Abstract
Diabetes and cancer are common diseases both with enormous impact on health burden globally. The increased risk of several types of cancer among people with type 2 diabetes mellitus has been indicated repeatedly. This study aimed at exploring and describing the association between type 2 diabetes and cancer incidence. A cohort of 428,326 people with type 2 diabetes was identified from the Finnish National Diabetes Register and followed up through a register linkage with the Finnish Cancer Registry for cancer incidence during 1988–2014. A total of 74,063 cases of cancer occurred in this cohort in 4.48 million person-years. This accounted for 16% more than the expected cancer incidence in the Finnish general population; the standardized incidence ratio (SIR) was 1.16 (95% confidence interval [CI] 1.15–1.16). There was a statistically significant excess of cancers of lip (SIR = 1.40, CI = 1.28–1.53), liver (SIR = 2.44, CI = 2.35–2.53), pancreas (SIR = 1.75, CI = 1.70–1.79), stomach (SIR = 1.22, CI = 1.18–1.26), colon (SIR = 1.22, CI = 1.19–1.25), gallbladder and bile ducts (SIR = 1.29, CI = 1.21–1.36), non-melanoma skin (SIR = 1.18, CI = 1.15–1.22), kidney (SIR = 1.42, CI = 1.37–1.47), bladder (SIR = 1.17, CI = 1.13–1.21), and thyroid (SIR = 1.22, CI = 1.12–1.31). There was a small statistically significant decrease in prostate cancer incidence (SIR = 0.95, CI = 0.93–0.96). This study showed an association between type 2 diabetes mellitus and the incidence of cancer at numerous sites in the Finnish population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous epidemiological studies have indicated strong positive associations between type 2 diabetes mellitus (T2DM) and the incidence of several cancer types [1, 2], and mortality [3, 4]. The strength of association depends on cancer site. The most considerable associations have been demonstrated for pancreatic cancers [5,6,7], and liver [8], but there are observations of increased risks of colorectal [9], breast [10], endometrial [11], and kidney cancers [12]. A reduced risk of prostate cancer in diabetic males have documented in many studies. This effect has noticed especially strong among diabetic men with high body mass index (BMI) [13, 14].
The connection between diabetes and cancer has been explored extensively, but the observed associations are not completely understood. It has been suggested, that possible underlying mechanisms could associate with insulin resistance and its accompanying hyperinsulinemia, hyperglycemia, and chronic inflammation [8, 15, 16]. Hormonal factors, low testosterone levels in particular, have been suggested to explain the reduced risk for prostate cancer among male diabetics [17].
In recent years, attention has also been paid on the potential role of several anti-diabetic drugs on cancer risk [18,19,20], but the results are inconsistent. Some studies but not all have suggested that insulin analogs may increase the cancer risk [20], and that the biguanide metformin apparently reduces the risk of many cancer types [21, 22], but these findings have not confirmed in all studies, and causality has not been proven.
The aim of this study was to describe the cancer risk pattern among the Finnish T2DM patients. Special attention was paid to the cancers with a priori suspected associations with diabetes.
Materials and methods
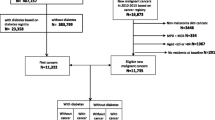
Persons diagnosed with diabetes in Finland during 1988–2007 were identified from the nationwide FinDM II database, which comprises persons with diabetes identified from: (1) the Register of Persons Eligible for Special Reimbursement of Medication for chronic conditions including diabetes (years 1964–2007); (2) the Prescription Register including all reimbursed medicines purchased from the Finnish pharmacies (years 1994–2007); (3) the National Hospital Discharge Registers including all inpatient care (years 1969–2007) and outpatient hospital visits (years 1998–2007); (4) the Causes-of-Death Register (1971–2007); and (5) the Medical Birth Register (1987–2007). The unique Finnish personal identity code assigned to all residents in Finland allowed the deterministic record linkage. More detailed description of the study population is available elsewhere [23].
Persons were considered to have diabetes if they had started antidiabetic medication or had been hospitalized with a diagnosis of diabetes. We excluded from our analyses women with gestational diabetes only and people, who were not permanent residents in the country. The high reimbursement level for antidiabetic medication has resulted in a high coverage of diabetic persons in the medication registers. The completeness of the Hospital Discharge Register is confirmed by the law for every hospital in the country to report on the inpatient episodes. A very good coverage of diabetic patients in the FinDM II database was demonstrated in a study, where the database was compared to a local Diabetes Register of the Helsinki metropolitan area; only some diabetic persons aged 65 years or more treated in outpatient primary care settings without antidiabetic medication were missing [24].
The precise etiological classification into type 1 (T1DM) and T2DM was not possible with the current register-based data. Therefore, we determined the diabetes type based on age at diagnosis of diabetes: persons diagnosed before the age of 30 years were considered to have T1DM and the rest T2DM.
The follow-up for cancer incidence through the files of the national Finnish Cancer Registry and for vital status and emigration through the Population Register was performed using the personal identity code as the key. The follow-up for cancer incidence started at the date of diagnosis of the diabetes or on 1st of January 1989, whichever was later, and ended at death or emigration, or on December 31, 2014, whichever occurred first. The follow-up of 204,724 male and 223,602 female T2DM patients produced altogether 4.48 million person-years (Table 1). The mean length of follow-up of a person was 10.5 years.
The numbers of observed cases and person-years at risk were counted by 5-year age groups, separately for calendar periods (1989–1994, 1995–1999, 2000–2004, 2005–2009 and 2010–2014). The expected numbers of cases for total cancer and specific cancer types were calculated by multiplying the number of person-years in each age group by the corresponding average cancer incidence in all of Finland during the period of observation. The specific cancer types selected a priori for the analysis included the cancer sites with known or suspected exceptional (increased or decreased) risk in T2DM patients in earlier studies, and other common cancer types to give the whole picture of the cancer situation among Finnish T2DM patients.
To calculate the standardized incidence ratio (SIR), the observed number of cases was divided by the expected number. The 95% confidence intervals (CI) for the SIR were based on the assumption that the number of observed cases followed a Poisson distribution.
Results
During the follow-up, 74,063 cases of cancer were diagnosed; the expected number was 63,870. Consequently, the total cancer incidence for adults with T2DM was 16% higher than expected (SIR = 1.16, CI = 1.15–1.16; Table 2).
The incidence of 16 cancer types was statistically significantly elevated; cancer of lip, stomach, colon, rectum, liver (SIR = 2.44, CI = 2.35–2.53), gallbladder, pancreas (SIR = 1.75, CI = 1.70–1.79), unspecified intestinal tract, non-melanoma skin, kidney, bladder, brain and central nervous system, thyroid, non-Hodgkin lymphoma, leukaemia, and ill-defined or unknown (Tables 2, 3).
For women with T2DM, 34,199 cancer diagnoses were observed, which was 18% higher than expected. We found statistically significant increase for cancers of lip, stomach, colon, rectum, liver (SIR = 1.90, CI = 1.77–2.03), gallbladder, pancreas (SIR = 1.72, CI = 1.65–1.78), unspecified intestinal tract, non-melanoma skin, kidney, non-Hodgkin lymphoma, corpus uteri, and other female genital organs (Table 3).
Among men with T2DM, 39,864 cancer diagnoses were observed, which was 14% more than expected. Statistically significant increase in cancer incidence were seen for cancers of lip, oral cavity, stomach, colon, rectum, liver (SIR = 2.79, CI = 2.66–2.92), gallbladder, pancreas (SIR = 1.78, CI = 1.71–1.85), unspecified intestinal tract, non-melanoma skin, kidney, bladder, thyroid, and other male genital organs (C60, C63). The inverse effect was seen for prostate cancer only (SIR = 0.95, CI = 0.93–0.96); 10,692 men with T2DM developed prostate cancer, which was 5% less than expected (Table 3).
Discussion
The total cancer incidence among the 428,326 T2DM patients was 16% higher among people with T2DM compared with the expected average cancer incidence in the Finnish population. The increase in cancer incidence was seen both in women (18%) and men (14%).
We found an almost threefold increase in liver cancer incidence among men with T2DM and nearly twofold increase among women with T2DM compared with the general population. Similar findings have been observed in several studies earlier [1, 8, 25]. It has been speculated whether diabetes is a direct risk factor for liver cancer or whether there are other common risk factors involved, such as non-alcoholic fatty liver disease (NAFLD), steatosis or cirrhosis [26, 27].
For pancreatic cancer we observed a more than 70% increased risk in both men and women with T2DM. This result applies to what is already known about the relationship between T2DM and cancer of the pancreas [5,6,7]. Suggestions of the possible underlying mechanisms between diabetes and pancreatic cancer include elevated insulin concentrations acting as a tumor-growth promoting factor in pancreatic cells, hyperglycemia, insulin resistance and hyperinsulinemia [5, 16]. It also has been questioned whether the association is caused by reverse causality and diabetes is caused by liver or pancreatic cancer [4]. However, this does probably not explain increased risks in our data because of the temporal relations. Pancreatic cancer is generally known as quickly progressive and fatal, elevated incidence may be due to reverse causality only in the very short time frame (less than 6 months) following diabetes onset. Sometimes pancreatic cancer is misdiagnosed as diabetes, due to similar symptoms. Continuous increased risk of pancreatic cancer after longer periods of follow-up (up to 10 years after diabetes onset) is unlikely due to reverse causality [7, 15].
We found an increased risk of cancer of urinary organs, particularly kidney cancer, in persons with T2DM. This finding is similar to a meta-analysis of studies investigating the association between diabetes and kidney cancer incidence and mortality [12]. The association has been tried to be described by common risk factors, such as obesity and cigarette smoking, or medication. Influence of possible confounding or detection bias can not put aside, neither. Because diabetic patients are under increased medical surveillance, and the measurement of urinary specimens is recommended (mainly for detection of signs of diabetic nephropathy) it is possible that kidney cancer tend to be found at an earlier stage in diabetic patients than in non-diabetic people [28].
Although higher risk of breast cancer among type 2 diabetic patients has been indicated in many studies [10, 29], the connection was not seen in our data. Instead, we found an increase in cancers of female reproductive organs, such as cancer of corpus uteri, also known as endometrial cancer (50%), and cancers of vulva and vagina in diabetic women (24%). Endometrial cancer risk has reported to be higher among women with diabetes [11, 30], but this relationship may be confounded by body weight [31]. Nevertheless, a positive association between T2DM and endometrial cancer has still been seen after adjusting for possible confounding factors: physical inactivity and obesity [11].
In contrast to the increased risk for many types of cancer, a 14% decreased risk of prostate cancer has been seen in pooled analysis of 45 studies [13]. In our study the diabetic males possessed 5% reduction in prostate cancer risk. This inverse relationship is not fully understood, but has been considered to result from lower testosterone levels in diabetic patients, as well as many more hormonal and metabolic factors related to lifestyle and medication. It has been suggested that diabetic men are more likely to be tested for prostate-specific antigen (PSA) levels, and this lead to an increased probability of detecting prostate cancer, opposite to the observed inverse association [14].
Cancer registration system in Finland is virtually complete nationwide and the computerized record linkage procedure based on the national personal identifier is precise [32]. Therefore, technical incompleteness of case ascertainment does not cause any bias in our results. However, confounding by lifestyle factors, such as physical inactivity, dietary habits and cigarette smoking, is possible [33]. The overall cancer incidence among working-aged Finnish men increases and among women decreases towards the lower social class [34]. Low socioeconomic status (SES) has been associated with late state diagnoses and T2DM is more common in people with lower SES, as well [35].
Other strengths of the study are, besides technical accuracy, relatively long-term follow-up and size of the data, due to record linkage. Finnish registers provide access to valuable data without time-consuming and expensive data collecting. Observed and expected frequencies of cancers are come from the same database. Most studies report associations between diabetes and cancer mortality. Compared to mortality, incidence is, nevertheless, a preferable indicator for cancer risk due to variation in cancer survival rates due to earlier diagnosis and better treatment.
Many studies investigating the association between diabetes and cancer incidence have not made a proper distinction between the two main types of diabetes. The precise etiological classification between T1DM and T2DM was not possible with our current register-based data, which might have led to misclassification, where group of T2DM includes persons who actually had other type of diabetes, but such a probability is small compared with the large number of people with T2DM [36].
It is very challenging to study associations between these heterogenous and complex diseases. T2DM shares several common risk factors with many cancer types, such as obesity, poor diet, ageing and physical inactivity. Thus confounding is likely to occur and the possibility of reverse causality could not be ignored, either. Risk factors for T2DM are similar to cardiovascular diseases (CVD), as well. Thus, a long follow-up shows negative correlation with cancer mortality, because of diabetic patients’ high mortality on CVD [37]. Additionally, it has been speculated that if cancer is more common in diabetic patients, the relation between diabetes and cancer might be underestimated since T2DM, in fact, is underdiagnosed disease. Also, due to possible undiagnosed individuals with T2DM the cancer risk in the non-diabetic population might be higher than observed [8].
It is still unclear, whether T2DM and its metabolic derangements (hyperglycemia, insulin resistance and hyperinsulinaemia) increase the risk of cancer directly or is the association indirect. There is a wide array of possible mechanisms related with cancer incidence in patients with T2DM. Due to the multiple and complex mitogenic effect of insulin, hyperinsulinaemia is a potential factor favouring cancer progression [38]. Increasing insulin levels activate the related insulin-like growth factor-I (IGF-I) that probably promotes cancer cell growth and proliferation. Impaired glucose tolerance is related to increased cancer risk without diabetes, too. Poor metabolic control leads to increasing oxidative stress causing a chronic pro-inflammatory state, resulting vulnerable cells predisposing to malignant transformation. Biological actions, common with degenerative diseases, such as T2DM, might accelerate carcinogenic processes [16].
We need more in-depth, well-designed follow-up studies to advance our knowledge of the complex association between T2DM and cancer incidence, and the underlying direct and indirect biological mechanisms. It is essential to explore the potential role of glucose-lowering treatments. The task is challenging, because of the diversity of therapies over time. A series of confounders and biases should be observed properly to ensure validity. Because of the growing, worldwide burden of diabetes, it is extremely important to acquire understanding to these questions to prevent cancer in diabetic patients.
References
Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analysis of observational studies. BMJ. 2015;350:g7607.
Noto H, Tsujimoto T, Sasazuki T, Noda M. Significantly increased risk of cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Endocr Pract. 2011;17:616–28.
Gallagher EJ, LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol Rev. 2015;95:727–48.
Harding JL, Shaw JE, Peeters A, Carstensen B, Magliano DJ. Cancer risk among people with type 1 and type 2 diabetes: disentangling truth associations, detection bias, and reverse causation. Diabetes Care. 2015;38:264–70.
Song S, Wang B, Zhang X, et al. Long-term diabetes mellitus is associated with an increased risk of pancreatic cancer: a meta-analysis. PLoS ONE. 2015;10:e0134321.
Zhang C, Yang G, Ling Y, Chen G, Zhou T. The early diagnosis of pancreatic cancer and diabetes: what’s the relationship? J Gastrointest Oncol. 2014;5:481–8.
Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83.
Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–85.
Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87.
Hardefeldt PJ, Edirimanne S, Eslick GD. Diabetes increases the risk of breast cancer: a meta-analysis. Endocr Relat Cancer. 2012;19:793–803.
Zhang ZH, Su PY, Hao JH, Sun YH. The role of preexisting diabetes mellitus on incidence and mortality of endometrial cancer: a meta-analysis of prospective cohort studies. Int J Gynecol Cancer. 2013;23:294–303.
Bao C, Yang X, Xu W, et al. Diabetes mellitus and incidence and mortality of kidney cancer: a meta-analysis. J Diabetes Complicat. 2013;27:357–64.
Bansal D, Bhansali A, Kapil G, Undela K, Tiwari P. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2013;16(151-8):S1.
Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomark Prev. 2006;15:2056–62.
Johnson JA, Carstensen B, Witte D, et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55:1607–18.
Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–23.
Xu H, Jiang HW, Ding GX, et al. Diabetes mellitus and prostate cancer risk of different grade or stage: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2013;99:241–9.
Tokajuk A, Krzyżanowska-Grycel E, Tokajuk A, Grycel S, Sadowska A, Car H. Antidiabetic drugs and risk of cancer. Pharmacol Rep. 2015;67:1240–50.
Ioannidis JP, Zhou Y, Chang CQ, Schully SD, Khoury MJ, Freedman AN. Potential increased risk of cancer from commonly used medications: an umbrella review of meta-analyses. Ann Oncol. 2014;25:16–23.
Karlstad Ø, Starup-Linde J, Vestergaard P, et al. Use of insulin and insulin analogs and risk of cancer—systematic review and meta-analysis of observational studies. Curr Drug Saf. 2013;8:333–48.
Yang T, Yang Y, Liu S. Association between metformin therapy and breast cancer incidence and mortality: evidence from a meta-analysis. J Breast Cancer. 2015;18:264–70.
Gandini S, Puntoni M, Heckman-Stoddard BM, et al. Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila). 2014;7:867–85.
Sund R, Koski S. FinDM II. On the register-based measurement of the prevalence and incidence of diabetes and its long-term complications. A technical report. Tampere: Finnish Diabetes Association; 2009.
Sund R, Harno K, Ranta S, Tolppala EP. Comparing the case inclusion in two population-based diabetes registers. Finn J eHealth eWelfare. 2010;2:136–46.
Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109–22.
Raff EJ, Kakati D, Bloomer JR, Shoreibah M, Rasheed K, Singal AK. Diabetes mellitus predicts occurrence of cirrhosis and hepatocellular cancer in alcoholic liver and non-alcoholic fatty liver diseases. J Clin Transl Hepatol. 2015;3:9–16.
Tudzarova S, Osman MA. The double trouble of metabolic diseases: the diabetes-cancer link. Mol Biol Cell. 2015;26:3129–39.
Larsson SC, Wolk A. Diabetes mellitus and incidence of kidney cancer: a meta-analysis of cohort studies. Diabetologia. 2011;54:1013–8.
De Bruijin KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100:1421–9.
Liao C, Zhang D, Mungo C, Tompkins DA, Zeidan AM. Is diabetes mellitus associated with increased incidence and disease-specific mortality in endometrial cancer? A systematic review and meta-analysis of cohort studies. Gynecol Oncol. 2014;135:163–71.
Luo J, Beresford S, Chen C, et al. Association between diabetes, diabetes treatment and risk of developing endometrial cancer. Br J Cancer. 2014;111:1432–9.
Pukkala E. Biobanks and registers in epidemiologic research on cancer. Methods Mol Biol. 2011;675:127–64.
Katzke VA, Kaaks R, Kühn T. Lifestyle and cancer risk. Cancer J. 2015;21:104–10.
Pukkala E, Martinsen JI, Lynge E, et al. Occupation and cancer—follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790.
Kim YJ, Jeon JY, Han SJ, Kim HJ, Lee KW, Kim DJ. Effect of socio-economic status on the prevalence of diabetes. Yonsei Med J. 2015;56:641–7.
Lammi N, Taskinen O, Moltchanova E, et al. A high incidence of type 1 diabetes and an alarming increase in the incidence of type 2 diabetes among young adults in Finland between 1992 and 1996. Diabetologia. 2007;50:1393–400.
Gordon-Dseagu VL, Mindell JS, Steptoe A, et al. Impaired glucose metabolism among those with and without diagnosed diabetes and mortality: a cohort study using Health Survey for England data. PLoS ONE. 2015;10:e0119882.
Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer Sci. 2013;104:9–14.
Acknowledgements
This study has been partly supported by the research Grants to JT from the Finnish Cancer Foundation, European Foundation for the Study of Diabetes and Sanofi-Aventis. JT is an advisory board member of Novo Nordisk and Merck KGaA. SH has received lecture fees from MSD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors KS, RS, IK and EP declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Saarela, K., Tuomilehto, J., Sund, R. et al. Cancer incidence among Finnish people with type 2 diabetes during 1989–2014. Eur J Epidemiol 34, 259–265 (2019). https://doi.org/10.1007/s10654-018-0438-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-018-0438-0