Abstract
The effect of heavy metals (HMs) and polycyclic aromatic hydrocarbons (PAHs) pollution on the microbiological status of soils on the coast of the Taganrog Bay and adjacent areas was studied. The content of total and exchangeable forms of HMs, the content of 16 priority PAHs and the abundance of several groups of culturable microorganisms was determined, namely copiotrophic, prototrophic, aerobic spore-forming bacteria, actinomycetes, molds and yeasts. The content of total and exchangeable forms of HMs in urban coastal soils in industrial zone significantly exceeded that in non-urban soils. The maximum concentrations of total forms of Mn, Cr, Ni, Cu, Zn, Pb and Cd are 1821, 871, 143, 89, 1390, 317 and 10 mg/kg, respectively. The median value of the total content of 16 PAHs in urban soils is 3 times higher than in the soils of natural areas and reached 4309 ng/g. The lowest numbers of copiotrophic bacteria, prototrophic bacteria and aerobic spore-forming bacteria were found in the soils of industrial zone: 6.8, 13.8 and 0.63 million CFU g−1 dry soil, respectively. The largest numbers of copiotrophic bacteria, prototrophic bacteria and aerobic spore-forming bacteria were recorded in the soils of natural areas—72.5, 136 and 5.73 million CFU g−1 dry soil, respectively. It was found that the abundance of copiotrophs, prototrophs, and aerobic spore-forming bacteria is more affected by the urbanization of coastal soils including the pollution of HMs and PAHs. Other groups of microorganisms (actinomycetes, molds and yeasts) turned out to be more resistant to anthropogenic factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic transformation of coastal areas is a global problem. Under the conditions of ongoing climate change, coastal areas are becoming more vulnerable and subject to the influence of human activities (Liu et al., 2020). The most significant anthropogenic impacts occur in highly industrialized regions (Sun et al., 2020). Due to the economic development of coastal cities, industrial growth and overpopulation, the pressure on the coastal ecosystem is increasing, the resilience to the impact of pollutants from land and the ability to self-repair is reduced (Liu et al., 2020).
Soils and their biotic component perform the most important ecosystem functions and ensure the sustainable functioning of coastal ecosystems. In addition, the soil is the main pollutant sink (Pan & Wang, 2012). Among the anthropogenic pollutants, heavy metals (HMs) and polycyclic aromatic hydrocarbons (PAHs) are the most significant (Minkina et al., 2021). They enter the soils of coastal ecosystems as a result of a number of anthropogenic activities, such as the development of industrial centers, tourism and transport, the excessive use of mechanized boats, the discharge of wastewater from large and small local enterprises (Dai et al., 2021; Liu et al., 2017). In world practice, for the control of the environment, including soils, the list of US priority pollutants (US EPA, 2020), which includes 16 representatives of polyarenes, is widely used. PAHs and HMs are often found together in contaminated soils and often come from the same sources, so combined PAHs and HMs contamination is of increasing concern (Liu et al., 2017). The joint effect of PAHs and HMs on microbiological activity in the environment is much more complex than their sole effects (the combined effects of HMs and PAHs on activities depend largely on their concentration ratios in soils (Wang et al., 2007)). Degradation of PAHs can be hindered in the presence of HMs (Moreira et al., 2013; Riis et al., 2002), while PAHs can reduce microbial activity and the ability to immobilize HMs. Thus, combined pollution can have a stronger effect on the activity of soil microorganisms than in soils contaminated with only one type of pollutant (Liu et al., 2017; Maliszewska-Kordybach & Smreczak, 2003).
At the moment, there is an insufficient number of studies devoted to studying the response of border ecosystems (coastal soils) to anthropogenic impact, despite the rapid growth of urbanization of such territories (Liu et al., 2022). At the same time, many aspects of the soil microbial communities response to long-term impacts are poorly understood. However, the study of microbial status appears a very useful indicator in monitoring the influence of pollution on the soil state (Brookes, 1995), since the negative impact of pollutants and urban environmental factors on the microbial community has a negative impact on all components of the ecosystem. Often, under the influence of pollutants, there is a decrease in the number of sensitive microorganisms, so many groups can act as indicators of pollution and the state of soils in general. Copiotrophic, oligotrophic and prototrophic bacteria were previously used as indicator microorganisms for soil health (Oliveira & Pampulha, 2006; van Bruggen et al., 2015). In addition to the above groups, spore-forming bacteria are also used as indicators of increasing concentrations of Cd, Pb, and Zn in soil (Smejkalova et al., 2003), micromycetes and actinomycetes were used to assess joint pollution with PAHs and Cd (Shen et al., 2005).
The purpose of this work was to study the effect of HMs and PAHs pollution on the microbiological status of soils on the coast of the Taganrog Bay and adjacent areas.
Materials and methods
Study area and soil sampling
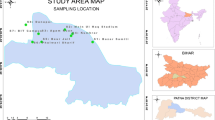
Non-urbanized soils monitoring sites were located in the main landscapes of the northern and southern coasts Taganrog Bay coasts and the Don River delta, including spits, estuaries, and estuarine areas of rivers flowing into the bay. The study of urbanized soils was carried out in the city of Taganrog, including industrial and recreational areas (beach) of the settlement (Fig. 1).
The properties of the studied soils are presented in Table 1. The soil cover of the study area is represented by Haplic Chernozem, Eutric Fluvisol, found in the floodplains of small rivers and especially in the Don River delta (Bezuglova et al., 2002) and the Urbic Technosols of the Taganrog city. The studied soils texture vary from medium to heavy loams. The organic carbon content is in the range of 1.04–3.94% (Table 1). Soil pH ranges from normal (7.45) to alkaline (8.68). However, the pH values are significantly higher in urban soils than in non-urbanized soils.
Eighteen soil samples were taken from the surface soil horizon (0–20 cm). Soil samples were thoroughly mixed and split in two parts. One part was taken immediately for microbiological analysis, the second part was air dried, crushed, and passed through a 1 mm sieve. Two groups of samples were identified: non-urbanized soils (plot numbers 1–9) and urban soils (plot numbers 10–18) located in the city of Taganrog (Table 1, Fig. 1). The most pronounced influence of anthropogenic factors was expected in the industrial zone of Taganrog city (the impact zone of «Krasny Kotelshchik» plant)—No. 10–16.
Various anthropogenic factors also affect non-urbanized soils, for example, site 2 is located in close proximity to the railway and the Bemit plant (automobile industry enterprise), site 8 is located at a distance of 90 m from the road bridge with intense traffic (Table 1).
Soil analysis
Determination of heavy metals and polyaromatic hydrocarbons
The total content of Cr, Mn, Ni, Cu, Zn, Cd, and Pb in soil was determined by X-ray fluorescence analysis on a Spectroscan MAX-GV spectrometer (Spectron, Russia) (OST 10-259-2000, 2001). In addition, the analysis of the content of exchangeable forms of metals was carried out (Minkina et al., 2008, 2013). Ammonium acetate buffer pH 4.5 (AAB) was used to extract exchangeable and soluble in weak acids forms, which characterize the current supply of the element in the soil. The concentrations of HMs in soil sample extracts were determined using the atomic absorption spectrophotometer (AAS) (KVANT 2-AT, Kortec Ltd, Russia).
Extraction of PAHs from soil samples was carried out with hexane in 3 replicates. The pre-interfering lipid fraction was removed by boiling 1 g of sample in 2% KOH solution. The concentration of PAHs in the extract was determined by high-performance liquid chromatography on an Agilent 1260 chromatograph (ISO 13877-2005, 2005). 16 priority PAHs included in the list of US priority pollutants were identified: naphthalene, biphenyl, phenanthrene, anthracene, acenaphthene, acenaphthylene, fluorene, pyrene, chrysene, benzo[a]anthracene, fluoranthene, benzo[b]fluoranthene benzo[k]fluoranthene, benzo[a]pyrene, dibenzo[a,h]antracene, benzo[g,h,i]perylene.
The overall degree of soil pollution by HMs and PAHs was assessed by the Total Pollution Index (TPI) based on the following formula:
where Kc is the concentration factor equal to the ratio of the actual content of mobile forms of HMs and PAHs in soil to its background value (Kc = Mj/Mb), and n is the number of chemical elements with Kc > 1 (Nevidomskaya et al., 2020). Alluvial soil located within the specially protected natural area of the Chumbur–Kosa farm, monitoring site No. 7, was used as a control comparison site.
The TPI is classified into the following pollution levels (Directive document 2.1.7.730-99, 1999; Konstantinova et al., 2020): no pollution (< 4), permissible level (4–8), low pollution (8–16), moderate pollution (16–32), considerable pollution (32–64), very high pollution (64–128), and extreme pollution (> 128).
Microbiological analyzes
To determine the abundance of microorganisms, soil dilutions prepared according to the generally accepted method (Blevins et al., 2020) were inoculated on agar media. The abundance of culturable bacteria and fungi was determined by the number of colonies formed on the nutrient medium. Nutrient agar was used to enumerate copiotrophic bacteria. To determine the abundance of prototrophic bacteria and actinomycetes, they were grown on starch-ammonium agar (ISP-4). Czapek-Dox medium was used to count molds and yeasts, and nutrient agar with the addition barley wort (final concentration of sugars 3%) was used to count aerobic spore-forming microorganisms. The latter were selected by heating soil suspension to 80 °C for 20 min to kill all cells except for the endospores. Aerobic spore-forming microorganisms were counted on days 2, colonies grown on nutrient agar—on days 3–5, colonies grown on ISP-4, Czapek-Dox medium—on days 5–7 from inoculation. Petri dishes with cultures were incubated at 30 °C (Bezuglova et al., 2019).
Statistical analysis
The software STATISTICA 8 (StatSoft, USA) was used to analyze the data. The normality of data distribution of HMs, PAHs was assessed using the Shapiro–Wilk test. As the distribution of some variables didn’t meet the normality criteria, the Mann–Whitney test was applied to assess the differences between independent samples. Spearman’s correlation coefficients were calculated to determine the relationship between microbiological parameters and physicochemical properties of soils. To determine factors influencing microbial communities, multi-way and one-way ANOVA analyzes of variance were applied, Tukey’s test was used as a post hoc test. For the application of one-way and multi-way ANOVA, the following grouping (factors) were identified: urban environment, HMs and PAHs. The total indicator of contamination with TPI was assessed as dangerous (if the TPI value is more than 32) and moderate (if the TPI value is less than 32). The hazardous and moderate degree of soil pollution with PAHs was distinguished according to the total concentration of PAHs and the concentration of benzo[a]pyrene. The soils with a total concentration of PAHs more than 1000 μg/kg, and/or the concentration of benzo[a]pyrene exceeding 100 μg/kg were considered hazardous, while the soils with lower levels of PAHs were considered moderately contaminated. The soils were also divided into urban and non-urban soils based on the presence of the urbic diagnostic horizon, as well as on the basis of the location of the site within the city borders and the degree of sealing of the adjacent territory.
Results
Soil pollution
In soils of natural areas, the median total content of HMs is close to the background value, except for Mn and Cr. At the same time, the content of mobile forms somewhat exceeds the median background concentrations of some metals in the soil. The excess reaches 2.3 times for both Cr and Zn (Fig. 2).
The content of total and mobile forms of HMs in urban soils is noticeably higher than in the soils of natural areas. There is an increase in the variability of HMs concentrations in the soil of the city, compared with the natural area. For most HMs, a significant excess of background concentrations was noted, which is especially pronounced for mobile forms of Zn. Thus, the median value of the concentration of mobile forms of HM Zn in urban soils exceeds the background concentrations by 23.5 times (Fig. 2).
Among various heavy metals, a statistically significant difference between HMs concentrations in the soils of natural and urban areas was found for the total forms of Mn, Cr, Zn, Cu, and for exchangeable Cr and Zn. The concentrations of other studied HMs were either slightly higher in urban soils or did not exceed the concentrations in non-urbanized soils at all (Fig. 2).
The total content of PAHs in the soils of natural and urban areas varies widely (Fig. 3). The median value of the total content of 16 PAHs in urban soils is 3 times higher than in the soils of natural areas. The maximum concentration of pollutants reaches 4309 ng/g.
The median content of individual PAHs compounds in the soils of natural areas does not exceed or is close to the background concentrations of pollutants in the soil. High-molecular PAHs predominate, such as fluoranthene and benzo(g,h,i)perylene, the median concentration of which exceeds 35 ng/g, and the maximum content is noted at the level of 372 ng/g and 680 ng/g, respectively (Fig. 4). The PAHs content in soils decreases in the following order: phenanthrene > benzo(g,h,i)perylene > fluoranthene > pyrene > benzo(b)fluoranthene > chrysene > benzo(a)pyrene > benzo(a)anthracene > fluorene > benzo(k)fluoranthene > dibenz(a,h)anthracene > acenaphthene = acenaphthylene > biphenyl > naphthalene > anthracene.
In the soils of the city, median values of PAHs concentrations in soils exceed the background values, which is especially pronounced for benzo(a)anthracene, benzo(a)pyrene, benzo(b)fluoranthene, dibenz(a,h)anthracene and benzo(g,h,i) perylene. The content of these pollutants in the soils of the city exceeds the background concentrations by more than five times. It is shown that the content of all high-molecular weight compounds in the soils of the city is significantly increased in comparison with the soils of natural areas. At the same time, a change in the predominant composition of individual PAHs compounds is observed, and the decreasing series in terms of the content of pollutants has the following form: (a)anthracene > benzo(k)fluoranthene > dibenz(a,h)anthracene > fluorene > acenaphthylene > acenaphthene > biphenyl > naphthalene > anthracene (Fig. 4).
To calculate the TPIHMs in soil, we used the data on their mobile forms concentrations. According to TPIHM, the soils of natural areas belong to the categories from no pollution to low pollution, and the soils of the city—from low pollution to extreme pollution. In general, the pollution category increases from the southern coast of the Taganrog Bay to the northern one, which is more clearly seen in TPIPAH. For the soils of natural areas, two locally contaminated sites were identified—No. 2 and No. 3, located within the northern coast of the Taganrog Bay. The pollution level of these sites was classified as very high, according to TPIPAH. In general, except for the monitoring sites No. 2 and No. 3, the category of soil pollution in natural areas varies from no pollution to considerable pollution, and urban soils from moderate pollution to extreme pollution (Fig. 5).
Microbial abundance and community structure
The data on the abundance of microorganisms are shown in Fig. 6. The lowest abundance of copiotrophic bacteria and prototrophic bacteria was found in the soil of site No. 14 (located in the industrial zone)—6.8 and 13.8 million CFU g−1 dry soil, respectively. The highest abundance of microorganisms was recorded in site No. 3. The abundance of prototrophic bacteria and copiotrophic bacteria was 136 and 72.5 million CFU g−1 dry soil, respectively. Among all non-urbanized soils, the lowest abundance of microbes was found in the soil of sites No. 4 (28.3 million CFU g−1 dry soil prototrophic bacteria) and No. 7 (31.7 million CFU g−1 dry soil copiotrophic bacteria).
It was found that the abundance of spore-forming microorganisms in urban soils is lower compared to non-urbanized soils (Fig. 7), since TPIHMs is higher in most urban soils as compared to non-urbanized soils. The lowest values of the abundance of spore-forming microorganisms were recorded in monitoring site No. 13 (630 thousand CFU g−1 dry soil), the highest—in the site No. 2 (5.73 million CFU g−1 dry soil). Actinomycetes turned out to be more resistant to the factors of the urban environment than aerobic spore-forming bacteria. Even at the extremely polluted sites in the city of Taganrog, there was no significant impact on actinomycetes abundance, which was as high, as in non-urban soils (about 8.9 million CFU g−1 dry soil (site No. 10)).
No distinctive patterns were identified in the distribution of the abundance of molds and yeasts (Fig. 8), which indicates the relative resistance of these groups of microorganisms to anthropogenic pressure. The maximum number of mold colonies was found in the soil of site 1 (282 thousand CFU g−1 dry soil), and yeasts—in soil 5 (144 thousand CFU g−1 dry soil). The minimum number of mold and yeast colonies was found in the soil of site 4 (15.8 thousand CFU g−1 dry soil and 14.7 thousand CFU g−1 dry soil, respectively).
Statistical analysis results
Correlation analysis results
During the correlation analysis, significant correlations were found between the abundance of copiotrophic bacteria, prototrophic bacteria, actinomycetes and the content of clay (r = − 0.75, at p < 0.01, r = − 0.53, r = − 0.47 at p < 0.05) (Table 2). There are also positive correlations between the concentrations of total forms of HMs (Mn, Zn, Cd) and the content of clay in the soil (r = 0.51, r = 0.55, r = 0.58 at p < 0.05).
Copiotrophic bacteria were the most sensitive to the HMs pollution group of microorganisms. Copiotrophs have shown a medium-strength negative correlation with total concentrations of Mn, Cr, Cu, Zn, Pb, Cd (p < 0.05). Of all the heavy metals considered, there was no correlation only with the total concentration of Ni. Moreover, there was a positive correlation between the concentration of the exchangeable form of Ni and the abundance of spore-forming bacteria. A positive medium-strength correlation was found between the abundance of molds and the concentration of the exchangeable form of Pb (r = 0.6, at p < 0.01). There were no significant correlations between the abundance of actinomycetes, yeasts and HMs concentrations. No correlations were found between the abundance of all studied groups of microorganisms and the content of PAHs in soils.
Analysis of variance
The results of the multi-way ANOVA test are presented in Table 3, the results of the ANOVA and Tukey’s test are presented in Supplementary Table 1.
Urbanization
The abundance of copiotrophic, prototrophic bacteria, spore-forming microorganisms in soils significantly decreases under the influence of the urban environment. At the same time, urban environmental factors had little effect on actinomycetes, molds, and yeasts.
Separate factors, such as the content of HMs and PAHs, made a smaller contribution to the microorganisms’ abundance patterns in soils, since only the abundance of yeasts significantly decreased in the soils heavily polluted with PAHs. The influence of HMs or PAHs on all other studied groups of microorganisms was not statistically significant. Much more effects arise from the interaction of different factors, reported below.
Urbanization*HMs The interaction between the urbanization factor and the content of HMs affected only the distribution of the abundance of molds. At the moderate level of HMs contamination, the numbers of molds in the soils of the city are less compared to non-urbanized soils. In the non-urbanized soils, the total contamination of HMs does not affect the abundance of mold, while in urban soils with a hazardous level of HMs contamination, their abundance is significantly higher than in less contaminated urban soils. In the case of a high level of HMs contamination, the abundance of fungi will be higher in urban soils than in non-urbanized soils.
Urbanization*PAHs The interaction between the urbanization factor and PAHs significantly affected only two groups of microorganisms: spore-forming bacteria and prototrophs. Within urban soils, regardless of the level of PAHs contamination, the abundance of both groups did not differ significantly. At the same time, outside the city, a higher abundance of prototrophs is observed in soils with high concentrations of PAHs. At high level of PAHs pollution, the abundance of aerobic spore-forming bacteria and prototrophs was higher in non-urbanized soils. In soils with lower concentrations of PAHs, a complex of urban environmental factors similarly affected only the abundance of spore-forming bacteria.
HMs*PAHs The combined effect of high concentrations of HMs and PAHs leads to a much more significant decrease in copiotrophs abundance, compared to the soils where the concentrations of PAHs and HMs are low. No effects on the other groups of microorganisms were significant.
In the soils with a low content of HMs, the presence of PAHs did not affect the abundance of all studied groups of microorganisms. At the same time, when the contamination with HMs was significant, microbial communities differ greatly depending on the concentration of PAHs. At the same time, the abundance of copiotrophs and actinomycetes is significantly lower in the soils heavily polluted with PAHs, while the abundance of spore-forming bacteria, on the contrary, is higher.
At high levels of PAHs pollution, the abundance of copiotrophs and prototrophs is lower in soils with high HMs content than in soils with low HMs concentrations. In soils contaminated with PAHs and HMs, the abundance of aerobic spore-forming bacteria and actinomycetes differs insignificantly from their abundance in soils with a low content of HMs but a high level of PAHs contamination.
At high level of HMs contamination, the abundance of spore-forming bacteria was significantly lower, and the abundance of actinomycetes, on the contrary, was higher than in soils with low HMs content. In the soil with a high content of HMs but a low content of PAHs, the abundance of actinomycetes is insignificantly higher than in soil with a low content of HMs but a high content of PAHs.
Urbanization*HMs*PAHs
The abundance of spore-forming bacteria in non-urbanized soils with high concentrations of PAHs and HMs significantly exceeds their abundance in uncontaminated non-urbanized soils. However, if non-urbanized soil is contaminated with HMs only, then the population of spore-forming microbes is suppressed and their abundance will be higher in uncontaminated non-urbanized soil. If the soil contains only high concentrations of PAHs, then the abundance of spore-forming microorganisms will be lower than in soils contaminated with both HMs and PAHs. The presence of PAHs had the strongest influence on the abundance of prototrophic microorganisms in non-urbanized soils. Thus, in soils contaminated with PAHs, their abundance was significantly higher than in uncontaminated soils.
In urban soils contaminated with HMs and PAHs, the abundance of prototrophs and spore-forming bacteria is insignificantly lower than in pristine non-urbanized soils.
HMs and PAHs pollution did not affect abundance the molds and yeasts in urban soils. The studied factors make a smaller contribution to the distribution of yeasts in the studied soils. In non-urbanized soil with a low level of HMs contamination, but a high content of PAHs, the abundance of molds is significantly higher than their abundance in soil contaminated with both pollutants.
In non-urbanized soils with high PAHs concentrations, the abundance of prototrophs increases. In soils with a high content of HMs and PAHs, the abundance of copiotrophs, prototrophs, and actinomycetes decreases, while spore-forming bacteria increase. In soil with a low content of PAHs and a high content of HMs, the abundance of spore-forming microbes decreases, while the abundance of actinomycetes, on the contrary, increases. Also, in urban soils with a dangerous level of HMs contamination, the abundance of molds is increased. Thus, there is a change in soil microbial communities depending on the type of pollution and the presence of other factors in the urban environment (Table 4).
To conclude, the combined effect of HMs and PAHs had a negative influence on more groups of microorganisms than separate pollutants in both urban and non-urban areas.
Discussion
The soils of a large industrial city, such as Taganrog, are characterized by the increased content of almost all pollutants studied in comparison with the soils of natural areas. Differences in the content of HMs are more pronounced for Cr and Zn concentrations. As for the PAHs content, benz(a)anthracene, benzo(a)pyrene, benzo(b)fluoranthene, dibenz(a,h)anthracene and benz(g,h,i)perylene are accumulated (Konstantinova et al., 2020). The predominant composition of PAHs, as well as an increased content of PAHs in urban soils, is characteristic of urbanized areas. The predominance of high-molecular PAHs in the soils of industrial zones may be associated with the intensity of production processes in the industrial zone of Taganrog. The use of coke ovens, electric arc furnaces, and heavy oil burning plants can lead to the release of large amounts of high-molecular weight PAHs (Lee, 2010). The predominance of 4- and 5-ring PAHs compounds in the soils of the impact zones of industrial enterprises indicates that the combustion of petroleum products is a possible source of PAHs (Kwon & Choi, 2014). The increased content of PAHs in the soil areas of the floodplain of the Mius River may be associated with oil spills in the Kerch Strait that happened previously (Kuznetsov & Fedorov, 2014). Some studies report that oil spills are a significant contributor as the main sources of PAHs (Pongpiachan et al., 2018).
It was found that the abundance of copiotrophic bacteria depends on the particle size distribution to a greater extent than that of prototrophic microorganisms. The other studied groups of microbes were not significantly affected by differences in the granulometric composition of soils. It is known that the sorption of HMs depends on particle size distribution (Nevidomskaya et al., 2021). This is because clay minerals absorb cations and anions through ion exchange or adsorption (Yi et al., 2017). Previous studies have shown that a significant contribution to the structure of the microbial communities is made by the granulometric composition, which determines pH, cation exchange capacity and organic matter content (Hamarashid et al., 2010). In addition, clay minerals are able to increase soil pH, reduce the bioavailability of heavy metals in soils (Yi et al., 2017). However, our study found a negative correlation between the abundance of microorganisms and the clay content. This may be due to a decrease in pore space and deterioration of soil aeration (Hamarashid et al., 2010), which creates unfavorable conditions for aerobic microorganisms.
It was found that copiotrophic bacteria are a more sensitive to HMs group of microorganisms, supported by a negative significant correlation. Positive correlations were observed only for Ni which can be explained by the stimulating effect of some HMs (Maliszewska et al., 1985). Previously, it was also shown that the mineralization and ammonification of nitrogen in the soil was stimulated by low cadmium concentrations, while the inhibitory effects were manifested at higher levels (Yang et al., 2005). The same happened with indicators of microbial biomass and basal respiration, which were stimulated at 50 and 100 mg kg−1 Ni, but were inhibited by a further increase in the content of Ni (Xia et al., 2018). Total concentrations of Ni in the soils we studied did not exceed 100 mg kg−1.
Actinomycetes, as well as yeast and molds, were more resistant to pollutants. Previous studies have shown that fungi and actinomycetes in soils are less sensitive than other culturable heterotrophic bacteria (Maliszewska et al., 1985; Oliveira & Pampulha, 2006; Lenart & Wolny-Koładka, 2013). In the work of Zhou et al. (2013), there were also no negative correlations between the concentration of metals and the abundance of fungi. Other studies have shown that the detected fungal CFU is less sensitive than the actual fungal biomass in the soil. HM-tolerant fungi tend to increase their numbers in polluted soils (Bååth, 1989). Various mechanisms of protection of actinomycetes from heavy metals are described, which allow the use of this group of microorganisms for soil phytoremediation (Taj & Rajkumar, 2016). It is also reported that the bacterial population in HM-contaminated sites mainly includes the representatives of Firmicutes, Proteobacteria and Actinobacteria phyla. Representatives of the genus Bacillus, Pseudomonas and Arthrobacter are often present in high abundance. They exhibit pollution tolerance even at high concentrations of Cd, Pb and Cu (Mishra et al., 2017). However, degradation of HMs is impossible; microbial communities can either adapt to their action or transform them into less bioavailable forms.
The absence of significant correlations between the abundance of microorganisms and PAHs concentrations may be due to the fact that PAHs contamination of the studied sites occurred gradually due to combustion of petroleum products, and not by a sharp influx of large amounts of hydrocarbons into the soil, whereas the abundance of microorganisms decreases with a single application of PAHs in model experiments (Singh & Haritash, 2019). It is known that microbial communities adapt to PAHs pollution and PAH-degrading representatives increase in numbers (Mangwani et al., 2017). The species with a limited adaptation capacity are gradually eliminated from communities (Salam et al., 2020). Thus, the studied microbial communities managed to adapt to the accumulation of PAHs. Similar results have been obtained in some studies where even sensitive nitrifiers have developed hydrocarbon tolerance with long-term pollution (Deni & Penninckx, 1999, 2004; Kurola et al., 2005). In addition, the literature describes microbial communities consisting of molds (genus Aspergillus, Penicillium, Fusarium, Trichoderma, Scedosporium and Acremonium) and bacteria (Pseudomonas, Klebsiella, Bacillus, Enterobacter, Streptomyces, Stenotrophomonas, Kocuria and Delftia) that have been isolated from soil contaminated with crude oil. Some isolates showed high tolerance to PAHs contamination, up to 6000 mg/l and were active PAHs degraders (Zafra et al., 2014). Some microbial consortia have been shown to be capable of surviving in the presence of toxic Cd and efficiently degrading high-molecular PAHs (Thavamani et al., 2012). Adapted communities were also found in heavily polluted soils of dried Lake Atamanskoe with extremely high levels of both PAHs and HMs. Despite the very high pollution, the structure of the Atamanskoe lake microbial community demonstrates a significant level of complexity and diversity (Gorovtsov et al., 2021).
The results of the multi-way analysis have led to a number of observations. The abundance of copiotrophs, spore-forming microorganisms and prototrophs was significantly higher in non-urbanized soils, while the abundance of molds, actinomycetes, and yeasts was at the same level. It is known that some yeast isolates are tolerant to 10–300 mM of Cu, Zn, Pb, Cd, Cr and k 0.1–0.5 mM of Hg (Aibeche et al., 2021). High concentrations of PAHs lead to a decrease in the abundance of yeast. The abundance of molds in urban soils with a dangerous level of HMs contamination is greater than in non-urbanized soils. In addition, it is known that the presence of metals can increase fungal activity. In a model experiment, the activity in contaminated soil was still higher than in control after a month of incubation (Kamal et al., 2010). In urban soils with a non-hazardous level of HMs contamination, their abundance is lower than with the same level of contamination in non-urbanized soils. Therefore, in the HM-contaminated soils of the city, the abundance of molds does not differ significantly from their abundance in non-urbanized soils with non-hazardous levels of contamination.
Based on our data, it was found that the combined effect of HMs and PAHs on spore-forming bacteria leads to increased abundance in polluted soils compared to uncontaminated soils. In heavily PAH-contaminated soil with a high HMs content, the abundance of spore-forming microorganisms is higher than in a heavily PAH-contaminated soil with a low HMs content. This dependence is observed both in urban soils and in non-urbanized soils. This is because the high concentrations of HMs and PAHs may inhibit other communities (prototrophs, copiotrophs, actinomycetes), while more resistant spore-forming bacteria survive and can even use PAHs as carbon and energy source. Selective inhibition of microorganisms by HMs and PAHs is also confirmed in previous studies (Thavamani et al., 2012). In addition, it is indicated that the existence of combined pollution (decabromodiphenyl and Cu (especially at high concentration)) in soils reduced microbial diversity compared to the controls (Zhang et al., 2012), It is known that various species of the Bacillus genus are capable of decomposing PAHs and using them as sources of carbon and energy, such as B. subtilis BMT4i (benzo[a] pyrene, naphthalene, anthracene and dibenzothiophene) (Lily et al., 2009). Bacillus pumilus 28–11 (naphthalene) (Calvo et al., 2004), B. thuringiensis (phenanthrene and imidacloprid) (Ferreira et al., 2016), Bacillus sp. SBER3 (anthracene, naphthalene, benzene, toluene and xylene) (Bisht et al., 2014), B. vallismortis JY3A (naphthalene, phenanthrene, anthracene, pyrene, fluorene, benzene, toluene) (Ling et al., 2011). There is also evidence that spore-forming bacteria are more resistant to Ni 2+, Zn 2+, Cd 2+ than other groups of bacteria (Roane & Kellogg, 1996). Ma et al. (2016) indicate that the presence of high concentrations of Mn2 + and fluoranthene enhances the growth of B. subtilis. However, at high concentrations of only HMs, the abundance of aerobic spore-forming bacteria, on the contrary, decreases, since there are other groups of microorganisms resistant to HMs (Molds, Actinomycetes).The opposite situation is observed in the case of the actinomycetes abundance. In the soils with high PAHs content, the level of HMs contamination does not have a significant effect on this group of bacteria. However, in slightly polluted soil with a high content of HMs, their abundance increases. Other studies also note the resistance of streptomycetes to HM. Different mechanisms of HMs neutralization are distinguished, for example, sorption by exopolymers, precipitation, biosorption and bioaccumulation (Timková et al., 2018). In addition, earlier studies showed that actinomycetes are more resistant to Cd than nonmycelial bacteria (Babich & Stotzky, 1977).
When comparing polluted urban and non-urbanized soils with a similar level of pollution, the abundance of fungi is significantly higher in the soils of the city. In urban soils, the other groups of microorganisms are suppressed, and resistant molds can survive. A decrease in the abundance of prokaryotes disrupts natural antagonistic relationships and removes the fungistatic effect of soils (Li et al., 2020). There are many works indicating the tolerance of molds (Oladipo et al., 2018), especially if the strains were isolated from soils contaminated with HMs (Iram et al., 2013).
Despite the important role of HMs and PAHs in shaping the soil microbial communities, it can be concluded that individual factors of HMs, PAHs or even their combined effects do not fully explain the impact of the urban environment on microbial communities, since anthropogenic pressure in the city is not limited by increased concentrations of the studied pollutants(increased pressure on the soil surface, heat island effect (Howard, 2017), fertilizers (Wakida & Lerner, 2005), salinization (Fay et al., 2008), more alkaline reaction compared to adjacent suburban areas (Yang & Zhang, 2015) and others factors). The previous studies emphasize the significant changes in microbial communities depending on the urbanization gradient, including their quantitative and qualitative composition (Chen et al., 2021; Yan et al., 2016; Zhao & Guo, 2010).
Conclusions
The coastal areas of the Taganrog Bay and adjacent territories are a vulnerable environment and the soils are subjected to a complex of factors of variable strength and origin. Despite the comparatively weak correlations of biological indicators and pollutant concentrations, it is worth noting significant differences in the abundance of microorganisms (copiotrophs, prototrophs, spore-formers) in non-urbanized soils and in urban soils. The combined pollution with HMs and PAHs and urbanization have a significant influence on the abundance of copiotrophs and aerobic spore-forming, prototrophic microorganisms. Other groups of microorganisms (actinomycetes, molds, yeasts) turned out to be more resistant to the negative factors of the urban environment. Under the combined influence of urbanization and HMs pollution, there is an increase in the abundance of molds.
In addition, it is worth noting a more pronounced inhibition of soil microbial communities by the combined presence of high concentrations of PAHs and HMs, compared with the effect of PAHs alone. This indicates that high concentrations of HMs can reduce the adaptive potential of microbial communities to the action of PAHs. Thus, communities change depending on the sensitivity of microorganisms to certain factors.
To determine the state of polluted soils that have been contaminated for a long time, it is advisable to determine the abundance of copiotrophs and aerobic spore-forming bacteria as they are the most sensitive to pollution. However, the changes in microbial communities lead to a displacement of sensitive species by more resistant ones. As a result, the total abundance of culturable bacteria may even increase, reflecting the high adaptive potential of soil microbial communities. Therefore, when conducting ecological monitoring based on the analysis of the abundance of microbial communities, it is necessary to take into account various biotic interactions, such as antagonism and synergism.
Availability of data and materials
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also form part of an ongoing study.
References
Aibeche, C., Selami, N., Zitouni-Haouar, F. E. H., Oeunzar, K., Addou, A., Kaid-Harche, M., & Djabeur, A. (2021). Bioremediation potential and lead removal capacity of heavy metal-tolerant yeasts isolated from Dayet Oum Ghellaz lake water (northwest of Algeria). International Microbiology. https://doi.org/10.1007/s10123-021-00191-z
Bååth, E. (1989). Effects of heavy metals in soil on microbial processes and populations (a review). Water, Air, and Soil Pollution, 47(3), 335–379. https://doi.org/10.1007/BF00279331
Babich, H., & Stotzky, G. (1977). Sensitivity of various bacteria, including actinomycetes, and fungi to cadmium and the influence of pH on sensitivity. Applied and Environmental Microbiology, 33(3), 681–695. https://doi.org/10.1128/aem.33.3.681-695.1977
Bezuglova, O. S., Gorovtsov, A. V., Polienko, E. A., Zinchenko, V. E., Grinko, A. V., Lykhman, V. A., & Demidov, A. (2019). Effect of humic preparation on winter wheat productivity and rhizosphere microbial community under herbicide-induced stress. Journal of Soils and Sediments, 19(6), 2665–2675. https://doi.org/10.1007/s11368-018-02240-z
Bezuglova, O. S., Privalenko, V. V., & Ostroborod’ko, N. P. (2002) Biochemical characterization of soils on the Taganrog Bay coast. In Ecosystemic studies of the Azov Sea water and coast (pp. 12–28) RAN (in Russian).
Bisht, S., Pandey, P., Kaur, G., Aggarwal, H., Sood, A., Sharma, S., & Bisht, N. S. (2014). Utilization of endophytic strain Bacillus sp. SBER3 for biodegradation of polyaromatic hydrocarbons (PAH) in soil model system. European Journal of Soil Biology, 60, 67–76. https://doi.org/10.1016/j.ejsobi.2013.10.009
Blevins, R. E., Feye, K. M., Dittoe, D. K., Bench, L., Bench, B. J., & Ricke, S. C. (2020). Aerobic plate count, Salmonella and Campylobacter loads of whole bird carcass rinses from pre-chillers with different water management strategies in a commercial poultry processing plant. Journal of Environmental Science and Health, Part B, 55(2), 155–165. https://doi.org/10.1080/03601234.2019.1670522
Brookes, P. C. (1995). The use of microbial parameters in monitoring soil pollution by heavy metals. Biology and Fertility of Soils, 19(4), 269–279. https://doi.org/10.1007/BF00336094
Calvo, C., Toledo, F. L., & González-López, J. (2004). Surfactant activity of a naphthalene degrading Bacillus pumilus strain isolated from oil sludge. Journal of Biotechnology, 109(3), 255–262. https://doi.org/10.1016/j.jbiotec.2004.01.009
Chen, Y., Martinez, A., Cleavenger, S., Rudolph, J., & Barberán, A. (2021). Changes in soil microbial communities across an urbanization gradient: A local-scale temporal study in the arid southwestern USA. Microorganisms, 9(7), 1470. https://doi.org/10.3390/microorganisms9071470
Dai, C., Han, Y., Duan, Y., Lai, X., Fu, R., Liu, S., & Zhou, L. (2021). Review on the contamination and remediation of polycyclic aromatic hydrocarbons (PAHs) in coastal soil and sediments. Environmental Research. https://doi.org/10.1016/j.envres.2021.112423
Deni, J., & Penninckx, M. J. (1999). Nitrification and autotrophic nitrifying bacteria in a hydrocarbon-polluted soil. Applied and Environment Microbiology, 65(9), 4008–4013. https://doi.org/10.1128/AEM.65.9.4008-4013.1999
Deni, J., & Penninckx, M. J. (2004). Influence of long-term diesel fuel pollution on nitrite-oxidising activity and population size of Nitrobacter spp. in soil. Microbiological Research, 159(4), 323–329. https://doi.org/10.1016/j.micres.2004.06.004
Directive document 2.1.7.730-799. (1999). Hygienic assessment of soil quality in populated areas. Ministry of Health of the Russian Federation, (in Russian).
Fay, L., Volkening, K., Gallaway, C., & Shi, X. (2008). Performance and impacts of current deicing and anti-icing products: User perspective versus experimental data. In 87th annual meeting of the transportation research board (pp. 1–22)
Ferreira, L., Rosales, E., Danko, A. S., Sanromán, M. A., & Pazos, M. M. (2016). Bacillus thuringiensis a promising bacterium for degrading emerging pollutants. Process Safety and Environmental Protection, 101, 19–26. https://doi.org/10.1016/j.psep.2015.05.003
Gorovtsov, A., Demin, K., Sushkova, S., Minkina, T., Grigoryeva, T., Dudnikova, T., & Kocharovskaya, Y. (2021). The effect of combined pollution by PAHs and heavy metals on the topsoil microbial communities of spolic technosols of the lake Atamanskoe, Southern Russia. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-021-01059-x
Hamarashid, N. H., Othman, M. A., & Hussain, M. A. H. (2010). Effects of soil texture on chemical compositions, microbial populations and carbon mineralization in soil. The Egyptian Journal of Experimental Biology, 6(1), 59–64.
Howard, J. (2017). Anthropogenic soils in urban settings. Anthropogenic soils (pp. 187–228). Springer. https://doi.org/10.1007/978-3-319-54331-4_10
Iram, S., Zaman, A., Iqbal, Z., & Shabbir, R. (2013). Heavy metal tolerance of fungus isolated from soil contaminated with sewage and industrial wastewater. Polish Journal of Environmental Studies, 22(3), 691–697.
ISO 13877-2005. (2005). Soil quality: Determination of polynuclear aromatic hydrocarbons-method using high-performance liquid chromatography. Retrieved at October 18, 2021.
Kamal, S., Prasad, R., & Varma, A. (2010). Soil microbial diversity in relation to heavy metals. In Soil heavy metals (pp. 31–63). Springer. https://doi.org/10.1007/978-3-642-02436-8_3
Konstantinova, E., Minkina, T., Konstantinov, A., Sushkova, S., Antonenko, E., Kurasova, A., & Loiko, S. (2020). Pollution status and human health risk assessment of potentially toxic elements and polycyclic aromatic hydrocarbons in urban street dust of Tyumen city, Russia. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-020-00692-2
Kurola, J., Salkinoja-Salonen, M., Aarnio, T., Hultman, J., & Romantschuk, M. (2005). Activity, diversity and population size of ammonia-oxidising bacteria in oil-contaminated landfarming soil. FEMS Microbiology Letters, 250(1), 33–38. https://doi.org/10.1016/j.femsle.2005.06.057
Kuznetsov, A. N., & Fedorov, Y. A. (2014). Oil components in the mouth area of the Don R. and in the Sea of Azov: Results of many-year studies. Water Resources, 41(1), 55–64. https://doi.org/10.1134/S0097807814010072
Kwon, H. O., & Choi, S. D. (2014). Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea. Science of the Total Environment, 470, 1494–1501. https://doi.org/10.1016/j.scitotenv.2013.08.031
Lee, B. K. (2010). Sources, distribution and toxicity of polyaromatic hydrocarbons (PAHs) in particulate matter. In Air pollution. IntechOpen. https://doi.org/10.5772/10045
Lenart, A., & Wolny-Koładka, K. (2013). The effect of heavy metal concentration and soil pH on the abundance of selected microbial groups within ArcelorMittal Poland steelworks in Cracow. Bulletin of Environmental Contamination and Toxicology, 90(1), 85–90. https://doi.org/10.1007/s00128-012-0869-3
Li, X., Garbeva, P., Liu, X., Klein Gunnewiek, P. J., Clocchiatti, A., Hundscheid, M. P., & De Boer, W. (2020). Volatile-mediated antagonism of soil bacterial communities against fungi. Environmental Microbiology, 22(3), 1025–1035. https://doi.org/10.1111/1462-2920.14808
Lily, M. K., Bahuguna, A., Dangwal, K., & Garg, V. (2009). Degradation of benzo[a]pyrene by a novel strain Bacillus subtilis BMT4i (MTCC 9447). Brazilian Journal of Microbiology, 40, 884–892. https://doi.org/10.1590/S1517-83822009000400020
Ling, J., Zhang, G., Sun, H., Fan, Y., Ju, J., & Zhang, C. (2011). Isolation and characterization of a novel pyrene-degrading Bacillus vallismortis strain JY3A. Science of the Total Environment, 409(10), 1994–2000. https://doi.org/10.1016/j.scitotenv.2011.02.020
Liu, L., Barberán, A., Gao, C., Zhang, Z., Wang, M., Wurzburger, N., & Zhang, J. (2022). Impact of urbanization on soil microbial diversity and composition in the megacity of Shanghai. Land Degradation & Development, 33(2), 282–293. https://doi.org/10.1002/ldr.4145
Liu, P., Hu, W., Tian, K., Huang, B., Zhao, Y., Wang, X., & Khim, J. S. (2020). Accumulation and ecological risk of heavy metals in soils along the coastal areas of the Bohai Sea and the Yellow Sea: A comparative study of China and South Korea. Environment International, 137, 105519. https://doi.org/10.1016/j.envint.2020.105519
Liu, S. H., Zeng, G. M., Niu, Q. Y., Liu, Y., Zhou, L., Jiang, L. H., & Cheng, M. (2017). Bioremediation mechanisms of combined pollution of PAHs and heavy metals by bacteria and fungi: A mini review. Bioresource Technology, 224, 25–33. https://doi.org/10.1016/j.biortech.2016.11.095
Ma, X. K., Ding, N., Peterson, E. C., & Daugulis, A. J. (2016). Heavy metals species affect fungal–bacterial synergism during the bioremediation of fluoranthene. Applied Microbiology and Biotechnology, 100(17), 7741–7750. https://doi.org/10.1007/s00253-016-7595-4
Maliszewska, W., Dec, S., Wierzbicka, H., & Woźniakowska, A. (1985). The influence of various heavy metal compounds on the development and activity of soil micro-organisms. Environmental Pollution Series A, Ecological and Biological, 37(3), 195–215. https://doi.org/10.1016/0143-1471(85)90041-8
Maliszewska-Kordybach, B., & Smreczak, B. (2003). Habitat function of agricultural soils as affected by heavy metals and polycyclic aromatic hydrocarbons contamination. Environment International, 28(8), 719–728. https://doi.org/10.1016/S0160-4120(02)00117-4
Mangwani, N., Kumari, S., & Das, S. (2017). Marine bacterial biofilms in bioremediation of polycyclic aromatic hydrocarbons (PAHs) under terrestrial condition in a soil microcosm. Pedosphere, 27, 548–558. https://doi.org/10.1016/S1002-0160(17)60350-3
Minkina, T., Konstantinova, E., Bauer, T., Mandzhieva, S., Sushkova, S., Chaplygin, V., & Maksimov, A. (2021). Environmental and human health risk assessment of potentially toxic elements in soils around the largest coal-fired power station in Southern Russia. Environmental Geochemistry and Health, 43(6), 2285–2300. https://doi.org/10.1007/s10653-020-00666-4
Minkina, T. M., Motuzova, G. V., Mandzhieva, S. S., Nazarenko, O. G., Burachevskaya, M. V., & Antonenko, E. M. (2013). Fractional and group composition of the Mn, Cr, Ni, and Cd compounds in the soils of technogenic landscapes in the impact zone of the Novocherkassk power station. Eurasian Soil Science, 46(4), 375–385. https://doi.org/10.1134/S1064229313040108
Minkina, T. M., Motuzova, G. V., Nazarenko, O. G., Kryshchenko, V. S., & Mandzhieva, S. S. (2008). Combined approach for fractioning metal compounds in soils. Eurasian Soil Science, 41(11), 1171–1179. https://doi.org/10.1134/S1064229308110057
Mishra, J., Singh, R., & Arora, N. K. (2017). Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Frontiers in Microbiology, 8, 1706. https://doi.org/10.3389/fmicb.2017.01706
Moreira, I. S., Amorim, C. L., Carvalho, M. F., Ferreira, A. C., Afonso, C. M., & Castro, P. M. (2013). Effect of the metals iron, copper and silver on fluorobenzene biodegradation by Labrys portucalensis. Biodegradation, 24(2), 245–255. https://doi.org/10.1007/s10532-012-9581-6
Nevidomskaya, D., Minkina, T., Fedorov, Y., Nazarenko, O., Kravtsova, N., & Litvinov, Y. (2020). Integral assessment of heavy metal pollution in Don River estuary soils. In E3S Web of Conferences (vol. 169, p. 01007). EDP Sciences. https://doi.org/10.1051/e3sconf/202016901007
Nevidomskaya, D., Minkina, T., Fedorov, Y., Litvinov, Y., Shcherbakov, A., Sherstnev, A., & Mazarji, M. (2021). Potentially toxic elements distribution in the contaminated bottom sediments by the industrial genesis within Lower Don river system. In E3S Web of conferences (vol. 265, p. 02018). EDP Sciences.
Oladipo, O. G., Awotoye, O. O., Olayinka, A., Bezuidenhout, C. C., & Maboeta, M. S. (2018). Heavy metal tolerance traits of filamentous fungi isolated from gold and gemstone mining sites. Brazilian Journal of Microbiology, 49, 29–37. https://doi.org/10.1016/j.bjm.2017.06.003
Oliveira, A., & Pampulha, M. E. (2006). Effects of long-term heavy metal contamination on soil microbial characteristics. Journal of Bioscience and Bioengineering, 102(3), 157–161. https://doi.org/10.1263/jbb.102.157
OST 10–259–2000. (2001). Soil. X-ray fluorescence determination of the total content of heavy metals. The Russian Federation Ministry of Agriculture (in Russian).
Pan, K., & Wang, W. X. (2012). Trace metal contamination in estuarine and coastal environments in China. Science of the Total Environment, 421, 3–16. https://doi.org/10.1016/j.scitotenv.2011.03.013
Pongpiachan, S., Hattayanone, M., Tipmanee, D., Suttinun, O., Khumsup, C., Kittikoon, I., & Hirunyatrakul, P. (2018). Chemical characterization of polycyclic aromatic hydrocarbons (PAHs) in 2013 Rayong oil spill-affected coastal areas of Thailand. Environmental Pollution, 233, 992–1002. https://doi.org/10.1016/j.envpol.2017.09.096
Riis, V., Babel, W., & Pucci, O. H. (2002). Influence of heavy metals on the microbial degradation of diesel fuel. Chemosphere, 49(6), 559–568. https://doi.org/10.1016/S0045-6535(02)00386-7
Roane, T. M., & Kellogg, S. T. (1996). Characterization of bacterial communities in heavy metal contaminated soils. Canadian Journal of Microbiology, 42(6), 593–603. https://doi.org/10.1139/m96-080
Salam, L. B., Obayori, O. S., Ilori, M. O., & Amund, O. O. (2020). Effects of cadmium perturbation on the microbial community structure and heavy metal resistome of a tropical agricultural soil. Bioresources and Bioprocessing, 7(1), 1–19. https://doi.org/10.1186/s40643-020-00314-w
Shen, G., Cao, L., Lu, Y., & Hong, J. (2005). Influence of phenanthrene on cadmium toxicity to soil enzymes and microbial growth (5 pp). Environmental Science and Pollution Research, 12(5), 259–263. https://doi.org/10.1065/espr2005.06.266
Singh, S. K., & Haritash, A. K. (2019). Polycyclic aromatic hydrocarbons: Soil pollution and remediation. International Journal of Environmental Science and Technology, 16(10), 6489–6512. https://doi.org/10.1007/s13762-019-02414-3
Smejkalova, M., Mikanova, O., & Boruvka, L. J. P. S. (2003). Effects of heavy metal concentrations on biological activity of soil microorganisms. Plant Soil and Environment, 49(7), 321–326.
Sun, M., Wang, T., Xu, X., Zhang, L., Li, J., & Shi, Y. (2020). Ecological risk assessment of soil cadmium in China’s coastal economic development zone: A meta-analysis. Ecosystem Health and Sustainability, 6(1), 1733921. https://doi.org/10.1080/20964129.2020.1733921
Taj, Z. Z., & Rajkumar, M. (2016). Perspectives of plant growth-promoting actinomycetes in heavy metal phytoremediation. In Plant growth promoting actinobacteria (pp. 213–231). Springer. https://doi.org/10.1007/978-981-10-0707-1_14
Thavamani, P., Megharaj, M., & Naidu, R. (2012). Bioremediation of high molecular weight polyaromatic hydrocarbons co-contaminated with metals in liquid and soil slurries by metal tolerant PAHs degrading bacterial consortium. Biodegradation, 23(6), 823–835. https://doi.org/10.1007/s10532-012-9572-7
Timková, I., Sedláková-Kaduková, J., & Pristaš, P. (2018). Biosorption and bioaccumulation abilities of actinomycetes/streptomycetes isolated from metal contaminated sites. Separations, 5(4), 54. https://doi.org/10.3390/separations5040054
van Bruggen, A. H., Sharma, K., Kaku, E., Karfopoulos, S., Zelenev, V. V., & Blok, W. J. (2015). Soil health indicators and Fusarium wilt suppression in organically and conventionally managed greenhouse soils. Applied Soil Ecology, 86, 192–201. https://doi.org/10.1016/j.apsoil.2014.10.014
US EPA (US Environmental Protection Agency). (2020). Integrated risk information system (IRIS). Office of Research and Development. Retrieved September 20, 2020, from https://cfpub.epa.gov/ncea/iris_drafts/AtoZ.cfm
Wakida, F. T., & Lerner, D. N. (2005). Non-agricultural sources of groundwater nitrate: A review and case study. Water Research, 39(1), 3–16. https://doi.org/10.1016/j.watres.2004.07.026
Wang, J., Lu, Y., & Shen, G. (2007). Combined effects of cadmium and butachlor on soil enzyme activities and microbial community structure. Environmental Geology, 51(7), 1221–1228. https://doi.org/10.1007/s00254-006-0414-y
Xia, X., Lin, S., Zhao, J., Zhang, W., Lin, K., Lu, Q., & Zhou, B. (2018). Toxic responses of microorganisms to nickel exposure in farmland soil in the presence of earthworm (Eisenia fetida). Chemosphere, 192, 43–50. https://doi.org/10.1016/j.chemosphere.2017.10.146
Xu, Y., Liang, X., Xu, Y., Qin, X., Huang, Q., Wang, L., & Sun, Y. (2017). Remediation of heavy metal-polluted agricultural soils using clay minerals: A review. Pedosphere, 27(2), 193–204. https://doi.org/10.1016/S1002-0160(17)60310-2
Yan, B., Li, J., Xiao, N., Qi, Y., Fu, G., Liu, G., & Qiao, M. (2016). Urban-development-induced changes in the diversity and composition of the soil bacterial community in Beijing. Scientific Reports, 6(1), 1–9. https://doi.org/10.1038/srep38811
Yang, J. L., & Zhang, G. L. (2015). Formation, characteristics and eco-environmental implications of urban soils: A review. Soil Science and Plant Nutrition, 61(sup1), 30–46. https://doi.org/10.1080/00380768.2015.1035622
Yang, Y., Chen, Y. X., Tian, G. M., & Zhang, Z. J. (2005). Microbial activity related to N cycling in the rhizosphere of maize stressed by heavy metals. Journal of Environmental Sciences, 17(3), 448–451.
Zafra, G., Absalón, Á. E., Cuevas, M. D. C., & Cortés-Espinosa, D. V. (2014). Isolation and selection of a highly tolerant microbial consortium with potential for PAH biodegradation from heavy crude oil-contaminated soils. Water, Air, & Soil Pollution, 225(2), 1–18. https://doi.org/10.1007/s11270-013-1826-4
Zhang, W., Zhang, M., An, S., Lin, K., Li, H., Cui, C., & Zhu, J. (2012). The combined effect of decabromodiphenyl ether (BDE-209) and copper (Cu) on soil enzyme activities and microbial community structure. Environmental Toxicology and Pharmacology, 34(2), 358–369. https://doi.org/10.1016/j.etap.2012.05.009
Zhao, Z., & Guo, H. (2010). Effects of urbanization on the quantity changes of microbes in urban-to-rural gradient forest soil. Agricultural Science & Technology-Hunan, 11(3), 118–122.
Zhou, D. N., Zhang, F. P., Duan, Z. Y., Liu, Z. W., Yang, K. L., Guo, R., & Li, C. F. (2013). Effects of heavy metal pollution on microbial communities and activities of mining soils in central Tibet, China. Journal of Food, Agriculture and Environment, 11(1), 676–681.
Funding
The research was financially supported by the Russian Science Foundation, Project No. 20-14-00317, at the Southern Federal University.
Author information
Authors and Affiliations
Contributions
EP: Conceptualization, Formulation of a Research Problem, Writing. FI: Data Curation, Writing—Reviewing. AG: Writing. TD: Data Processing, Data Performing. VZ: Conducting Experiments. TM: Experiments Design, Data Processing, Methodology, Discussion. SM: Writing—Review and Editing. AB: Methodology, Analytical work, Atomic Absorption. AS: Conducting Experiments, Data Creating. SS: Visualization, statistical processing, Analytical Work, HPLC.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest in this work.
Ethical approval
It is not applicable since the manuscript has not been involved in the use of any animal or human data or tissue.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pulikova, E., Ivanov, F., Gorovtsov, A. et al. Microbiological status of natural and anthropogenic soils of the Taganrog Bay coast at different levels of combined pollution with heavy metals and PAHs. Environ Geochem Health 45, 9373–9390 (2023). https://doi.org/10.1007/s10653-022-01405-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-022-01405-7