Abstract
Changes in the incidence of venous thromboembolism (VTE) during the last two decades have not been extensively studied. Therefore, we studied time trends in the incidence rates (IRs) of deep vein thrombosis (DVT) and pulmonary embolism (PE) in a cohort of 26,855 subjects, aged 25–97 years, enrolled in the Tromsø study in 1994/1995. The subject were followed-up throughout 2012, and all symptomatic, objectively confirmed, incident VTEs were identified using multiple sources (hospital discharge-, radiology procedure- and autopsy registry) and validated by review of medical records. Age-adjusted biennial IR per 100,000 person years (PY) with 95% confidence intervals (CI) were calculated using Poisson regression. Between January 1996 and December 2012, 693 VTEs occurred during 368,150 PY of follow up. The IR of VTE increased from 158 (95% CI 116–199) in 1996/1997 to 201 (95% CI 160–243) in 2010/2011. There was a marked increase in the rates of PE (with/without concurrent DVT) ranging from 45 (95% CI 23–67) in 1996/1997 to 113 (95% CI 82–144) in 2010/2011, whereas the rates of isolated DVT decreased (112, 95% CI 77–146 in 1996/1997 and 88, 95% CI 61–115 in 2010/2011). Despite advances in prophylaxis, the IR of VTE has increased slightly during the last 15 years, mainly due to an increase in PE. Although the introduction of better diagnostic tools to some extent may explain the increase in PE rates, our findings suggest that there is still a need for improvement in risk factor management and prevention strategies of first time VTE.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Venous thromboembolism (VTE) is the third most common life-threatening cardiovascular disease after myocardial infarction and stroke [1]. Lower limb deep vein thrombosis (DVT) and pulmonary embolism (PE) are the two most common disease manifestations of VTE [2]. Although VTE occurs more frequently in older individuals, it can affect individuals of any age and gender [3]. Due to high rates of complications such as the post thrombotic syndrome, recurrence and mortality among patients with VTE, the disorder remains a major public health challenge with a substantial disease burden [4].
Previously reported incidence rates of VTE varies considerably, and ranges between 62 and 143 per 100,000 individuals per year in the adult population [3, 5–10]. The variation may rely on population characteristics such as age distribution and ethnicity, available data sources, case definition and validation procedures, inadequate alienation of initial and recurrent episodes, study design and reliance on in-patient medical records only [3, 11–14].
Alterations of risk factor levels, prevention strategies, diagnostic tools and treatment modalities over time may alter the rate of VTE in a community. The more widespread use of low molecular weight heparins for VTE prophylaxis, as well as the introduction of multidetector row computed tomographic pulmonary angiography (CTPA) for detection of PE may have influenced the rates during the last 20 years. Moreover, the prevalence of VTE risk factors such as old age, obesity and cancer [14, 15] are increasing at the population level in Western countries [16]. Few studies have examined trends in the incidence of VTE during the last two decades. Using data from the Worcester study, Huang et al. [17] reported that the incidence rates (IR) of VTE increased by 82% from 73 per 100,000 to 133 per 100,000 in the period 1985–2009, primarily because of an increase in PE. Similarly, Wiener et al. [18] reported an 81% increase in the incidence of PE after the introduction of CTPA in the US. In contrast, Delluc et al. [19] reported a 28% reduction in the standardized annual incidence of VTE between 1998 and 2013 in the Brest District, Western France.
Reported data on the incidence of VTE vary considerably, and the majority of the population-based studies were carried out several decades ago [6, 11, 20, 21]. Furthermore, changes in the incidence of VTE have not been studied extensively during the last 20 years. Therefore, our aim was to describe the 16 years’ time trend (1996–2012) in the IRs of VTE in a large population-based cohort, and to describe the prevalence of clinical risk factors and provoking factors of VTE at the time of VTE diagnosis.
Methods
Study population
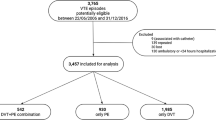
Participants were recruited from fourth survey of the Tromsø Study, conducted in 1994–1995. The Tromsø study is a single centre prospective, population based follow up study, with repeated health surveys of inhabitants in Tromsø, Norway. All inhabitants aged >24 years were invited, and 27,158 participated (response rate 77%). The study was approved by the regional committee for research ethics, and all participants gave their informed, written consent to participate. Subjects who did not consent to medical research (n = 300), and subjects not officially registered inhabitants of the municipality of Tromsø (n = 43), and those with a known history of VTE (n = 47) were excluded from the study. A total of 26,855 subjects were included in our study. Incident VTE events among the study participants were recorded through the end of follow up, 31st of December 2012.
Identification and validation of venous thromboembolism
All first lifetime events of VTE during follow up were identified by searching the hospital discharge registry, the autopsy registry, and the radiology procedure registry at the University Hospital of North Norway, from the date of enrolment in the Tromsø Study (1994–1995), through 31st of December 2012. The University Hospital of North Norway is the only hospital in the region, and all hospital care and relevant diagnostic radiology in the Tromsø community is provided exclusively by this hospital. We used a broad search strategy, and the relevant discharge codes were International Classification of Diseases (ICD-9) codes 325, 415.1, 451, 452, 453, 671.3, 671.4, 671.9 for the period 1994–1998, and ICD-10 codes I26, I67.6, I80, I81, I82, O22.3, O22.5, O87.1, O87.3 for the period 1999–2012 (Supplementary Table 1). The hospital discharge registry included both outpatient clinic visits and hospitalizations. An additional search through the computerized index of autopsy diagnoses was conducted, and cases diagnosed with VTE, either as a cause of death, or as a significant condition, were identified. We also searched the radiology database in order to identify potential cases of symptomatic objectively confirmed VTE that may have been missed because of coding errors in the hospital discharge registry. Trained personnel systematically reviewed all relevant diagnostic procedures performed at the Department of Radiology to diagnose VTE during the 16-year period, and cases with objectively confirmed VTE, were identified.
The medical records for each potential VTE case, derived from the hospital discharged registry, the autopsy registry, or the radiology procedure registry, were reviewed by trained personnel. For subjects derived from the hospital discharged registry and the radiology procedure registry, an episode of VTE was verified and recorded as a validated outcome when all four of the following criteria were fulfilled; (1) signs and symptoms consistent with DVT or PE were present (2) objectively confirmed by diagnostic procedures (compression ultrasonography of the whole leg, venography, CTPA, perfusion-ventilation scan [VQ-scan], pulmonary angiography or autopsy), (3) the medical record indicated that a physician had made a diagnosis of DVT or PE, and (4) the patient received treatment with anticoagulants (heparin, warfarin, or a similar agent) thrombolytics, or vascular surgery unless contraindications were specified. For subjects derived from the autopsy register, a VTE event was recorded as an outcome when the autopsy record (death certificate) indicated VTE as cause of death or as a significant condition contributing to death.
A VTE event was further classified as unprovoked or provoked, based on the presence of provoking factors at the time of VTE diagnosis. A VTE occurring without any provoking factor was defined as unprovoked, whereas a VTE occurring in the presence of one or more provoking factors was defined as provoked. The following were regarded as provoking factors: recent hospitalization, surgery or trauma, (within eight weeks before the event), acute medical condition (acute myocardial infarction, acute ischemic stroke, acute infections), active cancer, immobilization (bed rest >3 days or confinement to wheelchair within the last 8 weeks, or long distance travel ≥4 h within the last 14 days), or other factor specifically described as provoking by a physician in the medical record (e.g. intravascular catheter).
Statistical analyses
After the enrolment period (1994–1995) was completed, the participants were followed from the 1st of January 1996 to the 31st of December 2012. Thus, person years of follow up were accrued from the 1st of January 1996, until the date of an incident VTE, migration, death, or study end, whichever occurred first.
Statistical analyses were carried out using Stata version 14.0 (Stata corporation, College station, Texas, USA) and SPSS version 24 (IBM SPSS Statistics for Windows, New York, USA). The overall crude incidence rate of VTE was calculated and expressed as the number of events per 100,000 person years. We also calculated the incidence rates according to 10-year age groups in men and women, based on the event rate and total person time spent in each 10-year category. Moreover, for comparison purposes, we calculated the overall incidence rate of VTE standardised towards World Health Organization (WHO) standard population, 2000–2025 [22].
Since this was a closed cohort, the age distribution of the participants would change over time. Thus, for analyses of time trends, age-adjusted biennial incidence rates of VTE, DVT and PE were calculated for the periods 1996/1997, 1998/1999, 2000/2001, 2002/2003, 2004/2005, 2006/2007, 2008/2009 and 2010/2011 using Poisson regression. In order to investigate whether the change in incidence of PE and DVT could be particularly attributed to certain age groups, we compared the age-specific incidences in the first half period (i.e. 1996–2003) with the second half period (i.e. 2004–2012).
Results
Baseline characteristics of total cohort
Baseline characteristics of the 26,855 study participants are given in Table 1. The mean age was 47 ± 15 years, and the proportion of males was 47.5%. The mean BMI was 25.2 ± 3.9 kg/m2, and 0.6% had active cancer (i.e. cancer diagnosed <2 years before inclusion) at baseline.
Patient characteristics
There were 693 incident VTE events during a total of 368,150 PY of follow up between 1st of January 1996 and 31st of December 2012. Patients’ characteristics and risk factors at the time of VTE diagnosis are given in Table 2. The mean age at the time of VTE was 69 ± 13 years, and 53.6% (n = 372) were women. Moreover, 42.4% (n = 294) had PE with or without concurrent DVT, while 57.6% (n = 399) had DVT only. Among the DVTs, 43.0% were proximal leg DVT, 18.4% were calf vein DVTs and 4.6% were DVTs in other locations (e.g. upper extremities, visceral veins etc.). In total, 41.7% of the VTE events were classified as unprovoked, and correspondingly, 58.3% as provoked. In 33.3% of the patients, only one provoking factor was present, while 22.5% had two or more provoking factors. The most common provoking factors were active cancer (23.2%), immobilization (19.0%), recent surgery (14.7%) and acute medical conditions (14.5%). Among the clinical risk factors, recent hospitalization (34.8%) was the most common, followed by comorbid medical conditions (22.3%) and obesity (16.5%).
Overall incidence of VTE
The overall crude IR of VTE per 100,000 PY was 188 (95% CI 175–203), 108 (95% CI 98–119) for DVT and 80 (95% CI 71–89) for PE with or without DVT. The overall age-adjusted average IR of VTE per 100,000 PY (standardized towards WHO standard population, 2000–2025) was 86 (95% CI 59–113), 48 (95% CI 29–68) for DVT and 37 (95% CI 19–56) for PE with or without DVT. The age- and sex-specific IR of VTE per 100,000 PY increased considerably with age in both men and women (Fig. 1); from 12 (95% CI 4–35) in men aged 30–40 years to 723 (95% CI 556–943) in men aged 80–90 years, and correspondingly from 50 (95% CI 30–83) to 850 (95% CI 706–1006) in women of the same age groups. In the age group 70–80 years the incidence of VTE was higher in men than in women (536 vs. 410) although not statistically significant as the confidence intervals overlapped (Fig. 1).
Time trends in the incidence of VTE
The biennial age-adjusted IRs of VTE per 100,000 PY increased slightly (27%) during the 16-years study period from 158 (95% CI 117–199) in 1996/1997 to 201 (95% CI 160–243) in 2010/2011, with a peak of 219 (95% CI 175–263) in 2006/2007 (Fig. 2 and Supplementary Table 2). The age-adjusted IR per 100,000 PY of PE increased from 45 (95% CI 23–67) in 1996/1997 to 113 (95% CI 82–144) in 2010/2011. Contrary, the incidence of DVT showed a slightly decreasing trend from 112 (95% CI 77–146) to 88 (95% CI 61–115) per 100,000 PY. The rates of provoked and unprovoked VTEs showed similar increasing trends over the time period (Supplementary figure 1). The proportion of PE patients examined with CTPA increased during the study period, from 23.1% in 1998/1999 to 76.0% in 2010/2011 (Supplementary figure 2).
In order to investigate whether the change in incidence of PE and DVT could be particularly attributed to certain age groups, we compared the age-specific incidences in the first half period (i.e. 1996–2003) with the second half period (i.e. 2004–2012) (Fig. 3, panel a, b). When the second period was compared to the first period, the incidence of PE was slightly higher in those below 50 years (30, 95% CI 19–50 vs. 8, 95% CI 4–17), more or less similar in those 50–70 years (147, 95% CI 105–207 vs. 144, 95% CI 96–216), and markedly higher in those 70 years or older (355, 95% CI 298–424 vs. 144, 95% CI 105–197).
Discussion
The present study described trends in the incidence of VTE in a large population-based cohort from 1996 to 2012. The overall age-adjusted incidence of VTE increased slightly throughout the study period, from 158 per 100,000 person-years in 1996/1997 to 201 per 100,000 in 2010/2011. The majority of the increase was explained by a rise in PE, whereas the rate of DVT slightly decreased. Our findings indicate that despite advances in thromboprophylaxis during the last decades, VTE remains a major public health challenge.
In our study, the age-adjusted IR of VTE increased by 27% during the 16-year period. The highest peak was observed in 2006/2007 (38% higher than in 1996/1997). Other studies conducted in the same period, although different in the selection of VTE population and design, have reported similar trends [3, 20]. In the Worcester study, including 3040 patients with first-lifetime VTE, the IRs increased by approximately 45% from 92/100,000 to 133/100,000 in the period 1999–2009 [17]. In another US study, based on Medicare and commercial insurance databases, the annual VTE prevalence increased by 33% in the period 2002–2006 [23]. In our study, the rates of PE increased by 250% from 45 to 113 per 100 000, whereas the rates of DVT decreased during the study period. Strikingly similar trends in the incidence of PE and DVT were reported in the Worcester study [17]. In contrast, Delluc and co-workers reported a 28% reduction in the overall incidence of VTE from 1998 to 2013, and this reduction was mainly driven by a reduction in the incidence of DVT ([19]). Of note, this study included both first and recurrent VTEs in their IR calculations. Moreover, the study was based on two cross-sectional measurements which could make it more vulnerable to random fluctuations in the incidence rates.
Increased clinical awareness and extensive adoption of high-resolution multiple-detector CTPA to diagnose PE are possible explanations for the increasing incidence of VTE [17, 18]. The increasing age-adjusted incidence of PE corresponds well with the increasing use of CTPA at our hospital from around year 2000 and onwards, indicating that we are detecting more PEs than earlier. The question is whether these PEs are of clinical significance. In fact, the relatively constant mortality rate from PE during the last decades, combined with improved case-fatality rates, points towards over-diagnosing of PE with CTPA [18]. In our study, the age-adjusted IRs of PE increased in the period 1996–2006, and reached a plateau and stabilized in the period 2006–2012. This may indicate that we have reached the maximum detection rate of PEs with a more widespread use of CTPA. Alternatively, the introduction of pre-screening tools and a more restricted approach to CTPA may additionally explain this flattening of the curve to some extent. The apparent decrease in DVT incidence in the same period was presumably explained by the way PE was recorded in our study (i.e. PE with or without concurrent clinical DVT), as detection of more PEs in patients with concurrent symptomatic DVTs would be coded as PE rather than DVT. The rates of PE appeared to be particularly increased among those >70 years when the second half of the study period (2004–2012) was compared to the first half (1996–2003). This could potentially be explained by a lowered threshold to preform diagnostic procedures for PE detection in the elderly after the introduction of CTPA.
Although improved diagnostic tools may explain the observed increase in VTE incidence during the study period, VTE still remains a major health challenge, as the incidence is not decreasing in the society. This is in contrast to arterial cardiovascular diseases, such as myocardial infarction and ischaemic stroke, where the incidence has decreased substantially during the last decades despite improvement in diagnostic tools [24–26]. This decrease could, to a large extent, be attributed to more targeted prevention and favourable changes in modifiable cardiovascular risk factors [24]. Except for body mass index, few lifestyle factors have consistently been associated with VTE at the population level [27, 28]. Moreover, the age-adjusted incidence of provoked VTE did not appear to decrease during the time period, indicating a potential for more widespread use of thromboprophylaxis to prevent VTE in patients with a low bleeding risk. Identification and recognition of risk factors and improved prediction models to facilitate targeted prevention in high-risk situations is of importance to reduce the incidence of VTE in the society.
Strengths of our study included the well-defined source population with detailed information on person-time of follow-up for calculation of IRs. There is only one hospital serving the source population (the next nearest hospital is >250 km away), and all outpatient care for diagnostic assessment and treatment of VTE is exclusively provided by this hospital. To optimize the completeness of our case-registration, we used three different sources to identify VTE cases diagnosed and treated at this hospital. To minimize the chance of outcome misclassification, all potential cases were validated by comprehensive medical records review using well-defined criteria for outcome assessment. Our data also give information from a relatively recent time-period (up to 2012), compared to previous studies [22]. The standardization towards the WHO world population provides a possibility for future studies in other populations or countries to compare their rates to ours. Some limitations merits consideration. Although the detection of VTE cases were based on a broad search using multiple databases, it is possible that we missed some cases. Moreover, due to low autopsy rates in Norway, and limited validity of death certificate data, some cases of fatal PE could be missed. Information on VTE risk factors were limited to the content of the medical records, and therefore, the prevalence of risk factors at the time of VTE diagnosis could be underestimated. As presence of DVT was not routinely examined in patients presenting with PE, we could not distinguish between isolated PE and PE combined with DVT in our dataset.
In conclusion, the rates of VTE has not decreased during the last decades. Our findings indicate that VTE remains a major public health challenge. Future research should focus on improving risk stratification and prevention of VTE in order to reduce the burden of VTE in the society.
References
Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162(10):975–82. doi:10.1093/aje/kwi309.
Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–15.
Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158(6):585–93.
Raskob GE, Angchaisuksiri P, Blanco AN, et al. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34(11):2363–71. doi:10.1161/atvbaha.114.304488.
Naess IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrom J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. 2007;5(4):692–9. doi:10.1111/j.1538-7836.2007.02450.x.
Spencer FA, Emery C, Joffe SW, et al. Incidence rates, clinical profile, and outcomes of patients with venous thromboembolism. The Worcester VTE study. J Thromb Thrombolysis. 2009;28(4):401–9. doi:10.1007/s11239-009-0378-3.
Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Stormer J, Hansen JB. Family history of myocardial infarction is an independent risk factor for venous thromboembolism: the Tromso study. J Thromb Haemost. 2008;6(11):1851–7. doi:10.1111/j.1538-7836.2008.03102.x.
Johansson M, Johansson L, Lind M. Incidence of venous thromboembolism in northern Sweden (VEINS): a population-based study. Thromb J. 2014;12(1):6. doi:10.1186/1477-9560-12-6.
Isma N, Svensson PJ, Gottsater A, Lindblad B. Prospective analysis of risk factors and distribution of venous thromboembolism in the population-based Malmo Thrombophilia Study (MATS). Thromb Res. 2009;124(6):663–6. doi:10.1016/j.thromres.2009.04.022.
Ho WK, Hankey GJ, Eikelboom JW. The incidence of venous thromboembolism: a prospective, community-based study in Perth, Western Australia. Med J Aust. 2008;189(3):144–7.
Spencer FA, Emery C, Lessard D, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21(7):722–7. doi:10.1111/j.1525-1497.2006.00458.x.
Cushman M. Epidemiology and risk factors for venous thrombosis. Semin Hematol. 2007;44(2):62–9. doi:10.1053/j.seminhematol.2007.02.004.
White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4–8. doi:10.1161/01.CIR.0000078468.11849.66.
Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol. 2010;56(1):1–7. doi:10.1016/j.jacc.2010.01.057.
Kniffin WD Jr, Baron JA, Barrett J, Birkmeyer JD, Anderson FA Jr. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med. 1994;154(8):861–6.
Cohen AT, Agnelli G, Anderson FA, et al. Venous thromboembolism (VTE) in Europe. The number of VTE events and associated morbidity and mortality. Thromb Haemost. 2007;98(4):756–64.
Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985–2009). Am J Med. 2014;127(9):829–39. doi:10.1016/j.amjmed.2014.03.041.
Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831–7. doi:10.1001/archinternmed.2011.178.
Delluc A, Tromeur C, Le Ven F, et al. Current incidence of venous thromboembolism and comparison with 1998: a community-based study in Western France. Thromb Haemost. 2016;116(5):967–74. doi:10.1160/th16-03-0205.
Heit JA. Venous thromboembolism: disease burden, outcomes and risk factors. J Thromb Haemost. 2005;3(8):1611–7. doi:10.1111/j.1538-7836.2005.01415.x.
Heit JA. The epidemiology of venous thromboembolism in the community. Arterioscler Thromb Vasc Biol. 2008;28(3):370–2. doi:10.1161/ATVBAHA.108.162545.
Ahmad OB PC, Lopez AD, Murray CJL, Lozano R, Inoue M. Age standardization of rates: A new WHO standard. EIP/GPE/EBD World Health Organization; 2001.
Deitelzweig SB, Johnson BH, Lin J, Schulman KL. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. Am J Hematol. 2011;86(2):217–20. doi:10.1002/ajh.21917.
Mannsverk J, Wilsgaard T, Mathiesen EB, et al. Trends in modifiable risk factors are associated with declining incidence of hospitalized and nonhospitalized acute coronary heart disease in a population. Circulation. 2016;133(1):74–81. doi:10.1161/circulationaha.115.016960.
Mannsverk J, Wilsgaard T, Njolstad I, et al. Age and gender differences in incidence and case fatality trends for myocardial infarction: a 30-year follow-up. The Tromso Study. Eur J Prev Cardiol. 2012;19(5):927–34. doi:10.1177/1741826711421081.
Townsend N, Nichols M, Scarborough P, Rayner M. Cardiovascular disease in Europe–epidemiological update 2015. Eur Heart J. 2015;36(40):2696–705. doi:10.1093/eurheartj/ehv428.
Horvei LD, Braekkan SK, Mathiesen EB, Njolstad I, Wilsgaard T, Hansen JB. Obesity measures and risk of venous thromboembolism and myocardial infarction. Eur J Epidemiol. 2014;29(11):821–30. doi:10.1007/s10654-014-9950-z.
Braekkan SK, Hald EM, Mathiesen EB, et al. Competing risk of atherosclerotic risk factors for arterial and venous thrombosis in a general population: the Tromso study. Arterioscler Thromb Vasc Biol. 2012;32(2):487–91. doi:10.1161/atvbaha.111.237545.
Acknowledgements
K.G. Jebsen TREC is supported by an independent grant from Stiftelsen K.G. Jebsen.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arshad, N., Isaksen, T., Hansen, JB. et al. Time trends in incidence rates of venous thromboembolism in a large cohort recruited from the general population. Eur J Epidemiol 32, 299–305 (2017). https://doi.org/10.1007/s10654-017-0238-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-017-0238-y