Abstract
Volutella blight of boxwood, caused by the fungus Pseudonectria buxi, can cause extensive losses in commercial nurseries. To assess chemical methods for management, several fungicides commonly used to control diseases on ornamental plants were used in inhibition tests of fungal growth on amended media and on whole plants. Among 106 isolates from Ontario and 26 isolates from British Columbia, discriminatory threshold concentrations of propiconazole (1 μg/ml) and benomyl (10 μg/ml) fully inhibited growth, while iprodione (100 μg/ml) allowed minor hyphal growth of 4–11 % of the non-fungicidal control in agar tests. In pre-infection tests on whole plants, fungicides were applied and then 7 days later, leaf tips were cut, and the plants inoculated with a spore suspension of P. buxi. All treatments at 7 days after inoculation showed statistically significant disease control, all giving less than 13 % disease compared to the inoculated control at 91 % disease. Four of the treatments (chlorothalonil, copper-myclobutanil, thiophanate methyl, and propiconazole) were not significantly different from the non-inoculated control which showed no disease. In post-infection applications on whole plants where leaf tips were injured and immediately inoculated, followed 7 days later by fungicide application, all six fungicides (including mancozeb and iprodione) were found to significantly decrease sporodochial production by 57–89 % compared to the inoculated control, although diseased leaves did not recover. Further work is needed on the timing of applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Buxus L. species, known as boxwood or box, are important landscape plants. Pseudonectria buxi (DC.) Seifert, Gräfenhan & Schroers, also known as Volutella buxi (DC.) Berkeley, is the causal agent of Volutella leaf and stem blight on boxwood, commonly referred to as Volutella blight. Since the late 1990s, Volutella blight has been observed with increasing frequency in nurseries in Canada. In 2008 to 2010, several nurseries in Ontario had outbreaks of this boxwood disease causing economic losses. In 2011, a new highly virulent disease called box blight caused by Cylindrocladium buxicola Henricot was found in the USA (Ivors and LeBude 2011) and Canada (Elmhirst et al. 2013), but Volutella blight is still probably the most commonly observed disease of boxwood. Volutella blight has commonly been considered a secondary disease of boxwood, since the pathogen is often found on dead or previously killed tissues; however, the pathogen, P. buxi, has been found to cause extensive damage on live, apparently healthy plants, although wounding is a prerequisite (Shi and Hsiang 2014).
With Volutella blight, diseased leaves and stems turn yellow and die back, and although diseased plants can stay alive, the aesthetic appearance is adversely affected. In nurseries, management of the disease has required extensive culling, especially of recently rooted cuttings propagated under warm, humid conditions, favouring infection and disease spread. In addition to the necrotic leaves and twigs, pink spore masses of P. buxi may be found on stems and lower surfaces of leaves, and black streaks are also sometimes found on petioles and stems.
In Ontario, there are over a dozen fungicidal active ingredients used to control diseases of ornamental nursery plants (OMAFRA 2009), but no fungicides are registered for controlling Volutella blight of boxwood in Canada (PMRA 2012) and the USA (U.S. EPA 2014). In the literature, some older fungicides have been recommended for controlling Volutella blight, including Bordeaux mixture (White 1931), other copper fungicides, and lime sulphur (Hartman et al. 2000; Horst 2008; Malinoski and Davidson 2009). These older protectant fungicides have been used against Volutella blight, but do not cure diseased branches (White 1931). Although these fungicides are not currently registered in Canada for controlling Volutella blight of boxwood, Bordeaux mixture (Green earth BORDO), copper oxychloride (Copper Spray), and lime sulphur (Green earth lime sulphur) are readily available in Canada (PMRA 2012). In the USA, copper (Camelot, Kocide, etc.), chlorothalonil (Daconil, Echo Lite, ArmorTech, etc.) and manganese-zinc-ethylenebisdithiocarbamate (Protect DF) have been registered for controlling Volutella blight caused by Volutella pachysandra W. G. Hutch on pachysandra (Pachysandra terminalis Siebold & Zucc.). In Canada, only chlorothalonil (Daconil) is registered for controlling Volutella blight on pachysandra (PMRA 2012). Because P. buxi and V. pachysandra are closely related and may share similar disease cycles and sensitivity to fungicides, it is likely that Volutella blight of boxwood is also controlled by similar rates and conditions of chlorothalonil application.
In addition, there are some fungicides registered in Canada for controlling diseases of ornamentals caused by ascomycetous fungi, which may also have potential for managing this disease. For example, apple scab caused by Venturia inaequalis (Cooke) G. Winter is on the label of products containing the following active ingredients: propiconazole, mancozeb and myclobutanil (OMAFRA 2009). Black spot on rose (Rosa L.) caused by Diplocarpon rosae Wolf is on the label of products containing propiconazole, copper sulphate, chlorothalonil, and thiophanate-methyl (OMAFRA 2009). Phyton-27 (containing copper in the form of picro-cupric- ammonium formate) plus Heritage (containing azoxystrobin) have been used to manage leaf spot diseases caused by other ascomycetous pathogens of ornamentals such as species of Alternaria Nees, Colletotrichum Corda, Entomosporium Lév., and Myrothecium Tode (Chase 2000), and anecdotally, this is a combination that local nurseries in Ontario have been using in attempts to control Volutella blight of boxwood. In general, because no fungicides in Canada are specifically registered for use against Volutella blight of boxwood, fungicide applications have been limited. Moreover, the number and timing of applications for efficacious disease control have not been investigated. Some of these active ingredients registered for other ornamental plant diseases may have potential for management of this disease.
The purpose of this study was to assess sensitivity of P. buxi to several fungicides in amended media, and then assess the efficacy of specific fungicides to control Volutella blight on whole boxwood plants in commercial propagation rooms or greenhouses. In addition, the pre-infection (preventive) and post-infection efficacy of these fungicides against disease was also investigated.
Materials and methods
Plant materials, fungal isolation and inoculation
Intact 1-year-old plants of boxwood cv. Green Velvet with no visible signs or symptoms of Volutella blight were obtained from Ontario nurseries and grown in 3-in. (7.5-cm-diameter) pots. Like many commonly grown boxwood cultivars in Ontario, these are open-pollinated seedlings with the female parent B. sempervirens L. cv Suffruticosa and the male parent B. sinica var. insularis (Nakai) M. Cheng. Green Velvet has been found to be susceptible to Volutella blight in earlier cultivar tests (Shi and Hsiang 2014). All pots were kept at 25 °C and 80 % RH under 24 h fluorescent lighting (50 μmol/m2/s) before and during the trials. They were watered until soil saturation once a week with a 0.125 % solution of fertilizer 20-8-20 without supplemental micronutrients.
To obtain single-spore isolates, P. buxi was first isolated from samples collected from different nurseries in Ontario and British Columbia by plating 1 × 1 mm pieces on potato dextrose agar (PDA, Difco DF0013176, MD, USA) amended with 100 μg/ml each of tetracycline hydrochloride (Fisher AC23310, Ottawa, Canada) and streptomycin sulphate (Fisher BP910). Subcultures were made from colonies which resembled P. buxi, and these were grown on fresh PDA until pink droplets appeared on the agar surface (10 days at 25 °C). The droplets were then checked for the presence of characteristic conidia of P. buxi. Spore suspensions were made from these droplets, and dilutions were streaked on PDA to obtain isolated colonies. A single isolate per sample was retained for further study. To produce inoculum, pink droplets were gently pipetted into microfuge tubes, and concentrations were adjusted to 106 spores/ml. The spore suspensions with minimal mycelial fragments were applied by hand pumps until runoff to whole plants.
Fungicide-amended agar tests
To assess the efficacy of fungicides in controlling Volutella blight, the following three fungicides were used for a preliminary assessment with three Ontario isolates (08133, 08141 and 08143): Banner MAXX (containing 14.3 % propiconazole; Syngenta, Plattsville, Ontario, Canada), technical grade benomyl (95 % active ingredient; DuPont, Wilmington, Delaware, USA), and Rovral Green (containing 240 g/l iprodione; Bayer CropScience Inc., Calgary, Alberta, Canada). Propiconazole was added to molten PDA for final concentrations of 0, 0.01, 0.1 and 1 μg/ml. Benomyl and iprodione were added to molten PDA for final concentrations of 0, 0.1, 1 and 10 μg/ml. Each isolate by fungicide combination was repeated three times in separate 9-cm-diameter dishes. After the agar had solidified, PDA was cut into three 1-cm-wide strips with 0.5 cm spacing using a media strip cutter following Hsiang et al. (1997). Agar plugs of P. buxi were placed individually in the middle of the strips. All dishes were wrapped with Parafilm and incubated at 25 °C until mycelia of P. buxi had fully covered the strips of the non-amended control dishes. The extent of fungal growth was marked every day.
After analyzing inhibitory effects, discriminatory threshold fungicide concentrations for the presumed sensitive isolates were established for propiconazole, benomyl, and iprodione, while considering the differences between sensitive and resistant isolates for other species reported in the literature, and targeting near total inhibition by each fungicide for sensitive isolates. These threshold concentrations were then used to test 132 isolates from Ontario and British Columbia using the same strip agar assay. Each isolate by fungicide combination was repeated three times in separate dishes. Mycelial growth of P. buxi was measured and recorded every 2 days until non-amended PDA strips were fully covered by mycelia.
Fungicide tests on whole plants
Because wounding is required before infection of P. buxi (Henricot et al. 2000; Shi and Hsiang 2014), and to ensure consistent rates of infection, the tips of eight leaves per plant were cut off just before inoculation. Six fungicide treatments, one inoculation-only treatment and one water-only treatment (Table 1) were applied to 30-cm-tall boxwood plants until runoff (approximately 1.5 ml/pot). Spore suspensions (106 spores/ml, prepared as described above) of a single isolate (08147) were used as inoculum. A single isolate was chosen for inoculation because previous research (Shi 2011) has found limited variation between isolates from Ontario in terms of spore production, cultural morphology, and genetic variation. For both pre- and post-infection fungicide assessments, plants were placed into trays (28 × 53 cm) where treatments were randomized, and each flat of 18 plants was covered with a transparent plastic bag for 3 days after inoculation to maintain high humidity needed for infection.
To assess the pre-infection efficacy of fungicides, plants were inoculated 7 days after the six fungicidal treatments were applied, with three replicate plants per treatment. To assess the post-infection efficacy of fungicides, six fungicides were applied to plants in two ways: in group one, fungicides were applied at 7 days post-inoculation (dpi); and in group two, fungicides were applied at 7 dpi and again at 14 dpi. The combination of the six different fungicide treatments by application frequencies (single or double) resulted in a total of 14 different treatments (including untreated and inoculated controls) with three replicate plants per treatment during the same time.
Symptoms for pre-infection efficacy tests were rated based on the extent of foliar yellowing and shriveling, as a product of the number of symptomatic leaves (0 to 8 among the eight cut leaves) and the average proportion of symptomatic leaf area (ranging from 0 to 1 representing 0 to 100 %). The range of disease ratings was from 0 (no disease) to 8 (all eight leaves fully symptomatic). Prior to statistical analyses, the ratings were transformed to percent where 0 = 0 % disease to 8 = 100 % disease. In the pre-infection efficacy test, plants were rated 7 days after inoculation.
For post-infection efficacy tests, the effects of fungicides on sporodochial production were rated at 7 days after last fungicide treatment using a similar scale to the one described above, but based on the number of sporodochia rather than yellowing and shrivelling. For single 7 dpi treatments in the post-infection fungicide test, disease was rated 14 days after inoculation, and for the double application test, disease was rated at 21 days after inoculation which was 7 days after the last treatment.
Statistical analyses
For the preliminary inhibition test with a range of fungicide concentrations involving three isolates, EC50 values (the effective concentration required to inhibit diameter growth by 50 %) were calculated based on inhibition [=1–(the mean colony diameter on amended media divided by the mean colony diameter on unamended media)] in percent (Hsiang et al. 1997). The extent of mycelial growth between day 4 and day 5 was subjected to probit analysis using SAS 9.1 (SAS Institute, Cary, NC, USA) to produce EC50 values (SAS Probit program statements available on request). The EC50 values calculated from three replicates for each of the three isolates in this test were used to establish threshold inhibitory concentrations for each fungicide, and these threshold concentrations were tested on the remaining 129 isolates. For inhibition tests based on single threshold concentrations, the mycelial growth was measured between day 2 and day 4 and divided by two for a daily growth rate. The fungicide growth inhibition data and the disease ratings were subjected to analysis of variance using SAS PROC GLM. For the fungicide test on whole plants, disease ratings of the interactions between different fungicidal treatments and timing of fungicide application were analyzed using SAS PROC GLM. When a significant treatment effect was found, means were compared using Fisher’s least significant difference test (LSD) at p = 0.05.
Results
Threshold concentrations for fungicides
The overall mean EC50 values for the three isolates were as follows: propiconazole, 0.13 μg/ml; benomyl, 0.80 μg/ml; and iprodione, 14.5 μg/ml, with no significant differences between isolates (Table 2). Based on these results, threshold concentrations were established for each fungicide for subsequent tests against a larger number of isolates as follows: propiconazole, 1 μg/ml; benomyl, 10 μg/ml; and iprodione, 100 μg/ml. All isolates from Ontario (106) and British Columbia (32) were fully inhibited by the threshold concentrations of benomyl or propiconazole. On iprodione-amended media, minor hyphal growth of 4 to 11 % compared to the PDA control was observed.
Fungicide tests on whole plants
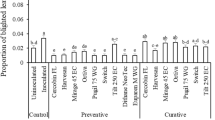
On control plants which were wounded and inoculated with no fungicide treatment, the first symptoms were observed 3 days after inoculation, but no symptoms were observed on non-wounded inoculated leaves. The wounded inoculated leaves became yellowed and were covered with sporodochia by day 7. In the pre-infection efficacy test where each fungicide was applied 7 days before inoculation, all six fungicidal treatments significantly suppressed disease development compared to the inoculated control (Table 3). Among the pre-infection treatments, significantly more disease was observed on plants treated with iprodione (13 %) or mancozeb (6.3 %), compared to the other four fungicides (1 to 5 % disease), which were not significantly different from the water check with no observable disease at day 7 (Table 3).
In post-infection assessments, because leaves were wounded and inoculated with spore suspensions before fungicide treatment 7 days later, they all became infected and started turning yellow and shrivelling by 7 days after inoculation which was the time of fungicide application. After the fungicide application, the number of sporodochia appeared fewer, but the yellow and shriveled leaves did not recover. Therefore, the extent of sporodochial production was used to indicate the disease level for post-infection treatments. For both types of post-infection treatments with either single (day 7) or double (day 7 and day 14) fungicide applications, significantly fewer sporodochia were observed on the plants treated with any of the six fungicides. The most efficacious treatments (Table 4) involved chlorothalonil (14 or 8 % disease) and propiconazole (16 or 11 % disease) for either the single or double applications, respectively; and the least efficacious were iprodione (38 or 29 % disease) and mancozeb (43 or 24 % disease), respectively. Significantly more sporodochia were seen with post-infection treatments of iprodione than other treatments.
Discussion
Pseudonectria buxi has commonly been considered a secondary pathogen, perhaps because it is widespread on dead boxwood tissues, and hence considered a saprophyte; but it is capable of killing boxwood tissues after wounding (Shi and Hsiang 2014). Before the advent of boxwood blight caused by C. buxicola, there were increasing concerns regarding Volutella blight, but there were no fungicides specifically registered for Volutella blight on boxwood in Canada and the USA. Amended agar containing propiconazole, benomyl, and iprodione were used in tests against 132 isolates of P. buxi, and all isolates were found to be sensitive to moderately sensitive to these fungicides. In whole plant treatments with six different fungicides or combinations, all six treatments were found to be significantly and highly effective against disease development when applied 7 days before wounding and inoculation. In post-infection tests, when treatments were applied 7 days after inoculation, curative or eradicative effects were not observed, but decreased sporodochial production was found, especially for chlorothalonil, propiconazole and thiophanate-methyl.
These fungicidal treatments were selected for this study based on their use with other ornamental plant diseases and their different mode of actions. Benomyl, a benzimidazole fungicide, was chosen because of its systemicity and efficacy against ascomycetous fungi (Corwin et al. 2007). Iprodione, a dicarboximide, which is a locally systemic fungicide that can move into treated leaves and redistribute to a limited degree within the treated portion of the plant (Mueller and Bradley 2008), is commonly used to control fungal diseases in field, fruit and ornamental crops. Propiconazole is another commonly used systemic fungicide, representing demethylation-inhibiting (DMI) fungicides which are commonly used for a variety of plant diseases. Copper, mancozeb and chlorothalonil are non-systemic protectant fungicides which target multiple sites in fungi. Chlorothalonil was chosen particularly because it is registered for Volutella blight on pachysandra in Canada and also in the USA (PMRA 2012). Ontario nurseries have previously been testing a mixture of 0.3 g/l Nova 40 W (containing myclobutanil) and Phyton-27 (containing copper in the form of picro-cupric-ammonium formate) to control Volutella blight on boxwood, and this combination was also selected for this test. In the literature, copper has been used to control Volutella blight on boxwood, and copper is also registered for controlling Volutella blight on pachysandra in the USA (White 1931; Malinoski and Davidson 2009; U.S. EPA 2014).
To obtain a baseline sensitivity analysis of fungicidal sensitivity, three fungicides (benomyl, propiconazole and iprodione) from commonly used fungicide families, were tested in amended agar tests with three isolates of P. buxi. These isolates were all presumed to be sensitive at the time of assessment because there was no historical record of long term fungicide use against Volutella blight where these isolates were collected. For propiconazole, the mean EC50 value in the fungus Pyrenophora tritici-repentis (Died.) Drechsler was found to be 0.4 μg/ml, and this was considered moderately sensitive (Beard et al. 2009). A mean EC50 of 0.005 μg/ml for propiconazole was considered to reflect high sensitivity in Sclerotinia homoeocarpa F.T. Benn., whereas, an EC50 of 0.103 μg/ml was thought to demonstrate reduced sensitivity resulting from extensive usage (Hsiang et al. 1997 and Golembiewski et al. 1995). The mean EC50 for propiconazole from three isolates in this study was 0.13 μg/ml. So this might mean that the P. buxi isolates tested were showing some reduced sensitivity to propiconazole, a DMI fungicide. Since nurseries have used Nova (containing myclobutanil, a DMI fungicide) to manage this disease, there might have been some selection for reduced sensitivity. However, there have been no reports or complaints of resistance to DMI fungicides for P. buxi from local nurseries or other locations.
For benomyl, the mean EC50 found in this study was 0.80 μg/ml, whereas for Cladobotryum spp. Nees, it was 0.14–0.97 μg/ml and these isolates were considered highly sensitive (Potočnik et al. 2007). For Microdochium nivale (Fr.) Samuels & I.C. Hallett, EC50 values ranged from 0.6 to 0.96 μg/ml from sensitive isolates and from 390 to 760 μg/ml for resistant isolates (Locke et al. 1987). This implies that sensitivity toward benzimidazole fungicides such as benomyl or thiophanate-methyl is very high in local populations of P. buxi.
For iprodione, the mean EC50 found in this study was 14.5 μg/ml, while for Cladobotryum spp., it was 0.35–2.30 μg/ml, and these isolates were considered highly sensitive (Potočnik et al. 2007). For Verticillium fungicola (Preuss) Hassebr., EC50 from 11.9 to 22.8 mg/l was considered moderately sensitive (Potočnik et al. 2008). Mean EC50 for Alternaria alternata (Fr.) Keissl. pv. citri on iprodione was 280 mg/l (Solel et al. 1996). These results imply that local isolates of P. buxi do not show resistance toward dicarboximide fungicides such as iprodione.
Based on these results of the sensitivity of the three isolates of P. buxi tested (all considered sensitive to slightly reduced sensitive to the fungicides tested), threshold discriminatory concentrations were established for testing of further isolates as follows: propiconazole at 1 μg/ml, benomyl at 10 μg/ml and iprodione at 100 μg/ml. Previous studies which have used threshold concentrations of these fungicides to assess sensitivity of filamentous ascomycetes include the following: benomyl against Botrytis elliptica (Berk.) Cooke (1 μg/ml) (Hsiang et al. 2001) and Fusarium proliferatum (Matsush.) Nirenberg ex Gerlach & Nirenberg (1 μg/ml) (Falk et al. 1996); iprodione against B. elliptica (10 μg/ml) (Hsiang et al. 2001); and propiconazole against Gloeosporium spp. (1 μg/ml) (Frost 2008).
In this study, benomyl was used at a higher threshold (10 μg/ml rather than 1 μg/ml in previous studies) because the EC50 for three isolates of P. buxi was found to be 0.80 μg/ml. Similarly, iprodione in this study was used at a higher threshold (100 μg/ml rather than 10 μg/ml in previous studies) because the EC50 for three isolates of P. buxi was found to be 14.5 μg/ml. All 132 isolates of P. buxi collected in 2009 and 2010 were found to be sensitive to these three fungicides, and no resistant isolates were found. The 106 isolates from Ontario were nearly all collected in Georgetown or St. Catharines in a geographical area less than 100 km apart, and the 32 isolates from British Columbia were similarly collected in a limited geographical area near Vancouver.
In fungicide tests on whole plants in the greenhouse, only inoculated plants showed any disease, even though control non-inoculated plants were also wounded. Six fungicidal treatments were tested on whole boxwood plants, and all showed inhibitory effects on disease development of P. buxi. Disease ratings were not significantly different between single or double post-inoculation fungicidal treatments, and multiple post-infection applications of fungicides may not necessarily reduce disease development or spore production further than a single application.
Daconil 2787 and Daconil Ultrex containing chlorothalonil are recommended for managing Volutella blight on pachysandra which is caused by the related pathogen V. pachysandra. In a growth room test on whole 1-year-old plants, Daconil 2787 showed strong inhibitory effects on disease development of Volutella blight of boxwood. In the propagation process in commercial nurseries, boxwood cuttings are sometimes dipped into fungicides such as Phyton-27 before planting. However, pink sporodochia of P. buxi can often be observed on these treated rooted cuttings after 2 months in production environments, probably because the copper active ingredient only has protectant activity on treated tissues or wears off within 2 months. In the fungicide tests on whole plants, the mixture of Phyton-27 and Nova 40 W showed efficacy in preventing and curing Volutella blight. Benomyl, which has been used in the past for managing many ornamental plant diseases, had inhibitory effects on reducing boxwood disease. Fungicide test data on whole plants were generally in agreement with the fungicide tests in vitro, showing that the fungi were sensitive to these fungicides and that the diseases could also be inhibited by these fungicides.
The systemic fungicides benomyl, propiconazole, and iprodione all showed inhibitory effects against P. buxi on amended agar and whole plants, and pre-infection fungicide use was more effective than post-infection fungicide use on whole plants in terms of inhibition of symptom expression. In vitro, conidia will start germinating on PDA within 12 h (Shi 2011), and symptoms and signs of Volutella blight on boxwood usually can be observed on inoculated leaves within 3 days (Shi and Hsiang 2014). After inoculation of whole plants, yellowing can be observed on the wounded inoculated leaves, as well as pink sporodochia of P. buxi. Applying fungicide 7 days after inoculation did not revive the infected leaves. However, post-infection fungicide use was found to decrease sporodochial production and hence assumed to have an effect on inoculum production. In future studies, the time of post-infection applications should be investigated more finely with first application before 7 days, and perhaps even before symptom development.
In these post-infection fungicide trials, the non-systemic fungicide, chlorothalonil, was found to not differ significantly from the systemic fungicides but had a significantly greater effect than the other non-systemic fungicide, mancozeb, and also more effective than iprodione, a locally systemic fungicide. The symptomatic infected tissues were disrupted 7 days after inoculation at the time of fungicide application, and this disruption could have prevented or reduced normal systemic movement of translocatable fungicides. However, the fungicides differ in their proprietary formulants, such as wetting agents and penetrants, which could have allowed differential penetration into disrupted tissues to contact fungal growth, and hence to inhibit sporodochial production. Other researchers have noted post-infection inhibitory effects of chlorothalonil (Veloukas et al. 2007), and mancozeb (Nagrale et al. 2012), but more research is needed.
Because the major propagation method for boxwood is by cuttings in moist, warm environments conducive to disease establishment and spread, further research is needed on efficacious disease controls where worker exposure risks are also minimized. In this study, any of the six fungicides tested could be used in an integrated control program to reduce infection by P. buxi, and losses due to Volutella blight, but further work is needed on timing of such applications and the duration of their effectiveness.
References
Beard, C., Loughman, R., Smith, A., & Speijers, J. (2009). Baseline sensitivity to three triazole fungicides in Pyrenophora tritici-repentis. Australasian Plant Pathology, 38, 168–172.
Chase, A. R. (2000). Fungicides for control of ornamental leaf spots. Western Connection, 2, 1–4.
Corwin, B., Tisserat, N., & Fresenburg, B. (2007). Identification and management of turfgrass diseases: plant protection programs (pp. 7). University of Missouri
Elmhirst, J. F., Auxier, B. E., & Wegener, L. A. (2013). First report of box blight caused by Cylindrocladium pseudonaviculatum (C. buxicola) in British Columbia, Canada. Plant Disease, 97, 559.
Falk, S. P., Pearson, R. C., Gadoury, D. M., Seem, R. C., & Sztejinberg, A. (1996). Fusarium proliferatum as a biocontrol agent against grape downy mildew. Phytopathology, 86, 1010–1017.
Frost, K. W. (2008). Identification and pathogenicity of some fungi associated with lowbush blueberry (pp. 81). The University of Marine. www.library.umaine.edu/theses/pdf/FrostKE2008.pdf. Accessed 11 May 2012.
Golembiewski, R. C., Vargas, J. M., Jr., Jones, A. L., & Detweiler, A. R. (1995). Detection of demethylation inhibitor (DMI) resistance in Sclerotinia homoeocarpa populations. Plant Disease, 79, 491–493.
Hartman, J. R., Pirone, T. P., & Sall, M. A. (2000). Pirone’s tree maintenance (No. Ed. 7). Oxford University Press. p341.
Henricot, B., Pérez Sierra, A., & Prior, C. (2000). A new blight disease on Buxus in the UK caused by the fungus Cylindrocladium. Plant Pathology, 49, 805.
Horst, R. K. (2008). Westcott’s plant disease handbook. Kluwer Academic Publishers. p22.
Hsiang, T., Yang, L., & Barton, W. (1997). Baseline sensitivity and cross-resistance to demethylation-inhibiting fungicides in Ontario isolates of Sclerotinia homoeocarpa. European Journal of Plant Pathology, 103, 409–416.
Hsiang, T., Hsieh, T. F., & Chastagner, G. A. (2001). Relative sensitivity to the fungicides benomyl and iprodione of Botrytis elliptica from Taiwan and the Northwestern USA. Plant Pathology Bulletin, 10, 93–95.
Ivors, K., & LeBude, A. (2011). A new pest to the U.S. Ornamental Industry: The “box blight” pathogen. North Carolina Pest Alert. www.hgic.umd.edu/content/documents/NCpestalertboxblight.pdf. Accessed 12 Dec 2012.
Locke, T., Moon, L. M., & Evans, J. (1987). Survey of benomyl resistance in Fusarium species on winter wheat in England and Wales in 1986. Plant Pathology, 36, 589–593.
Malinoski, M. K., & Davidson, J. A. (2009). IPM series: Boxwood. Maryland cooperative extension. Home and Garden Mimeo #HG52. http://extension.umd.edu/sites/default/files/_images/programs/hgic/Publications/HG52_IPM_Boxwood.pdf. Accessed 28 Apr 2014.
PMRA (Pest Management Regulatory Agency) (2012). Pesticide label search. http://pr-rp.hc-sc.gc.ca/ls-re/index-eng.php. Health Canada.
Mueller, D. S., & Bradley, C. A. (2008). Field crop fungicides for the north central United States. http://www.ncipmc.org/resources/Fungicide%20Manual4.pdf. Iowa State University. Accessed 08 Dec 2014.
Nagrale, D. T., Gaikwad, A. P., Goswami, S., & Sharma, L. (2012). Fungicidal management of Alternaria alternata (Fr.) Keissler causing blight of gerbera (Gerbera jamesonii H. Bolus ex JD Hook). Journal of Applied and Natural Science, 4, 220–227.
OMAFRA (Ontario Ministry of Agriculture, Food and Rural Affairs) (2009). Nursery and landscape plant production and IPM. Publication 383. Alberta: Queen’s Printer.
Potočnik, I., Milijašević, S., Rekanović, E., Todorović, B., & Stepanović, M. (2007). Sensitivity of Cladobotryum spp., a pathogen of the button mushroom (Agaricus bisporus), to some fungicides. Pesticidi Ifitomedicina, 22, 233–240.
Potočnik, I., Vukojević, J., Stajić, M., Tanović, B., & Todorović, B. (2008). Fungicide sensitivity of selected Verticillium fungicola isolates from Agaricus bisporus farms. Archives of Biological Sciences, 60, 151–157.
Shi, F. (2011). Causal agent, biology and management of the leaf and stem disease of boxwood (Buxus Spp.). M.Sc. Thesis, University of Guelph.
Shi, F., & Hsiang, T. (2014). Pseudonectria buxi causing leaf and stem blight on Buxus in Canada. European Journal of Plant Pathology, 138, 763–773.
Solel, Z., Timmer, L. W., & Kimchi, M. (1996). Iprodione resistance of Alternaria alternata pv. Citri from Minneola tangelo in Israel and Florida. Plant Disease, 80, 291–293.
U.S. EPA (U.S. Environmental Protection Agency) (2014). Pesticide Product Label System (PPLS). http://iaspub.epa.gov/apex/pesticides/f?p=PPLS:1/. Accessed 31 Jan 2014.
Veloukas, T., Bardas, G. A., Karaoglanidis, G. S., & Tzavella-Klonari, K. (2007). Management of tomato leaf mould caused by Cladosporium fulvum with trifloxystrobin. Crop Protection, 26, 845–851.
White, R. P. (1931). Diseases of Boxwood. New Jersey Agricultural Experiment Station Circular, 230, 230–244.
Acknowledgments
This research was supported by funding from Landscape Ontario and the Ontario Ministries of Agriculture, Food and Rural Affairs. Donation of plant material from nurseries in Ontario is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, F., Hsiang, T. Chemical management of Volutella leaf and stem blight of boxwood. Eur J Plant Pathol 142, 107–115 (2015). https://doi.org/10.1007/s10658-015-0595-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-015-0595-x