Abstract
The role of vaccination in the development of Guillain-Barré syndrome (GBS) is controversial, although cases of GBS have been reported following a wide range of vaccines. A nested case–control study was conducted between January 2011 and December 2015 in three Chinese cities. Four controls were matched to a case by gender, age, address and index date. An independent expert committee validated the diagnoses of cases and controls according to the Brighton Collaboration GBS case definition. Data on vaccinations were obtained from computerized vaccination records. Causal relations were assessed by conditional logistic regression. 1056 cases of GBS and 4312 controls were included in the analyses. Among paediatric and adult population, adjusted ORs for GBS occurrence within 180 days following vaccination were 0.94 (95% CI 0.54–1.62) and 1.09 (95% CI 0.88–1.32), respectively. No increased risk of GBS was detected for vaccination against hepatitis B, influenza, hepatitis A, varicella, rabies, polio(live), diphtheria, pertuss(acellular), tetanusis, measles, mumps, rubella, Japanese Encephalitis, and meningitis vaccines. Adjusted ORs for the recurrence of GBS after vaccination among paediatric and adult population were 0.85 (95% CI 0.07–9.50) and 1.18 (95% CI 0.49–2.65), respectively. In this large retrospective study, we did not find evidence of an increased risk of GBS and its recurrence among either paediatric (≤ 18 years) or adult (> 18 years) individuals within the 180 days following vaccinations of any kind, including influenza vaccination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Guillain-Barré syndrome (GBS) is an acute or subacute inflammatory polyradiculoneuropathy, which is characterized by symmetric flaccid paralysis of the extremities, sensory abnormalities, and cranial nerve palsy [1]. Although the underlying aetiology and pathophysiology of the GBS has not been clearly elucidated, it is thought to be an autoimmune process triggered by antigenic stimulation [2, 3], resulting in demyelination and destruction of peripheral nerves. Approximately two-thirds of GBS cases occur several days or weeks after an apparent infectious illness, commonly a gastrointestinal illness or upper respiratory tract infection [4]. Campylobacter enteritis, influenza, cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus (HIV), chikungunya, Zika virus, and Mycoplasma pneumoniae have been implicated to be the trigger [3, 5,6,7,8,9]. Malignancies and surgical procedures also have been suggested to increase the risk of GBS [10, 11].
Based primarily on biological plausibility and temporal associations, vaccinations have also been suggested to induce a small increased risk of GBS. In 1976, the risk of GBS increased significantly in the 6 weeks following the mass administration of the A/New Jersey/76 vaccine during the swine flu epidemic in the United States [12]. There have been case reports of GBS after other vaccinations [13], but there was no adequate evidence to draw clear conclusions for other vaccines [14,15,16,17,18].
Recently, the Expanded Program on Immunization (EPI) has been implemented in China. The number and type of vaccines administered to inhabitants increased. The possibility of catching a severe abnormal reaction such as GBS to vaccines is small, but the onset of such reactions usually attract extensive public concern, which may weaken public confidence in vaccines [19].
The purpose of this study was to evaluate in more detail the possible association between the risk of GBS including disease recurrence and vaccinations, using retrospective data accumulated 5 years.
Materials and methods
Setting
In China, most people receive medical service and specialized neurological care from public medical institutions. Generally, patients with suspected GBS are hospitalized as emergencies, and soon neurologists would be involved in their care. Patients less than 16 years old who catch neurological diseases are usually taken into pediatric hospitals.
Jiangsu Province is located in eastern China. It covers an area of 107.2 thousand km2 and has a mean population density of 767 inhabitants/km2. Xuzhou, Yancheng and Nantong, which are three cities in Jiangsu province, were included in this study. In all, 74 hospitals were involved, 33 in Xuzhou, 20 in Yancheng and 21 in Nantong, which all provided inpatient services. In these hospitals, any departments that might have admitted patients who meet the case definition were involved, especially neurology, pediatrics, internal medicine, and inpatient wards (including intensive care units). Informed consent was waived because it was a medical records review study without direct contact with patients. The study protocol was approved by the institutional review board of the Jiangsu Provincial Center for Disease Control and Prevention (JSCDC).
Case identification
We searched the Hospital Information Systems (HIS) for first mention of International Classification of Diseases, Tenth Revision (ICD-10), diagnostic codes (G61.0) for GBS from January 1, 2011, to December 31, 2015, for persons of any age. GBS diagnoses were confirmed by neurologists from clinical data, such as clinical manifestations, electromyogram (EMG, raw data), magnetic resonance imaging (MRI) and cerebrospinal fluid (CSF) examinations according to the Brighton Collaboration case definition criteria for GBS [20]. Cases with Fisher Syndrome, a variant of GBS, were excluded. Recurrent GBS was defined as 2 or more episodes of acute monophasic neuropathy followed by near complete recovery between episodes [21].
Control selection
Four control individuals randomly selected from the same hospital without history of GBS were matched to each GBS case according to year of birth (within 1 year), gender, and zip code (a surrogate measure for socioeconomic status) during the same period. The control participants were assigned the same index date as their matched case (the earliest date of first symptoms or diagnosis of GBS). Controls were selected among inpatients admitted for headache (except trigeminal neuralgia), migraine, vascular or other diseases incidence of which is not thought to be affected by vaccination. Patients with autoimmune diseases or chronic severe neurological diseases were excluded.
Vaccination records
Information on vaccinations was collected from Information Management System for Immunization Programming, in which anyone received vaccinations would be registered, matched with ID number and verified by paper vaccine administration certificates. Any vaccination was thought to be an exposure. We collected data on all vaccinations administered within 180 days.
Covariates
Additional data on the following covariates was obtained with a standardized data collection form: nationality (Han and others), occupation, marital status (married and single), allergy, familial diseases, past surgical interventions, and comorbid chronic diseases (such as malignancy, immunosuppression, and autoimmune disorders). Comorbid chronic diseases and history of infectious diseases were within 6 months before the index date.
Statistical analysis
Matched odds ratio (OR) and its corresponding 95% two-sided confidence intervals (CI) for the possible relation between GBS and vaccination were computed with conditional logistic regression following univariable analysis. “No vaccination” served as the reference category. For each case, the index date was the date of onset of the first symptoms of the CNS demyelinating event. For the controls, we assigned the time of the onset of GBS in the individual with whom the control was matched. The models were adjusted for nationality, occupation, marital status, allergy, familial diseases, comorbid chronic diseases, and history of infectious diseases within 6 months before the index date, and all variables with a P value less than 0.20 were considered for inclusion in the regression model and retained in the model according to a forward stepwise procedure based on a likelihood-ratio test [22]. The primary time window at risk was fixed at 4–42 days before the index date, a generally accepted risk interval between GBS onset and exposure to an antigenic stimulus (e.g. infection, vaccination), based on the biological plausibility for a causal relationship [20]. Secondly, 43-day to 90-day and 91-day to 180-day time windows were also considered, as reported by Stowe et al. [23].To estimate whether the paediatric was associated with any vaccine-associated GBS, the study population was stratified according to age (≤ 18 years and > 18 years) at the index date.
The means (SDs) of normally distributed variables were compared using 2-sample t tests; for binary or categorical variables, χ2 analysis with the Fisher exact test was used. For all analyses significance was accepted at a two sided P < 0.05. All analyses were conducted in 2018.
Results
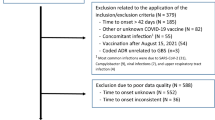
From a source population of about 23 million in the three cities, 1056 patients met Brighton level 1, 2, or 3 criteria and were included in the study after an independent diagnosis of two neurologists (Fig. 1). The main clinical features of the GBS cases are summarized in supplementary material (Table 1). The incident cases meeting the Brighton criteria were 686, 351 and 19 cases for levels 1, 2, and 3 of GBS, respectively. Most cases were men (n = 653, 61.84%), aged 19–60 (n = 581, 55.20%). The main characteristics of the selected cases (1056) with GBS and the controls (4312) are summarized in Table 1. Because of matching, cases and controls had similar distributions of sex and age. The comparison between cases and controls showed no meaningful difference with regard to nationality, marital status, place of residence, allergy history, family history of demyelinating diseases or other autoimmune diseases, and comorbid chronic diseases.
The analyses of the association between any vaccination and the risk of GBS by age and time since vaccination are shown in Table 2. Among paediatric population (≤ 18 years), the proportion of subjects who had been exposed to any vaccine within 180 days before the index date was 36.29% for GBS cases and 37.42% for controls, and the adjusted OR was 0.95 (95% CI 0.64–1.49). Among adult population (> 18 years), the corresponding proportion was 17.32% for cases and 16.48% for controls, and the adjusted OR was 1.09 (95% CI 0.91–1.34). When the data were pooled, no difference was found for GBS cases with preceding vaccination.
Within the time window at risk, hepatitis B was the most widely used vaccine in the study population, followed by influenza. Adjusted risk estimates for GBS with different vaccination ranged from 0.69 to 1.35. No increase in the risk of GBS was detected for vaccination against hepatitis B, influenza, hepatitis A, varicella, rabies, polio(live), diphtheria, pertuss(acellular), tetanusis, measles, mumps, rubella, Japanese Encephalitis, and meningitis vaccines (Table 3). There were no statistically significant interactions by sex for any of the associations between specific vaccines and GBS, indicating that the results were not different between male and female (Supplementary material Table 2).
Out of the 1056 initial GBS cases, 40 patients (3 ≤ 18 year-old vs . 37 > 18 year-old) have experienced at least once recurrence. The most often vaccination was a hepatitis B vaccination. Follow-up was a median of 24 months (25–75th percentile 6–65), 25 months (25–75th percentile 9–69) in children and 23 months (25–75th percentile 5–64) in adults, P = 0.43. Rate of recurrence was not significantly higher in patients> 18 year-old than ≤ 18 year-old (3.26% vs 2.80%, P = 0.80). Among both paediatric patients and adult patients, no relationship between vaccination status and GBS recurrence was detected (Table 4).
Discussion
In this nested case–control study spanning 5 years, we found no evidence of an increased risk of GBS within the 180 days after any vaccinations, as well as all vaccinations combined, among either paediatric (≤ 18 years) or adult (> 18 years) individuals.
Influenza vaccine is where the most researches conducted on regarding a possible association with GBS. Our results are consistent with several other studies from America, Australia, and Europe [24,25,26,27,28,29,30], which showed a lack of association between GBS following influenza vaccination. In contrary, three pooled analyses [31,32,33] reported increased risks of GBS following influenza vaccination. A Canadian study [34] and a German study [35] showed statistically significant associations between GBS and vaccination. Unlike other studies, Vellozzi et al. [36] suggested a protective effect of influenza A (H1N1) vaccination. They showed that at the end of the influenza season, the cumulative risk of GBS was lower among vaccinated than among unvaccinated individuals.
Cases of GBS have been reported following receipt of other multiple vaccines, including hepatitis B [16], polio [37], tetanus [15], Meningitis [38], and rabies [6]. However, it is difficult to interpret these associations because it is not possible to distinguish whether the observed event was causal or coincidental. Individual case reports or case series cannot address whether the frequency of cases is higher than the expected background rate and are less valuable for causality assessment. An American review concluded that there was insufficient evidence of an association between GBS and measles, mumps, rubella, varicella, hepatitis A or B, human papillomavirus, and diphtheria toxoid, tetanus toxoid, or acellular pertussis–containing vaccines [39]. Our study suggested that the observations in the published case reports probably represent coincidental temporal associations rather than causal associations.
Recurrence after an initial GBS diagnosis was in about 3.22% of patients in our study, which is consistent with previous reports in Japan [40], and lower than Netherland [41], Sweden [42], and Canada [43]. Children and adults experienced relapses in similar proportions in our study. No significant association between GBS recurrence and vaccination was found. This confirms another two studies that found the risk of developing another GBS episode following vaccination is small [44, 45].
Many identifiable infections are well known risk factors for GBS [13]. It is widely believed that immune stimulation plays a role in its pathogenesis. In the physiopathology of GBS following immunization, anti-GM1 (ganglioside) vaccine-induced antibodies were documented in mice [46]. Thus, it is biologically plausible that immunizations may lead to subsequent GBS in rare cases. Until now, however, with rare exceptions, causal associations between vaccination and GBS have not been substantiated.
The overall incidence in China was estimated to between 0.45/100,000/year and 1/100,000/year [19]. It was lower than that in Europe and North America, where prevalence was estimated to 1.1–1.8/100,000/year [47]. Phenotype of GBS is known to be influenced by geographical origins of patients. Acute inflammatory demyelinating polyradiculoneuropathy (AIDP) is more common in North America and Europe [48]. In other areas of the world a phenotype predominated by axonal damage is seen and has been termed acute motor axonal neuropathy (AMAN) [49].
Our study was a large population-based nested case–control study that included both paediatric and adult subjects. It adds to existing knowledge by providing information on several vaccines that had not been studied before. The retrospective nature of the case–control design may be affected by recall bias, but recall bias was avoided by using computerized vaccination records.
Our study has several limitations. It has been reported that cases with the outcomes of GBS (OR = 0.75, 95% CI 0.42–1.3) were no more likely than controls following administration of HPV4 vaccine [50]. We could not evaluate the association between GBS and HPV vaccine, because HPV vaccine is not available in Jiangsu until 2018. We could not examine the potential influence of (i) prior drugs, medications, herbal intake, (ii) vaccine preservatives and adjuvants, and further studies are needed to completely rule out the effect. The immunoglobulin treatment and response in cases which would reinforce the GBS diagnosis were not collected. Finally, additional laboratory and clinical research is needed to better understand the pathophysiology of (recurrent) GBS and the possible role of immunizations.
Conclusions
In summary, we found no evidence which demonstrate an association of vaccines with an increased risk of GBS and its recurrence among either paediatric (≤ 18 years) or adult (> 18 years) individuals within the 180 days following vaccinations. We found no association between influenza vaccination and an increased risk of GBS up to 180 days after vaccination, which is reassuring. Our results also suggest that previous case reports of GBS shortly after administration of several other vaccines probably represent coincidental temporal associations rather than real causal associations.
References
Park YS, Lee KJ, Kim SW, Kim KM, Suh BC. Clinical features of post-vaccination Guillain-Barré syndrome (GBS) in Korea. J Korean Med Sci. 2017;32:1154–9.
Hardy TA, Blum S, McCombe PA, Reddel SW. Guillain-Barré syndrome: modern theories of etiology. Curr Allergy Asthma Rep. 2011;11:197–204.
Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012;366:2294–304.
Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–66.
Lehmann HC, Hartung HP, Kieseier BC, Hughes RA. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect Dis. 2010;10:643–51.
Jacobs BC, Rothbarth PH, van der Meche FG, Herbrink P, Schmitz PI, de Klerk MA, et al. The spectrum of antecedent infections in Guillain-Barré syndrome: a case–control study. Neurology. 1998;51:1110–5.
Tam CC, O'Brien SJ, Rodrigues LC. Influenza, Campylobacter and Mycoplasma infections, and hospital admissions for Guillain-Barré syndrome, England. Emerg Infect Dis. 2006;12:1880–7.
Cao-Lormeau VM, Blake A, Mons S, Lastere S, Roche C, Vanhomwegen J, et al. Guillain-Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–9.
Stegmann-Planchard S, Gallian P, Tressieres B, Leparc-Goffart I, Lannuzel A, Signate A, et al. Chikungunya, a risk factor for Guillain-Barré syndrome. Clin Infect Dis. 2019. https://doi.org/10.1093/cid/ciz625.
Hiew FL, Rajabally YA. Malignancy in Guillain-Barré syndrome: a twelve-year single-center study. J Neurol Sci. 2017;375:275–8.
Rudant J, Dupont A, Mikaeloff Y, Bolgert F, Coste J, Weill A. Surgery and risk of Guillain-Barré syndrome: a French nationwide epidemiologic study. Neurology. 2018;91:e1220–e12271227.
Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barré syndrome following vaccination in the National Influenza Immunization Program, United States, 1976–1977. Am J Epidemiol. 1979;110:105–23.
Haber P, Sejvar J, Mikaeloff Y, DeStefano F. Vaccines and Guillain-Barré syndrome. Drug Saf. 2009;32:309–23.
Hughes R, Rees J, Smeeton N, Winer J. Vaccines and Guillain-Barré syndrome. BMJ. 1996;312:1475–6.
Newton N Jr, Janati A. Guillain-Barré syndrome after vaccination with purified tetanus toxoid. South Med J. 1987;80:1053–4.
Khamaisi M, Shoenfeld Y, Orbach H. Guillain-Barré syndrome following hepatitis B vaccination. Clin Exp Rheumatol. 2004;22:767–70.
Siddiqui A, Usmani RI, Anwer S, Afsar S. Guillain-Barré syndrome occurring after rabies vaccination. JPMA J Pak Med Assoc. 2005;55:87–8.
Greene SK, Rett M, Weintraub ES, Li L, Yin R, Amato AA, et al. Risk of confirmed Guillain-Barré syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink Project, 2009–2010. Am J Epidemiol. 2012;175:1100–9.
Chen Y, Ma F, Zhang J, Chu X, Xu Y. Population incidence of Guillain-Barré syndrome in parts of China: three large populations in Jiangsu province, 2008–2010. Eur J Neurol. 2014;21:124–9.
Sejvar JJ, Kohl KS, Gidudu J, Amato A, Bakshi N, Baxter R, et al. Guillain-Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612.
Ruts L, Drenthen J, Jacobs BC, van Doorn PA, Dutch GBSSG. Distinguishing acute-onset CIDP from fluctuating Guillain-Barré syndrome: a prospective study. Neurology. 2010;74:1680–6.
Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36.
Stowe J, Andrews N, Wise L, Miller E. Investigation of the temporal association of Guillain-Barré syndrome with influenza vaccine and influenza like illness using the United Kingdom General Practice Research Database. Am J Epidemiol. 2009;169:382–8.
Burwen DR, Sandhu SK, MaCurdy TE, Kelman JA, Gibbs JM, Garcia B, et al. Surveillance for Guillain-Barré syndrome after influenza vaccination among the Medicare population, 2009–2010. Am J Public Health. 2012;102:1921–7.
Greene SK, Rett MD, Vellozzi C, Li L, Kulldorff M, Marcy SM, et al. Guillain-Barré syndrome, influenza vaccination, and antecedent respiratory and gastrointestinal infections: a case-centered analysis in the vaccine safety datalink, 2009–2011. PLoS ONE. 2013;8:e67185.
Andrews N, Stowe J, Al-Shahi Salman R, Miller E. Guillain-Barré syndrome and H1N1 (2009) pandemic influenza vaccination using an AS03 adjuvanted vaccine in the United Kingdom: self-controlled case series. Vaccine. 2011;29:7878–82.
Verity C, Stellitano L, Winstone AM, Stowe J, Andrews N, Miller E. Pandemic A/H1N1 2009 influenza vaccination, preceding infections and clinical findings in UK children with Guillain-Barré syndrome. Arch Dis Child. 2014;99:532–8.
Dieleman J, Romio S, Johansen K, Weibel D, Bonhoeffer J, Sturkenboom M, et al. Guillain-Barré syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccine: multinational case-control study in Europe. BMJ. 2011;343:d3908.
Crawford NW, Cheng A, Andrews N, Charles PG, Clothier HJ, Day B, et al. Guillain-Barré syndrome following pandemic (H1N1) 2009 influenza A immunisation in Victoria: a self-controlled case series. Med J Aust. 2012;197:574–8.
Romio S, Weibel D, Dieleman JP, Olberg HK, de Vries CS, Sammon C, et al. Guillain-Barré syndrome and adjuvanted pandemic influenza A (H1N1) 2009 vaccines: a multinational self-controlled case series in Europe. PLoS ONE. 2014;9:e82222.
Salmon DA, Proschan M, Forshee R, Gargiullo P, Bleser W, Burwen DR, et al. Association between Guillain-Barré syndrome and influenza A (H1N1) 2009 monovalent inactivated vaccines in the USA: a meta-analysis. Lancet. 2013;381:1461–8.
Dodd CN, Romio SA, Black S, Vellozzi C, Andrews N, Sturkenboom M, et al. International collaboration to assess the risk of Guillain Barre syndrome following Influenza A (H1N1) 2009 monovalent vaccines. Vaccine. 2013;31:4448–58.
Martin Arias LH, Sanz R, Sainz M, Treceno C, Carvajal A. Guillain-Barré syndrome and influenza vaccines: a meta-analysis. Vaccine. 2015;33:3773–8.
De Wals P, Deceuninck G, Toth E, Boulianne N, Brunet D, Boucher RM, et al. Risk of Guillain-Barré syndrome following H1N1 influenza vaccination in Quebec. JAMA. 2012;308:175–81.
Prestel J, Volkers P, Mentzer D, Lehmann HC, Hartung HP, Keller-Stanislawski B, et al. Risk of Guillain-Barré syndrome following pandemic influenza A(H1N1) 2009 vaccination in Germany. Pharmacoepidemiol Drug Saf. 2014;23:1192–204.
Vellozzi C, Iqbal S, Stewart B, Tokars J, DeStefano F. Cumulative risk of Guillain-Barré syndrome among vaccinated and unvaccinated populations during the 2009 H1N1 influenza pandemic. Am J Public Health. 2014;104:696–701.
Kinnunen E, Farkkila M, Hovi T, Juntunen J, Weckstrom P. Incidence of Guillain-Barré syndrome during a nationwide oral poliovirus vaccine campaign. Neurology. 1989;39:1034–6.
Centers for Disease C, Prevention. Update: Guillain-Barré syndrome among recipients of Menactra meningococcal conjugate vaccine—United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep. 2006;55:1120–4.
Stratton K, Ford A, Rusch E, Clayton EW, editors. IOM (Institute of Medicine). Adverse effects of vaccines: evidence and causality. Washington, DC: National Academies Press; 2011. p 334.
Ishii J, Yuki N, Kawamoto M, Yoshimura H, Kusunoki S, Kohara N. Recurrent Guillain-Barré syndrome, Miller Fisher syndrome and Bickerstaff brainstem encephalitis. J Neurol Sci. 2016;364:59–64.
Kuitwaard K, van Koningsveld R, Ruts L, Jacobs BC, van Doorn PA. Recurrent Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2009;80:56–9.
Mossberg N, Nordin M, Movitz C, Nilsson S, Hellstrand K, Bergstrom T, et al. The recurrent Guillain-Barré syndrome: a long-term population-based study. Acta Neurol Scand. 2012;126:154–61.
Grand'Maison F, Feasby TE, Hahn AF, Koopman WJ. Recurrent Guillain-Barré syndrome. Clinical and laboratory features. Brain J Neurol. 1992;115(Pt 4):1093–106.
Pritchard J, Mukherjee R, Hughes RA. Risk of relapse of Guillain-Barré syndrome or chronic inflammatory demyelinating polyradiculoneuropathy following immunisation. J Neurol Neurosurg Psychiatry. 2002;73:348–9.
Kuitwaard K, Bos-Eyssen ME, Blomkwist-Markens PH, van Doorn PA. Recurrences, vaccinations and long-term symptoms in GBS and CIDP. J Peripher Nerv Syst JPNS. 2009;14:310–5.
Nachamkin I, Shadomy SV, Moran AP, Cox N, Fitzgerald C, Ung H, et al. Anti-ganglioside antibody induction by swine (A/NJ/1976/H1N1) and other influenza vaccines: insights into vaccine-associated Guillain-Barré syndrome. J Infect Dis. 2008;198:226–33.
McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009;32:150–63.
Asbury AK. New concepts of Guillain-Barré syndrome. J Child Neurol. 2000;15:183–91.
Griffin JW, Li CY, Ho TW, Xue P, Macko C, Gao CY, et al. Guillain-Barré syndrome in northern China. The spectrum of neuropathological changes in clinically defined cases. Brain J Neurol. 1995;118(Pt 3):577–95.
Geier DA, Geier MR. A case–control study of quadrivalent human papillomavirus vaccine-associated autoimmune adverse events. Clin Rheumatol. 2015;34:1225–311.
Acknowledgements
The authors greatly appreciated the fieldwork of the following institutions: Nantong CDC, Yancheng CDC, and Xuzhou CDC.
Funding
This work was supported by Science Project of the Jiangsu Commission of Health [Grant No. H201515] and the Science-Education Project of the Jiangsu Center for Disease Control and Prevention (CDC) [Grant No. JKRC2011023].
Author information
Authors and Affiliations
Contributions
YC, study concept and design, analysis and interpretation of data; JZ, study concept and design, acquisition of data; XC, acquisition of data, interpretation of data; YX, acquisition of data, interpretation of data; FM, study concept and design, study supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, J., Chu, X. et al. Vaccines and the risk of Guillain-Barré syndrome. Eur J Epidemiol 35, 363–370 (2020). https://doi.org/10.1007/s10654-019-00596-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-019-00596-1