Abstract
Fusarium oxysporum is a soil-borne fungus that causes vascular wilt and root rot. To compare infection and colonization by F. oxysporum f. sp conglutinans (Foc) on resistant and susceptible cabbage cultivars and to get more insights into interactions between Foc and cabbage, a green fluorescent protein (GFP)-expressing strain of Foc was inoculated onto resistant and susceptible cabbage varieties and monitored by laser scanning confocal microscopy (LSCM). Between 1 and 3 days post inoculation (dpi), conidia attached to root hairs and emergence sites of lateral roots, with hyphae penetrating into the epidermal tissues of roots. Few differences between susceptible and resistant cultivars were observed up to 1 to 3 dpi. From 4 to 6 dpi, hyphae progressed from the epidermis into cortical tissues and entered the xylem vessels in the susceptible cultivar, whereas such colonization was rarely observed in the resistant cultivar; these observations were further supported by statistical analysis. From 7 to 11 dpi, massive colonization and sporulation within infected xylem vessels were observed with hyphae extending into the upper hypocotyl of susceptible seedlings. Progressing symptoms of chlorosis, wilting, and death were observed on susceptible seedlings, whereas the resistant cultivar remained symptom-free. Foc was not reisolated from upper stems and petioles of the resistant cultivar, which confirmed our observations. This study provided a clear overview of the process of colonization of cabbage roots by Foc, and deepened our understandings on the details of compatible and incompatible interactions between Foc and cabbage cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The diseases caused by Fusarium include wilts, blights, rots and cankers on horticultural, field, ornamental, and forest crops in both agricultural and natural ecosystems (Ma et al. 2013). The pathogen Fusarium oxysporum is a soil-borne fungus that can infect more than 120 different plant species with various infection strategies (Armstrong 1981; Michielse and Rep 2009). The fungus invades the host roots and colonizes xylem tissues to cause wilt disease (Tjamos and Beckman 1989). Moreover, it shows an apparent gene-for-gene relationship with several hosts. Fusarium wilt in cabbage is only caused by Fusarium oxysporum f. sp conglutinans (Foc) (Tian 2009). Cabbage is widely planted in the North of China, where more than 800,000 ha are planted with cabbage every year. Cabbage plays a significant role in the year-round supply of vegetables, as well as in foreign exchange earnings through exports (Huang 2000). In the past few years, cabbage production in China has suffered from Fusarium wilt caused by Foc, resulting in significant production losses. Cabbage Fusarium wilt was discovered in the United States and first reported in China in 2001 (Li et al. 2003). To date, Fusarium wilt is considered a serious threat to the production and quality of cabbage.
The infection process of this pathogen mainly consists of five steps: root recognition, surface attachment, penetration and colonization of root cortex, proliferation and colonization in xylem vessels, and eventually rapid colonization in the host (Di Pietro et al. 2003). The soil-borne fungus F. oxysporum infects the root system, progresses through the root epidermis, the root cortex and colonizes the xylem vessels (Zvirin et al. 2010). Whilst resident in the xylem, the sporulation and germination of secondary mycelium progresses. The fungus moves upwards with the xylem stream and establishes itself throughout the plant (Bishop and Cooper 1983). Colonization by the fungus leads to a reduction in root mass, browning of the vascular system and eventual wilting and death of the cultivar. Disease symptoms in the leaves may be the most conspicuous during the early stages of infection. Once the fungus has colonized the plant, it is clearly visible that the vascular tissue turns brown in cross-sections of the stem. Wilting and death of the susceptible cultivars is inevitable (Di Pietro et al. 2003).
Research has focused on in vivo interactions between the pathogenic fungus and the plants to improve disease control. Visualization of plant infection and colonization by F. oxysporum using GFP was first conducted by Di Pietro et al. and Lagopodi et al. (Di Pietro et al. 2001a; Lagopodi et al. 2002). The processes of colonization, infection and disease development on host plant roots were clearly visualized using laser scanning confocal microscopy (LSCM). The most extensive studies on the interactions of F. oxysporum and host plants are between Fusarium oxysporum f. sp. lycopersici (Fol) and tomato (Di Pietro et al. 2001a; Lagopodi et al. 2002; Rodríguez-Molina et al. 2003). The differential colonization patterns of resistant and susceptible host plants infected by F. oxysporum were studied using melon and tomato plants (Zvirin et al. 2010; Rodríguez-Molina et al. 2003). The interaction between tomato and Fol was successfully used to study the molecular basis of disease susceptibility and resistance (Takken and Rep 2010). Despite our understanding of the infection process, the only practical measure for controlling Fusarium wilt is to grow resistant varieties (Di Pietro et al. 2003).
The aims of this study were to answer the following questions: 1) Why is invasion and colonization by F. oxysporum allowed in susceptible cultivars, 2) What are the effective defense barriers to F. oxysporum in resistant hosts, and 3) What differences exist between compatible and incompatible interactions between cabbage and Foc? In this study, GFP-labelled Foc was used to visualize and analyze the infection and colonization processes in susceptible and resistant cabbage cultivars by LSCM under in vivo conditions. Comparison of colonization patterns was conducted to determine the quantitative differences between compatible and incompatible interactions. Understanding these physiological interactions is of scientific and practical importance to manage the impact of F. oxysporum on the host plant cabbage.
Materials and methods
Host material
Cabbage cultivars “Zhong gan 21” and “Zhen qi” were chosen for their susceptibility and resistance, respectively, to Foc are well-characterized (Lv et al. 2011). The seeds were surface sterilized with 5.25 % sodium hypochlorite and pre-germinated on moist filter paper at 28 °C for 24–48 h. The seedlings were transferred to plastic pots filled with an autoclaved mixture of vermiculite and turf (1:1, v/v). Seedlings were incubated in the greenhouse at ~25 °C, with a 16 h photoperiod until the 2–3 true leaf stage.
Fungal transformation and maintenance
The Foc wild-type strain was obtained from Beijing Academy of Agriculture and Forestry Sciences and stored at −80 °C in 35 % glycerin. The isolate was cultured on potato dextrose agar (PDA) at 27 °C. The AGL-1 strain of Agrobacterium tumefaciens, which carried plasmid pCH-sGFP harbouring the gfp gene and the hygromycin B gene, was used to transform Foc (Li et al. 2011). A. tumefaciens pCH-sGFP (100 μl) and 100 μl Foc conidial suspension (106 conidia/ml) were mixed and plated onto the co-cultivation medium containing acetosyringone (40 μg/ml). Inoculated plates were covered with microporous filtering film and incubated at 27 °C for 48 h. The filtering film was transferred onto PDA selective medium containing cefotaxime (100 μg/ml) and hygromycin B (100 μg/ml). The filtering film was removed after 24 h. The selective medium plates were incubated for 5–6 days at 27 °C. All transformants were examined for gfp expression, morphology, growth rate and pathogenicity. Transformants were maintained on PDA amended with hygromycin B (50 μg/ml) and stored as for wild type strains as described above.
Inoculum preparation and inoculation procedure
The strains were grown on PDA at 28 °C in an illuminated incubator for 6–7 days. Fungal plugs (5 mm) were then transferred into flasks containing 300 ml autoclaved potato dextrose broth (PDB) and cultured at 28 °C on a shaker at 150 rpm for 72 h. Next, the mycelium was filtered through four layers of sterilized lens paper (“Shuangquan”, Q/XZJ 3028, Hangzhou, China) and the filtrate consisted of a conidial suspension. The inoculum concentration was adjusted to 106 conidia/ml. The roots of seedlings with 2–3 true leaves were gently washed in tap water and inoculated with the conidial suspension for 15 min (Lv et al. 2011; Van der Does et al. 2008). Seedlings grown under the same conditions were dipped in sterilized distilled water as non-inoculated controls. The control and inoculated seedlings were replanted in 16 cm diameter plastic pots and placed in the greenhouse under the same conditions as described above. For each cabbage genotype, more than 180 cabbage seedlings were used to observe colonization by LSCM and for reisolation of the fungus to compare colonization patterns. For each genotype, ten plants were sampled at each of 11 time points and all experiments were repeated three times.
Disease assessment and statistical analysis
The assessment of disease symptoms was performed when the cabbage seedlings showed obvious and severe wilting symptoms. According to pre-established rating scales, disease severity was divided into six levels. Rating scales of cabbage susceptibility to F. oxysporum f. sp. conglutinans were assessed for statistical analysis: index = 0–5, where “0” meant no evidence of disease symptoms; “1” represented only one piece of leaf showed slight or moderate yellowing; “2” indicated two leaves showing slight or moderate yellowing; “3” meant all leaves showed moderate yellowing or wilt except for interior leaves; “4” all leaves showed severe yellowing or wilt; “5” indicated that the seedling was dead (Lv et al. 2011). The disease index (DI) was calculated using the rating scale based on the following formula: DI = [Σ (each rating scale × the corresponding seedling numbers)/(the highest rating scale × the total numbers of seedlings)] × 100. Data from three separate experiments were gathered and the effects of inoculation by each isolate on different genotypes of cabbage were analyzed in SAS. Differences in DI were analyzed by the two-way ANOVA procedures using Duncan’s Multiple Range Test. Results were shown as the mean ± SE (standard error).
Microscopic examination
Microscopic examination commenced 24 h after inoculation, with observations conducted using different plants every 24 h for 11 days to capture all stages of disease progression. Inoculated and control plants were removed from the mixture of vermiculite and turf. Roots were gently washed in tap water to wash away particles and unattached conidia, sectioned into 1–2 mm slices (both cross and longitudinal sections) with a double-edged razor blade by hand. These were then placed directly on glass slides in drops of water and covered with a cover glass for microscopic analysis. For each seedling, several hand sections were prepared from four segments: the crown, the main roots, the lateral roots and the upper hypocotyls. Microscopic examinations were performed using a Zeiss LSM 700 laser scanning microscope with EC Plan-Neofluar objective lenses (20, 40, 63, and 100× magnification). Confocal settings were optimized for visualization of GFP with excitation initiated using a 488 nm Argon laser, detection of emitted light at 522 DF32 and auto-fluorescence detection at 605 DF32. Images were obtained from individual channels. The cabbage root cell walls produced red autofluorescent signals that were used to position the fungus. Ten seedlings were sampled at each time point.
Comparison of the colonization pattern between susceptible and resistant cabbage cultivars
We compared colonization patterns between susceptible and resistant cabbage cultivars infected by Foc. Colonization was determined by fungal reisolation from root, stem and petiole sections for each plant (Qiao et al. 2006). Cabbage cultivars were sampled at 3, 7, 9, 11 and 15 dpi. Plants were gently washed in tap water and cut at the roots, stem bases, upper stems and petioles. Plant sections were successively dipped into 70 % ethanol, 5.25 % sodium hypochlorite and sterile water. After surface sterilization, these samples were ground into a paste in 1 mL sterile-water and diluted 10- and 100-fold. Samples (200 μL) were cultured on PDA at 27 °C. The numbers of colony forming units were counted on the 8th day after cultivation. For each sample, three independent replicates were plated out for each of three or more seedlings.
Results
Disease symptoms of Fusarium wilt on susceptible and resistant cabbage seedlings
The most apparent and characteristic symptom was the yellowing of foliage as the disease progressed (Fig. 1a). At 3 dpi, there were no obvious foliar symptoms on either cultivar, but cultivar Zhong gan 21 displayed slight yellowing along the leaf vein. At 6 dpi, chlorotic foliar symptoms were present on the susceptible cultivar Zhong gan 21, but not on resistant cultivar Zhen qi. No disease symptoms were observed on resistant cultivar Zhen qi during the 11-day period after inoculation whilst foliar wilting and browning of vessel tissue were frequent on susceptible cultivar Zhong gan 21. The disease index was used to evaluate the susceptible and resistant cabbage cultivars. As shown in Fig. 1b, the disease indices were significantly different for susceptible cultivar Zhong gan 21 and resistant cultivar Zhen qi.
Disease symptoms on susceptible (Zhang gan 21) and resistant (Zhen qi) cabbage seedlings infected by Fusarium oxysporum f. sp. conglutinans (Foc). a. Disease symptoms on leaves of susceptible and resistant cabbage cultivars at 0, 3, 6 and 9 dpi; b. Disease index analysis of susceptible and resistant cabbage seedlings at 3, 7 and 15 dpi. Each disease index value was an average of three independent biological replicates with standard errors of the mean. Each disease index test incorporated more than 30 seedlings. Values followed by different letters were significantly different according to the Duncan’s Multiple Range Test at P < 0.01
Confirmation of GFP transformants
Eleven transformed fungal isolates were obtained and confirmed by PCR (Fig. 2a). Amplification of the 315 bp gfp fragment and the 517 bp hpt fragment indicated the successful genomic integration of exogenous T-DNA in the transformants. Furthermore, the gfp gene was stable after six successive subcultures. The mycelium and conidia of the transformed Foc fluoresced brightly (Fig. 2b). Analysis of growth rates and pathogenicity showed that there were no significant differences between the wild type isolate and transformant (Fig. 2c). After confirmation, we named the most brightly fluorescent transformant Foc-GFP. In this study, the brightest transformant Foc-GFP was used for visualization and analysis of the infection and colonization of susceptible and resistant cabbage cultivars Zhong gan 21 and Zhen qi, respectively.
Confirmation and analysis of Foc-GFP transformants. a. Amplification of the 315 bp and 517 bp gfp and hpt gene fragments by PCR to confirm the 11 transformants. M marker; W wild type isolate; b. Mycelium and conidia of Foc-GFP under fluorescence microscope; c. Analysis of growth rates and pathogenicity between the wild type isolate and transformant. Disease index (DI) of cabbage infected by Foc and Foc-GFP at 8 dpi. Values did not differ significantly between wild type and transformed isolates according to the Duncan’s Multiple Range Test
Initial interaction between Foc-GFP and cabbage seedlings: 1–3 dpi
Foc-GFP fluoresced brightly enough to be visible on cabbage roots even at a low (×100) magnification. No loss of fluorescence was detected during LSCM analyses. At 1 dpi, no mycelia were observed on the surface of roots,however, a small number of conidia were attached to the root hair (Fig. 3a) and the emergence site of the lateral root (Fig. 3b). At 2 dpi, hyphae were loosely attached to the main root surfaces (Fig. 3c) and were interwoven with root hairs (Fig. 3d). In addition, as Fig. 3d showed, most of the conidia on root surfaces had germinated. At 3 dpi, the hyphae attached to the central root surface (Fig. 3e) and individual hyphae started to grow along the junctions of the epidermal cells (Fig. 3f).
Early stages of infection (1–3 dpi) of susceptible cabbage seedlings inoculated with Foc-GFP. a. The conidia of Foc-GFP attached to the root hair; b. Germinated conidia of Foc-GFP attaching to the lateral root at the emergence site at 1 dpi; c. Intermingling of hyphae at the crown region at 2 dpi; d. Attachment of fungal hyphae to cabbage root hairs at 2 dpi; e. Attachment of hyphae setting in the epidermal cells at 3 dpi; f. Infection and colonization of the root surface by hyphae that were growing in epidermal cells at 3 dpi
Our observations showed that the contacts between Foc and roots were initiated at root hairs and the base of lateral roots. These areas seemed to be more easily infected by conidia and individual hyphae. Few differences in the initial infection and colonization of roots were observed between susceptible and resistant cabbage cultivars. At 1 dpi, regardless of cabbage genotype, the conidia adhered to the root epidermal cell borders, of which only a few showed signs of germination. At 3 dpi, hyphae had penetrated into the epidermal tissues of primary and lateral roots. During these 3 days after inoculation, there were no visible root or foliar disease symptoms on the susceptible and resistant cabbage cultivars.
Advanced infection of cabbage seedlings infected with Foc-GFP: 4–6 dpi
At 4 dpi, the cabbage seedlings looked healthy and symptom-free. Hyphae were observed in the first layer of epidermal cells (Fig. 4a). Sites where roots had been penetrated exhibited brown discoloration and were densely covered with mycelia. Growth of mycelia along the epidermal cell wall formed an expanding network that covered large areas in the root cortex at 5 dpi (Fig. 4b). Hyphae were also observed growing in one epidermal cell penetrated from the adjacent cell (Fig. 4c). At 6 dpi, the susceptible cabbage seedlings showed the early stages of disease symptoms. The leaves showed slight yellowing. Hyphae had entered the xylem vessels and begun to move upwards (Fig. 4d). At this time, hyphae oriented in parallel were often observed and no conidia were observed within infected xylem vessels. The hyphae established within root epidermal and cortical cells showed more elaborate and expanding networks for further colonization (Fig. 4e). During this time, necrotic lesions and tissue collapse were apparent at heavily infected sites and root tissues began to lose integrity (Fig. 4f). In Fig. 4g and h, the penetration sites of hyphae were clearly visible from one epidermal cell to the adjacent cell (indicated by white arrow).
Advanced infection (4–6 dpi) of susceptible cabbage seedlings inoculated with Foc-GFP. a. Root surface colonization by hyphae growing along the junction of the epidermal cells at 4 dpi; b. The expanding network of hyphae colonizing the root cortex at 5 dpi; c. A hypha growing in one epidermal cell penetrating into the adjacent cell; d. The hyphae entering the xylem vessels and beginning to move upwards; e. Hyphae growing in epidermal root cells at 6 dpi; f. Root tissue collapse at heavily infected sites; g and h. Growth of hyphae in root cell with a clearly visible penetration sites (indicated by white arrow) from one epidermal cell to the adjacent cell
Our observations showed that mycelia had extended from the epidermis into cortical tissues, proliferated rapidly in the cortical tissues and vessel tissues, and that some structures of colonized roots had collapsed in susceptible cabbage cultivars, whereas mycelia were rarely observed in cortical tissues of the resistant cultivars at this time. The difference in disease symptoms between the two genotypes became prominent. The typical and significant foliar yellowing symptoms were apparent on susceptible cabbage cultivars while resistant cultivars were symptom-free despite colonization.
Later stages of cabbage seedlings infected with Foc-GFP: 7–9 dpi
At 7–9 dpi, almost all the susceptible cabbage seedlings showed prominent foliar symptoms such as chlorosis and wilting, extensive damage to roots and vascular discoloration. At 7 dpi, hyphae had colonized the root system and begun to sporulate (Fig. 5a). Conidia, which colonized the root xylem vessel, had germinated and formed swollen hyphae for further colonization (Fig. 5b). At 9 dpi, the fully colonized root exhibited massive colonization with hyphae (Fig. 5c). During this time, the upper hypocotyl was colonized as well (Fig. 5d). Fig. 5e and f showed an eruption of hyphae from xylem vessels into the neighbouring parenchymal tissues and surrounding cortical tissues. Over the next few days, the disease progressed rapidly through the whole plant, eventually resulting in wilt and decay.
Later stages of infection and colonization (7–9 dpi) of susceptible cabbage seedlings inoculated with Foc-GFP. a. Foc-GFP conidia in the xylem vessel of a fully colonized root at 7 dpi; b. Germinated conidia of Foc-GFP growing in the xylem vessel of a colonized root at 7 dpi; c. Infection hyphae of Foc-GFP growing in a fully colonized root at 9 dpi; d. Hyphae growing in the upper hypocotyl of susceptible cabbage seedlings at 9 dpi; e. Fully colonized root of a seedling that exhibits extensive root rot at 9 dpi; f. The root of cabbage seedlings densely populated by Foc-GFP
Our observations showed that during the later stages of infection, large numbers of mycelia and conidia in the xylem vessels of the susceptible cultivar revealed massive colonization and sporulation, whereas this was not observed in xylem vessels of the resistant cultivar. Moreover, hyphae growing in the upper hypocotyl of susceptible cabbage seedlings were clearly visible, but this was also not observed in the resistant cultivar. The disease progressed rapidly on susceptible cabbage seedlings and resulted in wilting and death. The resistant cultivar remained symptom-free and grew well.
The colonization pattern of Foc in susceptible and resistant cabbage cultivars
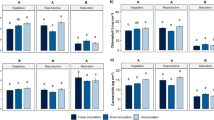
In the susceptible cabbage cultivar, roots, stems and petioles showed a gradual increase in colonization, reaching a maximum at 15 dpi (Fig. 6). Fungal abundance in the roots and stem bases of the susceptible cabbage cultivar was one order of magnitude higher compared with abundance in the upper stems and petioles. Three days after inoculation, fungi were recovered from the roots of the susceptible cabbage cultivars but not from the resistant cultivar, although we had observed the initiation of contact between Foc and roots for both cultivars. A gradual increase in colonization of the roots was observed from 7 to 15 dpi in susceptible cabbage cultivar Zhong gan 21 (Fig. 6a). In contrast, the fungal population in the roots of resistant cultivar Zhen qi was significantly lower and relatively stable. The results were consistent with our observations that colonization events were rarely seen in the resistant cabbage cultivar. From 3 to 15 dpi, fungal propagules were recovered from stems and petioles of the susceptible cabbage cultivar (Fig. 6b–d). The results agreed with the observation that hyphae had entered the xylem vessels and begun to move upwards at 6 dpi, and colonized the root system and begun to sporulate at 7 dpi. Significant upward stem colonization of susceptible cultivars occurred concurrently with the appearance of the first foliar disease symptoms. Rapid fungal distribution from the base to the top of the stem suggested faster stem colonization during the compatible interaction. However, little fungus was isolated from roots and stem bases of the resistant cabbage cultivar (Fig. 6a and b) and no fungus was isolated from the upper stems and petioles (Fig. 6c and d). Based on these results, it showed that the fungus was limited to the stem bases in the resistant cabbage cultivar. Furthermore, the resistant cabbage cultivar grew well and showed no disease symptoms. These results showed that the development of disease was closely linked with the extent of fungal colonization.
Comparison of colonization patterns between susceptible and resistant cabbage cultivars during 3–15 dpi. a, b, c and d. The colony forming units from each gram of roots, stem bases, upper stems and petioles in susceptible cabbage cultivar “Zhong gan 21” and resistant cabbage cultivar “Zhen qi” at 3, 7, 9, 11 and 15 dpi
Discussion
The GFP-marked fungus could be easily observed in and around the host root using LSCM. In 2001, Lorang et al. reported the transformation with gfp of 16 species belonging to 12 genera of filamentous fungi (Lorang et al. 2001). The first microscopic studies on the process of infection by F. oxysporum were conducted by Di Pietro et al. and Lagopodi et al. (Michielse and Rep 2009). They monitored invasion of tomato cultivars using F. oxysporum f. sp. lycopersici GFP strain (Di Pietro et al. 2001a; Lagopodi et al. 2002). A study was performed on Arabidopsis, and showed the dynamics of Fusarium colonization in time and space (Czymmek et al. 2007). Microscopic studies on Fusarium wilt were conducted on watermelon, banana and other species (Li et al. 2012; Liu 2012). Researchers have also compared the colonization patterns between susceptible and resistant cultivars of tomato, lettuce and melon inoculated with this fungus (Rodríguez-Molina et al. 2003; Vallad and Subbarao 2008; Zvirin et al. 2010). For the first time, we have observed and compared colonization of cabbage by a GFP-marked strain of Foc in terms of compatible and incompatible interactions. Our results were similar to colonization patterns previously reported. At 1 dpi, we observed conidia attached to the root hairs and the emergence sites of lateral roots. Olivain and Alabouvette (1999) observed the same results, with penetration events already occurring at 1 dpi between tomato and F. oxysporum (Olivain and Alabouvette 1999). At 3 dpi, Foc was only reisolated from the susceptible cabbage cultivar. We speculated that Foc was not recovered from the resistant cultivar because there was little attached fungus in this case. Most of the conidia on the root surface had germinated and begun to penetrate the epidermal cells in both susceptible and resistant cabbage seedlings. Germ tubes appeared from one or both ends of the conidia. In our observations, advanced colonization of cortical tissues frequently occurred on the susceptible cabbage cultivar, whereas mycelium was rarely observed in cortical tissues of the resistant cultivar at this time. Olivain et al. (2003) suggested that the resistant genotype could elicit a stronger response at the pre-vascular stage (Olivain et al. 2003). Zvirin et al. (2010) analyzed defense gene expression in the response of susceptible and resistant melon cultivars to Fusarium oxysporum f. sp. melonis inoculation to confirm that the resistant melon cultivar responded more efficiently at this stage (Zvirin et al. 2010). At 6 dpi, the differences in disease symptoms between the two genotypes became prominent. The typical and significant foliar yellowing symptoms were apparent on the susceptible cabbage cultivar while the resistant cultivar remained symptom-free despite colonization. At the later infection stage, large amounts of mycelium and conidia were observed in xylem vessels of the susceptible cultivar, revealing massive colonization and sporulation, whereas that was rarely observed in xylem vessels of the resistant cultivar. Similarly, the results were also supported by the statistical analysis. Mace and Veech (1971) and Beckman et al. (1972) reported the formation of secondary spores of F. oxysporum in the xylem of tomato cultivar (Mace and Veech 1971; Beckman et al. 1972). Beckman and Roberts (1995) reported that sporulation and germination of secondary mycelium were necessary for rapid upward colonization to account for the rapid colonization rates observed (Beckman and Roberts 1995). In our study, these events were clearly visible in Fig. 5a and b.
There were some differences observed in fungal colonization between the susceptible and resistant cabbage cultivars. The infection incidence of the resistant cabbage cultivar was lower than for the susceptible cabbage cultivar. For example, the initial counts of conidia attached to the surface of roots differed between the two cabbage cultivars. In addition, different kinds of expanding structures were observed during the colonization of the susceptible cultivar. “Small protuberances” developed on hyphae were used to describe intracellular infection during the infection of potato by V. dahlia (Perry and Evert 1983). Vallad and Subbarao (2008) observed germinating conidia with distinct appressoria on V. dahlia infected lettuce (Vallad and Subbarao 2008). In addition, the extending patterns in the susceptible cultivar were always systemic and the fungus in the susceptible cultivar easily formed elaborate network structures for further colonization, whilst the extending patterns were local on the resistant cultivar depending on individual hyphae. Foc extended upwards along the xylem vessel from the tip of the root. Infection and colonization in the vascular system of the susceptible cabbage cultivar seemed more efficient than in the resistant cultivar. The colonization rates and extent of Foc on roots, stems and petioles of susceptible and resistant cabbages also differed. At 9 dpi, the fungus was visible and reisolated from the upper hypocotyl of the susceptible cabbage cultivar and not visible or recoverable from the resistant cultivar. These results indicated that the resistant cabbage cultivar restricted further movement of the fungus in the xylem. Several processes including physical barriers and metabolite action of plants could prevent fungal growth and progress (Rodríguez-Molina et al. 2003). From this work, we concluded that the primary defence mechanism against Foc in resistant cabbage was localization of infection within basal vascular tissues. At 11 dpi, the susceptible cabbage cultivar plants were wilted, brown and dead while no disease symptoms were observed on the resistant cultivar plants. By microscopic examination, we observed that the vessel tissues of the susceptible cultivar were full of mycelium while the resistant cultivar remained stable and free of Foc infection.
Over all, during colonization of the susceptible cultivar, the abundance of Foc-GFP increased significantly after inoculation, reflecting rapid and continuous colonization of the plant tissues. Furthermore, the growth of seedlings was significantly restricted. In contrast, during colonization of the resistant cultivar, the plants showed no disease symptoms. The resistant cabbage cultivar was not completely immune from Foc, but could restrict pathogen progression to the stem bases. Few differences in penetration events were shown in the initial infection and colonization of roots between susceptible and resistant cabbage cultivars, but the isolated outgrowths were more abundant in the susceptible cultivars compared with the resistant cultivar. We speculated that physical barriers, pathogenesis-related proteins, pre-existing or inducible antimicrobial compounds, small antimicrobial peptides and reactive oxygen species might play crucial roles in the response of the resistant cabbage cultivar against Foc (Di Pietro et al. 2003). The root endodermis, vascular gels and tyloses in the resistant cabbage cultivar might act as structural barriers to block the advance of fungal hyphae.
This study provided a clear overview of the fungal colonization by Foc in the root system of cabbage seedlings. This has deepened our understanding of survival and infection of Foc on cabbage cultivars and on the details of the compatible and incompatible interactions between Foc and cabbage seedlings. Results from this study will contribute to investigation of disease-control measures against Fusarium oxysporum.
References
Armstrong, G. (1981). Formae speciales and races of Fusarium oxysporum causing wilt diseases. Fusarium: diseases, biology, and taxonomy.
Beckman, C. H., & Roberts, E. (1995). On the nature and genetic basis for resistance and tolerance to fungal wilt diseases of plants. Advances in Botanical Research, 21, 36–77.
Beckman, C., Elgersma, D., & MacHardy, W. (1972). The localization of fusarial infections in the vascular tissue of single-dominant-gene resistant tomatoes. Phytopathology, 62, 1256–1260.
Bishop, C., & Cooper, R. M. (1983). An ultrastructural study of vascular colonization in three vascular wilt diseases I. Colonization of susceptible cultivars. Physiological Plant Pathology, 23, 323–343.
Czymmek, K. J., Fogg, M., Powell, D. H., Sweigard, J., Park, S. Y., & Kang, S. (2007). In vivo time-lapse documentation using confocal and multi-photon microscopy reveals the mechanisms of invasion into the Arabidopsis root vascular system by Fusarium oxysporum. Fungal Genetics and Biology, 44, 1011–1023.
Di Pietro, A., Huertas-González, M. D., Gutierrez Corona, J. F., Martínez Cadena, G., Méglecz, E., & Roncero, M. I. G. (2001). Molecular characterization of a subtilase from the vascular wilt fungus Fusarium oxysporum. Molecular Plant-Microbe Interactions, 14, 653–662.
Di Pietro, A., Madrid, M. P., Caracuel, Z., Delgado-Jarana, J., & Roncero, M. I. G. (2003). Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Molecular Plant Pathology, 4, 315–325.
Huang, W. (2000). Cultivation techniques of Brassica vegetables for high yield and quality. Beijing: China Forestry Publishing House.
Lagopodi, A. L., Ram, A. F., Lamers, G. E., Punt, P. J., Van den Hondel, C. A., Lugtenberg, B. J., & Bloemberg, G. V. (2002). Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Molecular Plant-Microbe Interactions, 15, 172–179.
Li, M., Zhang, T., Li, X., & Yan, H. (2003). Fusarium wilt on Cruciferous vegetables and identification of their pathogens. Plant Protection, 29, 44–45.
Li, E., Wang, D., Yang, Y., & Xie, B. (2011). Agrobacterium tumefaciens mediated transformation of cabbage Fusarium wilt pathogen. China Vegetables, 14, 008.
Li, C., Liang, H., Xia, Y., & Peng, M. (2012). Visualization the colonization of watermelon infected by Fusarium oxysporum f. sp. niveum labeled by GFP. Journal of Tropical Crops, 32, 1935–1939.
Liu, Y. (2012). The use of green fluorescent protein marker to study infection of three different banana varieties with Fusarium oxysporum f. sp. cubense race4. Guangdong: Guangdong Ocean University.
Lorang, J., Tuori, R., Martinez, J., Sawyer, T., Redman, R., Rollins, J., Wolpert, T., Johnson, K., Rodriguez, R., & Dickman, M. (2001). Green fluorescent protein is lighting up fungal biology. Applied and Environmental Microbiology, 67, 1987–1994.
Lv, H., Fang, Z., Yang, L., Xie, B., Liu, Y., Zhuang, M., Zhang, Y., & Yang, Y. (2011). Research on screening of resistant resources to Fusarium wilt and inheritance of the resistant gene in cabbage. Acta Horticulturae Sinica, 5, 011.
Ma, L., Geiser, D. M., Proctor, R. H., Rooney, A. P., O’Donnell, K., Trail, F., Gardiner, D. M., Manners, J. M., & Kazan, K. (2013). Fusarium pathogenomics. Annual Review of Microbiology, 67, 399–416.
Mace, M. & Veech, J. (1971). Fusarium wilt of susceptible and resistant tomato isolines: host colonization. Phytochemistry.
Michielse, C. B., & Rep, M. (2009). Pathogen profile update: Fusarium oxysporum. Molecular Plant Pathology, 10, 311–324.
Olivain, C., & Alabouvette, C. (1999). Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici in comparison with a non-pathogenic strain. New Phytologist, 141, 497–510.
Olivain, C., Trouvelot, S., Binet, M. N., Cordier, C., Pugin, A., & Alabouvette, C. (2003). Colonization of flax roots and early physiological responses of flax cells inoculated with pathogenic and nonpathogenic strains of Fusarium oxysporum. Applied and Environmental Microbiology, 69, 5453–5462.
Perry, J. W., & Evert, R. F. (1983). Histopathology of Verticillium dahliae within mature roots of Russet Burbank potatoes. Canadian Journal of Botany, 61, 3405–3421.
Qiao, H. P., Huang, L. L. & Kang, Z. S. (2006). Inhibiting effect of endophytic bacteria of wheat on the main pathogenic fungi of root and stem. Chinese Journal of Applied Ecology, 17.
Rodríguez-Molina, M., Medina, I., Torres-Vila, L., & Cuartero, J. (2003). Vascular colonization patterns in susceptible and resistant tomato cultivars inoculated with Fusarium oxysporum f. sp. lycopersici races 0 and 1. Plant Pathology, 52, 199–203.
Takken, F., & Rep, M. (2010). The arms race between tomato and Fusarium oxysporum. Molecular Plant Pathology, 11, 309–314.
Tian, R. (2009). Integrative research on pathogen of Fusarium wilts and population structure of resistant germplasm in cabbage. Gansu: Gansu Agriculturial University.
Tjamos, E. & Beckman, C. H. (1989). Vascular wilt diseases of plants. Basic studies and control: Springer-Verlag.
Vallad, G., & Subbarao, K. (2008). Colonization of resistant and susceptible lettuce cultivars by a green fluorescent protein-tagged isolate of Verticillium dahliae. Phytopathology, 98, 871–885.
Van der Does, H. C., Duyvesteijn, R. G. E., Goltstein, P. M., Van Schie, C. C. N., Manders, E. M. M., Cornelissen, B. J. C., & Rep, M. (2008). Expression of effector gene SIX1 of Fusarium oxysporum requires living plant cells. Fungal Genetics and Biology, 45, 1257–1264.
Zvirin, T., Herman, R., Brotman, Y., Denisov, Y., Belausov, E., Freeman, S., & Perl-Treves, R. (2010). Differential colonization and defence responses of resistant and susceptible melon lines infected by Fusarium oxysporum race 1.2. Plant Pathology, 59, 576–585.
Acknowledgments
This research was supported by the Ministry of Agriculture Key Laboratory of genetic improvement of horticultural crops, the National Natural Science Foundation of China (No.31272003), the National Key Technology R&D Program (No.2012BAD19B06), the Special Fund for Agro-scientific Research in the Public Interest (No.200903049-04) and the National Staple Vegetable Industry Technology System Construction Project (No.CARS-25-B-01). The authors declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Erfeng Li and Gang Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, E., Wang, G., Yang, Y. et al. Microscopic analysis of the compatible and incompatible interactions between Fusarium oxysporum f. sp. conglutinans and cabbage. Eur J Plant Pathol 141, 597–609 (2015). https://doi.org/10.1007/s10658-014-0567-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-014-0567-6