Abstract
Fusarium graminearum is a hemibiotrophic plant fungal pathogen that causes head and seedling blight in wheat and other cereals; however little is known about the mechanisms involved in its pathogenicity. To examine the role of pathogen metabolites in pathogenecity, we studied the effects of F. graminearum crude extract on physiological and morphological responses of Falat and Sumai3, as respectively susceptible and resistant wheat cultivars to Fusarium head blight (FHB). Our results showed that seed germination, seedling growth and coleoptile cell development were highly affected by the pathogen crude extract in both cultivars, with Sumai3 growth being more affected than Falat. These results show little correspondence between wheat seedling tolerance to F. graminearum crude extract and resistance to FHB. Crude extract treatment resulted in significant increase of hydrogen peroxide (H2O2) and malondialdehyde (MDA) content in both cultivars which indicated an oxidative stress. Differential antioxidative responses to crude extract was observed; as activity of polyphenol oxidase (PPO), superoxide dismutase (SOD) and ascorbate peroxidases (APX) increased in Falat and decreased in Sumai3. In addition, a greater phenylalanine ammonia-lyase (PAL) activity was observed in treated seedlings of both cultivars. Quantitative Real- time PCR analysis showed that PAL gene expression in Falat was induced about 4 folds higher than Sumai3 under treatment. Taken together, our data suggest that a better employment of enzymatic and none enzymatic antioxidative systems in Falat could explain its higher degree of tolerance compared with Sumai3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ascomycete fungal pathogen Fusarium graminearum is one of the hemibiotrophic pathogen that can cause head (FHB) and seedling blight (FSB) in wheat and other cereals (Asran and Eraky Amal 2011). FHB infection is favored by warm and humid conditions during flowering and early stages of kernel development (Gilbert and Tekauz 2000). It occurs in the wheat spikes with chalky appearance and visible disease symptom (Foroud et al. 2012). Besides the negative effect on grain yield, Fusarium mycotoxins are directly accumulated in grains that are detrimental to both human and animals (Zhou et al. 2005). Shriveled grains contaminated with mycotoxins are commonly observed in susceptible cultivars infected by FHB (Bai and Shaner 2004). As for wheat FSB, infection takes place at germination stage throughout the succeeding development and can lead to poor plant establishment (Liu et al. 2012). The severity of seedling blight symptoms can vary from localised lesions to extensive necrosis of the coleoptile and roots and pre- and post-emergent seedling death (Haigh et al. 2009). Moreover, FSB can provide pathogens for the subsequent epidemics of FHB (Haigh et al. 2009; Li et al. 2010).
Total FHB resistance in wheat can be dissected into several resistance components (Mesterhazy et al. 1999): resistance to initial infection (type I), spread of FHB in the host (type II) (Schroeder and Christensen 1963), and insensitivity to toxin and ability to degrade deoxynivalenol (DON) (type III) (Wang and Miller 1988).
Study of both FHB and FSB resistance in wheat cultivars and their probable association with mycotoxins tolerance can help to develop new strategies for curtails disease caused by Fusarium spp.
Zhang et al. (2012) analyzed the gene expression profiles of F. graminearum hyphae growing inside wheat coleoptiles at three distinct stages of infection. They documented a number of stage-specific gene expression patterns in pathogen that were ascribed to distinct metabolic strategies including deployment of cell wall degrading enzymes, production of specific cell surface-interacting proteins, mitigation and production of ROS, shift in energy metabolism and production of phytotoxic secondary metabolite.
Tracking plant defense responses to all these pathogen metabolic changes is difficult because of difficulty of separating pathogen/ plant metabolic involvements as a result of pathogen hyphae penetration into plant cells in the final pathogenesis stages (Talas et al. 2011). To tackle this problem, one can use crude extract of pathogen, which contains all pathogen compounds to resemble the condition of living pathogen at its aggressive phase. Study of plant growth and development in response to F. graminearum crude extract and its probable correlation with resistance might clarify some unknown aspects of fungi metabolic toxicity. On the other hand, transcriptomic and proteomic analyses revealed that a key feature underlying successful pathogen recognition is the engagement of ROS, rapid production of reactive oxygen intermediates, primarily superoxide and hydrogen peroxide (H2O2) at the site of attempted infection (Shetty et al. 2008).
ROS accumulation is closely associated with the induction of defense response including enzymatic and non-enzymatic protective mechanisms to scavenge excess ROS. Several antioxidative enzymes including superoxide dismutase (SOD), catalase (CAT) and peroxidase (POX) are involved in detoxification of ROS (Lee and Lee 2000). In addition, phenolic and some other organic compounds serve as the potent non-enzymatic antioxidants in cells. Moreover, to confront stress, plants also induce secondary metabolites production as result of pathogen attack. These compounds help them to control pathogen penetration and spread. Phenylpropanoid metabolism sits across the boundary of primary and secondary metabolism. Phenylalanine ammonia-lyase (PAL) functions as a critical enzyme in the phenylpropanoid pathway and it is the key enzyme involved in synthesis of several secondary compounds such as phenolics and lignins (Hemm et al. 2004).
Here, we investigated the effect of F. graminearum crude extract on two wheat genotypes at germination and seedling stages to elucidate their effect on wheat growth and development, the mode of action and respective defensive response. We hypothesized that exposing wheat to a suite of F. graminearum metabolites may provide a good indication of resistance to the disease. We also tested the association between pathogen crude extract tolerance and seedling/head blight resistance in wheat. Further we investigated plant physiological and morphological reactions to F. graminearum crude extract to examine the correspondence between enzymatic and non-enzymatic ROS scavenging wheat potential and resistance to F. graminearum extract.
Materials and methods
Fungal growth condition
F. graminearum isolate F42 was derived from the wheat grains collected from wheat production areas in Gorgan County, Golestan Province, Iran. The isolate was cultured for 7 days in the dark at 25 °C on potato dextrose agar (PDA). Sporulation was induced by culturing the fungus in mung bean broth, 4 g of mung bean seed boiled in 100 ml of water for 2 days at 22 °C. The mixture of macroconidia with fungal mycelium was filtered through four layers of autoclaved cheesecloth. Spore concentration was determined using a hemocytometer.
Crude extract production
The crude extract production was carried out according to He et al. (2007). 100 grams of white rice (purchased from a local grocery store) was soaked in 43 ml distilled water for 6 h and autoclaved twice for 15 min at 121 °C. The resulting sterile rice was inoculated with 1 × 106 macroconidia and then incubated in dark at 28 °C for 18 days.
The rice culture (10 g) was homogenized in 20 ml methanol using polytron blender. The homogenates was allowed to stand for 2 h and filtered through Watman No. 1 filter paper (Watman, Maid stone, UK). The residue was re-extracted twice under constant stirring for 2 h, each time with 20 ml methanol. The combined filtrate was filtered through a 0.45 μm poly (vinylidene difluoride) syringe filter (whatman) before HPLC analysis.
Chromatographic separations were performed on an Agilent 1200 series high-performance liquid chromatography (HPLC) system including a quaternary pump and a degasser equipped with a G1315D diode array detector and a G1321A fluorescence detector. Separations were carried out on a C18 column (150 × 4.6 mm, with 3 mm particle size) from Waters Company (Massachusetts, USA). The flow rate of the mobile phase that consisted of acetonitril: water solution (10:90, v/v) was kept at 0.6 ml/min in 30 °C. Injection volume was 50 μl, and samples detection was performed at 218 nm for nivalenol (NIV), DON and its derivatives and at 275 nm for Zearalenol (ZEA) (Kotal and Radova 2002). Identification of DON, its derivatives and ZEA were achieved by comparing their retention time and UV- vis spectra with their standards. Quantification of DON was based on the calibration curve of DON standard.
Plant growth condition
Two wheat (Triticum aestivum) genotypes with contrasting levels of resistance and susceptibility to FHB were used in this study. An Iranian spring wheat cultivar, Falat, as a highly susceptible to FHB along with a Chinese originated FHB resistance cultivar, Sumai3 have been employed. Seeds of the two cultivars were surface sterilized with 15 % (v/v) sodium hypochlorite for 10 min and cultured on the main culture medium, a gelatinous water agar (8 g/l) medium, which had been amended with the toxic extract in treatments (Buerstmayr et al. 1997). Control media were free of phytotoxic semi-purified extract.
Plant growth condition and inoculation of wheat spikes
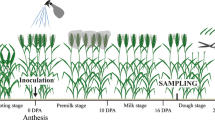
Seeds of two wheat cultivars Falat and Sumai3 were germinated in 2 L plastic pots (five per pot) filled with autoclaved composed-peat-based mixture, and plants were grown in a greenhouse at 22 °C/18 °C day/night cycle and a photoperiod of 16/8 h (day/night). For inoculation, the macronidia suspension of F. graminearum isolates F42 was adjusted to a concentration of 105 spore cm−3. At anthesis, 30 spikes of each cultivar, 1 spike per plant, were inoculated with 10 μl of F. graminearum suspension between palea and lemma. The central spikelets, one on either side of the rachis, were inoculated. After inoculation the plants were kept humid for 3 days and growth in the plastic house. Plants were evaluated for FHB disease for 28 days and at periodic intervals as percent spikelets infected with FHB (Makandar et al. 2012).
Inoculation of wheat seedlings
For seedling inoculation, wheat seeds were disinfected with 0.01 % HgCl2 for 1 min followed by two rinses of strille distilled water and were pre-germinated for 24 h in water. Then wheat seeds were placed in petri dishes with two layers of filter paper saturated with water. The same number of seeds was inoculated with discs of F. graminearum culture on PDA according to Wiśniewska and Chełkowski (1999). The disease rating of seedlings (roots and corresponding coleoptiles) was evaluated for 21 days and at periodic intervals after inoculation as a percentage of injuries: 0 – healthy plant, 100 % - total damage of roots (Grey and Mathre 1984).
Determination of H2O2 and MDA contents
H2O2 content in control coleoptile and in coleoptile exposed to F. graminearum extract was determined according to Velikova et al. (2000). Coleoptile tissues (0.4 g) were homogenized in an ice bath with 5 mL of 0.1 % (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000 g for 15 min and 0.5 ml of the supernatant was added to 0.5 ml of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M of potassium iodide (KI). The absorbance of the supernatant was measured at 390 nm. The content of H2O2 was calculated by comparison with a standard calibration curve previously made by using different concentrations of H2O2.
The lipid peroxidation was measured, following the method of Heath and Packer (1968). The coleoptile (0.5 g) was homogenized in 2.5 ml of 0.1 % (m/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 10, 000 × g for 20 min. To 1 ml aliquot of the supernatant, 4 ml of 0.5 % thiobarbituric acid (TBA) in 20 % TCA was added. The mixture was heated at 95 °C for 30 min and then quickly cooled in an ice bath. After centrifugation at 10, 000 g for 15 min, the absorbance of the supernatant was recorded at 532 and 600 nm. The value for non-specific absorption at 600 nm was then subtracted from that of 532 nm. The concentration of MDA was calculated using absorption coefficient of 155 mM−1cm−1.
Antioxidant enzyme activities
For estimation of total protein content and enzyme activity, plant material (coleoptile) was homogenized at 4 °C with a mortar and pestle in 0.1 M Tris–HCl buffer (pH 8.9) containing 10 mM mercapto ethanol and 4 % (m/v) polyvinylpolypyrrolidone (PVPP). The homogenates were centrifuged at 13, 000 × g for 30 min at 4 °C and resulting supernatants were kept at −70 °C and used for protein determination and enzyme assays. A high-speed centrifuge (J2-21 M, Beckman, Palo Alto, USA) and UV-visible recording spectrophotometer (UV-160, Shimadzu, and Tokyo, Japan) were used. The protein content was determined according to the method of Bradford (1976) using bovine serum albumin as a standard.
SOD (EC 1.15.1.1) activity was estimated by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT) as described by Giannopolitis and Ries (1977) in a reaction mixture containing 50 mM sodium phosphate buffer (pH 7.5), 13 mM methionine, 75 μM NBT, 75 μM riboflavin, 0.1 mM EDTA and 0.1 ml of enzyme extract. The reaction mixture was irradiated for 14 min and absorbance was read at 560 nm against the non-irradiated blank. One unit of SOD was defined as the amount of enzyme which caused 50 % inhibition of NBT reduction under the assay condition, and the results were expressed [Unit mg−1 (protein)].
CAT (EC 1.11.1.6) activity was assayed from the rate of H2O2 decomposition as measured by the decrease of absorbance at 240 nm, following the procedure of Aebi and Catalase (1974). The reaction mixture contained 0.625 ml 50 mM sodium phosphate buffer (pH 7.0), 0.075 ml H2O2 (3 %) and 0.01 ml enzyme extract. Activity was expressed as units (μmol of H2O2 decomposed per min per mg protein) [Unit mg−1 (protein)].
POX (EC 1.11.1.7) activity was measured according to the method of Abeles and Biles (1991). The reaction mixture contained 2 ml of 0.2 M acetate buffer (pH 4.8), 0.2 ml H2O2 (3 %), 0.1 ml of 20 mM benzidine and 0.1 ml enzyme extract. The increase of absorbance was recorded at 530 nm. The POX activity was defined as l μM of benzidine oxidized per min per mg protein [Unit mg −1 (protein)].
Polyphenol oxidase (PPO; EC 1.14.18.1) activity was determined according to the method of Raymond et al. (1993) at 40 °C. The reaction mixture contained 2.5 ml of 0.2 M sodium phosphate buffer (pH 6.8), 0.2 ml of 20 mM pyrogallol and 0.02 ml enzyme extract. The increase of absorbance was recorded at 430 nm. The PPO activity was defined as 1 μM of pyrogallol oxidized per min per mg protein [unit mg−1 (protein)].
Ascorbate peroxidase activity (APX; EC 1.11.1.11) was measured according to Jebara et al. (2005). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbic acid, 0.1 mM hydrogen peroxide and 10 μl of enzyme extract in a total volume of 1 ml. The concentration of oxidized ascorbate was determined by the decrease in absorbance at 290 nm. The concentration of oxidized ascorbate was calculated by using extinction coefficient (ε = 2.8 mM−1 cm−1). One unit of APX was defined as 1 μM oxidized ascorbate per min per mg protein [unit mg−1 (protein)].
Determination of phenolic and flavonoid contents
For estimation of phenolics and flavonoid contents 0.1 g powder of seedling tissues (coleoptile and emerging shoot) was extracted with boiling 80 % methanol for 3 h according to Conde et al. (1995) with minor modification. Total phenolics content were determined by using Folin-Ciocalteu reagent according to Akkol et al. (2008). One milliliter of methanolic extract or gallic acid (standard phenolic compound) was mixed with 5 ml Folin-Ciocalteu reagent and 4 mL sodium carbonate solution 7.0 %. The mixtures were allowed to stand for 2 h before its absorbance was measured at 765 nm. Gallic acid was used as a standard for the calibration curve. Total phenolic values are expressed in terms of mg equal gallic acid in 1 g FW. Total flavonoid content was estimated by the aluminum chloride method, according to Akkol et al. (2008). For total flavonoid 1 ml of methanolic extract, 250 μl of aluminum chloride solution and 250 μl of potassium acetate were mixed and the samples remained at room temperature for 30 min. The absorbance of the reaction mixture was measured at 415 nm with a spectrophotometer.
Estimation of total antioxidant activity by using DPPH scavenging assay
The free radical-scavenging activity of coleoptile was measured according to the method described by Shimada et al. (1992). Briefly, 0.1 g of coleoptile tissues was extracted by 1 ml methanol. Then 0.1 ml of plant extract was added to 3.9 ml of 80 ppm of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) solution. The mixture was shaken vigorously and allowed to stand for 30 min in the dark. The absorbance was then measured at 517 nm. Free radical scavenging activity was calculated by the following equation.
Determination PAL activity
PAL activity was determined based on the rate of cinnamic acid production as described by Ochoa-Alejo and Gómez-Peralta (1993). Briefly, 1 ml of the extraction buffer, 0.5 ml of 10 mM L-phenylalanine, 0.4 ml of double distilled water and 0.1 ml of enzyme extract were incubated at 37 °C for 1 h. The reaction was terminated by the addition of 0.5 ml of 6 M HCl, and the product was extracted with 15 ml ethyl acetate, followed by evaporation to remove the extracting solvent. The solid residue was suspended in 3 ml of 0.05 M NaOH and the cinnamic acid concentration wherein was quantified with the absorbance measured at 290 nm. One unit of PAL activity is equal to 1 μmol of cinnamic acid produced per min.
PAL gene expression analyses
Total RNA were extracted from control and treated plants using RNX-plus kit (RN7713C, CinnaGen, Iran) according to the manufacturer’s instruction with slight optimization. The quality and concentration of RNA samples were examined by EB-stained agarose gel electrophoresis and spectrophotometer analysis. RNA was treated with DNaseI (Fermentase TM, Germany) to remove DNA contamination before cDNA synthesis according to manufactures instructions. Three microgram of DNase-treated RNA was reverse transcribed into complementary DNA (cDNA) using Revert Aid TM Reverse Transcriptase (Fermentas, Canada), oligo dT18 and random hexamer primers (MWG, Germany) in a total volume of 20 μl reaction mixture, according to the manufacturer’s instructions.
mRNA expression levels of PAL was measured with appropriate primers which were designed using PRIMER EXPRESS software (Applied Biosystems). Primers used for qRT-PCR are listed in Table 1. The relative expression levels of PAL gene were quantified in comparison with the house keeping gene β- actin as an internal control. Quantitative real-time PCR was performed using Applied Biosystems 7500 Real Time PCR System (Applied Biosystem/MDS SCIEX, Foster City, CA, USA), with 10 ng cDNA, 10 μl of SYBR Green I master mix (Takara, Shiga, Japan) and 200 nM of forward and reverse primers up to final reaction volumes of 20 μl, according to the manufacturer’s instructions. The PCR was performed through following instruction: an initial denaturation at 95 °C for 5 min, followed by 40 cycles of denaturation at 95 °C for 10 s, annealing at 60 °C for 30 s and extension at 72 °C for 30 s. The specificity of the PCR products was examined by melting curve analysis, restriction endonuclease digestion followed by 12 % polyacrylamide gel electrophoresis. The mean of ΔCT for two cultivars after treatment and control condition was calculated and finally the relative expression of PAL gene was estimated by ratio formula (ratio = 2-ΔΔCt) as described by (Livak and Schmittgen 2001). PAL gene expression level was expressed relative to the non-treated control plants. All experiments were at least, duplicated and a serially diluted cDNA was examined to obtain a standard curve for each primer.
Anatomical methods
Cross-sections of coleoptiles were taken by hand. Sections were cleared in sodium hypochlorite and stained by carmine-vest (1 % w/v in 50 % ethanol) and methyl green (1 % w/v, aqueous) and mounted in gelatin. Then well-stained sections were photographed with an Olympus BH2 and all the measurements and observations were performed 10 times on different sides were performed by measurement software with five repeats at each part.
Statistical analysis
Each experiment was repeated three times to confirm the reproducibility of the data and reduce errors. Comparison between control and treated plants was carried out by Student’s independent t test using SPSS-16 (SPSS, Chicago, IL, USA). Differences were considered significant at a P-value <0.05, unless otherwise noted.
Results
HPLC analysis confirmed the presence of DON, 3-Acetyl DON, 15 Acetyl DON, Nivalenol and ZEA in the crude extract (Supplementary Fig. S1). DON was selected as internal control to determine crude extract concentration. The extract contained 20 ppm DON expressed the physiological effect (between 40 and 60 % reduction) on seed germination in both genotypes. The crude extract contains 20 ppm of DON decreased the germination rate to approximately 63 % in Sumai3 and 41 % in Falat (Supplementary Fig. S2a). Thus this concentration was used for further analysis.
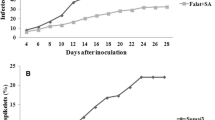
Results of spike inoculation with F. graminearum strain F42 showed a distinctly different resistance reaction in two wheat cultivars. In spikes after single-floret inoculation Sumai3 displayed more resistance (21 % infected spikelets) compared with Falat (100 %) 28 days after inoculation (Supplementary Fig. S2c). In seedlings after inoculation, differences in resistance were not significant between Sumai3 and Falat and both cultivars exhibited about 95 % disease rating on their roots (Supplementary Fig. S2b). Thus a resistance inversion between FHB and FSB was apparently present in Sumai3. In spite of its FHB resistance Sumai3 was sensitive to F. graminearum and F. graminearum crude extract at seedling stage. Falat showed susceptibility to all FHB, FSB and F. graminearum crude extract at seedling stage but it exhibited better function (germination and growth) than Sumai3 when exposed to crude extract.
F .graminearum extract significantly affected the coleoptile length and fresh weight of both genotypes compared with the control (P < 0.05), with Sumai3 being more affected than Falat (Fig. 1). The anatomical analysis of the treated coleoptile with crude extract revealed a decrease in the cortex thickness of both cultivars. Moreover, the vascular bundles in treated coleoptile showed a decrease in size in both cultivars compared to the control (Fig. 2).
Phytotoxic effect of F. graminearum crude extract on coleoptile length and fresh weight of two wheat cultivars. Vertical bars indicate Means ± SE based on three replicates. At minimum 25 plants per genotype were tested for each treatment. An asterisk (*) denotes a value that is significantly different (p < 0.05; t test) from control
A reduction in amylase activity was observed in both cultivars after crude extract treatment (Fig. 3); however this reduction in Sumai3 was more pronounced than Falat.
Lipid peroxidation was assessed as MDA content. To determine ROS scavenging capacity, the H2O2 content of both susceptible and resistant genotypes under pathogen extract were investigated. According to our observation, seed treatment with F. graminearum extract significantly increased H2O2 and MDA content in both genotypes. Our results also, showed that under F. graminearum crude extract treatment the fold increase of H2O2 and MDA is higher than in Sumai3 when compared to Falat (Fig. 4).
The fungi extract significantly increased activity of CAT in both cultivars (Fig. 5). PPO activity in Sumai3 decreased under treatment. However, the decrease in PPO activity was not significant. In contrast, significant increase of PPO activity (about 1.5 fold) occurred in Falat under treatment. SOD and APX activity in Falat were higher than those of Sumai3 under control condition. In Falat, SOD and APX activities were increased in the presence of extract. In contrast, crude extract decreased SOD and APX activities in Sumai3. Significant enhancement of POX activity was observed in Falat (about 2.5 fold), but not in Sumai3 due to F. graminearum extract treatment. Protein content in Falat significantly increased (about 1.5 fold) in contrast to Sumai3 under treatment as compared to control (Fig. 5).
Changes in protein content (mg/g F.W.) and specific activities of Superoxide Dismutase (SOD), Catalase (CAT), Peroxidase (POX), Ascorbat Peroxidase (APX) and Polyphenol Oxidase (PPO) in Falat and Sumai3 treated with F. graminearum crude extract. Vertical bars indicate Means ± SE of three replicates. An asterisk (*) denotes a value that is significantly different (p < 0.05; t test) from control
Phenolic and flavonoid content significantly enhanced in both cultivars, but enhancement in phenolic content was higher in Falat (about 3.5 fold) in comparison with Sumai3 (Fig. 6 a and b). Our data revealed that F. graminearum extract caused a significant increase in DPPH radical scavenging activity in both cultivars; however this induction was higher in Falat compared to Sumai3 (Fig. 6c).
Total content of phenolic (a), flavonoid (b) and DPPH scavenging activity (c) in the coleoptile of Falat and Sumai3 treated by F. graminearum crude extract. Vertical bars indicate Means ± SE based on three replicates. An asterisk (*) denotes a value that is significantly different (p < 0.05; t test) from control
F. graminearum extract caused significant increase in gene expression and PAL activity in both cultivars (Fig. 7). Our results further showed that induction of PAL gene expression under treatment was more pronounced in Falat compared to Sumai3.
a Activity of Phenylalanine ammonia-Lyase (PAL) in two wheat cultivars Falat and Sumai3 treated by F. graminearum crude extract. b: RT-qPCR analyses of PAL gene transcripts in Falat and Sumai3 under treatment. Columns represent average induction (±SE) of gene transcripts in treated plants compared to control plants at the same time for three biological replicates. Values normalized to wheat β-actin normalization factor. An asterisk (*) denotes a value that is significantly different (p < 0.05; t test) from control
Discussion
In this study, we examined the effect of F. graminearum crude extract on wheat germination and seedling growth. This is the first report on the physiological and developmental responses of wheat cultivars to F. graminearum crude extract.
Our results revealed a direct relation between germination rate and crude extract concentration in media culture. This shows the toxic effect of crude extract as the presence of at least five mycotoxines was confirmed by HPLC analysis. F. graminearum extract caused a significant reduction in germination rate, fresh weight and seedling height in both cultivars. Moreover, the anatomical studies on the treated coleoptiles of both cultivars revealed a decrease in the cortex thickness and vascular bundles, in accordance with plant growth inhibition due to extract toxicity. It seems that the extract negatively affects plant cell division and expansion, as observed in microscopic studies total cell area and numbers of coleoptiles in the treated seedlings were significantly less than control. Our observations showed that Sumai3 seedlings were more sensitive to F. graminearum crude extract than Falat.
Positive correlation between growth and amylase activity in both genotypes and significant decrease of amylase activity in treated seedling of both cultivars (Fig. 8) suggest that reduction in germination and growth in treated seeds could be attributed to reduction in seedling amylase activity. The current findings hypothesized the direct or indirect effect of pathogen metabolites (toxins) on seedling amylase activity may be the main target of pathogen effect in Fusarium seedling blight. Phytotoxic study of mycotoxins on cereal seedling has shown that mycotoxins can inhibit seed germination and growth by affecting amylase activity and inhibition of starch hydrolysis (Hasan 1999). According to our observations, decrease in seedling amylase activity was more pronounced in Sumai3 than Falat, which could explain the higher sensitivity of Sumai3 seedlings to F. graminearum crude extract than Falat.
Schematic diagrams of the physiological response of Falat (a) and Sumai3 (b) wheat to F. graminearum crude extract. Color intensity is proportional to activity or content. Size of shapes adjusted based on physiological response of treated plant in comparison to control. Abbreviations: APX ascorbat peroxidase, CAT catalase, MDA malondialdehyde, PAL phenylalanine ammonia-Lyase, PC phenolic compound, POX peroxidase, PPO polyphenol oxidase, SOD superoxide dismutase
Several studies reported that Fusarium metabolites, including mycotoxins, could affect plant growth. For example, Wang and Miller (1988) showed that Fusarium metabolites significantly decreased growth of the coleoptile tissues in wheat. Similarly treatment of cell culture of Linum album by F. graminearum culture filtrate caused decline in cell growth and reproduction (Tahsili et al. 2014).
Many studies have been performed using pathogen produced toxic metabolites as selection agents to find plants with the increased levels of resistance to diseases (Van den Bulk 1991). The present study investigated the possibility of in vitro selections for more resistant cultivar of wheat through finding relation between crude extract tolerance and resistance to FHB or FSB. We found a resistance inversion between FHB and FSB in Sumai3 as reported before (Li et al. 2010). In spite of its FHB resistance Sumai3 showed susceptibility to F. graminearum crude extract at seedling stage. Buerstmayr et al. (1997) reported similar results about Sumai3 when exposed to F. graminearum crude extract. Falat showed susceptibility to all FHB, FSB and F. graminearum crude extract at seedling stage but it exhibited more tolerance than Sumai3 when exposed to crude extract. Analyzing the relationship between the results of seedling response to crude extract, FHB and FSB infection revealed no significant relation between non-germinated seeds percentage and disease rating. Sumai3 resistance against FHB was suggested as type II resistance (Jayatilake et al. 2011) which involves a combination of structural features that slow fungal spread and the activation of a systemic response in uninfected tissues adjacent to the site of infection to prevent and minimize secondary infection (Makandar et al. 2012).
Our data suggest that even resistant wheat at seedling stage might not be able to detoxify mycotoxins. Lemmens et al. (2005) describe a significant correlation between field FHB resistance and toxin resistance, whereas Bruins et al. (1993) reported no association between the two variables. Poppenberger et al. (2003) reported the isolation and characterization of a gene from Arabidopsis thaliana encoding a UDP-glycosyltransferase that is able to detoxify DON. They showed that expression of the glucosyltransferase is developmentally regulated and induced by DON as well as salicylic acid, ethylene and jasmonic acid. Lemmens et al. (2005) believed that resistance to DON is correlated with resistance to spread of FHB (Type III resistance). They showed that in resistant wheat lines, the applied DON was converted to DON-3-O-glucoside as the detoxification product. They concluded that resistance to DON is important in the FHB resistance complex and hypothesize that the resistant wheat cultivar encodes a DON-glucosyltransferase or regulates the expression of such enzyme. In the present study Sumai3 could not tolerate crude extract indicating that detoxifying enzymes could not functionally be activated to confront mycotoxines at seedling stage. Of course in our study F.graminareum crude extract contains a broader range of biomolecules than only DON in other studies. On the other hand this results emphasis that type II resistance can be activated at Sumai3 head but not at seedling or germination stage. Of course considering results obtained by others (Poppenberger et al. 2003; Lemmens et al. 2005) it seems that type III resistance can be activated in company with type II at flowering. This shows a probable developmental dependence of resistance to F.graminareum in wheat.
The observed increased content of H2O2 and MDA in our experiment provides support for the detrimental effect of Fusarium mycotoxins through promoting cell death by induction of hydrogen peroxide production thereby disturbing balance between production and removal of ROS in cellular components (Desmond et al. 2008).
It is well known that oxidative burst as an earlier plant reaction to necrotrophic fungal pathogens can support necrotrophic growth of the pathogen whereas also stimulating an antimicrobial defense response in the host (Dixon and Lamb 1990). Promotion of H2O2 accumulation may presumably be related to increased SOD activity or inhibition of enzymes responsible for H2O2 scavenging (Chao et al. 2010; Hayat et al. 2010; G. Kang et al. 2003; Krantev et al. 2008).
In plants, antioxidative system controls the production of ROS. In this respect, SOD, POX and CAT play important roles. SOD protects cells from oxidative stress by converting superoxide to H2O2 that can be scavenged by POX and CAT (Bowles 1990).
Our results indicate significant differences in the scavenging enzyme activities between treated and control plants in the both cultivars. Moreover, we observed differential antioxdative responses to crude extract in the both cultivars. According to our results activity of SOD, CAT, POX, PPO and APX induced after treatment in Falat. In contrast, crude extract decrease SOD, APX and PPO activity in Sumai3 (Fig. 8).
In light of the fact that decrease in activity of SOD can lead to increased concentration of free radical, our findings of increased activity of SOD in Falat, can be interpreted as an attempt to overcome the oxidative stress. Increased activity of SOD in response to Fusarium infection was reported by García-Limones et al. (2002).
There is compelling evidence showing that PPO and POX play important role in cell wall reinforcement and lignifications in response to pathogen attack (Mohammadi and Kazemi 2002). Hence, increases in activity of PPO and POX in Falat can lead to increase of plant resistance. Similarly, we observed a positive correlation between PPO and POX activity with Falat resistance, suggesting that these enzymes might be involved in seedling viability within pathogen crude extract.
Besides antioxidative enzymes, plants develop efficient non-enzymatic antioxidants system to scavenge ROS and mitigating oxidative stress. Phenolic compounds can protect plants by scavenging of ROS and reducing their toxicity in cytoplasmic structures. (Mishra et al. 2012; Shaheen et al. 2013). In our study, total phenolics and falvonoids content and DPPH radical scavenging activity were increased in response to the F. graminearum extract in both cultivars (Fig. 8), which could be linked to their antioxidant capacity (Li et al. 2007). Higher content of phenolic compounds in Falat in response to crude extract treatment were in accordance with changes in PAL activity and gene expression. PAL by deamination of L-phenylalanine to trans-cinnamic acid is considered as an important regulation point between primary and secondary metabolism (Dixon and Paiva 1995). Greater induction of PAL gene expression of Falat in response to the pathogen crude extract with considering its substantial role in biosynthesis of secondary metabolites including phenolics support the idea of its crucial role in wheat resistance against pathogen (Hill-Ambroz et al. 2006; Guo et al. 2007). PAL activity may be involved in the plant defense system through biosynthesis of active metabolites, such as phytoalexins, phenolics, lignins and salicylic acid in plant defense pathways (Mandal et al. 2009). Moreover, Skadhauge et al. (1997) indicated that barely seeds deficient in flavonoid production, show increased penetration of F. graminearum. Similarly, Tahsili et al. (2014) showed that culture filtrate of F. graminearum could increase content of total phenolics, falvonoids and PAL activity in cell culture of Linum album.
Conclusion
Wheat cultivars with different genetic background showed different level of resistance to pathogenic fungi like F. graminearum. Considering wheat seedling growth and development under the crude extract of F. graminearum proved that Sumai3 in spite of its resistance to FHB is sensitive to crude extract and susceptible to seedling blight. Falat showed susceptibility to FHB, FSB and sensitivity to crude extract but it was more tolerant to crude extract than Sumai3. Apparently there was no association between wheat resistance to FHB, FSB and F. graminearum crude extract. This shows a dependency between resistance and development. We also observed that pathogen metabolites could affect plant metabolism and ROS production. Moreover, we observed substantial differences in physiological responses between Falat and Sumai3 in response to F. graminearum crude extract. A set of antioxidative enzymes and antioxidant compounds was activated in Falat which can control reactive oxygen species. Falat also employs an unknown strategy to prevent blocking protein biosynthesis by mycotoxins.
References
Abeles, F. B., & Biles, C. L. (1991). Characterization of peroxidases in lignifying peach fruit endocarp. Plant Physiology, 95(1), 269–273.
Aebi, H., & Catalase, B. H. (1974). Methods of enzymatic analysis (pp. 673–677). New York: Academic.
Akkol, E. K., Göger, F., Koşar, M., & Başer, K. H. C. (2008). Phenolic composition and biological activities of Salvia halophila and Salvia virgata from Turkey. Food Chemistry, 108(3), 942–949.
Asran, M., & Eraky Amal, M. (2011). Aggressiveness of certain Fusarium graminearum isolates on wheat seedlings and relation with their trichothecene production. Plant Pathology Journal, 10(1), 36–41.
Bai, G., & Shaner, G. (2004). Management and resistance in wheat and barley to Fusarium head blight 1. Annual Review of Phytopathology, 42, 135–161.
Bowles, D. J. (1990). Defense-related proteins in higher plants. Annual Review of Biochemistry, 59(1), 873–907.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1), 248–254.
Bruins, M., Karsai, I., Schepers, J., & Snijders, C. (1993). Phytotoxicity of deoxynivalenol to wheat tissue with regard to in vitro selection for Fusarium head blight resistance. Plant Science, 94(1), 195–206.
Buerstmayr, H., Lemmens, M., Grausgruber, H., & Ruckenbauer, P. (1997). Breeding for scab resistance in wheat: inheritance of resistance and possibilities for in vitro selection. In Fusarium head scab: global status and future prospects: Proceedings of a workshop held at CIMMYT, El Batan, Mexico, 13–17 October, 1996 (pp. 52). CIMMYT.
Chao, Y.-Y., Chen, C.-Y., Huang, W.-D., & Kao, C. H. (2010). Salicylic acid-mediated hydrogen peroxide accumulation and protection against Cd toxicity in rice leaves. Plant and Soil, 329(1–2), 327–337.
Conde, E., Cadahia, E., & Garcia-Vallejo, M. (1995). HPLC analysis of flavonoids and phenolic acids and aldehydes in eucalyptus spp. Chromatographia, 41(11–12), 657–660.
Desmond, O. J., Manners, J. M., Stephens, A. E., Maclean, D. J., Schenk, P. M., Gardiner, D. M., et al. (2008). The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defence responses in wheat. Molecular Plant Pathology, 9(4), 435–445.
Dixon, R. A., & Lamb, C. J. (1990). Molecular communication in interactions between plants and microbial pathogens. Annual Review of Plant Biology, 41(1), 339–367.
Dixon, R. A., & Paiva, N. L. (1995). Stress-induced phenylpropanoid metabolism. The Plant Cell, 7(7), 1085.
Foroud, N., Ouellet, T., Laroche, A., Oosterveen, B., Jordan, M., Ellis, B., & Eudes, F. (2012). Differential transcriptome analyses of three wheat genotypes reveal different host response pathways associated with Fusarium head blight and trichothecene resistance. Plant Pathology, 61(2), 296–314.
García-Limones, C., Hervás, A., Navas-Cortés, J. A., Jiménez-Díaz, R. M., & Tena, M. (2002). Induction of an antioxidant enzyme system and other oxidative stress markers associated with compatible and incompatible interactions between chickpea (Cicer arietinum L.) and Fusarium oxysporum f. sp. ciceris. Physiological and Molecular Plant Pathology, 61(6), 325–337.
Giannopolitis, C. N., & Ries, S. K. (1977). Superoxide dismutases I. Occurrence in higher plants. Plant Physiology, 59(2), 309–314.
Gilbert, J., & Tekauz, A. (2000). Review: recent developments in research on Fusarium head blight of wheat in Canada. Canadian Journal of Plant Pathology, 22(1), 1–8.
Grey, W., & Mathre, D. (1984). Reaction of spring barleys to common root rot and its effect on yield components. Canadian Journal of Plant Science, 64(2), 245–253.
Guo, X., Chen, Y., Li, C., & Ren, H. (2007). Effects of Fusarium graminearum crude toxin on MDA contents and SOD and PAL activities in the seedlings of different wheat varieties.
Haigh, I., Jenkinson, P., & Hare, M. (2009). The effect of a mixture of seed-borne Microdochium nivale var. majus and Microdochium nivale var. nivale infection on Fusarium seedling blight severity and subsequent stem colonisation and growth of winter wheat in pot experiments. European Journal of Plant Pathology, 124(1), 65–73.
Hasan, H. A. H. (1999). Phytotoxicity of pathogenic fungi and their mycotoxins to cereal seedling viability. Mycopathologia, 148(3), 149–155.
Hayat, Q., Hayat, S., Irfan, M., & Ahmad, A. (2010). Effect of exogenous salicylic acid under changing environment: a review. Environmental and Experimental Botany, 68(1), 14–25.
He, J., Yang, R., Zhou, T., Tsao, R., Young, J. C., Zhu, H., et al. (2007). Purification of deoxynivalenol from Fusarium graminearum rice culture and mouldy corn by high-speed counter-current chromatography. Journal of Chromatography A, 1151(1), 187–192.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125(1), 189–198.
Hemm, M. R., Rider, S. D., Ogas, J., Murry, D. J., & Chapple, C. (2004). Light induces phenylpropanoid metabolism in Arabidopsis roots. The Plant Journal, 38(5), 765–778.
Hill-Ambroz, K., Webb, C. A., Matthews, A. R., Li, W., Gill, B. S., & Fellers, J. P. (2006). Expression analysis and physical mapping of a cDNA library of Fusarium head blight infected wheat spikes. Crop Science, 46(Supplement_1), S-15–S-26.
Jayatilake, D., Bai, G., & Dong, Y. (2011). A novel quantitative trait locus for Fusarium head blight resistance in chromosome 7A of wheat. Theoretical and Applied Genetics, 122(6), 1189–1198.
Jebara, S., Jebara, M., Limam, F., & Aouani, M. E. (2005). Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. Journal of Plant Physiology, 162(8), 929–936.
Kang, G., Wang, C., Sun, G., & Wang, Z. (2003). Salicylic acid changes activities of H 2 O 2-metabolizing enzymes and increases the chilling tolerance of banana seedlings. Environmental and Experimental Botany, 50(1), 9–15.
Kotal, F., & Radova, Z. (2002). A simple method for determination of deoxynivalenol in cereals and flours. Czech Journal of Food Sciences, 20(2), 63–68.
Krantev, A., Yordanova, R., Janda, T., Szalai, G., & Popova, L. (2008). Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. Journal of Plant Physiology, 165(9), 920–931.
Lee, D. H., & Lee, C. B. (2000). Chilling stress-induced changes of antioxidant enzymes in the leaves of cucumber: in gel enzyme activity assays. Plant Science, 159(1), 75–85.
Lemmens, M., Scholz, U., Berthiller, F., Dall’Asta, C., Koutnik, A., Schuhmacher, R., et al. (2005). The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Molecular Plant-Microbe Interactions, 18(12), 1318–1324.
Li, H.-B., Cheng, K.-W., Wong, C.-C., Fan, K.-W., Chen, F., & Jiang, Y. (2007). Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chemistry, 102(3), 771–776.
Li, X., Zhang, J., Song, B., Li, H., Xu, H., Qu, B., et al. (2010). Resistance to Fusarium head blight and seedling blight in wheat is associated with activation of a cytochrome P450 gene. Phytopathology, 100(2), 183–191.
Liu, Z.-W., Li, H.-P., Cheng, W., Yang, P., Zhang, J.-B., Gong, A.-D., et al. (2012). Enhanced overall resistance to Fusarium seedling blight and Fusarium head blight in transgenic wheat by co-expression of anti-fungal peptides. European Journal of Plant Pathology, 134(4), 721–732.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods, 25(4), 402–408.
Makandar, R., Nalam, V. J., Lee, H., Trick, H. N., Dong, Y., & Shah, J. (2012). Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Molecular Plant-Microbe Interactions, 25(3), 431–439.
Mandal, S., Mallick, N., & Mitra, A. (2009). Salicylic acid-induced resistance to Fusarium oxysporum f. sp. lycopersic in tomato. Plant Physiology and Biochemistry, 47(7), 642–649.
Mesterhazy, A., Bartok, T., Mirocha, C., & Komoroczy, R. (1999). Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant Breeding, 118(2), 97–110.
Mishra, K., Ojha, H., & Chaudhury, N. K. (2012). Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food Chemistry, 130(4), 1036–1043.
Mohammadi, M., & Kazemi, H. (2002). Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Science, 162(4), 491–498.
Ochoa-Alejo, N., & Gómez-Peralta, J. E. (1993). Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper (Capsicum annuumL.). Journal of Plant Physiology, 141(2), 147–152.
Poppenberger, B., Berthiller, F., Lucyshyn, D., Sieberer, T., Schuhmacher, R., Krska, R., et al. (2003). Detoxification of the Fusarium mycotoxin deoxynivalenol by a UDP-glucosyltransferase from Arabidopsis thaliana. Journal of Biological Chemistry, 278(48), 47905–47914.
Raymond, J., Rakariyatham, N., & Azanza, J. (1993). Purification and some properties of polyphenoloxidase from sunflower seeds. Phytochemistry, 34(4), 927–931.
Schroeder, H., & Christensen, J. (1963). Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology, 53(7), 831–838.
Shaheen, S., Naseer, S., Ashraf, M., & Akram, N. A. (2013). Salt stress affects water relations, photosynthesis, and oxidative defense mechanisms in Solanum melongena L. Journal of Plant Interactions, 8(1), 85–96.
Shetty, N. P., Lyngs Jørgensen, H. J., Jensen, J. D., Collinge, D. B., & Shekar Shetty, H. (2008). Roles of reactive oxygen species in interactions between plants and pathogens. European Journal of Plant Pathology, 121(3), 267–280.
Shimada, K., Fujikawa, K., Yahara, K., & Nakamura, T. (1992). Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry, 40(6), 945–948.
Skadhauge, B., Thomsen, K. K., & Wettstein, D. (1997). The role of the barley testa layer and its flavonoid content in resistance to Fusarium infections. Hereditas, 126(2), 147–160.
Tahsili, J., Sharifi, M., Safaie, N., Esmaeilzadeh-Bahabadi, S., & Behmanesh, M. (2014). Induction of lignans and phenolic compounds in cell culture of Linum album by culture filtrate of Fusarium graminearum. Journal of Plant Interactions, 9(1), 412–417.
Talas, F., Parzies, H. K., & Miedaner, T. (2011). Diversity in genetic structure and chemotype composition of Fusarium graminearum sensu stricto populations causing wheat head blight in individual fields in Germany. European Journal of Plant Pathology, 131(1), 39–48.
Van den Bulk, R. (1991). Application of cell and tissue culture and in vitro selection for disease resistance breeding—a review. Euphytica, 56(3), 269–285.
Velikova, V., Yordanov, I., & Edreva, A. (2000). Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Science, 151(1), 59–66.
Wang, Y., & Miller, J. (1988). Effects of Fusarium graminearum metabolites on wheat tissue in relation to Fusarium head blight resistance. Journal of Phytopathology, 122(2), 118–125.
Wiśniewska, H., & Chełkowski, J. (1999). Influence of exogenic salicylic acid on Fusarium seedling blight reduction in barley. Acta Physiologiae Plantarum, 21(1), 63–66.
Zhang, X.-W., Jia, L.-J., Zhang, Y., Jiang, G., Li, X., Zhang, D., et al. (2012). In planta stage-specific fungal gene profiling elucidates the molecular strategies of Fusarium graminearum growing inside wheat coleoptiles. The Plant Cell, 24(12), 5159–5176.
Zhou, W., Kolb, F. L., & Riechers, D. E. (2005). Identification of proteins induced or upregulated by Fusarium head blight infection in the spikes of hexaploid wheat (Triticum aestivum). Genome, 48(5), 770–780.
Acknowledgments
We thank two anonymous reviewers for constructive comments on an earlier version of this paper. The financial support of this research was equally provided by College of Science, University of Tehran and Gorgan University of Agricultural Sciences and Natural Resources.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sorahinobar, M., Niknam, V., Ebrahimzadeh, H. et al. Lack of association between Fusarium graminearum resistance in spike and crude extract tolerance in seedling of wheat. Eur J Plant Pathol 144, 525–538 (2016). https://doi.org/10.1007/s10658-015-0792-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0792-7