Abstract
Dye wastewater possess immense toxicity with carcinogenic properties and they persist in environment owing to their stability and resistance to chemical and photochemical changes. The bio degradability of dye-contaminated wastewater is low due to its complex molecular structure. Nano-photocatalysts based on zinc oxide are reported as one of the effective metal oxides for dye remediation due to their photostability, enhanced UV and visible absorption capabilities in an affordable manner. An electron–hole pair forms when electrons in the valence band of ZnO nano-photocatalyst transfer into the conduction band by absorbing UV light. The review article presents a detailed review on ZnO applications for treating acidic and basic dyes along with the dye degradation performance based on operating conditions and photocatalytic kinetic models. Several acidic and basic dyes have been shown to degrade efficiently using ZnO and its nanocomposites. Higher removal percentages for crystal violet was reported at pH 12 by ZnO/Graphene oxide catalyst under 400 nm UV light, whereas acidic dye Rhodamine B at a pH of 5.8 was degraded to 100% by pristine ZnO. The mechanism of action of ZnO nanocatalysts in degrading the dye contamination are reported and the research gaps to make these agents in environmental remediation on real time operations are discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The expansion of textile and dye industries is in faster pace attributed by rapid development of industries and urban infrastructure in recent decades. Organic dyes and azo dyes are more prevalent in these industries but with usage of highly toxic chemicals particularly in textile manufacturing (Ahmadi & Ganjidoust, 2021; Mohammadzadeh et al., 2015; Parthipan et al., 2021; Satdeve et al., 2019; Sekaran et al., 2022; Vidya et al., 2017; Xie et al., 2010). They are applied in different processes, especially polyvinyl chloride for sizing clothes, chlorine bleach for bleaching fabrics, benzedrine and toluidine for coloring (Bai et al., 2022; Ong et al., 2018; Singhal et al., 2022; Weldegebrieal, 2020; Zarrabi et al., 2022).Therefore, dyeing and finishing chemicals represent 20% of worldwide water contamination (Le et al., 2022; Moslehnejad et al., 2022; Oskoei et al., 2016; Sathishkumar et al., 2011). Textile industries release around 1 million tons of dyes into the environment every year. It is possible for sensitive individuals to develop skin irritations, allergic reactions, or dermatitis as a result of direct contact with acid dyes. Inhalation of dust or fumes released by acid dyes, especially in poorly ventilated areas, asthmatic symptoms, coughing, shortness of breath and respiratory distress can result (Chung, 2016; Soares et al., 2015). They also bioaccumulate in living cells and disrupts homeostasis. Reduced azo dyes produce highly mutagenic aromatic compounds, even more than their original counterparts. Since water loses its transparency due to textile effluent, it induces turbidity and prevents sunlight from penetrating, which in turn inhibits the photosynthesis process of many aquatic species. Air–water networks are disrupted, which leads to oxygen transfer failure (Karri et al., 2021). The most extreme consequence of textile waste is the depletion of dissolved oxygen (DO) in the water, which is crucial for aquatic life. Water cannot also self-purify due to this. It clogs soil pores and results in soil productivity loss. Moreover, when such effluents are allowed to circulate in the fields, root penetration is inhibited as soil texture is strengthened. Drinking water quality in groundwater is negatively affected by this wastewater, which flows in drainage systems and rivers (Sharma et al., 2021; Tkaczyk et al., 2020). The direct dyes used in textile processing are acid, basic, vat and mordant substances. Those substances are utilized to color textiles, paper, leather, and other chemical production materials (Bai et al., 2022; Khataee et al., 2016; Mohammadzadeh et al., 2015; Ong et al., 2016). Acid dyes are mainly used to color protein fibers and nylon because they have almost equal numbers of amino and carboxyl groups (H2N-W-COOH) like wool, silk, and polyamide fibers. In addition, acidic dyes contain sulphonic and carboxylic acids, which are used to color various textile materials. The available forms of acid dyes are red-4092, acid orange-7, acid blue-113, and acid yellow-3 dyes. (Mohamed et al., 2019b; Sekaran et al., 2022; Siddiqui et al., 2021) A basic dye is a cationic water soluble dye used mainly on fabrics, while an acid dye is a hydrophilic anionic dye used primarily on fabrics. They mainly contain a dialkylamino group that imparts capability for coloring polyester fabrics. Common examples of basic dyes are: Methyl Orange, Methylene Blue and Crystal Violet (Habibollahi et al., 2022; Kumar et al., 2021; Mohamed et al., 2019b; Shubha et al., 2022; Zarrabi et al., 2019).
Earlier research focused on dye removal, elimination and degradation processes using various removal methods have been superseded by the invention of nanoscience since 20 years with innovative approaches in material synthesis and operations pertaining to dye removal. In addition to electronic applications, metal oxide nanoparticles have also been used in biomedical applications, biohydrogen/bioethanol production (Bosu et al., 2022a), heavy metal and toxic contaminant remediation from wastewater effluents (Bosu & Rajamohan, 2022; Mano et al., 2015; Rajamohan et al., 2022b). It has been widely reported that nanomaterial activates oxidation\reduction reactions which support the elimination of dyes from sewage. However, it is critical to choose a suitable nano-photocatalyst for the degradation process (Giannakis et al., 2017; Wu et al., 2006). Nevertheless, considering the nature of conductor materials due to their free electrons they only undergo oxidation reactions. Insulators overall are ineffective because only one reduction reaction occurs within the process. On the other hand, semiconductors can perform both oxidation and reduction reactions. As a result, it’s fair to say that semiconductors are the most efficient materials to use (Ahsaine et al., 2018). The most salient features of nano photo catalysts over bulk materials is the surface area-to-volume ratio, which enhances reactivity, improves light absorption, facilitates electron and hole separation and migration, prevents their recombination and increases photocatalytic efficiency (Rajamohan et al., 2022a). The shape, size, and crystal facets of nanomaterials can influence their photocatalytic activity exponentially. Enhanced durability and longer lifespan of catalysts can be achieved by using nanostructured materials in place of bulk materials that can agglomerate, degrade, or leach (Bosu et al., 2022b; Santosh et al., 2016; Yaqoob et al., 2020). Natural dyes degradation methods mainly depend on natural absorbents such as orange peel, banana peel, grapefruit peel, etc. as these organic materials contain carbon-rich compounds such as cellulose and hemicellulose (Khezrianjoo et al., 2019; Qi et al., 2022; Zarrabi et al., 2018) Ahmadi & Ganjidoust, 2021). The degradation of natural dyes using organic fruit peels has been reported in several published articles. A study claimed that banana peel is one of the effective natural organic materials to synthesize nanoparticles for dye degradation, which can absorb upto 40% of the total weight of toxic dyes. The standard industrial techniques are chemical degradation, chemical adsorption, and nano-filtration (Habibollahi et al., 2022; Heidari-Asil et al., 2022; Khataee et al., 2015). In the Nano photocatalytic waste treatment process, nanoparticles can absorb energy and use it to degrade or break down a broad spectrum of organic pollutants. Nano-photo-catalysts refer to a small semiconductor particle with atleast one dimension of a few nanometers used for active chemical reactions such as oxidation and reduction processes, and for degrading dyes using light energy. Nano-photocatalysis can be accomplished using organic, inorganic, and carbon-based nanoparticles (Ahmad et al., 2022; Hammad & Abdel-wahab, 2022; Tai et al., 2020). Metal oxide nanomaterials are inorganic substances that can be used on the dye surfaces to initiate the oxidation and reduction reactions. The commonly known metals oxides with such properties are titanium oxide (TiO2), zinc oxide (ZnO), and iron II oxide (Fe2O3). Metal oxidation reaction occurs where OH− is produced which is used to remove the acidic and basic dyes in simultaneous reduction reactions (Subbulakshmi et al., 2023; Elsabahy & Wooley, 2012; Li et al., 2013; Bai et al., 2014; C Thomas et al., 2015; Roy et al., 2015; Zhang et al., 2017). The suitability of metal oxides is based on several processing conditions, such as the photo-activity, light source stability, process cost, and environmental and human safety during the degradation process. Regarding this consciousness, zinc oxide and titanium oxide are reported as the most common nanomaterials for application in dye degradation. However, previous studies claim that zinc oxide is more effective than titanium oxide in terms of economy, non-toxicity and efficiency in dye absorption on a wider range of application (Junlabhut et al., 2014; Lee et al., 2016; Moma & Baloyi, 2019; Qiu et al., 2011; Satdeve et al., 2019).
A lack of specific guidelines for chemical dye degradation caused previous researchers to use individual dye degradation methods, leading to unclear investigation setups to determine criteria for acid and basic dye breakdown. Additionally, most of the previous studies failed to demonstrate the efficiency of various primary and acidic dye degradation techniques (Heidari-Asil et al., 2022; Ong et al., 2018; Sudarshan et al., 2023). Therefore, a comprehensive review of the use of acids and basic dyes degradation techniques is presented so as to enable the readers to identify the applicability of ZnO based nanoparticles and the challenges in real-time applications in textile industries. In this study, an elaborate review on the degradation techniques of chemical dyes using ZnO photocatalysts is presented. The detailed discussion focusing on the removal performances, operating conditions and mechanisms is presented.
Classification of nano photocatalyst
Classifying the metal oxide nano catalysts aid in comparing their performance and mechanism of actions in degrading the dye complex. Figure 1 illustrates various types of nanophotocatalysts: organic nanoparticles, inorganic nanoparticles and carbon-based nanoparticles. A polymeric nanoparticle can either be classified as a nanosphere or a nanocapsule and lipid nanoparticles can be divided into lipids or polymeric nanocatalysts (Elsabahy & Wooley, 2012; Thool et al., 2014). Inorganic compounds include metal oxides, metal nanoparticles, and ceramic nanoparticles. When oxides of metals such as ZnO and CuO are used to remove dyes from wastewater, the removal efficacy of dye increases significantly. Metal oxide-based nanomaterials exhibit wide band gap semiconductor properties due to their geometrical, crystallographic, and morphological characteristics. Moreover, they exhibit excellent hydrophilic stability and toxic-free properties making metal oxides great photocatalytic materials that are suitable for various energy and environmental fields (Eskizeybek et al., 2012; Kadi et al., 2016; Shen et al., 2018). A pure metal such as nickel or gold is not as efficient and stable as silicon due to its band gap and inability to absorb light (Giannakis et al., 2017; Roy et al., 2015). Similarly, ceramic nanoparticles are fabricated by employing oxides, phosphates, and carbonates, which are generally classified as inorganic compounds (Thomas et al., 2015). Likewise, a carbon-based nanoparticle consists of a carbon nanotube, a carbon fullerene, or a graphene sheet and the graphene sheets are rolled up in a tubular structure to form carbon nanotubes (Li et al., 2013; Zhang et al., 2017).
Degradation of basic dyes by ZnO nano-photocatalysis
There is a wide use of cationic dyes in textile industries that are soluble in water. This section focusses on the degradation of basic dyes using ZnO based nanophotocatalysts under various operating conditions with special emphasis on the efficiency and mechanism of action of various nanophotocatalysts.
Research was conducted on the preparation of (CoZn)–Metal–Organic Framework (MOF)-nanoparticles (NPs) and their calcination in air with sunlight to produce bi-oxide metal peaks with higher energies, showing electron interaction Al2O3 ZnO TiO2 and transmission between ZnO and Co3O4. The study investigated the impact of operating conditions, specifically pH, dye concentration, and temperature. The dye removal efficiency decreased from 52% when the starting concentration of CoZn was 30 (mg/L). Kinetic experiments revelated that the best fit model is Pseudo-1st order kinetics. The residual amount of methyl orange was used to express the intensity variations at 464 nm absorbance, which was used to determine the photocatalytic efficiency of the samples. Researchers claimed the production of Co3O4-ZnO spectra for the first time, showing the bending of the imidazole rings in the ZnO crystal structure between 1139 and 998 cm (Tran et al., 2021).
On kapok fiber paper, methyl orange dye removal was investigated using a hybrid nanocomposite made of zinc oxide (ZnO) and polyaniline (PANI) under the influence of pH (2.0–6.0) and initial dye concentration (0.6–25 mg/L) conditions. As the number of PANI deposition cycles increased, methyl orange removal efficiency increased as well. With a thicker PANI sheet, there might be more methyl orange adsorption sites available. However, the removal efficiency did not significantly change even after 50 cycles. The removal efficiency decreased from 97.33 to 45.84% when the initial concentration of dye was 25 mg/L. The degradation mechanism was reported as pseudo-2nd order model (kinetics) and Freundlich model (isotherm). The breakdown of the methyl orange dye was 10 percent more effective during the adsorption test under the influence of UV light. Furthermore, FT-IR examination of the adsorption mechanism demonstrated that it was purely the outcome of chemical interactions inside the atomic structure of the nanocomposite (Lacuesta et al., 2018).
In a study, water-revolving coat was employed to maintain a constant temperature in a Pyrex light-based reactor aimed with acid dye treatment using ZnO catalyst. The study investigated the influence of operating conditions, specifically pH, dye concentration, and temperature. First-order kinetic model fitted the experimental values and the equilibrium studies were discovered to fit the Langmuir isotherm. A strong synergy between UV photons and ZnO nanophotocatalyst was observed for the dye remediation. The methyl orange dye photodegradation used ZnO nanoparticles as a photocatalyst and an incident photon with a wavelength of 390 nm in a ZnO photocatalyst initiated a bound electron–hole pair and excited the valence electrons (Kumar et al., 2015).
An investigation utilized ZSM-5/ZnO/Ag nanoparticles to develop a photocatalyst for the degradation of methyl orange present in aqueous solutions and to increase the photocatalytic efficiency of the nanocomposite. The maximum dye removal (90%) was reported in basic conditions at a pH of 11 with the initial concentration of 100 mg/l. Under these conditions the corresponding dye uptake was found as 20 mg/g. Kinetic experiments were conducted and the best fit model was reported as the pseudo 2nd order. The result indicated that light being absorbed by dye molecules in large quantities rather than photocatalyst particles lessened the catalytic reaction (Vaez & Javanbakht, 2020).
Production and surface analysis of Co − ZnO were conducted to the test the photocatalytic properties for breaking down methyl orange in the presence of visible light. With an initial dye concentration of 100 mg/l and a pH of 10, highest dye removal of 93% was recorded. Under these optimal conditions, the maximum dye uptake was found as 0.05 g /50 mg. After conducting kinetic experiments, the pseudo-second order was determined as the best-fitting model. As the concentration of MO was raised, the photocatalytic activity of 10% Co-ZnO dropped. Due to dye molecule absorption at increasing dye concentrations, photons were unable to reach the catalyst's surface. In the case of cobalt-doped zinc oxide composite, the calculated band gap is 2.16 eV, compared to 3.37 eV for zinc oxide. Due to this, the photoreduction of biotoxins can be catalyzed by Co-ZnO when exposed to visible light (Adeel et al., 2021).
Co-precipitation method was used to prepare Ag-ZnO nanocomposites for dye degradation in varied pH, dye concentration, and temperature conditions. With an initial dye concentration of 35 mg/l, pH 6.0 was observed to have the highest dye removal of 98.4%. The isotherm modeling studies proved that the Freundlich isotherm fitted well with the experimental data. Due to ZnO nanoparticles' UV absorption peak, which prevents it from being utilized in the visible spectrum of sunlight, ZnO has poor photocatalytic effectiveness. The degrading efficiency of methylene blue, rhodamine b, and methyl orange were determined as 98.7%, 99.4%, 92.9%, and 92%, respectively, after four recycling (Stanley et al., 2021).
Adsorbents based on optically active metal-oxide nanoparticles were used as treatment agents for color and heavy metal removal from effluents. Using 100 mg/L of dye concentration and a pH of 6.0 98% of dye was removed as per the reports which corresponds to an uptake of 0.3 mg/g. The experimental data were found to fit the second order, while the equilibrium studies were able to fit the Langmuir isotherm. PVA's UV–vis spectra revealed adsorption at wavelengths below 300 nm. Trypan Blue dye has the highest degradation percentage when compared to methylene blue and crystal Violet, according to the results of the UV and FTIR characterization (Kathiresan et al., 2021).
An in-depth study was conducted, optimizing photo mineralization of aqueous methyl orange using a nano-ZnO catalyst. More crucially, the method can be employed in naturally occurring water with a pH that is often lower than 7, and only a smaller quantity of catalysts is sufficient to breakdown the contaminants. The outcomes also demonstrated the detrimental effects on water filtration process as potentially high CO2 concentrations. The effects of other gaseous fluxes that were added to the reaction mixtures are also covered. A dye concentration of 100 mg/l with a pH of 7.0 removed 95% of the dye with an uptake capacity of 70 mg/g. Observations were said to be consistent with Langmuir isotherms, while experimental data fitted with pseudo 2nd order kinetics. By altering the working conditions, methyl orange photodegradation in water with solar light's photocatalytic effectiveness of nano-ZnO powders was reduced (Zyoud et al., 2015).
Cu2O functionalized ZnO nanoparticles were synthesized for the mineralization of methylene blue in the presence of UV photons. The maximum dye removal of 95% was reported at pH 6.0 with an initial dye concentration of 100 mg/l. Under optimal conditions, the maximum dye uptake was found as 40 mg/g. Cu2O showed the IR vibrational region at around 628 cm−1 as a distinct peak. After 45 min of illumination, about 1.7% of the methylene blue molecules were removed by photolysis. It confirms that dye particles were robust enough to resist the irradiation of UV photons. Due to this, there are inadequate free radicals in the applied state to trigger oxidation. Additionally, preliminary trials showed that the surface sorption method could remove about 1% of MB molecules. To attain a balanced sorption/desorption state, all suspensions were agitated at dark for 5 min, then the photodegradation process was restarted (Norouzi et al., 2021).
To accelerate the breakdown of organic methylene blue dye, CuO/ZnO nanophotocatalysts with visible light and green biosynthesis were employed. Hybrid composites integrated with two or more semiconductors that have interdependent properties were proven to be more functional than individual semiconductors. Using biomass filtrate of Penicillium corylophilum strain As-1, copper, and zinc intermediates, a biological CuO/ZnO nanocomposite was fabricated, which has enhanced heat resistance than either ZnO or CuO nanoparticles. Dilute NaOH was added as the mixture was agitated, bringing the pH of the mixture to 10. The highest dye degradation efficiency was recorded as 96% at pH 10 and 100 mg/l dye concentration with the highest sorption capacity of 50 mg/g. To determine the chemical and morphological structures of the generated CuO/ZnO nanocomposites, they were further refined and analysed using Fourier transform infrared (FT-IR), X-ray diffraction (XRD), Scanning electron micrscopy- Energy dispersive X-ray (SEM–EDX), X-ray photoelectron spectroscopy (XPS), and Transmission electron microscopy (TEM) (Fouda et al., 2020).
Studies were conducted on the elimination of methylene blue by employing pristine zinc oxide and 2% iron functionalized zinc oxide nanopowder under UV illumination. Under the ideal conditions of initial dye concentration of 10 mg/L and pH 2, the highest degradation with zinc oxide was 86%, while for 2% functionalized zinc oxide it was found to be 92%. Kinetic experiments were conducted, and the best-fit model was reported as pseudo-first-order model. Compared to earlier studies, this experiment found that 2% Fe/ZnO is more suitable and superior photocatalyst to ZnO. By doping iron, best photocatalytic characteristics were attained. The fabricated semiconductor photocatalyst's crystalline structure increased its efficiency by minimizing the loss of electron–hole pairs brought by the immobilization of two or more charge carriers in an impaired state (Isai & Shrivastava, 2019).
A simple one-pot combustion method was used to fabricate a zinc oxide-ternary heterostructure Mn3O4/ZnO/Eu2O3 nanocomposites. The highest dye elimination of 98% was reported at pH 7.0 with an initial dye concentration of 100 mg/l and the corresponding dye uptake was 50 mg/g. Mn3O/ZnO/Eu2O3 photocatalysts were reported to give 98 and 96% of degradation in 150 min of natural sunlight and illumination, respectively. Based on the kinetic and equilibrium studies, it was reported that the active photocatalytic sites in the solution can produce more radicals when the catalyst dose is increased; however, the solution becomes opaque as the catalyst dose increases and light cannot pass through it (Shubha et al., 2021b).
Researchers found that toxic dyes could be degraded by photocatalytic means under coordinated daylight with the assistance of a cost-effective catalyst. The maximum dye removal of 95% was reported at pH 3.0 with an initial dye concentration of 100 mg/l with an adsorption capacity of 20 mg/g. Kinetic experiments were conducted and the best-fit model was reported as a pseudo-first-order model. The catalyst was removed through filtration using a 0.2 L PVDF syringe filter after the suspension was subjected to radiation for a prescribed amount of time. The concentration of MB in the filtrate was then measured using UV–vis spectroscopy. Under sunlight, ZnO-Ag nanostructures were tested for their photochemical function toward the remediation of methylene blue (Kaviya & Prasad, 2015).
The photocatalytic activity and statistical analysis of methylene blue dye were studied using zinc oxide nanoparticles with a spherical shape. Removal of dye using nano photocatalyst was studied under a wide range of pH (6.9–12.50) and dye concentration (10–100 mg/L). The highest methylene blue elimination efficiency was 72.37% at pH 12.50 with its initial concentration of 100 mg/l and sorption capacity 10 mg/g. The experimental data were reported to fit the first-order kinetic model, and the equilibrium studies were discovered to fit the Langmuir isotherm. Different morphologies of ZnO, such as nanorods, nano-disks nanoparticles, and microstructures resembling cauliflower and mushrooms, were employed for the photodegradation of methyl orange, methylene blue, and rhodamine B under UV irradiation (Wahab et al., 2017).
Crystal violet dye degradation was examined utilizing zinc acetate as a stimulant in the alkaline solution and used as a catalyst for ZnO-flower synthesis in the presence of UV photons with highest wavelength of absorption for dye occurring at 590 nm. The crystal violet absorbance intensity constantly declined with exposure time, indicating a drop in crystal violet dye concentration and demonstrating the mineralization or removal of dye by adsorption using ZnO-flowers. According to the photocatalytic activity, crystal violet dye got rapidly degraded by as-produced ZnO-flowers, with a degradation rate of 96% within 80 min. The quick conversion of crystal violet to lesser harmful organic/ mineral compounds was significantly accelerated by the rapid production of functioning oxygen-rich particles on the outer layer of ZnO. Mass spectrometry indicated that crystal violet dye got fully mineralized in 80 min and is predominant at m/z 14,372 (Ameen et al., 2013).
A novel composite comprising cobalt-doped zinc oxide (ZCo) embedded in natural river silt was utilized to explore the removal of crystal violet dye through adsorption and photocatalysis under visible light. The study examined the impact of three operating parameters on the percentage of crystal violet dye elimination, including the aqueous medium pH, dye concentration, and cobalt impregnation percentage. Maximum dye removal of 20% was reported at pH 8.0 with an initial dye concentration of 1 g/l. Under optimal conditions, the maximum dye uptake was found as 10 mg/g. The kinetic model was reported to fit the experimental data and the Langmuir isotherm was found to represent the equilibrium studies. It was demonstrated that the presence of river silt makes it easier for dye molecules to bind to surfaces and is degraded by the ZCo catalyst. The dyedeterioration is highly significant (0.01 percent) and influenced favorably by the amount of ZCo incorporated into the sediment (Fahoul et al., 2022).
Thermal breakdown of (Zn-Ac) nanoparticles produced by the supercritical antisolvent (SAS) precipitation method resulted in the formation of ZnO photocatalyst which was employed for dye removal. Maximum dye removal of 92% was reported at pH 8.0 with the sorption capacity of 60 mg/g crystal violet. Additionally, it was shown that ZnO is superior to TiO2 in removing contaminants from wastewater, including organic dyes (Franco et al., 2019). In a similar study, effective precipitation process was employed to create zinc oxide nanoparticles where the highest removal of 92% methyl violet was recorded with highest sorption rate of 30 mg/g. Kinetic experiments were conducted and the best fit model was reported as pseudo-2nd order model. The UV–vis experiments showed that after 80 min, methyl violet was successfully degraded. The antibacterial activity of the synthesized zinc oxide nanoparticles against P. aeruginosa, E. coli, and S. aureus was assessed. The zinc oxide nanoparticles are shielded from the gram-positive S. aureus bacteria by their thicker peptidoglycan cell membrane (Jeyasubramanian et al., 2015).
An analysis on photonic elimination of methyl orange using zinc oxide loaded on mesoporous silica was reported. Maximum dye removal of 90% was reported at pH 8 with an initial dye concentration of 100 mg/l. Under optimum conditions, the maximum dye uptake was found as 10 mg/g. Kinetic experiments were conducted and the best fit model was reported as pseudo-1st order model. In addition, two physical parameters mostly govern the elimination of methyl orange. Salicylaldimine's strong coordination function suggests that its alteration does aid in the capture of additional zinc ions (Shen et al., 2018).
The effects of spinach on the photocatalytic degradation of paraquat and methyl orange under sunlight and a UV lamp, as well as the formation of zinc oxide nanoparticles, were investigated in an experiment. The highest dye removal of 91% was reported at pH 11 with an initial concentration of 50–100 ppm. Under ideal circumstances, the maximum dye uptake was found as 100 mg/g. Kinetic experiments were conducted and the best fit model was reported as pseudo-1st an order model. The photocatalytic degradation of methyl orange was studied by periodically measuring the absorbance using an ultraviolet and visible spectrophotometer with a wavelength of 465 nm (Munshi et al., 2018).
Cellulose/PVC/ZnO composites irradiated with photocatalytic UV light were used for the degradation of congo red and crystal violet dyes. The most efficient dye removal of 90% was observed at pH 10 with the highest sorption capacity measured as 30 mg/g. Kinetic experiment studies reveal that the best fit model was pseudo-first order model. To determine photocatalytic activity of the cellulose/PVC/ZnO nanocomposite, the degradation rate of an aqueous solution containing 3 ppm of the dyes congo red and crystal violet were determined. As the time interval increased, the crystal violet dye concentration decreased. Similar, the 530 nm intensity peaks in congo red solution are noticed which declined over time and started vanishing by UV irradiation (Linda et al., 2016).
Textile effluents containing organic dye components were successfully treated using heterogeneous photocatalysis. It was reported that 97% of dye was removed at pH 11 with a sorption capacity of 15 mg/g. Kinetic experiments were conducted and the best fit model was reported as pseudo-first order model. ZnO nanoparticles typically have bandgap energies of 3.3 eV and can be employed to explore the photocatalytic reaction when UV light is present. Ag and graphene oxide incorporated to ZnO surfaces resulted in a steady reduction in bandgap energy, which may indicate improved photocatalytic activity when exposed to UV radiation (Al-Mamun et al., 2021).
In an experiment using industrial wastewater from tanneries and sunlight-activated carbon from noxious plants, researchers removed chromium and methylene blue efficiently from aqueous solutions. The highest methylene blue remediation of 93% was reported at pH 10.0. Kinetic experiments were conducted and the best fit model was reported as pseudo-first order model. Biologically produced ZnO nanoparticles can perform better photodegradation when exposed to direct sunlight between 8 am and 4 pm since there are fewer variations in solar intensity in these hours. With tungsten lamps being expensive and dangerous, the present endeavor ensures cheap photodynamics and thus can be a viable alternative (Kamaraj et al., 2020).
ZnO-based ternary heterostructure nanoparticles produced the highest methylene blue elimination rate of 97% at pH 10 and 30 mg/l initial dye concentration. Kinetic experiments were conducted, and the best fit model was reported as pseudo-2nd order model. To explore the removal strategy, the variation of the absorption peak intensity measured at 663 nm was monitored. ZnO/ Eu2O3/NiO nanoparticles are used to photo catalyze the breakdown of MB in three different environments: sunshine, UV ray irradiation, and fluorescent light at a wavelength of 254 nm (Shubha et al., 2021a).
Researchers conducted a series of tests on the development of WO3-ZnO binary nanostructures over 2D rGO nanosheets which led to the production of brand-new ternary nanocomposites supported by graphene (WO3-ZnO/rGO). The highest dye removal of 94% was reported at pH 7.0 with an initial dye concentration of 40 mg/l. Kinetic experiments’ data showed that the best fit model is pseudo- 1st order model. UV–vis study was carried out for optical and photocatalytic investigation in the 200–800 nm wavelength range on a Carry-60 dual beam UV–vis spectrophotometer. The UV–vis spectrum of methylene blue dye was observed in the presence of WO3, ZnO, WO3-ZnO, and WO3-ZnO@rGO photocatalysts (max = 633 nm) (Chaudhary et al., 2020).
An experimental finding proved that Fe2O3 functionalised ZnO nanoparticles were capable of carrying out methylene blue mineralization using photocatalysis. It was reported that at a pH of 7.0 and an initial dye concentration of 0.4 g/l, the maximum dye removal was 50%. The band gap energy values of Fe2O3, ZnO, and Fe2O3–ZnO samples were calculated to be approximately 2.59, 3.16, and 2.73 eV, respectively. The methylene blue photodegradation experiment was conducted at various intervals between 0 and 120 min to evaluate the kinetics of the process which revealed the fit of pseudo first order kinetics (Noruozi & Nezamzadeh-Ejhieh, 2020). Table 1 presents the performance of various ZnO based nano photocatalyst on degradation of basic dyes.
Degradation of acidic dyes by ZnO nano-photocatalysis
Acid dyes are generally used to dye textiles in immersion, predominantly those containing sulphonic or carboxylic acid salt groups. In this section, a detailed discussion on how ZnO nano-photocatalysts degrade acid dyes by photocatalysis, with specific focus on the conditions that yield maximum degradation rate. Removal of acid yellow-3 dye by the application of pure zinc oxide and zinc oxide doped with neodymium as nano-photo catalysts was reported. The experimental study reported that the degradation of acid yellow-3 dye followed first-order kinetics and the Eley–Rideal mechanism. In addition, high degradation of acid yellow-3 dye was noted at a low concentration of 10 ppm, and the dye effectively degraded at temperatures greater than 50 ℃ and pH 8. The findings revealed that neodymium(Nd)-ZnO could be recycled more than pure ZnO. Similarly, after 160 min, the percentage of degradation of acid yellow-3 dyes by Nd-ZnO and pure zinc oxide were 91% and 80%, respectively, indicating that ZnO doped with Nd is more effective and stable than pure ZnO (Khan et al., 2022). The breakdown of acid violet-7 from aqueous solutions by green ZnO and Fe3O4 was investigated using Camellia sinensis leaves. The experimental results showed that the Langmuir model fitted better than the Freundlich and Temkin models and the pseudo-first-order model applied for kinetic model. This study established that lower pH aided in dye removal effectiveness.The efficiency decreased as the initial concentration of acid violet-7 increased. The highest removal efficiency was observed as 93.25% at 365 nm after 120 min (Roy et al., 2022).
Experiments aimed to eliminate congo red dye, used ZnO in combination with three different solvents: ethanol (E), methanol (M), and water (W). In the study, it was found that the Congo red deteriorated more rapidly as the weight of ZnO increased. The experiment was conducted using the sol–gel method. Degradation occurred more effectively under basic conditions. The removal efficiency of (ZnO)E, (ZnO)M, and (ZnO)W, respectively, were 81.33%, 65.31%, and 70.04%. ZnO with ethanol as the solvent reported as the most effective approach for removing Congo red dye from wastewater. The (ZnO)E response rate grew (0.022 min−1) faster than (ZnO)M and (ZnO)W forms (Ong et al., 2016). The breakdown of acid red dye was demonstrated using ZnO as a nano-photocatalyst influenced by ultraviolet irradiation. The experiment used dye doses of 0.5, 1, 1.5, and 2 mg/L and examined the efficacy of acid red dye removal between pH 5 and 10. As a result, a raise in the dye concentration resulted in a slower rate of deterioration. At 0.5 mg/L, pH 5 and a radiation time of 12 min, the removal efficiency was peaked at its highest (Dehghani & Mahdavi, 2015). An experiment was conducted on the breakdown of reactive red-11 dye from water using pure cellulose acetate-polyurethane, and doped with ZnO as a nano-photocatalyst. With initial dye concentrations ranging from 50 to 250 mg/L, the dye uptake mechanism followed pseudo-first-order kinetics. According to the studies, raising the initial dye concentration reduced the degradation rate. Furthermore, the highest degradation of reactive red-11 was recorded at pH 7, 50 mg/L, and 40 min of radiation exposure (Rajeswari et al., 2017).
A set of data was collected on the important parameters involved in the degradation of congo red dye from wastewater using a nanocomposite ZnO/Fe3O4/PANI. This study analyzed the effect of dye concentration, pH, visible light, and nanocomposite dose in the dye removal. The removal kinetics was found to fit with the pseudo-second-order mechanism. Furthermore, the Temkin and Freundlich isotherms revealed best fits with minimum standard errors. The produced nanocomposite was isolated from the reaction mixture by a magnetic rod. At pH 7, 1 g/L photocatalyst dose, and 30 mg/L, the highest degradation of congo red was reported as 86%. In addition, recycling tests found that ZnO can be reused up to five times (Zare et al., 2022).
Under UV light, acid red 206 dye was degraded using nano-photocatalysts ZnO/ Fe3O4/bentonite. The investigation indicated that acid red 206 degradation follows pseudo-first-order kinetic model with a maximum degradation efficiency of 92% under optimized conditions of pH 3, catalyst dosage of 0.25 g/L and a dye concentration of 40 mg/L, and UV light of 0.154 nm in a time period of 120 min. Furthermore, the report claimed that the nanocomposites can be recycled six times. (Rahimi et al., 2022).
A nano photocatalyst based on zinc oxide was fabricated to eliminate acid blue 113 dye from contaminated sources. The removal mechanism was reported to follow pseudo-first-order kinetics. After 30 min at pH 4 and 0.7 catalyst dose, 580 nm and 100 mg/L, a removal efficiency of 98.78% of acid blue 113 dye was attained. An enhanced degradation efficiency of 98.78% was achieved for 0.7 g of the photocatalyst loaded in the system. Nevertheless, the degradation efficiency remains stable and decreases by 0.5% (98.2%) with catalyst dosages greater than 0.7 g/L. As a result of the clumped molecules, the efficiency of the catalyst decreased at high doses of the catalyst. The findings of the recycling experiments demonstrated that ZnO nanoparticles can be recycled five times with 85.19% recovery rate. Excess photocatalyst scatters light onto the catalyst's active site, causing dye molecules to bind to the catalyst (Sekaran et al., 2022).
The removal of acid blue 25 dye utilizing zinc oxide as a nano photocatalyst and banana peel waste was studied. The degradation efficiency of acid blue 25 dye was 94% at optimum conditions of pH 5, initial dye concentration of 150 mg/L, nanocomposite concentration of 0.6 g/L, and UV irradiation of 16 W. As a result of photocatalytic degradation, the average oxidation state of acid blue 25 solution and the carbon oxidation state increased, indicating an improvement in biodegradability. A significant part of the degradation process was reported to be caused by the production of holes by photolysis and the formation of superoxide radicals. Multiple successive experiments were conducted to determine the reusability of the nanocomposite, and the degradation efficiency decreased from 94 to 82% after four cycles (Ahmadi & Ganjidoust, 2021).
The treatment of acid blue 113 dye from aqueous solutions was examined using thermally activated persulfate with ZnO—GAC (Granular Activated Carbon). The highest degradation of acid blue113 occurred at pH 4.7, 50 min, initial dye concentration of 2.5 g/L. Increasing the amount of catalyst from 0.5 to 2.5 g/L improved the removal efficiency from 78.5% to about 87% at a constant persulfate concentration and solution temperature of 70 °C. Additionally, increasing the ZnO-GAC dosage led to PS activation and radical species production, in addition to AB113 adsorption. The absorption of thermal energy by hot spots at ZnO active sites excited the electrons in the valence band. At the ZnO surface, holes and free electrons were generated when electrons got transferred to the conduction band. As a result, AB113's maximum degradation was determined to be 94.2%. ZnO doped with GAC showed a remarkable reusability, allowing it to be recycled 5 times with only a 3.5% efficiency loss (Samarghandi et al., 2020).
A semi-batch reactor was used to determine the photocatalytic activity of Ag-grafted ZnO nanoparticles by degrading acid blue 113 under UV illumination. ZnO-Ag had greatly superior photocatalytic properties when compared to ZnO synthesized using the same method. Photogenerated electrons and holes recombined less frequently, leading to enhanced photocatalytic activity. Optimal amounts of Ag (3.5 mol%) were needed to be doped with ZnO to improve ZnO photocatalytic activity. Decolorization of acid blue 113 solution containing 20 mg/L was optimal at pH 7.5 and at a catalyst dose of 0.15 g/L. ZnO-Ag photocatalysts synthesized in this study showed reasonable photostability and kinetic studies show pseudo-first-order decolorization (Mohammadzadeh et al., 2015).
An azo dye, acid blue 92, was sonocatalytically degraded in an aqueous solution using samarium (Sm)-doped ZnO nanoparticles prepared by sonochemical means. The kinetic model was best fitted by pseudo-first-order mechanism. After 150 min, a degradation rate of 90.1% was observed at a dye concentration of 10 mg/L. The experimental results demonstrated that Sm-ZnO can be recycled four times with a recovery rate of 87.41% and 88.54%. ZnO crystal lattices was prevented from aggregating by Sm incorporation. Doped samples were found to contain Sm as determined by XPS spectra. The doped sonocatalyst demonstrated higher degradation efficiency and the efficiency increased when peroxydisulfate was added, and degradation efficiency decreased when chloride was added. In this study, free radicals were found to control sonochemical degradation more than any other mechanism. The Gas chromatography- mass spectrometric analysis detected five degradation intermediates after four consecutive degradation experiments using the prepared sonocatalyst (Khataee et al., 2016).
ZnO doped with gadolinium (Gd) was used as a nano-photocatalyst in studies on acid orange-7 dye degradation. The best depolarization effectiveness was discovered in the Gd-ZnO photocatalyst. According to the results of the experiments, acid orange-7 dye degradation followed the "hot spot" and "sonoluminescence" pathways. The initial acid orange-7 dosage was 5 mg/L, but increasing dosage reduced acid orange-7 dye degradation from 90.9 to 44%. When a pH of 6.34 and UV radiation between 200 and 700 nm were utilized, the maximum efficiency of 90% was attained after 90 min. ZnO doped with Gd had four times the reusability potential of pure ZnO, according to recycling tests (Khataee et al., 2015).
Experimental data obtained on the pure cellulose acetate-polyurethane (CA-PU) was compared with CA-PU doped with ZnO as a nano-photocatalyst to break down reactive orange-84 dye from water. A 50 mL volume of dye suspension with a concentration of 100 mg/L was used to conduct adsorption experiments on a membrane surface at different pH values. By adding 1 M HCl (aq) or NaOH (aq) to the solution, the pH of the solution was adjusted to 3–11 and the initial dye concentrations for the experimentations ranged from 50 to 250 mg/L. The removal process was reported to follow pseudo-first-order kinetics. It was observed that, raising the initial dye concentration resulted in a significant drop in degradation rate. At a pH of 7, 50 mg/L of initial concentration and a radiation time of 40 min, maximal degradation (99.28%) of reactive orange-84 was observed. (Rajeswari et al., 2017).
ZnO nanorods were used in experimental studies to explore the decolorization of acid orange-7 dye. ZnO nanorods produced a large number of electric charges when bent under outward oscillation, generating a piezoelectric charge. Through electrochemical redox reactions, piezoelectrically induced electric charges were able to initiate discoloration of dye solution. Furthermore, ZnO nanorods performed well for six cycles tested with low decrement in efficiency comparted to the pristine form and the degradation of acid orange-7 achieved nearly 80% in 50 min (Xu et al., 2018).
The elimination of acid orange-7 by UV light with cadmium oxide (CdO) doped with ZnO was investigated using an ultrasound-assisted co-precipitation method. The reports stated that after 140 min, the rate of decolorization of the dye increased with pH 7 when the initial dye concentration was 20 mg/L. Morphological characterization analysis and photocatalytic performance experiments performed on samples synthesized with ultrasound irradiation showed better results. A uniform morphology tends to be obtained following ultrasound irradiation, according to scanning electron microscope images. In energy dispersive X-ray analysis of sonicated samples, dispersion enhancement was demonstrated. Meanwhile, Brunauer–Emmett–Teller analysis revealed a significant increase in the surface area of the ZnO catalyst after CdO addition. Light absorption during the visible spectrum got shifted to the visible region when CdO content was added. In the study, acid orange-7 was degradation was reported as 69% degradation (Margan & Haghighi, 2018).
Rhodamine-B was removed from aqueous solutions using the ZnO nanophotocatalyst. The pseudo-first-order kinetics was determined to be the best fit from experiment data. At a pH of 5.8 and a reaction time of 180 min and initial dye concentration of 10 mg/L maximum elimination of the dye was reported. To achieve an equilibrium of the dye adsorption and desorption on the catalyst surface, the suspension was loaded into a Pyrex-type reactor, shielded with aluminum foil, and stirred at 350 rpm for 60 min in the dark without irradiation. Following 60 min of centrifugation, the supernatant was analyzed using a spectrophotometer. The recycling test was used to investigate ZnO stability, and it was discovered that ZnO may be recycled four times (Lops et al., 2019). Table 1 presents the performance of various ZnO based nano photocatalyst on degradation of acid dyes.
Mechanism of dye removal by ZnO based nanophotocatlyst
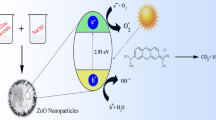
A nanophotocatalyst can be used as a sensitizer for light-stimulated redox processes due to its electronic structure. The electrical structure can be defined by the filled valence and vacant conduction bands (Lee et al., 2016; Samarghandi et al., 2020) Nano-photo catalysis reactions include migration, photoexcitation, charge separation, and oxidation–reduction reactions. The degradation mechanisms of acidic and basic dyes are shown in Fig. 2 in a fitting sequence Studies on degradation of acid dyes by various ZnO based nano photocatalysts is presented in Table 2.
The adsorption of light photons by Ag/ Graphene Oxide (GO) /ZnO nanocomposites was used to demonstrate a plausible mechanism for the photodegradation of methylene blue dye. In a study it was stated that resonance and conduction bands, excited methylene blue dye, silver component, and nanographene sheets were reported to have energies of 7.25, 4.05, 3.60, 4.28, and 4.42 eV, respectively. Electrons will be excited from the valence band of ZnO to the conduction band when the semiconductor ZnO surface is exposed to ultraviolet light. The photon energy is greater than the bandgap energy in this light. Because the GO nanosheets are potential conductors and planar structures, the excited electrons are transferred to the GO nanosheets, which reduces the recombined charge carrier. By generating free electrons, superoxide anion generation effectively degraded the methylene blue dye. The addition of Ag into the ZnO surface reduced the rate of charge carrier recombination. Ag nanoparticles can accept photogenerated holes to obtain e-/h + pairs to react with water to form hydroxide radicals. CO2 and H2O are nontoxic byproducts of the superoxide and hydroxide radicals produced. By transferring electrons from the dye solution to graphene, which has a higher electron mobility, superoxide is generated by reacting with oxygen molecules in the dye solution. Radicals produced during the reaction have a high potential to degrade the dye into nontoxic CO2 and H2O (Rashid al mamun et al., 2021).
Using aqueous solutions of crystal violet and congo red dyes, cellulose/PVC/ZnO nanocomposite was tested for its photocatalytic activity under UV light irradiation. As electrons are transferred from the conduction band to the valence band of the catalyst, hydroxyl radicals are produced as a result of photocatalytic degradation. Dye molecules are affected by hydroxyl radicals produced during oxidation. Nevertheless, the molecular conjugation system reduced at 530 and 320 nm, mainly due to the azo group. After each 15-min interval of irradiation, FTIR studies confirmed that the bond breaks first in the lower energy of the azo group, followed by the higher energy bond in the benzene and naphthalene rings. Accordingly, the higher energy bond broke, causing damage to the conjugated system (azo group). By adding more catalysts to the same concentration, the process was enhanced aggregated, leading to a reduction in the catalyst's surface area, making the catalyst inaccessible to the absorbing dye. Compared to other photocatalysts, it is more active (Linda et al., 2016).
The ZnO nano-photocatalyst absorbs UV photons and generates electron–hole pairs (Alamgholiloo et al., 2022; Ong et al., 2018; Rudolph & Henry, 1964). Furthermore, photo-generated electrons and hole pairs can migrate to the catalyst surface and react with oxygen to form the superoxide radical O2-, which functions as a reducing agent. O2- will then react with H2O to form hydroxyl radical OH. Moreover, valance band holes H+ can react with H2O to form OH (Lee et al., 2016; Nam et al., 2018). The formed holes on the catalyst captures water molecules and oxidizes them into hydroxyl radicals which have extremely powerful oxidizing properties. Additionally, electrons deplete the reactant while holes oxidize it. The valence band and conduction band levels are also important considerations (Aghaei et al., 2020; Dihom et al., 2022; Mohamed et al., 2019a). The conduction band of the catalyst should be more negative than the level of hydrogen production for effective hydrogen generation. Similarly, for the formation of oxygen, the valence band must be more positive than the water oxidation level. The acidic and basic dyes are decomposed using the OH and O.2− produced by the oxidation and reduction reactions (Nemiwal et al., 2021; Saravanan et al., 2016)
The mechanism involved in photocatalysis by ZnO based nanophotocatalysts is presented in Fig. 2.
Conclusion and future perspectives
Dye removal involves numerous processes, including oxidation and reduction reactions, in which zinc oxide aids in activating and producing superoxide oxygen and OH. The findings of this investigation revealed that raising the fraction of zinc oxide in the resultant compounds can increase the average particle size of the compounds and the removal effectiveness reduced when the initial dye concentration was increased. Degrading dyes with metal oxide nanoparticles depends on various factors such as nanoparticle size, surface area, concentration, pH, and temperature, as well as the dye itself. Optimizing these parameters can be challenging and may vary for different dyes, making it challenging to achieve optimal conditions for degradation. In dye degradation processes, metal oxide nanoparticles can be affected by their stability in the treatment process as well as their tendency to aggregate or deactivate over time. In addition to reducing the active surface area available for reactions, aggregates may also limit the efficiency of those reactions. Large-scale water treatment can be expensive because metal oxide nanoparticles have to be made and implemented. Some industries or regions might find this method economically unfeasible due to the cost of synthesizing nanoparticles and the energy and resources needed to produce them (Patil et al., 2023; Pattnaik et al., 2023). Different dye molecules have different chemical structures, compositions, and complexity, so these photocatalysts can degrade various dyes differently. The degradation of some dyes may take longer or require higher catalyst concentrations if they are more resistant to degradation. Chemical and structural modifications of metal oxides are necessary by engineering techniques to widen band gaps, dope heteroatoms, and prepare nanocomposites. By using advanced techniques, metal oxide semiconductors will be able to utilize the entire solar electromagnetic spectrum, allowing for increased efficiency by delaying electron–hole recombination. Nanoencapsulation techniques present new opportunities for research and development. For efficient photocatalysis, low catalyst dosages and high reusability are helpful for their utility in dye degradation treatment. The future of modification techniques will include green and economical modifiers controlled by thermodynamics and kinetics. It is possible to predict the structural properties of catalysts based on understanding molecular-level mechanisms and introducing theoretical investigations. There is a need for more research on ZnO functionality as a stimulant in the future. Compared with other types of pollution, these types of pollutants are the least studied, and there have been few discoveries as to the effective solar-active methods for removing stimulants. For dye degradation in wastewater treatment, integrating artificial intelligence with metal oxide nanophotocatalysts presents several compelling possibilities. Utilize machine learning algorithms to enhance dye degradation by predicting and optimizing metal oxide nanophotocatalyst properties. As a result of the use of artificial intelligence, large datasets can be analyzed to find the most effective catalyst compositions, structures, and surface modifications, leading to the rapid development of high-performance catalysts. The use of artificial intelligence algorithms can help us predict and understand how metal oxide nanophotocatalysts degrade dye molecules. By using predictive models, we can identify the most efficient degradation routes, enabling us to design targeted catalysts and optimize processes (Bhagat et al., 2023; Zhang et al., 2023).
Data availability
No datasets were generated or analysed during the current study.
References
Adeel, M., Saeed, M., Khan, I., et al. (2021). Synthesis and characterization of Co–ZnO and evaluation of its photocatalytic activity for photodegradation of methyl orange. ACS Omega, 6, 1426–1435. https://doi.org/10.1021/acsomega.0c05092
Aghaei, H., Ghavi, M., Hashemkhani, G., & Keshavarz, M. (2020). Utilization of two modified layered doubled hydroxides as supports for immobilization of Candida rugosa lipase. International Journal of Biological Macromolecules, 162, 74–83. https://doi.org/10.1016/j.ijbiomac.2020.06.145
Ahmad, I., Shukrullah, S., Naz, M. Y., et al. (2022). Recent progress in rare earth oxides and carbonaceous materials modified ZnO heterogeneous photocatalysts for environmental and energy applications. Journal of Environmental Chemical Engineering, 10, 107762. https://doi.org/10.1016/j.jece.2022.107762
Ahmadi, S., & Ganjidoust, H. (2021). Using banana peel waste to synthesize BPAC/ZnO nanocomposite for photocatalytic degradation of Acid Blue 25: Influential parameters, mineralization, biodegradability studies. Journal of Environmental Chemical Engineering, 9, 106010. https://doi.org/10.1016/j.jece.2021.106010
Ahsaine, H. A., Slassi, A., Naciri, Y., et al. (2018). Photo/electrocatalytic properties of nanocrystalline ZnO and La–Doped ZnO: Combined DFT fundamental semiconducting properties and experimental study. ChemistrySelect, 3, 7778–7791. https://doi.org/10.1002/slct.201801729
Alamgholiloo, H., Nazari, S., Asgari, E., et al. (2022). Facile fabrication of Z-scheme TiO2/ZnO@MCM-41 heterojunctions nanostructures for photodegradation and bioactivity performance. Journal of Molecular Liquids, 364, 119990. https://doi.org/10.1016/j.molliq.2022.119990
Al-Mamun, M. R., Islam, M. S., Hossain, M. R., Kader, S., Islam, M. S., & Khan, M. Z. H. (2021). A novel and highly efficient Ag and GO co-synthesized ZnO nano photocatalyst for methylene blue dye degradation under UV irradiation. Environmental Nanotechnology, Monitoring & Management, 16, 100495. https://doi.org/10.1016/j.enmm.2021.100495
Ameen, S., Akhtar, M. S., Nazim, M., & Shin, H.-S. (2013). Rapid photocatalytic degradation of crystal violet dye over ZnO flower nanomaterials. Materials Letters, 96, 228–232. https://doi.org/10.1016/j.matlet.2013.01.034
Bai, L., Guo, R., Chen, Z., et al. (2022). Chemically fabricated ZnO/Ag/AgmMonOl/ZnxMoyOz heterojunction nanophotocatalysts for abating organic dyes in wastewater. Journal of Cleaner Production, 363, 132481. https://doi.org/10.1016/j.jclepro.2022.132481
Bai, X., Wang, L., Wang, Y., et al. (2014). Enhanced oxidation ability of g-C3N4 photocatalyst via C60 modification. Applied Catalysis, B: Environmental, 152, 262–270. https://doi.org/10.1016/j.apcatb.2014.01.046
Bhagat, S. K., Pilario, K. E., Babalola, O. E., Tiyasha, T., Yaqub, M., Onu, C. E., & Yaseen, Z. M. (2023). Comprehensive review on machine learning methodologies for modeling dye removal processes in wastewater. Journal of Cleaner Production, 385, 135522. https://doi.org/10.1016/j.jclepro.2022.135522
Bosu, S., & Rajamohan, N. (2022). Nanotechnology approach for enhancement in biohydrogen production-review on applications of nanocatalyst and life cycle assessment. Fuel, 323, 124351. https://doi.org/10.1016/j.fuel.2022.124351
Bosu, S., Pooja, R. P., & Rajasimman, M. (2022a). Role of nanomaterials in enhanced ethanol production through biological methods: Review on operating factors and machine learning applications. Fuel, 320, 123905. https://doi.org/10.1016/j.fuel.2022.123905
Bosu, S., Rajamohan, N., & Rajasimman, M. (2022b). Enhanced remediation of lead (II) and cadmium (II) ions from aqueous media using porous magnetic nanocomposites: A comprehensive review on applications and mechanism. Environmental Research, 213, 113720. https://doi.org/10.1016/j.envres.2022.113720
Chakraborty, U., Bhanjana, G., Kaur, N., et al. (2021). Microwave-assisted assembly of Ag2O–ZnO composite nanocones for electrochemical detection of 4-Nitrophenol and assessment of their photocatalytic activity towards degradation of 4-Nitrophenol and Methylene blue dye. Journal of Hazardous Materials, 416, 125771. https://doi.org/10.1016/j.jhazmat.2021.125771
Chaudhary, K., Shaheen, N., Zulfiqar, S., et al. (2020). Binary WO3-ZnO nanostructures supported rGO ternary nanocomposite for visible light driven photocatalytic degradation of methylene blue. Synthetic Metals, 269, 116526. https://doi.org/10.1016/j.synthmet.2020.116526
Chung, K. T. (2016). Azo dyes and human health: A review. Journal of Environmental Science and Health, Part C, 34(4), 233–261. https://doi.org/10.1080/10590501.2016.1236602
Dehghani, M. H., & Mahdavi, P. (2015). Removal of acid 4092 dye from aqueous solution by zinc oxide nanoparticles and ultraviolet irradiation. Desalination and Water Treatment, 54, 3464–3469. https://doi.org/10.1080/19443994.2014.913267
Dihom, H. R., Al-Shaibani, M. M., Radin Mohamed, R. M. S., et al. (2022). Photocatalytic degradation of disperse azo dyes in textile wastewater using green zinc oxide nanoparticles synthesized in plant extract: A critical review. Journal of Water Process Engineering, 47, 102705. https://doi.org/10.1016/j.jwpe.2022.102705
Elsabahy, M., & Wooley, K. L. (2012). Design of polymeric nanoparticles for biomedical delivery applications. Chemical Society Reviews, 41, 2545–2561. https://doi.org/10.1039/C2CS15327K
Eskizeybek, V., Sarı, F., Gülce, H., et al. (2012). Preparation of the new polyaniline/ZnO nanocomposite and its photocatalytic activity for degradation of methylene blue and malachite green dyes under UV and natural sun lights irradiations. Applied Catalysis B: Environmental, 119–120, 197–206. https://doi.org/10.1016/j.apcatb.2012.02.034
Fahoul, Y., Tanji, K., Zouheir, M., et al. (2022). Novel River Sediment@ ZnOCo nanocomposite for photocatalytic degradation and COD reduction of crystal violet under visible light. Journal of Molecular Structure, 1253, 132298. https://doi.org/10.1016/j.molstruc.2021.132298
Fouda, A., Salem, S. S., Wassel, A. R., et al. (2020). Optimization of green biosynthesized visible light active CuO/ZnO nano-photocatalysts for the degradation of organic methylene blue dye. Heliyon, 6, e04896. https://doi.org/10.1016/j.molstruc.2021.132298
Franco, P., Sacco, O., de Marco, I., & Vaiano, V. (2019). Zinc oxide nanoparticles obtained by supercritical antisolvent precipitation for the photocatalytic degradation of crystal violet dye. Catalysts, 9, 346. https://doi.org/10.3390/catal9040346
Giannakis, S., Liu, S., Carratalà, A., et al. (2017). Iron oxide-mediated semiconductor photocatalysis vs. heterogeneous photo-Fenton treatment of viruses in wastewater. Impact of the oxide particle size. Journal of Hazardous Materials, 339, 223–231.
Habibollahi, Z., Peyravi, M., Khalili, S., & Jahanshahi, M. (2022). ZnO-based ternary nanocomposite for decolorization of methylene blue by photocatalytic dynamic membrane. Materials Today Chemistry, 23, 100748. https://doi.org/10.1016/j.mtchem.2021.100748
Hammad, A. H., & Abdel-wahab, MSh. (2022). Photocatalytic activity in nanostructured zinc oxide thin films doped with metallic copper. Physica B Condens Matter, 646, 414352. https://doi.org/10.1016/j.physb.2022.414352
Heidari-Asil, S. A., Zinatloo-Ajabshir, S., Alshamsi, H. A., et al. (2022). Magnetically recyclable ZnCo2O4/Co3O4 nano-photocatalyst: Green combustion preparation, characterization and its application for enhanced degradation of contaminated water under sunlight. International Journal of Hydrogen Energy, 47, 16852–16861. https://doi.org/10.1016/j.ijhydene.2022.03.157
Isai, K. A., & Shrivastava, V. S. (2019). Photocatalytic degradation of methylene blue using ZnO and 2% Fe–ZnO semiconductor nanomaterials synthesized by sol–gel method: A comparative study. SN Applied Science, 1, 1–11. https://doi.org/10.1007/s42452-019-1279-5
Jeyasubramanian, K., Hikku, G. S., & Sharma, R. K. (2015). Photo-catalytic degradation of methyl violet dye using zinc oxide nano particles prepared by a novel precipitation method and its anti-bacterial activities. Journal of Water Process Engineering, 8, 35–44. https://doi.org/10.1016/j.jwpe.2015.08.007
Junlabhut, P., Mekprasart, W., Noonuruk, R., Chongsri, K., & Pecharapa, W. (2014). Characterization of ZnO: Sn nanopowders synthesized by co-precipitation method. Energy Procedia, 56, 560–565. https://doi.org/10.1016/j.egypro.2014.07.193
Kadi, M. W., McKinney, D., Mohamed, R. M., Mkhalid, I. A., & Sigmund, W. (2016). Fluorine doped zinc oxide nanowires: Enhanced photocatalysts degrade malachite green dye under visible light conditions. Ceramics International, 42(4), 4672–4678. https://doi.org/10.1016/j.ceramint.2015.11.052
Kamaraj, M., Srinivasan, N. R., Assefa, G., Adugna, A. T., & Kebede, M. (2020). Facile development of sunlit ZnO nanoparticles-activated carbon hybrid from pernicious weed as an operative nano-adsorbent for removal of methylene blue and chromium from aqueous solution: Extended application in tannery industrial wastewater. Environmental Technology & Innovation, 17, 100540. https://doi.org/10.1016/j.eti.2019.100540
Karri, R. R., Ravindran, G., & Dehghani, M. H. (2021). Wastewater—sources, toxicity, and their consequences to human health. In Soft computing techniques in solid waste and wastewater management (pp. 3–33). Elsevier. https://doi.org/10.1016/B978-0-12-824463-0.00001-X
Kathiresan, G., Vijayakumar, K., Sundarrajan, A. P., Kim, H. S., & Adaikalam, K. (2021). Photocatalytic degradation efficiency of ZnO, GO and PVA nanoadsorbents for crystal violet, methylene blue and trypan blue dyes. Optik, 238, 166671. https://doi.org/10.1016/j.ijleo.2021.166671
Kaviya, S., & Prasad, E. (2015). Biogenic synthesis of ZnO–Ag nano custard apples for efficient photocatalytic degradation of methylene blue by sunlight irradiation. RSC Advances, 5, 17179–17185. https://doi.org/10.1039/C4RA15293J
Khan, U., Jan, F. A., Ullah, R., Wajidullah, Ullah, N., & Salman. (2022). Comparative photocatalytic performance and therapeutic applications of zinc oxide (ZnO) and neodymium-doped zinc oxide (Nd–ZnO) nanocatalysts against acid yellow-3 dye: Kinetic and thermodynamic study of the reaction and effect of various parameters. Journal of Materials Science: Materials in Electronics, 1–20. https://doi.org/10.1007/s10854-021-07483-0
Khataee, A., Saadi, S., Vahid, B., Joo, S. W., & Min, B. K. (2016). Sonocatalytic degradation of Acid Blue 92 using sonochemically prepared samarium doped zinc oxide nanostructures. Ultrasonics Sonochemistry, 29, 27–38. https://doi.org/10.1016/j.ultsonch.2015.07.026
Khataee, A., Soltani, R. D. C., Karimi, A., & Joo, S. W. (2015). Sonocatalytic degradation of a textile dye over Gd-doped ZnO nanoparticles synthesized through sonochemical process. Ultrasonics Sonochemistry, 23, 219–230. https://doi.org/10.1016/j.ultsonch.2014.08.023
Khezrianjoo, S., Lee, J., Kim, K. H., & Kumar, V. (2019). Eco-toxicological and kinetic evaluation of TiO2 and ZnO nanophotocatalysts in degradation of organic dye. Catalysts, 9(10), 871. https://doi.org/10.3390/catal9100871
Kumar, A., Dixit, U., Singh, K., Gupta, S. P., & Beg, M. S. J. (2021). Structure and properties of dyes and pigments. Dyes and Pigments-Novel Applications and Waste Treatment, 131.
Kumar, R., Kumar, G., Akhtar, M. S., & Umar, A. (2015). Sonophotocatalytic degradation of methyl orange using ZnO nano-aggregates. Journal of Alloys and Compounds, 629, 167–172. https://doi.org/10.1016/j.jallcom.2014.12.232
Lacuesta, A. C., Herrera, M. U., Manalo, R., & Balela, M. D. L. (2018). Fabrication of kapok paper-zinc oxide-polyaniline hybrid nanocomposite for methyl orange removal. Surface and Coatings Technology, 350, 971–976. https://doi.org/10.1016/j.surfcoat.2018.03.043
Le, A. T., Le, T. D. H., Cheong, K. Y., & Pung, S. Y. (2022). Immobilization of zinc oxide-based photocatalysts for organic pollutant degradation: A review. Journal of Environmental Chemical Engineering, 10(5), 108505. https://doi.org/10.1016/j.jece.2022.108505
Lee, K. M., Lai, C. W., Ngai, K. S., & Juan, J. C. (2016). Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Research, 88, 428–448. https://doi.org/10.1016/j.watres.2015.09.045
Li, G., Jiang, B., Li, X., Lian, Z., Xiao, S., Zhu, J., & Li, H. (2013). C60/Bi2TiO4F2 heterojunction photocatalysts with enhanced visible-light activity for environmental remediation. ACS Applied Materials & Interfaces, 5(15), 7190–7197. https://doi.org/10.1021/am401525m
Linda, T., Muthupoongodi, S., Shajan, X. S., & Balakumar, S. (2016). Photocatalytic degradation of congo red and crystal violet dyes on cellulose/PVC/ZnO composites under UV light irradiation. Materials Today: Proceedings, 3(6), 2035–2041. https://doi.org/10.1016/j.matpr.2016.04.106
Lops, C., Ancona, A., Di Cesare, K., Dumontel, B., Garino, N., Canavese, G., & Cauda, V. (2019). Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro-and nano-particles of ZnO. Applied Catalysis B: Environmental, 243, 629–640. https://doi.org/10.1016/j.apcatb.2018.10.078
Mano, T., Nishimoto, S., Kameshima, Y., & Miyake, M. (2015). Water treatment efficacy of various metal oxide semiconductors for photocatalytic ozonation under UV and visible light irradiation. Chemical Engineering Journal, 264, 221–229. https://doi.org/10.1016/j.cej.2014.11.088
Margan, P., & Haghighi, M. (2018). Sono-coprecipitation synthesis and physicochemical characterization of CdO-ZnO nanophotocatalyst for removal of acid orange 7 from wastewater. Ultrasonics Sonochemistry, 40, 323–332. https://doi.org/10.1016/j.ultsonch.2017.07.003
Mohamed, A. A., Fouda, A., Abdel-Rahman, M. A., Hassan, S. E. D., El-Gamal, M. S., Salem, S. S., & Shaheen, T. I. (2019a). Fungal strain impacts the shape, bioactivity and multifunctional properties of green synthesized zinc oxide nanoparticles. Biocatalysis and Agricultural Biotechnology, 19, 101103. https://doi.org/10.1016/j.bcab.2019.101103
Mohamed, M. M., Ghanem, M. A., Khairy, M., Naguib, E., & Alotaibi, N. H. (2019b). Zinc oxide incorporated carbon nanotubes or graphene oxide nanohybrids for enhanced sonophotocatalytic degradation of methylene blue dye. Applied Surface Science, 487, 539–549. https://doi.org/10.1016/j.apsusc.2019.05.135
Mohammadzadeh, S., Olya, M. E., Arabi, A. M., Shariati, A., & Nikou, M. K. (2015). Synthesis, characterization and application of ZnO-Ag as a nanophotocatalyst for organic compounds degradation, mechanism and economic study. Journal of Environmental Sciences, 35, 194–207. https://doi.org/10.1016/j.jes.2015.03.030
Moma, J., & Baloyi, J. (2019). Modified titanium dioxide for photocatalytic applications. Photocatalysts-Applications and Attributes, 18, 10–5772. https://doi.org/10.5772/intechopen.79374
Moslehnejad, N., Jahangiri, M., Vafaee, F., & Salavati-Niasari, M. (2022). Synthesis and characterization of ZnO–Ce nanophotocatalyst and their application for the removal of dye (Reactive red 198) by degradation process: Kinetics, thermodynamics and experimental design. International Journal of Hydrogen Energy, 47, 23980–23993. https://doi.org/10.1016/j.ijhydene.2022.05.189
Munshi, G. H., Ibrahim, A. M., & Al-Harbi, L. M. (2018). Inspired preparation of zinc oxide nanocatalyst and the photocatalytic activity in the treatment of methyl orange dye and paraquat herbicide. International Journal of Photoenergy. https://doi.org/10.1155/2018/5094741
Nam, S. N., Cho, H., Han, J., Her, N., & Yoon, J. (2018). Photocatalytic degradation of acesulfame K: Optimization using the Box-Behnken design (BBD). Process Safety and Environmental Protection, 113, 10–21. https://doi.org/10.1016/j.psep.2017.09.002
Nemiwal, M., Zhang, T. C., & Kumar, D. (2021). Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Science of the Total Environment, 767, 144896. https://doi.org/10.1016/j.scitotenv.2020.144896
Norouzi, A., Nezamzadeh-Ejhieh, A., & Fazaeli, R. (2021). A Copper (I) oxide-zinc oxide nano-catalyst hybrid: Brief characterization and study of the kinetic of its photodegradation and photomineralization activities toward methylene blue. Materials Science in Semiconductor Processing, 122, 105495. https://doi.org/10.1016/j.mssp.2020.105495
Noruozi, A., & Nezamzadeh-Ejhieh, A. (2020). Preparation, characterization, and investigation of the catalytic property of α-Fe2O3–ZnO nanoparticles in the photodegradation and mineralization of methylene blue. Chemical Physics Letters, 752, 137587. https://doi.org/10.1016/j.cplett.2020.137587
Ong, C. B., Mohammad, A. W., Rohani, R., Ba-Abbad, M. M., & Hairom, N. H. H. (2016). Solar photocatalytic degradation of hazardous Congo red using low-temperature synthesis of zinc oxide nanoparticles. Process Safety and Environmental Protection, 104, 549–557. https://doi.org/10.1016/j.psep.2016.04.006
Ong, C. B., Ng, L. Y., & Mohammad, A. W. (2018). A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renewable and Sustainable Energy Reviews, 81, 536–551. https://doi.org/10.1016/j.rser.2017.08.020
Oskoei, V., Dehghani, M. H., Nazmara, S., et al. (2016). Removal of humic acid from aqueous solution using UV/ZnO nano-photocatalysis and adsorption. Journal of Molecular Liquids, 213, 374–380. https://doi.org/10.1016/j.molliq.2015.07.052
Patil, Y., Attarde, S., Dhake, R., Fegade, U., & Alaghaz, A. N. M. (2023). Adsorption of Congo red dye using metal oxide nano-adsorbents: Past, present, and future perspective. International Journal of Chemical Kinetics, 55(10), 579–605. https://doi.org/10.1002/kin.21675
Parthipan, P., Cheng, L., Rajasekar, A., Govarthanan, M., & Subramania, A. (2021). Biologically reduced graphene oxide as a green and easily available photocatalyst for degradation of organic dyes. Environmental Research, 196, 110983. https://doi.org/10.1016/j.envres.2021.110983
Pattnaik, A., Sahu, J. N., Poonia, A. K., & Ghosh, P. (2023). Current perspective of nano-engineered metal oxide based photocatalysts in advanced oxidation processes for degradation of organic pollutants in wastewater. Chemical Engineering Research and Design. https://doi.org/10.1016/j.cherd.2023.01.014
Puneetha, J., Kottam, N., & Rathna, A. (2021). Investigation of photocatalytic degradation of crystal violet and its correlation with bandgap in ZnO and ZnO/GO nanohybrid. Inorganic Chemistry Communications, 125, 108460. https://doi.org/10.1016/j.inoche.2021.108460
Qi, K., Zhuang, C., Zhang, M., Gholami, P., & Khataee, A. (2022). Sonochemical synthesis of photocatalysts and their applications. Journal of Materials Science & Technology, 123, 243–256. https://doi.org/10.1016/j.jmst.2022.02.019
Qiu, G., Dharmarathna, S., Genuino, H., Zhang, Y., Huang, H., & Suib, S. L. (2011). Facile microwave-refluxing synthesis and catalytic properties of vanadium pentoxide nanomaterials. ACS Catalysis, 1, 1702–1709. https://doi.org/10.1021/cs200437x
Rahimi, S. M., Panahi, A. H., Allahyari, E., & Nasseh, N. (2022). Breaking down of low-biodegradation Acid Red 206 dye using bentonite/Fe3O4/ZnO magnetic nanocomposite as a novel photo-catalyst in presence of UV light. Chemical Physics Letters, 794, 139480. https://doi.org/10.1016/j.cplett.2022.139480
Rajamohan, N., Bosu, S., Ngo, G. H., & Al-Shibli, N. (2022a). Fabrication of modified carbon nano tubes based composite using ionic liquid for phenol removal. Molecular Catalysis, 533, 112792. https://doi.org/10.1016/j.mcat.2022.112792
Rajamohan, N., Bosu, S., Rajasimman, M., & Varjani, S. (2022b). Environmental remediation of selenium using surface modified carbon nano tubes: Characterization, influence of variables, equilibrium and kinetic analysis. Environmental Research. https://doi.org/10.1016/j.envres.2022.114629
Rajeswari, A., Vismaiya, S., & Pius, A. (2017). Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chemical Engineering Journal, 313, 928–937. https://doi.org/10.1016/j.cej.2016.10.124
Roy, K., Sarkar, C. K., & Ghosh, C. K. (2015). Photocatalytic activity of biogenic silver nanoparticles synthesized using yeast (Saccharomyces cerevisiae) extract. Applied Nanoscience, 5, 953–959. https://doi.org/10.1007/s13204-014-0392-4
Roy, N., Alex, S. A., Chandrasekaran, N., Kannabiran, K., & Mukherjee, A. (2022). Studies on the removal of acid violet 7 dye from aqueous solutions by green ZnO@ Fe3O4 chitosan–alginate nanocomposite synthesized using Camellia sinensis extract. Journal of Environmental Management, 303, 114128. https://doi.org/10.1016/j.jenvman.2021.114128
Rudolph, G., & Henry, M. C. (1964). The thermal decomposition of zinc acetylacetonate hydrate. Inorganic Chemistry, 3, 1317–1318.
Samarghandi, M. R., Tari, K., Shabanloo, A., et al. (2020). Synergistic degradation of acid blue 113 dye in a thermally activated persulfate (TAP)/ZnO-GAC oxidation system: Degradation pathway and application for real textile wastewater. Separation and Purification Technology, 247, 116931.
Santhosh, C., Velmurugan, V., Jacob, G., Jeong, S. K., Grace, A. N., & Bhatnagar, A. (2016). Role of nanomaterials in water treatment applications: A review. Chemical Engineering Journal, 306, 1116–1137. https://doi.org/10.1016/j.cej.2016.08.053
Saravanan, R., Sacari, E., Gracia, F., Khan, M. M., Mosquera, E., & Gupta, V. K. (2016). Conducting PANI stimulated ZnO system for visible light photocatalytic degradation of coloured dyes. Journal of Molecular Liquids, 221, 1029–1033.
Satdeve, N. S., Ugwekar, R. P., & Bhanvase, B. A. (2019). Ultrasound assisted preparation and characterization of Ag supported on ZnO nanoparticles for visible light degradation of methylene blue dye. Journal of Molecular Liquids, 291, 111313. https://doi.org/10.1016/j.molliq.2019.111313
Sathishkumar, P., Sweena, R., Wu, J. J., & Anandan, S. (2011). Synthesis of CuO–ZnO nanophotocatalyst for visible light assisted degradation of a textile dye in aqueous solution. Chemical Engineering Journal, 171, 136–140. https://doi.org/10.1016/j.cej.2011.03.074
Sekaran, C., Vishnu, D., Dhandapani, B., Alagesan, T., & Balaji, G. (2022). Facile synthesis of zinc oxide nanoparticles using glycerol as cross-linker and the kinetic studies for the photocatalytic degradation of acid blue 113 dye. Results Chem, 4, 100377. https://doi.org/10.1016/j.rechem.2022.100377
Sharma, J., Sharma, S., & Soni, V. (2021). Classification and impact of synthetic textile dyes on aquatic flora: A review. Regional Studies in Marine Science, 45, 101802. https://doi.org/10.1016/j.rsma.2021.101802
Shen, Z., Zhou, H., Chen, H., Xu, H., Feng, C., & Zhou, X. (2018). Synthesis of nano-zinc oxide loaded on mesoporous silica by coordination effect and its photocatalytic degradation property of methyl orange. Nanomaterials, 8(5), 317. https://doi.org/10.3390/nano8050317
Shubha, J. P., Adil, S. F., Khan, M., Hatshan, M. R., Kavalli, K., & Shaik, B. (2021a). Facile fabrication of a ZnO/Eu2O3/NiO-Based ternary heterostructure nanophotocatalyst and its application for the degradation of methylene blue. ACS Omega, 6, 3866–3874. https://doi.org/10.1021/acsomega.0c05670
Shubha, J. P., Savitha, H. S., & Adil, S. F. (2021b). Straightforward synthesis of Mn3O4/ZnO/Eu2O3-based ternary heterostructure nano-photocatalyst and its application for the photodegradation of methyl orange and methylene blue dyes. Molecules, 26, 4661. https://doi.org/10.3390/molecules26154661
Shubha, J. P., Sushma, N. V., Adil, S. F., Khan, M., Assal, M. E., Hatshan, M. R., & Shaik, B. (2022). ZnO/La2O3/NiO based ternary heterostructure nano-photocatalyst: Preparation, characterization and its application for the degradation of methylene blue. Journal of King Saud University-Science, 34, 101738. https://doi.org/10.1016/j.jksus.2021.101738
Siddiqui, V. U., Ansari, A., Ansari, M. T., Akram, M. K., Siddiqi, W. A., Alosaimi, A. M., Hussein, M. A., & Rafatullah, M. (2021). Optimization of facile synthesized ZnO/CuO nanophotocatalyst for organic dye degradation by visible light irradiation using response surface methodology. Catalysts, 11, 1509. https://doi.org/10.3390/catal11121509
Singhal, N., Selvaraj, S., Sivalingam, Y., & Venugopal, G. (2022). Study of photocatalytic degradation efficiency of rGO/ZnO nano-photocatalyst and their performance analysis using scanning Kelvin probe. Journal of Environmental Chemical Engineering, 10(2), 107293. https://doi.org/10.1016/j.jece.2022.107293
Soares, B. M., Araújo, T. M. T., Ramos, J. A. B., Pinto, L. C., Khayat, B. M., Bahia, M. D. O., & Khayat, A. S. (2015). Effects on DNA repair in human lymphocytes exposed to the food dye tartrazine yellow. Anticancer Research, 35(3), 1465–1474.
Stanley, R., Jebasingh, J. A., Stanley, P. K., Ponmani, P., Shekinah, M. E., & Vasanthi, J. (2021). Excellent photocatalytic degradation of methylene blue, rhodamine b and methyl orange dyes by Ag–ZnO nanocomposite under natural sunlight irradiation. Optik, 231, 166518. https://doi.org/10.1016/j.ijleo.2021.166518
Sudarshan, S., Harikrishnan, S., RathiBhuvaneswari, G., Alamelu, V., Aanand, S., Rajasekar, A., & Govarthanan, M. (2023). Impact of textile dyes on human health and bioremediation of textile industry effluent using microorganisms: Current status and future prospects. Journal of Applied Microbiology, 134(2), 064. https://doi.org/10.1093/jambio/lxac064
Subbulakshmi, A., Durgadevi, S., Anitha, S., Govarthanan, M., Biruntha, M., Rameshthangam, P., & Kumar, P. (2023). Biogenic gold nanoparticles from Gelidiella acerosa: Bactericidal and photocatalytic degradation of two commercial dyes. Applied Nanoscience, 13(6), 4033–4042. https://doi.org/10.1007/s13204-022-02693-2
Tai, X. H., Lai, C. W., Juan, J. C., & Lee, K. M. (2020). Nano-photocatalyst in photocatalytic oxidation processes. In Nanomaterials for air remediation (pp. 151–165). Elsevier.
Thomas, S. C., Kumar Mishra, P., & Talegaonkar, S. (2015). Ceramic nanoparticles: Fabrication methods and applications in drug delivery. Current Pharmaceutical Design, 21, 6165–6188.
Thool, G. S., Singh, A. K., Singh, R. S., Gupta, A., & Susan, M. A. B. H. (2014). Facile synthesis of flat crystal ZnO thin films by solution growth method: A micro-structural investigation. Journal of Saudi Chemical Society, 18, 712–721. https://doi.org/10.1016/j.jscs.2014.02.005
Tkaczyk, A., Mitrowska, K., & Posyniak, A. (2020). Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Science of the Total Environment, 717, 137222. https://doi.org/10.1016/j.scitotenv.2020.137222
Tran, V. A., Phung, T. K., Vo, T. K., Nguyen, T. T., Nguyen, T. A. N., Viet, D. Q., Hieu, V. Q., & Vo, T. T. T. (2021). Solar-light-driven photocatalytic degradation of methyl orange dye over Co3O4-ZnO nanoparticles. Materials Letters, 284, 128902. https://doi.org/10.1016/j.matlet.2020.128902
Vaez, Z., & Javanbakht, V. (2020). Synthesis, characterization and photocatalytic activity of ZSM-5/ZnO nanocomposite modified by Ag nanoparticles for methyl orange degradation. Journal of Photochemistry and Photobiology, a: Chemistry, 388, 112064. https://doi.org/10.1016/j.jphotochem.2019.112064
Vidya, C., Manjunatha, C., Chandraprabha, M. N., Rajshekar, M., & Mal, A. R. (2017). Hazard free green synthesis of ZnO nano-photo-catalyst using Artocarpus Heterophyllus leaf extract for the degradation of Congo red dye in water treatment applications. J Environmental Chemical Engineering, 5, 3172–3180. https://doi.org/10.1016/j.jece.2017.05.058
Wahab, R., Khan, F., Lutfullah, & Al-Khedhairy, A. A. (2017). Photocatalytic activity and statistical determination of ball-shaped zinc oxide NPs with methylene blue dye. Inorganic and Nano-Metal Chemistry 47, 536–542. https://doi.org/10.1080/15533174.2016.1186082
Weldegebrieal, G. K. (2020). Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorganic Chemistry Communications, 120, 108140. https://doi.org/10.1016/j.inoche.2020.108140
Wu, L., Wu, Y., Pan, X., & Kong, F. (2006). Synthesis of ZnO nanorod and the annealing effect on its photoluminescence property. Optical Materials, 28(4), 418–422. https://doi.org/10.1016/j.optmat.2005.03.007
Xie, W., Li, Y., Sun, W., Huang, J., Xie, H., & Zhao, X. (2010). Surface modification of ZnO with Ag improves its photocatalytic efficiency and photostability. Journal of Photochemistry and Photobiology a: Chemistry, 216, 149–155. https://doi.org/10.1016/j.jphotochem.2010.06.032
Xu, X., Jia, Y., Xiao, L., & Wu, Z. (2018). Strong vibration-catalysis of ZnO nanorods for dye wastewater decolorization via piezo-electro-chemical coupling. Chemosphere, 193, 1143–1148. https://doi.org/10.1016/j.chemosphere.2017.11.116
Yaqoob, A. A., Parveen, T., Umar, K., & Mohamad Ibrahim, M. N. (2020). Role of nanomaterials in the treatment of wastewater: A review. Water, 12(2), 495. https://doi.org/10.3390/w12020495
Zare, N., Kojoori, R. K., Abdolmohammadi, S., & Sadegh-Samiei, S. (2022). Ultrasonic-assisted synthesis of highly effective visible light Fe3O4/ZnO/PANI nanocomposite: Thoroughly kinetics and thermodynamic investigations on the Congo red dye decomposition. Journal of Molecular Structure, 1250, 131903. https://doi.org/10.1016/j.molstruc.2021.131903
Zarrabi, M., Haghighi, M., & Alizadeh, R. (2018). Sonoprecipitation dispersion of ZnO nanoparticles over graphene oxide used in photocatalytic degradation of methylene blue in aqueous solution: Influence of irradiation time and power. Ultrasonics Sonochemistry, 48, 370–382. https://doi.org/10.1016/j.ultsonch.2018.05.034
Zarrabi, M., Haghighi, M., & Alizadeh, R. (2019). Enhanced sono-dispersion of Bi5O7I and Bi2ClHO3 oxides over ZnO used as nanophotocatalyst in solar-light-driven removal of methylene blue from water. Journal of Photochemistry and Photobiology a: Chemistry, 370, 105–116. https://doi.org/10.1016/j.jphotochem.2018.10.007
Zarrabi, M., Haghighi, M., Alizadeh, R., & Mahboob, S. (2022). Hybrid sonoprecipitation fabrication of magnetic ZnO-GO-Fe3O4 nanophotocatalyst for solar-light-driven degradation of dyes in water. Materials Research Bulletin, 153, 111907. https://doi.org/10.1016/j.materresbull.2022.111907
Zhang, X., Cao, S., Wu, Z., Zhao, S., & Piao, L. (2017). Enhanced photocatalytic activity towards degradation and H2 evolution over one dimensional TiO2@ MWCNTs heterojunction. Applied Surface Science, 402, 360–368. https://doi.org/10.1016/j.apsusc.2017.01.096
Zhang, W., Huang, W., Tan, J., Huang, D., Ma, J., & Wu, B. (2023). Modeling, optimization and understanding of adsorption process for pollutant removal via machine learning: Recent progress and future perspectives. Chemosphere, 311, 137044. https://doi.org/10.1016/j.chemosphere.2022.137044
Zheng, K., Liu, H., Nie, C., Zhang, X., Hu, H., Ma, G., Wang, H., & Huo, J. (2019). Controllable synthesis of honeycomb-structured ZnO nanomaterials for photocatalytic degradation of methylene blue. Materials Letters, 253, 30–33. https://doi.org/10.1016/j.matlet.2019.06.017
Zyoud ,A., Zu’bi, A., Helal, M. H., Park, D., Campet, G., & Hilal, H. S. (2015). Optimizing photo-mineralization of aqueous methyl orange by nano-ZnO catalyst under simulated natural conditions. Journal of Environmental Health Science and Engineering 13, 1–10. https://doi.org/10.1186/s40201-015-0204-0
Funding
This research received no specific funding.
Author information
Authors and Affiliations
Contributions
KRSEL, REM, and SB ( research assistants) took part in writing, data preparation and processing. NR (professor) took part in supervision, conceptual design formation, validation, writing and editing. MR (professor) took part in supervision, writing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saif Al Essai, K.R., Moheyelden, R.E., Bosu, S. et al. Enhanced mitigation of acidic and basic dyes by ZnO based nano-photocatalysis: current applications and future perspectives. Environ Geochem Health 46, 139 (2024). https://doi.org/10.1007/s10653-024-01935-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10653-024-01935-2