Abstract
Several case–control and prospective cohort studies have examined the association between the consumption of nuts and legumes and the risk of colorectal cancer. For the quantitative assessment of this association, we conducted a meta-analysis of observational studies. We searched PubMed and Web of Science databases along with hand searches for eligible studies published up to January 2022. A total of 13 studies (8 cohort studies and 5 case–control studies) on nuts consumption and 29 studies (16 cohort studies and 13 case–control studies) on legumes consumption were included in the meta-analysis. The pooled relative risks (RRs) of colorectal cancer for the highest versus lowest categories of nuts consumption and legumes consumption were 0.84 (95% CI: 0.71–0.99) and 0.90 (95% CI: 0.83–0.98), respectively. Based on the dose–response analysis, a 28 g/day (1 serving/day) increment of nut consumption was associated with a 33% lower risk of colorectal cancer, and 100 g/day (1 serving/day) increment of legumes consumption was associated with a 21% lower risk of colorectal cancer. By geographic region for nuts consumption, however, the inverse association for the highest versus lowest categories was only observed in Asia (RR = 0.44; 95% CI: 0.29–0.68) from 3 studies, and no association was found in America (RR = 1.01; 95% CI: 0.92–1.11) (Pdifference = 0.003) or Europe (RR = 1.02; 95% CI: 0.84–1.25) (Pdifference = 0.003). In addition, the associations tended to be weak when stratified by adjustment for confounders. Our findings suggest that the evidence for an association is currently weak, and thus further well-designed prospective studies are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer is the third most common cancer worldwide, with more than 1.93 million new cases of colorectal cancer incidence, and more than 935,000 deaths from colorectal cancer in 2020 [1]. In addition, the World Health Organization (WHO) estimated that in 2040, the global incidence of colorectal cancer rises to more than 3.15 million new cases and more than 1.62 million deaths from colorectal cancer [2]. The trends in colorectal cancer incidence and mortality are related to the current level of human development and might be due to adopting more Western diets and lifestyles [3].
Nuts and legumes may be considered key components of healthy diet patterns. Nuts and legumes play an important role in plant foods characterized by the Mediterranean diet due to their favorable nutrient profile [4]. Nuts included not only tree nuts, but also a wide range of nuts including peanuts. Peanuts are actually legumes, but they are often identified by consumers as part of the nuts. Nuts are a rich source of vegetable proteins, unsaturated fatty acids, vitamin B-6, vitamin E, selenium, fiber, folic acid, and other phytochemicals [5,6,7]. Legumes also are good sources of phytochemicals, protein fiber, and some micronutrients [4, 8]. In addition, legumes are generally low in fats, except for soybean [4]. Several previous observational studies reported that nuts and legumes were associated with a lower risk of colorectal cancer [9,10,11,12,13,14,15,16,17].
Three meta-analyses on nuts consumption and cancer risk have been previously conducted and included some results for the association between nuts consumption and colorectal cancer risk as part of subgroup analysis by cancer type [7, 18, 19]. However, no comprehensive meta-analysis was conducted to quantitatively assess the association between nuts consumption and the risk of colorectal cancer. For legumes consumption, there was a previous meta-analysis of colorectal cancer risk, which also included studies of legume fiber consumption [20]. In addition, the World Cancer Research Fund International/American Institute for Cancer Research (WCRF-AICR) conducted a meta-analysis of legumes consumption and colorectal cancer [21], which included a few studies only.
Thus, we systematically reviewed and performed a comprehensive meta-analysis of all observational studies to quantitatively evaluate the association between the consumption of nuts and legumes and risk of colorectal cancer.
Methods
Literature search and study selection
Studies published up to January 2022 were searched from PubMed and ISI Web of Science electronic databases, and the searches were limited to articles published as written in English and full-length. The search strategy included the following keywords: “(nut OR almond OR cashew OR tree nut OR peanut OR pecan OR pine nut OR pistachio OR macadamia OR hazelnut OR walnut OR brazil nut OR legume OR soy OR bean OR pea OR soybean OR tofu OR soymilk OR pulse OR lentils OR miso OR natto) AND (colorectal OR colon OR rectal OR rectum) AND (cancer OR neoplasm OR carcinoma OR tumor)”. In addition, we supplemented by a manual search of reference lists of retrieved articles and reviews to identify additional qualified studies. The searches were limited to articles published as written in English and full-length. Inclusion criteria were as follows: (1) case–control or cohort studies; (2) studies that reported the association between the consumption of nuts or legumes and the risk of colorectal cancer; (3) studies that reported relative risks (RRs) and confidence intervals (CIs). If more than one article reported the results from the same study, we selected the study which included more cases. In addition, we excluded a study that had no adjustment for any confounder [9].
Data extraction
Data extraction was conducted independently by two investigators (S.J. and Y.J.) using the meta-analysis of observational studies in epidemiology (MOOSE) guidelines [22], and any disagreements were addressed by checking the original reports and discussion. The following information was extracted from each study: first author’s last name, year of publication; country and study name; study design; follow-up period or study period; baseline age; sex; number of cases and controls/participants or person-time; each category of nuts or legumes consumption; RRs and 95% CIs for all categories of nuts and legumes consumption; adjustment for potential confounders. If studies reported several RRs, we used the RR that reflected maximally adjusted for potentially confounding variables. If some studies assessed more than one type of legumes products, we used the RR that was the most representative of overall legumes consumption and legumes that were the most commonly consumed.
Statistical analysis
To estimate pooled RRs and its 95% CIs for the highest versus lowest category of nuts and legumes consumption, we combined a natural logarithm of the RR from the original study, using the random-effects models by DerSimonian and Laird, which incorporate both within- and between-study variations [23]. If the study separately reported by cancer site, we combined the two results using a fixed-effect model to obtain an overall estimate of colorectal cancer or colon cancer first and then combined with other studies [10, 16, 17, 24,25,26,27,28]. In addition, if the study separately reported according to sex [11, 14, 16, 24, 26, 29,30,31,32,33], different ages [34], race [35] or family history [36, 37], we also combined the two results using a fixed-effect model to obtain an overall estimate first and then combined with other studies. To investigate whether the association between consumption of nuts and legumes and risk of colorectal cancer differed by study design (cohort/case–control), sex, cancer site (colon/proximal colon/distal colon/rectal), geographical region (America/Europe/Asia/Oceania), we conducted a subgroup analysis when separated data were available.
Linear dose–response analyses using the generalized least-squares trend (GLST) estimation method by Greenland and Longnecker were conducted to estimate study-specific slopes across categories of nuts and legumes consumption [38,39,40]. We used the median value for each exposure category of nuts and legumes consumption. If the upper category was open-ended, we assumed the same amplitude as the previous category. Studies with less than 3 exposure categories or missing data on the number of cases and participants for each exposure category were excluded from the dose–response analysis. For studies that reported exposure units other than grams per day, we defined one serving as 28 g for nuts and 100 g for legumes according to the standard of the previous study converting these into grams per day [18, 41].
Statistical heterogeneity across the included studies was assessed using the Q statistic [42], and inconsistency was quantified by I2 statistic [43]. Sensitivity analyses were conducted by excluding each study at a time. Potential publication bias was evaluated with Begg’s [44] and Egger’s tests [45]. To detect the effect of possible missing studies on the overall effect, we used Duval and Tweedie trim-and-fill methods [46]. A two-tailed P value < 0.05 was considered statistically significant. All statistical analyses were performed by using Stata/SE version 14.2 Software (StataCorp, College Station, TX, USA).
Results
Study characteristics
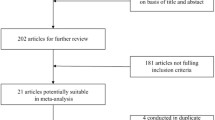
For nuts consumption, a total of 13 studies including 8 prospective cohort studies [12, 14, 29, 47,48,49,50] with 9546 cases and 5 case–control studies [10, 17, 34, 35, 51] with 2914 cases were included in the meta-analyses (Fig. 1). For legumes consumption, a total of 29 studies including 16 prospective cohort studies [12, 14, 15, 26, 28, 30,31,32, 36, 52,53,54,55,56,57] with 13,631 cases and 13 case–control studies [10, 11, 13, 16, 24, 25, 27, 33, 35, 37, 51, 58,59,60] with 7275 cases were included in the meta-analyses. The characteristics of the included studies are summarized in Tables 1 and 2. By geographic region, regarding nuts consumption, seven studies were performed in America, three studies in Europe, and three studies in Asia. Regarding legumes consumption, ten studies were performed in America, four studies in Europe, fourteen studies in Asia, and one study in Oceania. Most of the studies adjusted for age, total energy intake, alcohol consumption, smoking, body mass index (BMI, kg/m2), and physical activity.
Nuts consumption and colorectal cancer
A total of thirteen studies including 12,460 cases and 926,327 participants investigated the association between nuts consumption and risk of colorectal cancer (Table 1). The pooled RR for highest versus lowest categories of nuts consumption was 0.84 (95% CI: 0.71–0.99), with some evidence of heterogeneity (I2 = 82.4%, P < 0.001) (Fig. 2, Table 3). No significant associations were found when stratified by study design or sex, and the meta-regression analysis showed no significant differences (Pdifference > 0.5 for all comparisons). By cancer site, a significant inverse association was shown in colon (RR = 0.78; 95% CI: 0.63–0.96) and rectal cancer (RR = 0.71; 95% CI: 0.51–0.98). Based on the meta-regression analyses, there was no significant difference with cancer site (Pdifference > 0.7 for all comparisons). By geographic region, however, there was some difference in RRs. The inverse association between nut consumption and colorectal cancer risk was only observed in Asia (RR = 0.44; 95% CI: 0.29–0.68), while no association was found in America (RR = 1.01; 95% CI: 0.92–1.11) (Pdifference for America vs. Asia = 0.003) or Europe (RR = 1.02; 95% CI: 0.84–1.25) (Pdifference for Europe vs. Asia = 0.003). Furthermore, no heterogeneity was observed in the studies stratified by region. Regarding adjustment for confounders, there was no significant difference with energy intake, alcohol intake, smoking, BMI, physical activity, or dietary factors (Pdifference > 0.4 for all comparisons). Nine studies [12, 17, 29, 35, 47, 48, 50] were included in the dose–response analysis for nuts consumption and risk of colorectal cancer. A 28 g/day (1 serving/day) increment of nuts consumption was associated with a 33% lower risk of colorectal cancer (RR = 0.67; 95% CI: 0.45–0.98).
Legumes consumption and colorectal cancer
A total of twenty-nine studies including 20,906 cases and 1,688,603 participants investigated the association between legumes consumption and risk of colorectal cancer (Table 2). The pooled RR of colorectal cancer for the highest versus lowest categories of legumes intake was 0.90 (95% CI: 0.83–0.98), with some evidence of heterogeneity (I2 = 56.5%, P < 0.001) (Fig. 3, Table 4). In the stratified analysis by study design, we found no significant association, and the meta-regression analysis showed no significant differences (Pdifference = 0.31). By sex, a significant inverse association was found in women (RR = 0.86; 95% CI: 0.75–0.99), but not in men (RR = 0.93; 95% CI: 0.84–1.02) (Pdifference = 0.64). By cancer site, we found a significant inverse association in colon (RR = 0.89; 95% CI: 0.82–0.96) and rectal cancer (RR = 0.82; 95% CI: 0.70–0.94). By geographic region, there was a significant inverse association in Asia (RR = 0.88; 95% CI: 0.77–0.999) and Oceania (RR = 0.59; 95% CI: 0.35–0.98), while there was no significant association between in America (RR = 0.90; 95% CI: 0.78–1.04) and Europe (RR = 1.00; 95% CI: 0.85–1.19). In the meta-regression analysis, however, the pooled RRs were not significantly different with geographic region (Pdifference for America, Europe, or Oceania vs. Asia = 0.84, 0.30, and 0.24, respectively). By adjustment for confounders, we found no significant difference with energy intake, alcohol intake, smoking, BMI, physical activity, or dietary factors (Pdifference > 0.1 for all comparisons). Fourteen studies [12, 15, 16, 27, 28, 30, 35, 36, 53, 56,57,58, 60] were included in the dose–response analysis for legumes consumption and risk of colorectal cancer. A 100 g/day (1 serving/day) increment of legumes consumption was associated with a 21% lower risk of colorectal cancer (RR = 0.79; 95% CI: 0.64–0.97).
Publication bias
For the analysis of nuts consumption and risk of colorectal cancer, there was no evidence of publication bias in Egger’s (P value for bias = 0.06) and Begg’s test (P value for bias = 0.09). For the analysis of legumes consumption and risk of colorectal cancer, Egger’s test suggested some evidence of bias (P value for bias = 0.02), but not in Begg’s test (P value for bias = 0.21). When we used the trim-and-fill method to examine the influence of potential publication bias, the pooled RR did not alter, indicating that the results were not affected by publication bias.
Discussion
The present meta-analysis of observational studies assessed the association between nuts and legumes consumption and colorectal cancer risk. This meta-analysis indicated that people in the highest category of nuts and legumes consumption had a decreased risk of colorectal cancer by 16% and 10%, respectively, compared with those in the lowest category. In addition, we found the inverse association tends to be stronger in Asia, especially for nuts consumption. The results of the dose–response analysis also supported these associations.
A few previous meta-analyses have been conducted to assess the association between nuts consumption and cancer risk, and the association of nuts consumption with colorectal cancer risk was examined in the form of subgroup analysis only [7, 18, 19]. For legumes consumption, two meta-analyses were conducted to examine the association with colorectal cancer risk [20, 21]. One of the meta-analyses from the WCRF-AICR continuous update project included six studies, and due to the limited number of studies, it could not conduct a stratified analysis [21]. Another meta-analysis of legumes consumption also included studies of legume fiber consumption [20]. In the present meta-analysis, we included twenty-nine studies, and thus could conduct a stratified analysis. In addition, we included studies with legumes consumption only, and also conducted a dose–response analysis of nuts and legumes consumption.
There was some evidence of heterogeneity among the studies in the meta-analysis of nuts and legumes consumption and the risk of colorectal cancer overall. For the results of nuts consumption, the observed heterogeneity among the studies tended to disappear when stratified by geographic region. The International Nut and Dried Fruit Council Foundation (INC) reported that consumption of nuts varies by region, depending on the type of nuts [61]. Europe was the largest consumer of tree nuts, but in terms of peanut consumption, per capita, peanut consumption in Nigeria and China was much higher than in other countries [61]. In addition, in many countries, nuts were not depicted in food guides or not mentioned in brief guides or other available descriptions of food classification [62], so the consumption patterns of nuts in different countries would be different. We observed an association between nuts consumption and colorectal cancer risk only in Asia. Although it is difficult to make the definitive explanation for this result, it may be because Asians have a lower risk of nut allergy than people in Western countries [17, 63], which leads to different dietary patterns. For the type of nuts, American and European studies contained more variety of nut types than Asian studies. One Asian study only included peanut products [14], and the other Asian study only included pine nuts, peanuts, and almonds [17], while the study by Hoshiyama et al. did not mention the type of nuts [10]. The different types of nuts consumption among the studies might also explain the different results by region. However, the inverse association of nuts consumption found in Asian studies came from only three studies, and most of them were case–control studies, which are susceptible to methodological biases. For the results of legumes consumption, although the observed heterogeneity tends to disappear in colon cancer when stratified analysis by cancer site, heterogeneity could still be observed in rectal cancer. We cannot clearly explain the reasons for the observed heterogeneity, but we speculate that it may be due to the diversity of legumes. We have usually eaten dried legumes, which have matured and dried on the plant, but there are also several legumes eaten as a vegetable when they are green or sprouted, such as green beans, runner beans, and beansprouts, etc. [64]. In addition, legumes can be consumed as soy products such as tofu, soy milk, and natto, etc. Dry legumes are higher in protein than most other plant foods and are typically high in carbohydrates and dietary fiber [64], although legumes are low in fat, except for soybeans [4, 64]. On the other hand, it may be because the consumption of legumes varies by region. In East Asian diets, soybeans are the main source of phytoestrogen isoflavones, and the average isoflavone intake of the Asian population is almost 10 times higher than in Western countries [65, 66]. Therefore, more studies are needed to determine subgroups of nuts and legumes consumption in relation to colorectal cancer risk.
Several potential mechanisms of nuts and legumes consumption may reduce the risk of colorectal cancer. Nuts and legumes are a dietary source of fiber, and dietary fiber could bind and excrete potential luminal carcinogens, such as secondary bile acids and reduce fecal pH in the colon, thereby playing a critical role in colorectal cancer prevention [4, 5, 67]. In addition, high-fiber diets may decrease insulin resistance, which is a risk factor for colorectal cancer [64]. Nuts contain phytochemicals, such as resveratrol and quercetin, which can decrease the inflammatory process and prevent inflammation-induced tumors [5, 68]. Legumes also contain phytochemicals, which are isoflavones, representative anti-cancer components. Isoflavones have a structure similar to estrogen and selectively bind to estrogen receptors (ER) [66, 69]. When estrogen is deficient, phytoestrogens can exert an estrogenic effect [66]. Moreover, a previous meta-analysis reported that the ER-β protein protects against carcinogenesis and colorectal cancer development when activated by estrogen, indicating the potential for ER-β to act as a tumor suppressor [66, 70].
The present study had some advantages. It is the first meta-analysis to assess the association between nuts consumption and the risk of colorectal cancer. In addition, a relatively large number of studies allowed us to conduct subgroup analyses by study design, sex, cancer site, and geographic region, as well as the linear dose–response meta-analysis. The present meta-analysis included the most recent prospective cohort and case–control studies and the largest number of study participants. In addition, most of the studies included in the analysis were adjusted for confounding factors such as age, sex, energy intake, alcohol intake, smoking, BMI, and physical activity.
Despite these advantages, several potential limitations of our meta-analysis should be considered. First, the current meta-analysis included several case–control studies in addition to cohort studies, and therefore, potential methodological biases, including selection bias or recall bias, might be considered. The inverse association of nuts consumption found in three Asian studies came mostly from case–control studies, so we should be interpreted with caution. Second, some misclassification of nuts and legumes consumption may exist, which influences the results of individual studies and thus pooled estimates in this meta-analysis. In addition, the types of nuts and legumes consumed throughout each study varied widely. This wide range includes peanuts and peanut butter, which are legumes, but often identified by consumers as part of the nut, and some studies did not report detailed types of nuts and legumes consumption. Third, the cut-offs for the consumption of nuts and legumes in the highest and lowest categories varied among the studies. To address this limitation, we conducted a linear dose–response meta-analysis as well. Fourth, for the analysis of legumes consumption and risk of colorectal cancer, we observed evidence of bias from Egger’s test, but it was not observed in Begg’s test. The difference in the results obtained by the two methods might be because of the greater statistical power of the regression method [71]. In addition, we conducted the trim-and-fill method, which showed that the results were not affected by publication bias. Finally, when we conducted the stratified analysis by the adjustment for confounders, it tended to show weaker associations when limited to studies with strong adjustment. Moreover, the number of well-adjusted cohort studies are still limited at present.
In conclusion, the results of the current meta-analysis suggest that the evidence for an association between nuts and legumes consumption and the risk of colorectal cancer is currently weak. We observed that the association tended to be weak when stratified by adjustment for confounders. Thus, further well-designed prospective cohort studies, especially well-adjusted for confounders, on different types of nuts and legumes consumption and different types of study populations are warranted.
Code availability
Available from the corresponding author on reasonable request.
References
Ferlay JEM, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer. 2020. https://gco.iarc.fr/today. Accessed 23 July 2021
Ferlay JLM, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observatory: cancer tomorrow. Lyon, France: International Agency for Research on Cancer. 2020. https://gco.iarc.fr/tomorrow. Accessed 23 July 2021
Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–91. https://doi.org/10.1136/gutjnl-2015-310912.
Martini D, Godos J, Marventano S, et al. Nut and legume consumption and human health: an umbrella review of observational studies. Int J Food Sci Nutr 2021;1–8. https://doi.org/10.1080/09637486.2021.1880554
González CA, Salas-Salvadó J. The potential of nuts in the prevention of cancer. Br J Nutr. 2006;96(Suppl 2):S87-94. https://doi.org/10.1017/bjn20061868.
Alasalvar C, Bolling BW. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br J Nutr. 2015;113(Suppl 2):S68-78. https://doi.org/10.1017/s0007114514003729.
Naghshi S, Sadeghian M, Nasiri M, Mobarak S, Asadi M, Sadeghi O. Association of total nut, tree nut, peanut, and peanut butter consumption with cancer incidence and mortality: a comprehensive systematic review and dose-response meta-analysis of observational studies. Adv Nutr (Bethesda, Md.). 2021;12(3):793–808. https://doi.org/10.1093/advances/nmaa152
Messina M. Soy and health update: evaluation of the clinical and epidemiologic literature. Nutrients. 2016;8(12). https://doi.org/10.3390/nu8120754
Hu JF, Liu YY, Yu YK, Zhao TZ, Liu SD, Wang QQ. Diet and cancer of the colon and rectum: a case-control study in China. Int J Epidemiol. 1991;20(2):362–7. https://doi.org/10.1093/ije/20.2.362.
Hoshiyama Y, Sekine T, Sasaba T. A case-control study of colorectal cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Tohoku J Exp Med. 1993;171(2):153–65. https://doi.org/10.1620/tjem.171.153.
Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study. I. Vegetables and fruit. Int J Cancer. 1993;53(5):711–9. https://doi.org/10.1002/ijc.2910530502
Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148(8):761–74. https://doi.org/10.1093/oxfordjournals.aje.a009697.
Deneo-Pellegrini H, Boffetta P, De Stefani E, Ronco A, Brennan P, Mendilaharsu M. Plant foods and differences between colon and rectal cancers. Eur J Cancer Prev Off J Eur Cancer Prev Org (ECP). 2002;11(4):369–75. https://doi.org/10.1097/00008469-200208000-00009.
Yeh CC, You SL, Chen CJ, Sung FC. Peanut consumption and reduced risk of colorectal cancer in women: a prospective study in Taiwan. World J Gastroenterol. 2006;12(2):222–7. https://doi.org/10.3748/wjg.v12.i2.222.
Yang G, Shu XO, Li H, et al. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am J Clin Nutr. 2009;89(2):577–83. https://doi.org/10.3945/ajcn.2008.26742.
Shin A, Lee J, Lee J, et al. Isoflavone and soyfood intake and colorectal cancer risk: a case-control study in Korea. PLoS ONE. 2015;10(11): e0143228. https://doi.org/10.1371/journal.pone.0143228.
Lee J, Shin A, Oh JH, Kim J. The relationship between nut intake and risk of colorectal cancer: a case control study. Nutr J. 2018;17(1):37. https://doi.org/10.1186/s12937-018-0345-y.
Zhang D, Dai C, Zhou L, et al. Meta-analysis of the association between nut consumption and the risks of cancer incidence and cancer-specific mortality. Aging. 2020;12(11):10772–94. https://doi.org/10.18632/aging.103292
Long J, Ji Z, Yuan P, et al. Nut consumption and risk of cancer: a meta-analysis of prospective studies. Cancer Epidemiol Biom Prev. 2020;29(3):565–73. https://doi.org/10.1158/1055-9965.epi-19-1167.
Zhu B, Sun Y, Qi L, Zhong R, Miao X. Dietary legume consumption reduces risk of colorectal cancer: evidence from a meta-analysis of cohort studies. Sci Rep. 2015;5:8797. https://doi.org/10.1038/srep08797.
Vieira AR, Abar L, Chan DSM, et al. Foods and beverages and colorectal cancer risk: a systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR continuous update project. Ann Oncol. 2017;28(8):1788–802. https://doi.org/10.1093/annonc/mdx171.
Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology. A proposal for reporting. JAMA. 2000;283(15):2008–12. https://doi.org/10.1001/jama.283.15.2008.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. https://doi.org/10.1016/0197-2456(86)90046-2.
Inoue M, Tajima K, Hirose K, et al. Subsite-specific risk factors for colorectal cancer: a hospital-based case-control study in Japan. Cancer Causes Control CCC. 1995;6(1):14–22. https://doi.org/10.1007/bf00051676.
Nishi M, Yoshida K, Hirata K, Miyake H. Eating habits and colorectal cancer. Oncol Rep. 1997;4(5):995–8. https://doi.org/10.3892/or.4.5.995.
Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJ, van den Brandt PA. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: the Netherlands cohort study on diet and cancer. Am J Epidemiol. 2000;152(11):1081–92. https://doi.org/10.1093/aje/152.11.1081.
Budhathoki S, Joshi AM, Ohnaka K, et al. Soy food and isoflavone intake and colorectal cancer risk: the Fukuoka colorectal cancer study. Scand J Gastroenterol. 2011;46(2):165–72. https://doi.org/10.3109/00365521.2010.522720.
Michels KB, Edward G, Joshipura KJ, et al. Prospective study of fruit and vegetable consumption and incidence of colon and rectal cancers. J Natl Cancer Inst. 2000;92(21):1740–52. https://doi.org/10.1093/jnci/92.21.1740.
Nieuwenhuis L, Simons C, Weijenberg MP, van den Brandt PA. Nut and peanut butter intake and the risk of colorectal cancer and its anatomical and molecular subtypes: the Netherlands Cohort Study. Carcinogenesis. 2020;41(10):1368–84. https://doi.org/10.1093/carcin/bgaa080.
Oba S, Nagata C, Shimizu N, et al. Soy product consumption and the risk of colon cancer: a prospective study in Takayama. Japan Nutr Cancer. 2007;57(2):151–7. https://doi.org/10.1080/01635580701274475.
Park Y, Subar AF, Kipnis V, et al. Fruit and vegetable intakes and risk of colorectal cancer in the NIH-AARP diet and health study. Am J Epidemiol. 2007;166(2):170–80. https://doi.org/10.1093/aje/kwm067.
Akhter M, Inoue M, Kurahashi N, Iwasaki M, Sasazuki S, Tsugane S. Dietary soy and isoflavone intake and risk of colorectal cancer in the Japan public health center-based prospective study. Cancer Epidemiol Biom Prev. 2008;17(8):2128–35. https://doi.org/10.1158/1055-9965.epi-08-0182.
Le Marchand L, Hankin JH, Wilkens LR, Kolonel LN, Englyst HN, Lyu LC. Dietary fiber and colorectal cancer risk. Epidemiology. 1997;8(6):658–65. https://doi.org/10.1097/00001648-199710000-00008
Young TB, Wolf DA. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer. 1988;42(2):167–75. https://doi.org/10.1002/ijc.2910420205.
Williams CD, Satia JA, Adair LS, et al. Dietary patterns, food groups, and rectal cancer risk in Whites and African-Americans. Cancer Epidemiol Biom Prev. 2009;18(5):1552–61. https://doi.org/10.1158/1055-9965.epi-08-1146.
Sellers TA, Bazyk AE, Bostick RM, et al. Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa, United States). Cancer Causes Control CCC. 1998;9(4):357–67. https://doi.org/10.1023/a:1008886715597.
Huang XE, Hirose K, Wakai K, et al. Comparison of lifestyle risk factors by family history for gastric, breast, lung and colorectal cancer. Asian Pacific J Cancer Prev APJCP. 2004;5(4):419–27.
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. https://doi.org/10.1093/oxfordjournals.aje.a116237.
Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4(3):218–28. https://doi.org/10.1097/00001648-199305000-00005
Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stand Genomic Sci. 2006;6(1):40–57. https://doi.org/10.1177/1536867X0600600103.
Bechthold A, Boeing H, Schwedhelm C, et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019;59(7):1071–90. https://doi.org/10.1080/10408398.2017.1392288.
Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–29. https://doi.org/10.2307/3001666.
Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557. https://doi.org/10.1136/bmj.327.7414.557.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101. https://doi.org/10.2307/2533446.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629. https://doi.org/10.1136/bmj.315.7109.629.
Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. https://doi.org/10.1111/j.0006-341x.2000.00455.x.
Jenab M, Ferrari P, Slimani N, et al. Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biom Prev. 2004;13(10):1595–603.
Lin J, Zhang SM, Cook NR, Lee IM, Buring JE. Dietary fat and fatty acids and risk of colorectal cancer in women. Am J Epidemiol. 2004;160(10):1011–22. https://doi.org/10.1093/aje/kwh319.
Yang M, Hu FB, Giovannucci EL, et al. Nut consumption and risk of colorectal cancer in women. Eur J Clin Nutr. 2016;70(3):333–7. https://doi.org/10.1038/ejcn.2015.66.
Fang Z, Wu Y, Li Y, et al. Association of nut consumption with risk of total cancer and 5 specific cancers: evidence from 3 large prospective cohort studies. Am J Clin Nutr. 2021;114(6):1925–35. https://doi.org/10.1093/ajcn/nqab295.
Evans RC, Fear S, Ashby D, et al. Diet and colorectal cancer: an investigation of the lectin/galactose hypothesis. Gastroenterology. 2002;122(7):1784–92. https://doi.org/10.1053/gast.2002.33659.
Flood A, Velie EM, Chaterjee N, et al. Fruit and vegetable intakes and the risk of colorectal cancer in the Breast Cancer Detection Demonstration Project follow-up cohort. Am J Clin Nutr. 2002;75(5):936–43. https://doi.org/10.1093/ajcn/75.5.936.
Lin J, Zhang SM, Cook NR, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States). Cancer Causes Control CCC. 2005;16(3):225–33. https://doi.org/10.1007/s10552-004-4025-1.
Butler LM, Wang R, Koh WP, Yu MC. Prospective study of dietary patterns and colorectal cancer among Singapore Chinese. Br J Cancer. 2008;99(9):1511–6. https://doi.org/10.1038/sj.bjc.6604678.
Bamia C, Lagiou P, Buckland G, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28(4):317–28. https://doi.org/10.1007/s10654-013-9795-x.
Vogtmann E, Xiang YB, Li HL, et al. Fruit and vegetable intake and the risk of colorectal cancer: results from the Shanghai Men’s Health Study. Cancer Causes Control CCC. 2013;24(11):1935–45. https://doi.org/10.1007/s10552-013-0268-z.
Jones P, Cade JE, Evans CEL, Hancock N, Greenwood DC. The Mediterranean diet and risk of colorectal cancer in the UK Women’s Cohort Study. Int J Epidemiol. 2017;46(6):1786–96. https://doi.org/10.1093/ije/dyx155.
Seow A, Quah SR, Nyam D, Straughan PT, Chua T, Aw TC. Food groups and the risk of colorectal carcinoma in an Asian population. Cancer. 2002;95(11):2390–6. https://doi.org/10.1002/cncr.10971.
Abu Mweis SS, Tayyem RF, Shehadah I, et al. Food groups and the risk of colorectal cancer: results from a Jordanian case-control study. Eur J Cancer Prev. 2015;24(4):313–20. https://doi.org/10.1097/cej.0000000000000089.
Azzeh FS, Alshammari EM, Alazzeh AY, et al. Healthy dietary patterns decrease the risk of colorectal cancer in the Mecca Region, Saudi Arabia: a case-control study. BMC Public Health. 2017;17(1):607. https://doi.org/10.1186/s12889-017-4520-4.
International Nut and Dried Fruit Council Foundation. Nuts & dried fruits statistical yearbook 2020/2021. Reus, Spain; 2021.
Herforth A, Arimond M, Álvarez-Sánchez C, Coates J, Christianson K, Muehlhoff E. A global review of food-based dietary guidelines. Adv Nutr 2019;10(4):590–605. https://doi.org/10.1093/advances/nmy130
Shek LP, Cabrera-Morales EA, Soh SE, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immunol. 2010;126(2):324–31, 31.e1–7. https://doi.org/10.1016/j.jaci.2010.06.003
World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Export Report 2018. Wholegrains, vegetables and fruit and the risk of cancer.London; Arlington (VA): World Cancer Research Fund International; American Institute for Cancer Research; 2018. https://www.wcrf.org/dietandcancer/wholegrains-vegetables-and-fruit/. Accessed 1 September 2021
Yu J, Bi X, Yu B, Chen D. Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients. 2016;8(6). https://doi.org/10.3390/nu8060361
Khankari NK, Yang JJ, Sawada N, et al. Soy intake and colorectal cancer risk: results from a pooled analysis of prospective cohort studies conducted in China and Japan. J Nutr. 2020;150(9):2442–50. https://doi.org/10.1093/jn/nxaa194.
Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol. 2014;6(2):41–51. https://doi.org/10.4251/wjgo.v6.i2.41.
Falasca M, Casari I. Cancer chemoprevention by nuts: evidence and promises. Front Biosci. 2012;4:109–20. https://doi.org/10.2741/254.
Kuiper GG, Lemmen JG, Carlsson B, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–63. https://doi.org/10.1210/endo.139.10.6216.
Niv Y. Estrogen receptor β expression and colorectal cancer: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2015;27(12):1438–42. https://doi.org/10.1097/meg.0000000000000471.
Sterne JA, Gavaghan D, Egger M. Publication and related bias in meta-analysis: power of statistical tests and prevalence in the literature. J Clin Epidemiol. 2000;53(11):1119–29. https://doi.org/10.1016/s0895-4356(00)00242-0.
Funding
This work was supported by the National Research Foundation of Korea, funded by Ministry of Science, ICT and Future Planning (Grant number: NRF-2021R1F1A1050847).
Author information
Authors and Affiliations
Contributions
Study concept and design: SJ and YJ, data collection and statistical analysis: SJ, writing—original draft: SJ, writing—review and editing: YJ, study supervision: YJ, Interpretation of the data, critical revision of the paper for important intellectual content and approval of the final paper for submission: SJ and YJ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jin, S., Je, Y. Nuts and legumes consumption and risk of colorectal cancer: a systematic review and meta-analysis. Eur J Epidemiol 37, 569–585 (2022). https://doi.org/10.1007/s10654-022-00881-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-022-00881-6