivative induces systemic acquired resistance in tobacco. The Plant Journal 10 (1), 61–70.
Ishii, H., Tomita, Y., Horio, T., Narusaka, Y., Nakazawa, Y., Nishimura, K., & Iwamoto, S. (1999). Induced resistance of acibenzolar-S-methyl (CGA 245704) to cucumber and Japanese pear diseases. European Journal of Plant Pathology, 105, 77–85.
Katz, V., Thulke, O. U., & Conrath, U. A. (1998). Benzothiadiazole primes parsley cells for augmented elicitation of defense responses. Plant Physiology, 117(4), 1333–1339.
Klosterman, S. J., Atallah, Z. K., Vallad, G. E., & Subbarao, K. V. (2009). Diversity, pathogenicity, and management of verticillium species. Annual Review of Phytopathology, 47, 39–62.
Li, L., & Steffens, J. C. (2002). Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta, 215(2), 239–247.
Louws, F. J., Wilson, M., Campbell, H. L., Cuppels, D. A., Jones, J. B., Shoemaker, P. B., Sahin, F., & Miller, S. A. (2001). Field control of bacterial spot and bacterial speck of tomato using a plant activator. Plant Disease, 85(5), 481–488.
Mandal, B., Mandal, S., Csinos, A. S., Martinez, N., Culbreath, A. K., & Pappu, H. R. (2008). Biological and molecular analyses of the acibenzolar-S-methyl-induced systemic acquired resistance in flue-cured tobacco against tomato spotted wilt virus. Phytopathology, 98(2), 196–204.
Masia, A., Ventura, M., Gemma, H., & Sansavini, S. (1998). Effect of some plant growth regulator treatments on apple fruit ripening. Plant Growth Regulation, 25(2), 127–134.
Matheron, M. E., & Porchas, M. (2002). Suppression of phytophthora root and crown rot on pepper plant treated with acibenzolar-S-methyl. Plant Disease, 86(3), 292–297.
Moerschbacher, B., Heck, B., Kogel, K. H., Obst, O., & Reisener, H. G. (1986). An elicitor of the hypersensitive lignification response in wheat leaves isolated from the rust fungus Puccinia graminis f. sp. tritici. II. Induction of enzymes correlated with the biosynthesis of lignin. Zeitschrift fur Naturforschung 41c: 839–844.
Muñoz, Z., & Moret, A. (2010). Sensitivity of botrytis cinerae to chitosan and cibenzolar-S-methyl. Pest Management Science, 66(9), 974–979.
Nicholoson, R. L., & Hammerschmidt, R. (1992). Phenolic compounds and their role in resistance. Annual Review of Phytopathology, 30, 369–389.
Pappu, H. R., Csinos, A. S., McPherson, R. M., Jones, D. C., & Stephenson, M. G. (2000). Effect of acibenzolar-S-methyl and imidacloprid on suppression of tomato spotted wilt tospovirus in flue-cured tobacco. Crop Protection, 19(5), 349–354.
Rekanovic, E., Milijasevic, S., Todorovic, B., & Potocnik, I. (2007). Possibilities of biological and chemical control of verticillium wilt in pepper. Phytoparasitica, 35(5), 436–441.
Soylu, S., Baysal, O., & Soylu, E. M. (2003). Induction of disease resistance by the plant activator, acibenzolar-S-methyl (ASM), against bacterial canker (Clavibacter michiganensis subsp. Michiganensis) in tomato seedlings. Plant Science, 105(5), 1069–1075.
Talboys, P. W. (1984). Chemical control of verticillium wilts. Phytopathologia Mediterranea, 23, 163–175.
Tian, L., Wang, K. R., & Lu, J. Y. (1998). Effect of carbendazim and tricyclazole on microsclerotia and melanin formation of Verticillium dahliae. Acta Physica Sinica, 28, 263–268.
Tripathi, D., & Pappu, H. R. (2015). Evaluation of acibenzolar-S-methyl-induced resistance against iris yellow spot tospovirus. European Journal of Plant Pathology, 142(4), 855–864.
Wendehenne, D., Durner, J., Zhixiang, C., & Klessig, D. F. (1998). Benzothiadiazole, an inducer of plant defenses, inhibits catalase and ascorbate peroxidase. Phytochemistry, 47(4), 651–657.
Wilhel, M., & Taylor, J. B. (1965). Control of verticillium wilt of olive through natural recovery and resistance. Phytopathology, 55, 310–316.
Acknowledgments
This work was supported by the Moroccan Ministry of Higher Education and Research. Many thanks to Dr. S. Qsaib for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zine, H., Rifai, L.A., Faize, M. et al. Duality of acibenzolar-S-methyl in the inhibition of pathogen growth and induction of resistance during the interaction tomato/vertcillium dahliae . Eur J Plant Pathol 145, 61–69 (2016). https://doi.org/10.1007/s10658-015-0813-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0813-6