Abstract
Whether and how coffee use influences glucose metabolism is still a matter for debate. We investigated whether baseline coffee consumption is longitudinally associated with risk of impaired fasting glucose in a cohort of 18-to-45 year old subjects screened for stage 1 hypertension and whether CYP1A2 polymorphism modulates this association. A total of 1,180 nondiabetic patients attending 17 hospital centers were included. Seventy-four percent of our subjects drank coffee. Among the coffee drinkers, 87 % drank 1–3 cups/day (moderate drinkers), and 13 % drank over 3 cups/day (heavy drinkers). Genotyping of CYP1A2 SNP was performed by real time PCR in 639 subjects. At the end of a median follow-up of 6.1 years, impaired fasting glucose was found in 24.0 % of the subjects. In a multivariable Cox regression coffee use was a predictor of impaired fasting glucose at study end, with a hazard ratio (HR) of 1.3 (95 % CI 0.97–1.8) in moderate coffee drinkers and of 2.3 (1.5–3.5) in heavy drinkers compared to abstainers. Among the subjects stratified by CYP1A2 genotype, heavy coffee drinkers carriers of the slow *1F allele (59 %) had a higher adjusted risk of impaired fasting glucose (HR 2.8, 95 % CI 1.3–5.9) compared to abstainers whereas this association was of borderline statistical significance among the homozygous for the A allele (HR 1.7, 95 % CI 0.8–3.8). These data show that coffee consumption increases the risk of impaired fasting glucose in hypertension particularly among carriers of the slow CYP1A2 *1F allele.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A large number of epidemiologic studies have demonstrated that type 2 diabetes mellitus (T2DM) is a major risk factor for the development of all manifestations of cardiovascular disease (CVD), and recent data suggest that the proportion of CVD attributable to T2DM is increasing [1]. Thus identifying subjects at risk is a key issue in order to prevent the development of the disease. Mounting evidence suggests that early lifestyle interventions are more effective and cost saving for T2DM prevention compared with pharmacologic approaches [2]. A number of prospective cohort studies have reported a negative association between increased coffee consumption and risk of development of T2DM [3, 4]. According to a recent meta-analysis, the incidence of T2DM decreases by 12 % for every 2 cups/day increment in coffee intake, by 11 % for every 2 cups/day increment in decaffeinated coffee, and by 14 % for every 200 mg/day increment in caffeine intake [4]. A protective effect of coffee for T2DM and the metabolic syndrome has been observed also in cross-sectional studies [5–8]. However, in short-term intervention trials caffeine intake or caffeinated coffee have been shown to reduce insulin sensitivity and glucose tolerance [9–11], an effect not seen with decaffeinated coffee [9]. These data cast many doubts on a longitudinal causal link between coffee intake and prevention of diabetes and no information on coffee use has been included by ADA guidelines among the lifestyle measures suitable to the diabetic patient [12]. Impaired fasting glucose is a precursor to type 2 diabetes in most individuals [12], and studying the longitudinal association between coffee consumption and impaired fasting glucose might help clarify this controversial issue. The aim of the present study was to examine the long term effect of coffee drinking on the risk of developing impaired fasting glucose in the participants of the Hypertension and Ambulatory Recording VEnetia Study (HARVEST), a prospective longitudinal study of young subjects screened for stage 1 hypertension [13, 14]. As the clinical effects of coffee drinking may depend on the genetic background of an individual, another purpose of the present analysis was to ascertain the effect of regular coffee intake on fasting glucose within subjects stratified by CYP1A2 genotype, which has been shown to modulate the association of coffee use with cardiovascular outcomes [15, 16].
Methods
Subjects
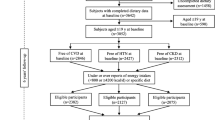
The study was carried out in 1,180 white patients aged from 18 to 45 years who took part in the HARVEST [13, 14]. The study participants were never treated patients screened for stage 1 hypertension. Patients with T2DM, nephropathy, and CVD were excluded. The 639 subjects taking part in the genetic subproject were all those recruited and followed up in the 4 HARVEST centres which agreed to participate in the genetic study (Padova, Vittorio Veneto, San Daniele del Friuli, and Trento) [16]. Their characteristics were similar to those of the rest of the population. In particular, the frequency of coffee drinkers (73.7 %) and abstainers (26.3 %) was exactly the same in the two subgroups. In addition, 17 participants who developed diabetes during the follow-up (14 coffee drinkers and 3 abstainers) were excluded. The higher prevalence of men among our study participants confirmed previous observations of a much higher prevalence of men in the young, stage 1 segment of the hypertensive population [17]. The HARVEST study is conducted in 17 hypertension units in Italy. Patients’ recruitment was obtained with the collaboration of the local general practitioners who were instructed during local meetings. Consecutive patients with the above mentioned clinical characteristics seen in the offices of the general practitioners and willing to participate in the study were eligible for recruitment and were sent to the referral centers. Patient files, blood and urine samples are periodically collected by five monitors and taken to the coordinating office in Padova, where they are processed.
Procedures
The procedures followed were in accordance with institutional guidelines. At baseline, all subjects underwent physical examination, anthropometry, blood chemistry, and urine analysis. Participants completed questionnaires about their medical history, family history of hypertension, physical activity, and dietary habits including coffee intake, alcohol use, and cigarette smoking. Coffee consumption was defined according to the number of caffeine-containing coffees drunk per day. The caffeine content per cup of ‘espresso’ Italian coffee, which was the most abundantly consumed type of coffee by the HARVEST participants, averages 100 mg [18, 19]. Decaffeinated coffee, tea and other caffeinated drinks were not taken into account in the present study, being unusual and irregular in this area of Italy [20]. A positive family history of hypertension was defined as one or two parents having hypertension and/or taking antihypertensive treatment. More details about the interview, life style assessment, and criteria used for subjects’ classification according to life style have been reported elsewhere [13, 14, 16, 19]. Twenty-four-hour ambulatory blood pressure and heart rate monitoring was performed with the A&D TM-2420 model 7 (Tokyo, Japan), or the ICR Spacelabs 90207 (Redmond, WA, USA). Both devices were previously validated [21, 22]. Clinic blood pressure was the mean of six readings obtained during two visits performed 2 weeks apart. Body mass index (BMI) was considered as an index of adiposity (weight divided by height squared). In 631 participants, urine was collected over 24 h for catecholamine assessment. Adequacy of the 24-h urine collections was assessed by self-report of missed or spilled collections as well as by creatinine excretion per kg body weight [19]. Immediately after completion volumes were measured and urine specimens were frozen (−20 °C) and sent to the Coordinating Office at the University of Padova, where epinephrine and norepinephrine were measured by a high-performance liquid chromatography method [16]. The study was approved by the Ethics Committee of the University of Padova, and written informed consent was given by the participants.
Follow up
In the HARVEST study, office blood pressure and life style habits are assessed monthly during the first 3 months of follow up, then after 6 months, and every 6 months thereafter. After baseline examination, subjects are given general information about non pharmacological measures by the HARVEST investigators, following the suggestions of current guidelines on the management of hypertensive patients. To ensure homogeneous counseling by doctors participating in the study, training in current international guidelines was provided to them throughout the study duration. HARVEST participants are followed until they develop sustained hypertension requiring antihypertensive treatment according to the guidelines available at the time. If patients develop sustained hypertension, the investigators perform a final clinical assessment including biochemical tests before treatment is initiated. Subjects who do not meet the criteria for treatment continue to be checked at 6 month intervals. Routine blood tests including glucose were carried out at yearly intervals and at study end. Enrolment of subjects with *1A/*1A genotype versus those with *1A*1F + *1F/*1F genotype and of coffee drinkers versus abstainers was equally distributed throughout the years. During a median follow-up of 6.1 years (interquartile range 2.5–9.9 years), hypertension was developed by 683 out of the 1,180 patients. In the subjects who remained normotensive, the last available clinical assessment was used. For subjects lost to follow-up, the last available glucose values were taken into account. Impaired fasting glucose was defined as a fasting plasma glucose between 100 and 125 mg/dL measured at the final available visit [12]. All data used for the present study were collected from untreated patients.
Genotyping
Genomic DNA was extracted from whole blood through the NucleoSpin® Blood kit (Macherey–Nagel, Düren, Germany). Primers and probes for allelic discrimination analysis of CYP1A2 polymorphism, designed from sequences derived from the GenBank database using Primer 3 (provided by the Whitehead Institute Cambridge, Massachusetts,USA) and Operon’s Oligo software (Operon Technologies Inc., Alameda, California, USA), were as follows: forward primer AGAGAGCCAGCGTTCATGTT, reverse primer CTGATGCGTGTTCTGTGCTT, CYP1A2*1F probe (FAM-labelled)-5′-TCTGTGGGCCCAGGA-3′ (BLACK HOLE1), CYP1A2*1A (TEXAS RED labelled)-5′-TCTGTGGGCACAGGA-3′ (BLACK HOLE2), as described by dbSNP reference number (rs762551) on the National Center for Biotechnology Information (NCBI) Web site [23] and we defined the allele containing the “A” nucleotide as CYP1A2*1A. Purified DNA (2 μL) was amplified in a real-time PCR reaction in the iCycler iQ™ system (BIO-RAD, Hercules, CA). All the reactions were performed in 96-well plates, using the iQ™ Supermix (BIO-RAD, Hercules, CA). Positive controls, genotyped by direct sequencing, were included in each run, together with a negative control containing no DNA template. Taqman reactions were thermocycled as follows: 95 °C for 3 min to denature, 40 cycles at 95 °C for 30 s for denaturing and 60 °C for 1 min for annealing and extension.
Data analysis
The present analysis was performed in the 1,180 subjects for whom biochemical data and information on lifestyle habits were available both at baseline and final assessments and who had at least 6 months of follow-up. Subjects were grouped according to their habitual consumption of coffee into three categories of coffee drinking, non drinkers (none), moderate drinkers (1–3 cups daily) and heavy drinkers (4 or more cups daily), a classification used in previous analyses [14, 16, 19]. Subjects were divided into four categories of alcohol use (0, <50, 50–100, >100 g of alcohol/day) [20]. As there were only seven subjects in the >100 g/day alcohol class, the two upper classes of alcohol were subsequently analyzed together. Smokers were classified into 4 categories according to the daily number of cigarettes smoked: non smokers, 1–5, 6–10, >10 cigarettes/day. The distribution of clinical variables was compared across classes of coffee consumption by analysis of covariance adjusting for age and sex. The significance of differences in categorical variables was assessed with the Chi square test. The cumulative incidence of impaired fasting glucose associated with coffee consumption at baseline was calculated using Kaplan–Meier analysis. The difference in impaired fasting glucose incidence between coffee drinking levels was tested using the log-rank test. Coffee intake was also modeled as a time-dependent categorical variable in Cox proportional hazards analysis. No violations to the proportional hazards assumption were detected by inspection of survival curves. Multivariate Cox proportional hazards models were developed to adjust for possible confounding variables. The variables found to be associated with outcome at univariable survival analysis and/or believed to be of prognostic importance were sex, age, BMI, parental hypertension, physical activity, coffee intake, smoking status, alcohol consumption, baseline plasma glucose, ambulatory blood pressure and ambulatory heart rate. Another explanatory variable included in the models was the change in body weight at the end of the follow-up. In the subgroup with genetic data, the risk of impaired fasting glucose related to coffee intake was also assessed within each CYP1A2 group, adjusting for the same variables as above. Estimates of relative risk and corresponding two-sided 95 % confidence intervals (CIs) relating coffee consumption to risk of impaired fasting glucose were computed from the Cox models. To make sure about the robustness of our results, data were also included in a multivariable logistic model using the same variables as in the Cox model. A two-tailed probability value <0.05 was considered significant. Data are presented as mean ± SD unless specified. For statistics, epinephrine and norepinephrine were logarithmically (base 10) transformed owing to their skewed distribution. Analyses were performed using Statistica version 6 (Stat Soft, Inc., Tulsa, OK, USA), Systat version 11 (SPSS Inc., Evanston, IL, USA), and MedCalc version 12.5.0.0 (MedCalc Software, Ostend, Belgium).
Results
Seventy-four percent of our subjects drank coffee (Table 1). Among the coffee drinkers, 87 % drank 1–3 cups/day, and 13 % drank over 3 cups/day. Compared to abstainers, coffee drinkers were older, heavier, had worse lifestyle habits and had a higher level of urinary epinephrine. No between-group differences were found for sex, clinic or ambulatory blood pressure, biochemical data, and urinary norepinephrine. Among the subjects with CYP1A2 polymorphism data, genotype frequencies (*1A*1A = 41.9 %, *1A*1F = 43.7 %, *1F*1F = 14.4 %) were in agreement with the Hardy–Weinberg equilibrium (χ 2 = 0.80, p = 0.37). The proportion of genotypes did not differ between the categories of coffee intake (p = 0.47).
Follow-up
During the follow-up only 45 subjects (3.8 %) changed their coffee habits. Of these, 21 subjects started to drink coffee or increased coffee consumption, whereas 24 subjects quitted or reduced coffee use. For these 45 participants we also calculated average coffee intake. At the end of follow-up, 283 subjects (24.0 %) had impaired fasting glucose. Participants with impaired fasting glucose were older and more frequently male, had higher BMI and systolic blood pressure, had a worse metabolic profile and higher urinary epinephrine than those who were normoglycemic (Table 2). A higher follow-up increase in plasma glucose but not in body weight was found in the former. Development of sustained hypertension needing treatment was more common among the subjects with impaired fasting glucose at study end than among those with normal glucose (70.0 vs. 57.0 %, p < 0.001).
Association of coffee intake with risk of impaired fasting glucose
At follow-up end, impaired fasting glucose was more common among coffee drinkers than abstainers (abstainers, 18.7 %; moderate drinkers, 25.0 %; heavy drinkers, 31.6 %; p for trend = 0.003). Kaplan–Meier analysis confirmed that impaired fasting glucose was developed more frequently by coffee drinkers compared with abstainers (Fig. 1, log-rank p < 0.0001). In an age-and-sex-adjusted Cox analysis, the association between impaired fasting glucose and coffee intake remained significant in both classes of coffee drinking (Table 3). As several subjects had increased plasma glucose already at baseline, data were further adjusted for baseline glucose + parental hypertension, physical activity, smoking status, and alcohol intake, which caused only a slight attenuation of the coffee-impaired fasting glucose relationship among the moderate drinkers (Table 3). After inclusion of BMI, and ambulatory blood pressure and heart rate at baseline, the association of coffee drinking with impaired fasting glucose remained statistically significant only in the heavy drinkers. Inclusion of change in body weight during the follow-up did not materially change these findings (Table 3). Inclusion of average coffee intake instead of baseline coffee intake in the regression caused a decline of the overall p value from 0.0005 to 0.0025. When the multivariable analysis (model 5) was repeated after excluding the subjects who had impaired fasting glucose at baseline, the longitudinal association of coffee use with impaired fasting glucose was still significant for the heavy drinkers (p = 0.0029) and was non significant for the moderate drinkers (p = 0.26). Similar results were found when the data were included in a logistic regression. Also in a fully adjusted logistic model coffee was an independent predictor of future impaired fasting glucose though to a lower level of statistical significance. The odds ratios (95 % CI) were 1.77 (1.02–3.08) for heavy coffee drinkers, and 1.27 (0.87–1.86) for moderate drinkers. In this model time was a strong predictor of impaired fasting glucose (p < 0.001).
Subgroup with genetic data
In this subgroup (n = 639), impaired fasting glucose at study end was detected in 160 participants (25.0 %). In Fig. 2, the curves for risk of impaired fasting glucose according to coffee consumption in the participants stratified by CYP1A2 genotype are shown. After taking into account age, sex, baseline glucose, parental hypertension, physical activity, smoking status, alcohol intake, BMI, and ambulatory blood pressure and heart rate at baseline, the risk of impaired fasting glucose associated with coffee intake among carriers of the slow *1F allele was 1.42 (CI 0.91–2.22, p = 0.13) for moderate drinkers and was 2.78 (1.32–5.88, p = 0.0076) for heavy drinkers. Among the participants who were homozygous for the rapid *1A allele, the corresponding HRs were 1.33 (CI 0.67–2.67, p = 0.42) and 1.71 (0.76–3.84, p = 0.20), respectively.
Discussion
The present results show that among a sample of young-to-middle-age adults screened for stage 1 hypertension coffee consumption had independent prospective linear association with impaired fasting glucose. Coffee use remained a significant predictor of impaired fasting glucose also when many other clinical variables including baseline plasma glucose and body weight changes during the follow-up were taken into account and when subjects with impaired fasting glucose at baseline were excluded. Another important feature of the present study is that the association between coffee intake and impaired fasting glucose was stronger in the carriers of the *1F variant of the CYP1A2 gene especially for the heavy coffee drinkers. To our knowledge, this is the first prospective study that shows a longitudinal relationship of baseline coffee drinking with risk of prediabetes in hypertension.
Previous studies
These observations are seemingly contradictory to the findings suggesting a reduced incidence of T2DM with habitual coffee consumption. Indeed, a large body of evidence has shown that higher habitual coffee intake is associated with higher insulin sensitivity and decreased rate of T2DM in both cross-sectional [5–8] and longitudinal studies [3, 4]. Interestingly, in longitudinal studies similar associations with risk of T2DM have been found for caffeinated coffee, decaffeinated coffee, and caffeine [4]. However, short-term interventional studies do not confirm evidence from observational studies showing that caffeine intake can acutely lower insulin sensitivity [24, 25] and increase glucose concentrations in both diabetic and non diabetic individuals [26–28]. An unfavorable effect of caffeinated coffee and caffeine on glucose metabolism has been shown also after several days of coffee consumption. Van Dam et al. [10] found that high coffee consumption for 4 weeks increased fasting insulin concentrations compared with coffee abstinence. In addition, in clinical studies caffeinated coffee showed less pronounced adverse effects on glucose tolerance and insulin sensitivity than pure caffeine, and decaffeinated coffee even exerted a beneficial effect on glucose metabolism indicating divergent effects of caffeine and non-caffeine components of coffee on glucose management [29, 30]. The conflicting results obtained in epidemiological studies and clinical research have been attributed to the tolerance to caffeine’s action that may be developed following chronic consumption. However, according to a recent meta-analysis the negative association between coffee and risk of T2DM was stronger in studies with shorter follow-up duration [4]. As mentioned above, another possible explanation is that non-caffeine coffee components may reduce or antagonize the adverse effects of caffeine on glucose metabolism. Coffee is a complex ‘blend’ of a vast number of different bioactive chemicals, any of which may have different effects on glucose management [31]. Among these, the polyphenols chlorogenic acid and dihydrocaffeic acid (both found in coffee) have been shown to have an antagonistic effect on glucose transport in the intestine thereby eliciting a positive effect on glucose homeostasis [32]. In rats with diet-induced whole-body insulin resistance, Shearer et al. [33] found that decaffeinated coffee improved insulin-stimulated whole body glucose disposal during a hyperinsulinemic–euglycemic clamp and that this effect was abolished in the animals that received decaffeinated coffee with caffeine. If caffeine, caffeinated coffee, and decaffeinated coffee elicit differential metabolic effects, it is difficult to explain why decaffeinated coffee and caffeinated beverages had similar protective effects on the incidence of T2DM in longitudinal epidemiologic studies [4]. The divergent results of the present and previous reports may be due to a different metabolic response to coffee of hypertensive patients compared to normotensive subjects. Another possible explanation is that the coffee–T2DM association found in observational studies is factitious rather than causal being due to dietary restrictions in patients at high risk of diabetes who later developed overt T2DM. Patients with metabolic abnormalities are recommended to avoid consumption of sugar containing beverages which may include coffee and caffeinated beverages. This was less likely to occur in our study that focused on impaired fasting glucose rather than on T2DM.
Genetic subgroup analysis
The adverse effect of caffeinated coffee on glucose level observed in the present study was confirmed by the results obtained in the subgroup with genetic data. CYP1A2 polymorphism has important influences on caffeine metabolism [34–36]. Individuals homozygous for the *1A/*1A genotype are fast caffeine metabolizers, and in these subjects the adverse effects of coffee on glucose metabolism may be attenuated by the relatively higher concentration of non-caffeine components. Carriers of the *1F allele are slow metabolizers and are therefore more exposed to the effects of caffeine on glucose metabolism. Indeed, among our heavy coffee drinkers, carriers of the *1F allele had a 178 % increase in risk of impaired fasting glucose compared to a 71 % increase in homozygous for the *1A allele.
Pathophysiologic mechanisms
Caffeine may elevate glucose level by increasing the concentrations of gluco-regulatory hormones. In the present study we found a linear relationship between coffee consumption and urinary epinephrine. Caffeine stimulates the release of epinephrine, which exerts actions opposite to that of insulin via adrenergic stimulation [37]. In the presence of a β-adrenergic receptor antagonist, the insulin antagonistic effects of caffeine are abolished, suggesting that the negative effects of caffeine on insulin sensitivity are mediated via epinephrine secretion [38]. Another reason for the detrimental effect of coffee on glucose management may be the adenosine receptor antagonism induced by caffeine [39].
Methodological issues
In the present article, mainly data from Cox models were presented because according to most authors Cox analysis is superior to logistic analysis for longitudinal data especially when the observation time length differs considerably among participants [40, 41]. In the HARVEST study protocol the end-point is hypertension and not impaired fasting glucose. However, by using the actual time at which impaired fasting glucose was identified we satisfied the Cox proportional hazards model assumption and in this condition the Cox approach is usually preferable to other approaches since it generally provides estimates that are more robust [40–42]. However, to make sure that our results about the relationship of coffee intake with impaired fasting glucose were robust, we also included these variables in a multivariable logistic regression obtaining consistent results. It should be noted that in the logistic model time was a strong predictor of impaired fasting glucose (p < 0.001) supporting the concept that time to event was a crucial parameter for establishing the likelihood of developing impaired fasting glucose.
Study limitations
In the present study, misclassification of coffee consumption could be of concern, because some participants might change their habits during follow-up. However, in agreement with previous reports our data showed that coffee use was a constant and well-reported habit [43]. Another possible limitation is that results obtained with Italian coffee may not apply to other types of coffee. Similar results were found for filtered and instant coffee In the Nurses’ Health Study II [44] and for boiled and other types of coffee in the Norway health survey [45]. However, in the E3N/EPIC study filtered coffee was found to be associated with a reduced risk of T2DM whereas this was not true for instant coffee [46]. Finally, coffee consumption is often associated with unhealthy lifestyle and dietary habits. In the present study, we adjusted for baseline BMI, body weight changes and lifestyle habits which were actually poorer among the coffee drinkers. However, our data were not adjusted for the possible confounding effect of other dietary habits such as saturated fat intake or lower fiber intake which may have influenced the association of coffee with impaired fasting glucose.
Conclusions
The present results suggest that in hypertension caffeinated coffee should be considered a dietary risk factor for impaired fasting glucose especially in carriers of the CYP1A2 slow *1F allele. Our findings contradict previous epidemiologic studies that have advocated high coffee consumption as a means to lower risk for T2DM. Long-term interventional studies are needed before implementing guidelines regarding coffee consumption for hypertensive patients at risk of diabetes.
References
Fox CS, Coady S, Sorlie PD, D’Agostino RB Sr, Pencina MJ, Vasan RS, et al. Increasing cardiovascular disease burden due to diabetes mellitus: the Framingham Heart Study. Circulation. 2007;115:1544–50.
Tuomilehto J, Eriksson J. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50.
Huxley R, Lee CM, Barzi F, Timmermeister L, Czernichow S, Perkovic V, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–63.
Jiang X, Zhang D, Jiang W. Coffee and caffeine intake and incidence of type 2 diabetes mellitus: a meta-analysis of prospective studies. Eur J Nutr. 2014;53:25–38.
van Dam RM, Dekker JM, Nijpels G, Stehouwer CD, Bouter LM, Heine RJ. Coffee consumption and incidence of impaired fasting glucose, impaired glucose tolerance, and type 2 diabetes: the Hoorn Study. Diabetologia. 2004;47:2152–9.
Agardh EE, Carlsson S, Ahlbom A, Efendic S, Grill V, Hammar N, et al. Coffee consumption, type 2 diabetes and impaired glucose tolerance in Swedish men and women. J Intern Med. 2004;255:645–52.
Yamaji T, Mizoue T, Tabata S, Ogawa S, Yamaguchi K, Shimizu E, et al. Coffee consumption and glucose tolerance status in middle-aged Japanese men. Diabetologia. 2004;47:2145–51.
Rebello SA, Chen CH, Naidoo N, Xu W, Lee J, Chia KS, et al. Coffee and tea consumption in relation to inflammation and basal glucose metabolism in a multi-ethnic Asian population: a cross-sectional study. Nutr J. 2011;10:61–70.
Moisey LL, Kacker S, Bickerton AC, Robinson LE, Graham TE. Caffeinated coffee consumption impairs blood glucose homeostasis in response to high and low glycemic index meals in healthy men. Am J Clin Nutr. 2008;87:1254–61.
van Dam RM, Pasman WJ, Verhoef P. Effects of coffee consumption on fasting blood glucose and insulin concentrations: randomized controlled trials in healthy volunteers. Diabetes Care. 2004;27:2990–2.
Gavrieli A, Fragopoulou E, Mantzoros CS, Yannakoulia M. Gender and body mass index modify the effect of increasing amounts of caffeinated coffee on postprandial glucose and insulin concentrations; a randomized, controlled, clinical trial. Metabolism. 2013;62:1099–106.
American Diabetes Association. Executive summary: standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S4–10.
Sartori M, Semplicini A, Siffert W, Mormino P, Mazzer A, Pegoraro F, et al. G-protein beta3-subunit gene 825T allele and hypertension: a longitudinal study in young grade I hypertensives. Hypertension. 2003;42:909–14.
Palatini P, Dorigatti F, Santonastaso M, Cozzio S, Biasion T, Garavelli G, et al. Association between coffee consumption and risk of hypertension. Ann Med. 2007;39:545–53.
Cornelis MC, El-Sohemy A, Kabagambe EK, Campos H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA. 2006;295:1135–41.
Palatini P, Ceolotto G, Ragazzo F, Dorigatti F, Saladini F, Papparella I, et al. CYP1A2 genotype modifies the association between coffee intake and the risk of hypertension. J Hypertens. 2009;27:1594–601.
Wang Y, Wang QJ. The prevalence of prehypertension and hypertension among US adults according to the new joint national committee guidelines: new challenges of the old problem. Arch Intern Med. 2004;164:2126–34.
Casiglia E, Paleari CD, Petucco S, Bongiovì S, Colangeli G, Baccilieri MS, et al. Haemodynamic effects of coffee and purified caffeine in normal volunteers: a placebo-controlled clinical study. J Hum Hypertens. 1992;6:95–9.
Palatini P, Canali C, Graniero GR, Rossi GP, De Toni R, Santonastaso M, et al. Relationship of plasma renin activity with caffeine intake and physical training in mild hypertensive men. Eur J Epidemiol. 1996;12:485–91.
Winnicki M, Bonso E, Dorigatti F, Longo D, Zaetta V, Mattarei M, et al. Effect of body weight loss on blood pressure after 6 years of follow-up in stage 1 hypertension. Am J Hypertens. 2006;19:1103–9.
Palatini P, Penzo M, Canali C, Pessina AC. Validation of the A&D TM-2420 model 7 for ambulatory blood pressure monitoring and effect of microphone replacement on its performance. J Ambul Monit. 1991;4:281–8.
O’Brien E, Mee F, Atkins N. O’ Malley K. Accuracy of the Spacelabs 90207 determined by the British Hypertension Society protocol. J Hypertens. 1991;9:573–5.
National Center for Biotechnology Information. dbSNP Home Page. http://www.ncbi.nlm.nih.gov/SNP/index.html. Accessibility verified on Feb 04, 2014.
Keijzers GB, De Galan BE, Tack CJ, Smits P. Caffeine can decrease insulin sensitivity in humans. Diabetes Care. 2002;25:364–9.
Thong FS, Derave W, Kiens B, Graham TE, Ursø B, Wojtaszewski JF, et al. Caffeine-induced impairment of insulin action but not insulin signaling in human skeletal muscle is reduced by exercise. Diabetes. 2002;51:583–90.
Pizziol A, Tikhonoff V, Paleari CD, Russo E, Mazza A, Ginocchio G, et al. Effects of caffeine on glucose tolerance: a placebo controlled study. Eur J Clin Nutr. 1998;52:846–9.
Mougios V, Ring S, Petridou A, Nikolaidis MG. Duration of coffee- and exercise-induced changes in the fatty acid profile of human serum. J Appl Physiol. 2003;94:476–84.
Lane JD, Barkauskas CE, Surwit RS, Feinglos MN. Caffeine impairs glucose metabolism in type 2 diabetes. Diabetes Care. 2004;27:2047–8.
Battram DS, Arthur R, Weekes A, Graham TE. The glucose intolerance induced by caffeinated coffee ingestion is less pronounced than that due to alkaloid caffeine in men. J Nutr. 2006;136:1276–80.
Moisey LL, Robinson LE, Graham TE. Consumption of caffeinated coffee and a high carbohydrate meal affectspostprandial metabolism of a subsequent oral glucose tolerance test in young, healthy males. Br J Nutr. 2010;103:833–41.
Bonita JS, Mandarano M, Shuta D, Vinson J. Coffee and cardiovascular disease: in vitro, cellular, animal, and human studies. Pharmacol Res. 2007;55:187–98.
Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr. 2003;78:728–33.
Shearer J, Farah A, de Paulis T, Bracy DP, Pencek RR, Graham TE, et al. Quinides of roasted coffee enhance insulin action in conscious rats. J Nutr. 2003;133:3529–32.
Gu L, Gonzalez FJ, Kalow W, Tang BK. Biotransformation of caffeine, paraxanthine, theobromine and theophylline by cDNA-expressed human CYP1A2 and CYP2E1. Pharmacogenetics. 1992;2:73–7.
Sachse C, Brockmoller J, Bauer S, Roots I. Functional significance of a C to A polymorphism in intron 1 of the cytochrome P450 1A2 (CYP1A2) gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–9.
Han XM, Ou-Yang DS, Lu PX, Jiang CH, Shu Y, Chen XP, et al. Plasma caffeine metabolite ratio (17X/137X) in vivo associated with G-2964A and C734 polymorphisms of human CYP1A2. Pharmacogenetics. 2001;11:429–35.
Avogaro A, Toffolo G, Valerio A, Cobelli C. Epinephrine exerts opposite effects on peripheral glucose disposal and glucose-stimulated insulin secretion: a stable label intravenous glucose tolerance test minimal model study. Diabetes. 1996;45:1373–8.
Thong FS, Graham TE. Caffeine-induced impairment of glucose tolerance is abolished by beta-adrenergic receptor blockade in humans. J Appl Physiol. 2002;92:2347–52.
Fredholm BB. Astra Award Lecture: adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76:93–101.
Green MS, Symons MJA. Comparison of the logistic risk function and the proportional hazards model in prospective epidemiologic studies. J Chronic Dis. 1983;36:715–24.
Peduzzi P, Holford T, Detre K, Chan YK. Comparison of the logistic and Cox regression models when outcome is determined in all patients after a fixed period of time. J Chronic Dis. 1987;40:761–7.
Annesi I, Moreau T, Lellouch J. Efficiency of the logistic regression and Cox proportional hazards models in longitudinal studies. Stat Med. 1989;8:1515–21.
van Dam RM, Feskens EJ. Coffee consumption and risk of type 2 diabetes mellitus. Lancet. 2002;9344:1477–8.
van Dam RM, Willett WC, Manson JE, Hu FB. Coffee, caffeine, and risk of type 2 diabetes: a prospective cohort study in younger and middle-aged US women. Diabetes Care. 2006;2:398–403.
Hjellvik V, Tverdal A, Strom H. Boiled coffee intake and subsequent risk for type 2 diabetes. Epidemiology. 2011;3:418–21.
Sartorelli DS, Fagherazzi G, Balkau B, Touillaud MS, Boutron-Ruault MC, de Lauzon-Guillain B, et al. Differential effects of coffee on the risk of type 2 diabetes according to meal consumption in a French cohort of women: the E3N/EPIC cohort study. Am J Clin Nutr. 2010;4:1002–12.
Acknowledgments
The study was funded by the University of Padova, Padova, Italy, and by the Associazione “18 Maggio 1370”, San Daniele del Friuli, Italy.
Conflict of interest
The authors have no financial interest in the subject matter or materials discussed in the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palatini, P., Benetti, E., Mos, L. et al. Association of coffee consumption and CYP1A2 polymorphism with risk of impaired fasting glucose in hypertensive patients. Eur J Epidemiol 30, 209–217 (2015). https://doi.org/10.1007/s10654-015-9990-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-015-9990-z