Abstract
Owing to the importance of urinary stones as one of the biominerals in the human body, it is necessary to investigate their chemical composition and mineralogy. In this matter, a mineralogical study using X-ray diffraction and scanning electron microscopy indicated that urinary stones in Lorestan Province were divided into 5 groups of calcium oxalate, urate, cysteine, phosphate and mixed stones (Whewellite, uric acid, phosphate). In this regard, the microscopic studies revealed that Whewellite was the most important mineral phase among various phases. In the following, the major and rare elements of each group were determined by inductively coupled plasma mass spectrometry (ICP-MS) and X-ray fluorescence analysis. The obtained results demonstrated that Ca was found the most abundant element in urinary stones. In the analysis results of the major oxides, compared to other major oxides, CaO had the highest frequency in urinary stones. The reason was due to the role of calcium in most of the basic functions in cell metabolism. The average values of isotope 13C and 16O in the studied urinary stones were obtained − 33.71 and − 20.57, respectively. Overall, the values of 13C isotope in urinary stones were lower than those in the similar stones and human hard tissues in other countries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urinary stone illness is the third most common complication among diseases of the urinary system (Chandrajith et al., 2006). It is a biomineral formation in the kidneys, bladder and urinary tract and is among the painful diseases that can have adverse effects on the kidneys and the urinary system if not cured appropriately (Wijayarathna & Abeygunasekera, 2013). Urinary stones have approximately 40 different compounds, and the most common compounds include calcium oxalate (CaC2O4), phosphate Ca5(PO4)3(OH) and uric acid (C5H4N4O3) (Daudon et al., 2010; Hesse et al., 1988). Many of these stones are a combination of two or more mineral phases (Zarasvandi et al. 2014). These complex structures have several causes and are determined by physicochemical factors of the urinary system (Kuta, 2012). In addition, 70–80% of urinary stones are calcium oxalate-type stones (Frackowiaka et al., 2010; Singh et al., 2009). The main components of these stones can be a combination of organic, mineral materials with crystalline or amorphous structures (Hesse et al., 2009). The percentage of patients with urinary stones in diverse areas of the world varies between 20 and 30%, and the frequency of urinary stones is lower in women than in men (Saffarinejad, 2007; Keshavarzi et al., 2016; Zarasvandi et al., 2014; Sood et al., 2010). In recent years, the cause of the increasing prevalence of this disease has been strictly related to changes in lifestyle, industrialization and changes in the diet (Paolo et al., 2013). These changes in environmental parameters have been associated with exercise and physical activity, hot and dry climates and a diet program containing calcium, salt, low potassium, no fiber, excessive animal protein and alcohol (Gupta & Kesarwani., 2002; Holmes et al., 2001). Among environmental factors, water hardness is the most important factor in causing kidney stones (Kohri et al., 1989). The ratio of magnesium (Mg) to calcium (Ca) has also been shown to correlate with urinary stones (Bellizzi et al., 1998; Paolo et al., 2013). Bellizzi et al. (1998) have reported that increasing the hardness level of drinking water can increase the amount of urinary calcium by 50% if oxalate is not excreted. Mineralogical studies of the physicochemical properties of urinary stones in different parts of the world indicated that the incidence of urinary stones is affected by a set of biological and environmental parameters (Abboud, 2008b; Afaj & Sultan, 2005; Ancharov et al., 2005; Chandrajith et al, 2019; Qaader et al., 2006). In fact, other investigations cannot identify a specific parameter as the key cause of the disease (Kerr & Laing, 1992; Al-Eisa et al., 2002; Basiri & Shakhssalim, 2010a, 2010b). Stable isotope analysis can be performed on human leftovers and other species of animal communities such as fish, oysters and mice (Chandrajith et al., 2019). Such an analysis involves identification of isotopes and interpretation of their association with diet, metabolism, environment situation and geography (Chandrajith et al., 2019). The presence of carbon isotopes in human urinary stones is a more helpful tool to determine eating behaviors rather than other human tissues such as hair and urine (Athanasiadou et al., 2017). In human tissues, carbon isotope ratios can vary from person to person and geographical conditions due to different eating habits and lifestyles (Athanasiadou et al., 2017). Tracing isotopes in hard human tissues such as teeth and bones is a useful tool in clinical and forensic medicine (Chandrajith et al., 2019). In general, the isotopic composition of carbon (13C) and oxygen (18O) in human tissues reflects the isotopic composition of consumed food (Krouse & Levinson, 1984; Krouse et al., 1987). Iran is one of the critical regions located in the Africa-Asia urinary stone belt with different climatic conditions (Zarasvandi et al., 2013). The frequency of kidney stones varies with an average of 5.7% in diverse regions of Iran (Zarasvandi et al., 2014). Despite the prevalence of this disease, few studies have been conducted in this area. Mineralogical, geochemical and isotopic properties of kidney stones can help to understand the interactions of geographical, geological and biological factors with the frequency and composition of urinary stones (Athanasiadou et al., 2017; Zarasvandi et al., 2014). In addition, such studies are necessary to find ways to prevent and rehabilitate this disease as well as provide more information about that field. Therefore, the main purpose of this investigation is to explore the mineralogy, geochemical composition and stable isotope compositions in urinary stones of Lorestan Province in southwestern Iran.
The case study
Lorestan with an area of approximately 29,308 km2 is a province located in western Iran (the latitude of 32°37′ -34° 22′ N and longitude of 46° 51′-50° 3′ E). This region has four diverse climates (semidry, semimoist temperate, semimoist cold and altitude climates), which is completely evident from the northeast to the southwest. The minimum and maximum height above sea level is 330 m in Pole-Zal and 4050 m in the Oshtoran Mountains, respectively (Delfan et al., 2014). The location of the province in the geographical area of Zagros has caused the province to have a favorable climate. Lorestan is a mountainous land, and except for a few plains, most areas of the province are covered with dense mountains and narrow valleys of the Zagros Mountains (Gholami et al., 2011). Figure 1 presents the map of Lorestan Province (Gholami et al., 2011).
Material and methods
In this study, 53 urinary stone samples were collected from hospitals in different cities of Lorestan Province in southwestern Iran (Fig. 2). After collecting the samples, the patients' personal information such as age, sex, weight, medical history and address before surgery was recorded in a questionnaire. To remove urine, blood and organic matter residues, each specimen was washed several times by distilled water and after drying, it was kept in a polyethylene bag in the laboratory before crushing and conducting chemical analysis. To measure the concentration of major oxides and trace elements in the selected samples, 10 samples of kidney stones were measured in the geochemistry laboratory of Zarmazma Mineral Studies Co. using the ICP-MS method. In this analysis, 0.25 g of each sample was heated with HF-HClO4-HNO3 acids at 200 °C for 4–5 h. Then, for complete digestion, the samples were dissolved in HCl, and the element concentrations were measured. The accuracy of the results of this method was 5%. The chemical composition of the major oxides was determined in the laboratory of Kansaran Binalood company in Tehran Science and Technology Park using XRF (Philips Magix Pw1480 model). Considering the importance of mineralogical investigations of kidney stones to find relation with the obtained chemical composition and optical properties of urinary stones, 10 samples were selected to prepare thin sections with a polarizing microscope in the mineralogical laboratory of Ahvaz University. Also, 10 samples were sent to Razi Metallurgical Studies Center for X-ray diffraction (XRD) analysis to study the crystalline phase and scanning electron microscopy (SEM) to investigate the size, system, shape and name of the constituent minerals as well as the elemental composition studied urinary stones. In addition, 13 urinary stones samples were analyzed for isotopic characteristics of 13C and 16O by inductively coupled plasma mass spectrometry (ICP-MS) in Actlabs, Canada. The carbon dioxide emitted by the Thermo Fisher Delta V-Plus mass spectrometer was measured after reacting a total of 0.5 mg of the sample powder with 100% phosphoric acid at 70 °C by Gasbench II. The isotopic measurement error of the device is 0.1%. The mixture of oxygen and carbon isotopes of the samples is reported in terms of VPDB and in terms of fraction per thousand.
Results and discussion
The subjects’ age and sex are important factors playing a key role in the formation and diversity of urinary stones (Talwar et al., 1991; Basiri & Shakhssalim, 2010a, 2010b). The urinary stones formation and the predominant chemical composition of stones depend on age and sex (Knoll, 2010). The prevalence rate reported by the age group continuously shows the pattern of increase and decrease with increase in the population age (Afaj & Sultan, 2005). The peak age of prevalence in Iran, Japan and the USA is similar, ranging from 40 to 49 years (Romero et al., 2010). Due to the small number of samples analyzed (n = 53), which can be considered a limitation in this study, all the conclusions made in this section are considered experimental. Furthermore, 60% of the subjects were male, and 40% were female. Table 1 shows the distribution of urinary stones according to the subjects’ age and sex. The prevalence of urinary stones was higher in patients aged 40–60 years. The prevalence of calcium oxalate and uric acid stones in men was more than that in women. Daudon et al. (1993) also showed that calcium oxalate and urate stones were more common in men than in women. Phosphate and cystine stones account for a small percentage of urinary stones. External morphology, color, texture, structure and size of crystals are important factors providing useful information about age, mineralogical composition and possible crystallization processes in biominerals (Afaj & Sultan, 2005; Zarasvandi et al., 2014). In addition, the study of thin sections under polarizing microscopic in geological and geochemical studies is important to understand how urinary stones are formed (Zarasvandi et al., 2014). Microscopic study of the nucleus and radial layers of the studied urinary stones is highly important to identify its morphological characteristics and metabolic illnesses that are responsible for nucleation or growth procedure of stone. Sekkoum et al. (2011a, b) ,Chandrajith (2019) and Abboud (2008a, b, c) investigated the thin sections on the agenda urinary stones and showed concentric and radial layer structures, which is a common feature of calcium oxalate minerals. As demonstrated by Daudon et al. (1993), a very radial structure of Whewellite confirms the crystalline growth and a highly active lithogenic process. Moreover, uric acid stones are formed in the form of pebbles with smooth surfaces, but without polished surfaces. In addition, rough and spherical surfaces have been observed in several urinary specimens. The cross section of Whewellite urinary stones under a polarized microscope shows separate rings with a radial structure around a nucleus (Fig. 2a). Furthermore, oncoids (larger than 2 mm) are comparable in limestone. In some urinary stones, elliptical branching is visible in several thin sections, which may be due to compositional changes with microfilament structures in the delocalized nucleus (Fig. 2b and c). Mineral crystals grow with different cores, which may reflect domains of different urinary composition conditions (Chandrajith et al., 2019). Sedimented layers usually appear in a curved form and show a prominent band pattern. The width of the sheets often differs from the alternative bands of dark and light layers. Narrow layers and radial and hollow structures are communal structural properties in stones appeared under isotropic polarizing light. Cavities in stones differ in terms of shape and size and may be created by air, liquid or both being trapped in the biomass (Sekkoum et al. 2011a, 2011b). Cavities were more communal in the midline between the peripheral tangential layers and the central nucleus (Chandrajith et al., 2019). Examination of the morphological structure of the selected urinary stones using polarizing light has shown that in all studied samples, stratification and radial strictures are clearly visible. (Fig. 3) Another point is that the repetition of the nucleus alternately in the stone structure, in addition to the existence of different stages of formation, highlights the role of multi-nucleation in the creation of urinary compounds. In other words, what was clearly seen in the microscopic study of the thin sections is that the distribution of the constituents of the core is not limited to the presence in this part and in most cases alternately forms a large part of the stone structure (Zarasvandi et al., 2014). Calcite also plays such a role in the studied thin sections and was found in the core of all studied urinary stones (Fig. 4). The presence of an amorphous nucleus in the center of concentric layers is another feature of the calcium oxalate group (Abboud., 2008b). Figure 4a and b shows a mineral with a radial texture growing from the margin to the inside with a high amount of iron oxide. The existence of iron in calcium oxalate stones may be due to the trapping of iron ions on the surface of crystal (Bazin et al., 2007). The existence of calcium oxalate mineral properties in the studied thin sections indicates the fact that oxalate minerals, especially Whewellite mineral, can be considered the most significant minerals in the studied urinary stones (Zarasvandi et al., 2013). Additionally, the presence of concentric and radial morphology, which is the important feature of the calcium oxalate group, can also be observed (Abboud, 2008a, 2008b, 2008c). There are many different types of biominerals, and one of the most common one is calcium carbonate with calcite polymorphs (McGrath, 2001). Figure 4c shows calcite biominerals with an amorphous-shaped texture. The presence of these biominerals indicates that in addition to Whewellite minerals, biominerals are also effective in the formation of urinary stones. Calcium minerals constitute around 50% of known biominerals (Lowenstam & Weiner, 1989). This is due to the role of Ca in most of the basic functions of the cellular metabolism (Lowenstam & Margulis, 1980; Simkissand Wilbur, 1989; Berridge et al., 1998). Ca is a major component of various urinary stones, and the highest amount of sulfur (S) is observed in cystine stones (C6H12N2O4S2) (Zarasvandi et al., 2014). Sulfur is also a major component of cystine stones (Hesse et al., 2009).
X-ray diffraction (XRD) test results
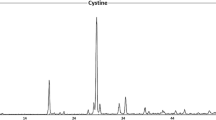
Table 1 presents the mineralogical results of urinary stones, which have been determined using the X-ray diffraction (XRD) method. X-ray diffraction results show that the main minerals in the studied urinary stones are: Whewellite (C2CaO4.H2O) (monoclinic), Weddellite (C2CaO4.2H2O), Uricite (C4 (NH)2O2C (NH)2O (monoclinic), Cysteine (C6H12N2O4S2) and Hydroxyapatite (Ca10 (PO4)6 (OH)2) (Fig. 5). Calcium oxalate minerals existing in the form of monohydrate (Whewellite) and dihydrate (Weddellite) are known for their abundance of Ca (Fig. 5a) (Abboud, 2008a, 2008b, 2008c). Like all urinary stones, uric acid is formed when uric acid levels in the urine increase and remain undissolved. Under these conditions, a very short period is required for uric acid crystals to be formed. Lack of water in the urinary system also increases the rate of formation of these stones (Becker, 2007). Urate stones, commonly referred to as uric acid stones, have two types of aqueous (uric acid and anhydrous (uricite)) (Abboud, 2008a, 2008b, 2008c). One of the most important reasons for the formation of urate stones can be related to the presence of high levels of uric acid and thus the lower pH level of urine (pH > 5.6)). Urine can be used for various reasons such as metabolic processes as well as the type of food used by patients, but the patient's increased age can also be another important reason to increase the prevalence of uric acid minerals in patients with urinary stones (Hesse et al., 2009). In general, uric acid minerals are formed when acidic urine and calcium oxalate minerals are formed when the urine is alkaline (Fig. 5b and c) (Hesse et al., 2009). The presence of these compounds together indicates a change in urine pH. In the sample of the studied stones, the calcium oxalate–urate group with Weddellite-oricite phases was also found (Fig. 5c). Cystine-type stones are dense amino acid stones formed in certain tissues, such as nerves and muscles (Fig. 5d) (Becker, 2007). To prevent an increase in the amount of cystine in the urine and forming cystine-type urinary stones, a diet with very little amino acid can be used (Zarasvandi et al., 2014). Unfortunately, choosing such a diet may significantly reduce patients' quality of life (Zarasvandi et al., 2014). In addition, high levels of sodium in the organs of the body, especially the urinary tract, are one of the important reasons for the formation of cystine stones (Zarasvandi et al., 2014). Since cystine secretion is directly associated with an increase in urinary sodium, sodium chloride uptake should also be limited. Obviously, since sodium is one of the most common elements in drinking water, high-hardness drinking water (containing high amounts of sodium and calcium) can also be another reason for the increased formation of cystine stones (Zarasvandi et al., 2013). Additionally, due to the presence of sulfur in the composition of these stones (Chandrajith et al., 2006), the presence of high amounts of sulfur can also be effective in the prevalence of cystine-type urinary minerals, so that drinking water from calcareous and carbonate formations can raise the risk of cystine stones. The mineral is found in pure form in the studied stones. The most common group (35%) is calcium oxalate stones. The second largest group is calcium oxalate/uric acid (20%) mixed stones, while 15% belong to the uric acid group. Calcium oxalate/phosphate stones (15%) and cystine (10%) also form urinary stones (Fig. 6). To investigate the urinary stones diversity in Lorestan Province, the results of the present study were compared to the data reported from other countries such as Iraq (Afaj & Soltan, 2005), Jordan (Abboud, 2008a, b, c), Sri Lanka (Chandrajith et al., 2019) and Khuzestan (Iran) (Zarasvandi et al., 2013) and Fars (Iran) (Keshavarzi et al., 2015) provinces, which are presented in Fig. 7. The choice of Jordan and Iraq, in addition to the high frequency of urinary stones in these areas, due to the location of these countries in the Middle East and their climatic conditions is almost similar to Lorestan Province. Sri Lanka has also been selected as an Asian country in the Asia-Africa Kidney Belt. The high distribution of urate group minerals in the study area (35%), in addition to hot and dry climatic conditions in some parts of the province, is probably related to the diet of residents in these areas. Furthermore, since drinking water with high hardness and a diet rich in protein are the causes of cystine stones, the abundance of these stones in the study area can be attributed to the diet of the inhabitants of the area (Zarasvandi et al., 2014). The results of this comparison indicate that the frequency of oxalate calcium urinary stones in Lorestan Province, with other countries except Jordan, shows a relative similarity. The very high abundance of calcium oxalate can also be related to the amount of calcium in the diet and drinking water used (Abboud, 2008b).
Scanning electron microscopy (SEM) studies
Calcium oxalate minerals like Whewellite, which can be detected by the monoclinic system and plate morphology, can be clearly seen in the SEM images of urinary stones (Fig. 8a and b) (Abboud, 2008a, 2008b, 2008c). In SEM images, uric acid crystals with a monoclinic system are polymorphically and prismatic recognizable (Giannossi & Summa, 2012a, 2012b). In some samples, uricite crystals are visible in a background of fine and compact crystals (Fig. 8c). This difference in crystals indicates a difference in the concentration of uric acid in the urine as well as a different period in the formation of urinary crystals. Large crystals exhibit the slow formation of biomineralization, but the compression of the crystals may be due to the presence of excessive urate compounds in the urinary system (Hesse et al., 2002).
The cystine (C6H12N2O4S2) biomineral also shows the hexagonal crystal system well (Fig. 8d). In some cases, due to changes in urine pH, crystals of uric acid and calcium oxalate are seen together in the urinary stone. The association of uricite with Whewellite indicates a change in the pH of the urinary system. In the urinary system, uric acid crystals precipitate at pH > 6 and lead to formation of uric acid urinary stones, but if the urinary system becomes alkaline, uric acid remains in the solution and does not precipitate; therefore, the conditions are favorable for the formation of calcium oxalate crystals like Whewellite (Giannossi & Summa, 2012a, 2012b). These changes can be owing to environmental factors such as nutrition and drinking and metabolic water (Hesse et al., 2002). Large crystalline and prismatic cystine crystals are found among calcium oxalate minerals (Fig. 8d). In addition to calcium oxalate and urate minerals, phosphate minerals like struvite, which are formed under alkaline conditions similar to calcium oxalate minerals, are also observed in SEM images with the Whewellite mineral. Irregular morphology is specific to phosphate minerals, which can be observed in SEM images like spherical and semi-spherical shapes of specific phosphate minerals on calcium oxalate monoclinic crystals (Fig. 8f).
Status of elemental composition of the studied urinary stones and their comparison to other regions of the world
Determination of the concentrations of major oxides and trace elements is one of the important factors in environmental studies (Zarasvandi et al., 2014). In addition, concerning the study of urinary stones, the concentration of elements such as Na, Cu, Mg, S, Ca, P and Mn in the urinary stones is important. The presence or absence of some elements in the human body is one of the factors that can affect the formation of urinary stones (Touryan et al., 2004). The effect of trace elements on kidney disease is not yet known, but scientists have progressively concentrated on the effect of trace elements on urinary stone formation (Keshavarzi et al., 2015; Zarasvandi et al., 2013; Chandrajith, 2006). In the current research, the concentration of the elements selected from 10 urinary stone samples was investigated and is presented in Table 2. Ca is the most abundant existing in major compounds in urinary stones compared to other elements. This is due to the role of Ca in most of the basic functions in cell metabolism (Abboud, 2008a, 2008b, 2008c). Ca affects urinary stones depending on the water and the type of food, such as milk, dairy products, eggs, tea and hard water (Zarasvandi et al., 2013; Robertson et al., 1980; Sobhi, 2006). The Na, P and S are also abundant in urinary stones. Relatively high amounts of Mg, Fe and K are present in all of the studied urinary stone samples (Table 3). The Fe in the urine is usually derived from the human urinary cells, and its percentage rises in the urinary system (Chandrajith, 2006). Other elements such as Al, Cu, Ni and Sr produce weakly soluble salts with phosphate and oxalate ions and therefore play a crucial role in the formation of urinary stones (Bazin, 2007). Munoz and Valiente (2005) indicated that such elements accelerated the biocrystallization and recrystallization procedure and affected the external morphology of growing crystals. Bazin (2007) indicated the inhibitory role of Al, Fe and Cu in the growth of calcium oxalate at trace amounts. These elements display a strong affinity for alkaline elements and become a second alternative in organic and inorganic structures that could potentially affect kidney stone disease (Bazin, 2007). The main cause of the presence of Na and K in urine samples is their amount in water or food. The average concentrations of Na, K and Mg in the studied samples are 616.5, 1770.7 and 374 ppm, respectively. Table (4) compares the average concentrations of metal elements in the present study to samples taken in different geological regions of other places. Differences between the concentration of elements in urinary stones sampled from diverse areas are likely related to geology, quality of drinking water, diet and treatment facilities (Abboud, 2008b; Golovanova et al., 2006). Thus, it is not surprising that urine stones sampled from Lorestan Province are different from other regions of the world such as Jordan (Abboud, 2008a, 2008b, 2008c), Sri Lanka (Chandrajith et al., 2019) and Italy (Giannossi et al., 2013). Comparison of the results of the present study to those of other studies conducted in Iran, including Khuzestan (Zarasvandi et al., 2014) and Shiraz (Keshavazi et al., 2015), and the northern part of the world such as Jordan (Abboud, 2008a, 2008b, 2008c), Sri Lanka (Chandrajith et al., 2019), Czech Republic (Kuta et al., 2013) and Basilicata (Italy) (Giannossi et al. 2013, shows that the major elements such as Ca, Mg and K are less concentrated in the studied urinary stones than in other regions. The Fe, Al and Pb in the studied urinary stones in Lorestan Province have higher values compared to other places compared (Table 4). Furthermore, other parameters such as race (Akoudad et al., 2010), gender, age, diet and geographical conditions directly affect urinary stone formation (Atan et al., 2005; Mohamed Farook et al., 2006). The main environmental factors are geology and lithology (Kohri et al., 1989), water-soluble salts such as Mg, CO32−, Ca (Siener, 2004) and water hardness (Schwartz et al., 2002). These factors are important in determining the mineralogical composition, especially in different types of calcium oxalate minerals. According to the presented cases, the elemental composition results of urinary stones in Lorestan Province well show that drinking water quality and diet considerably affect the composition of kidney stones. Differences in the chemical composition of urinary stone samples collected in Lorestan Province with other regions of the world especially in Jordan and cities like Shiraz (Iran) confirm the result. Divalent ions (for example, Zn and Sr) are more probable to be substituted in stones containing calcium (Bazin et al., 2007). The amount of metals (such as Zn and Sr) among calcium oxalate stones is higher than that of Whewellite (Joost & Tessadri 1986). Concentrations of Zn and Sr in the calcium oxalate group with the mineral phases of Weddellite and Whewellite (208 ppm and 230 ppm, respectively) were higher than those of the mineral phase of pure Weddellite and Whewellite (69 ppm and 73 ppm, respectively) (Fig. 9).
In fact, the amount of elements in urinary stones depends on the mineral phase (Golovanova et al., 2006). The amount of Zn in Weddellite is higher than Whewellite, owing to substitution of Zn for ions with similar valances formed during the crystallization process, and then is converted to Whewellite (Giannossi, 2012a; 2012b). Therefore, trace elements can help to stabilize calcium oxalate dihydrate (Weddellite) (Hesse et al., 1976). The amount of Zn and Sr in mixed stones indicates different phases within the stone (Perk et al., 2002). The amount of Fe in non-calcium stones (uric acid and cystine) is less than that in calcium stones (calcium oxalate) (Bazin et al., 2007). High levels of Fe may be due to the inhibitory property of Fe+3 during the crystallization of calcium oxalate. Muñoz et al. (2010) and Meyer and Angino (1997) showed the inhibitory effect of Cu on calcium phosphate stones, but did not indicate effects on the crystalline growth of calcium oxalate. The results of the current study show that the major elements such as Mg and P are less concentrated in the studied urinary stones than in other areas. The metals of Fe and Al in the samples of Lorestan Province have higher values than those of other cities. According to the results, CaO ranged from 0.05 to 37.33% with an average of 10.81% has higher concentration than that of other major oxides in the studied urinary stones. Ca is a major component of various urinary stones. Alkali metal oxides such as Na2O and K2O are present in urinary samples with a mean of 0.21 and 0.76%, respectively. The important cause of the presence of Na and K in the urinary stones is related to diet habit, including consumed water or food. The average amount of MgO and SO3 in the studied urinary samples is 0.37 and 0.41%, respectively. The key source of MgO is its high amount in water. MgO is one of the most important compounds in the process of biological calcification, but its main effect on the formation of kidney stones has not yet been determined. Laboratory studies have proven the role of MgO in reducing the nucleation of calcium oxalate crystals in the existence of Mg (Singh et al., 2009). S is one of the main components of cystine stones (C6H12N2O4S2). The Al2O3 levels in the studied stones varied from 0.33 to 0.36%. The concentration of Al+3 in phosphate urinary stones like apatite has the highest concentration. Low amounts of Al+3 cause oxalate ions to escape, thereby preventing the formation of CaO compounds (Abboud, 2008a, 2008b, 2008c). The value of LOI in all of the studied samples varies from 55.17 to 99.86% with an average of 86.25%. It should also be noted that determination of LOI values in this experiment is highly important (Zarasvandi et al., 2014). The highest and lowest levels of LOI are uric acid and phosphate stones, respectively. LOI is a volatile substance, and HCO and carbon dioxide are carbonates and considered a part of elemental and oxidative analyses and test quality, including the amount of organic materials and carbonate in lake sediments (Heiri et al., 2001).
Correlation of elements in urinary stones
Apart from environmental and biological factors, one of the key parameters affecting the composition of urinary stones is to investigate the inter-elemental relationship that can be geochemically substituted for each other (Zarasvandi et al., 2013). To investigate the controlling role of elements in determining the chemical composition of urinary stones in Lorestan Province, the correlation coefficient among the major oxides as well as between the major oxides and trace elements is presented in Tables 5 and 6. Correlation coefficients corresponding to the major oxides indicate no significant relationship between CaO and P2O5, as the two major oxides in urinary stones, which can be related to mineralogical studies (Zarasvandi et al., 2013). In other words, urinary stones create calcium and phosphate in the same form, but generally CaO and P2O5 oxides form the minerals phosphate and calcium oxalate separately. CaO forms calcium oxalate, while significant concentrations of P2O5 oxides are not able to replace mineral compounds. In other words, the concentration of CaO in phosphate stones and the concentration of P2O5 in calcium oxalate stones are low. The results also demonstrate that both P2O5 and CaO phases are located in the structure of phosphate and calcium minerals, and consequently, the amount of these oxides increases with the increase in both mentioned mineral phases (Zarasvandi et al., 2013). The data also show the R2 value between MgO and P2O5 (0.95), revealing the tendency of MgO to participate in the phosphate structure. Positive correlation (0.92) of Na2O with P2O5 indicates that it is due to the high affinity of NaO to P2O5 (Chandrajith et al., 2006; Zarasvandi et al., 2013). MgO does not positively correlate with CaO. Table 6 presents the correlation coefficients of the major oxides with several trace metal elements (6) and displays that all other elements except Sr are not associated with other major oxides such as P2O5, CaO and MgO. This can be assigned to the presence of these trace metal elements in high amounts in drinking water and nutrition of the study area. Therefore, geochemical controllers do not play a major role in their amount (Zarasvandi et al., 2013 ). Table 7 presents the correlation coefficient among the metal elements. A positive relation between Na and Ca (R2 = 0.47) indicates that Na substitutes Ca due to the similarity of the ionic radius in the crystal structure (Abboud, 2008a, 2008b, 2008c). The positive correlation between Mg and P (R2 = 0.8) indicates the tendency of Mg to enter the structure of phosphate minerals. Mg is one of the key metals in biological classification, and its existence in urinary stones is the result of increasing the Mg concentration in the human body ((Atakan et al., 2007; Deeming & Webu, 1977).
The exact role of Mg in the formation of kidney stones has not been completely elucidated thus far (Siner et al., 2004. Kohri et al., 1988). Evidently, food and hard water are responsible for the amounts of Mg, along with some drugs, and Mg is one of the main components of phosphate stones (Giannossi et al., 2013). In such stones, Mg is deposited with ammonium and phosphate in alkaline urine (Keshavarzi et al., 2015). A negative correlation between Ca and K (R2 = − 0.04) indicates that these two elements have different geochemical properties. A positive correlation between Na and P can be considered a strong affinity of alkali metals and phosphorus in urinary stones (Keshavarzi et al., 2015). The positive correlation of P and Ca with Sr can be due to substitution of this element for Ca and P in oxalate-type urinary stones. Ca and Sr compete for their place in the calcium oxalate structure due to similarities in the metabolic process (Abboud, 2008a, 2008b, 2008c). The correlation between Mg and Sr is 0.64 due to placement of high amounts of Sr in calcium oxalate minerals.
Isotopic studies
Stable isotopes of carbon and oxygen (16O and13C) are extensively applied as biogeochemical traces, but the composition of isotopes in the urinary stones is not known completely (Chandrajith et al., 2019). The average isotopes 13C and 16O in the analyzed samples are − 33.71 ‰ (− 24.87 to − 39.56) and − 20.57 (− 15.78 to − 25.98)‰, respectively. Calcium oxalate/hydroxyapatite stones are 16O rich in isotope compared to uric acid stones with an average of − 21.52 and Whewellite stones with an average of − 23.75, as reported by Chandrajith et al. (2019) (Table 8). The isotope values of 13C and 16O are higher in calcium oxalate/hydroxyapatite samples than in uric acid and calcium oxalate urinary stones. Calcium oxalate/hydroxyapatite and Whewellite stones have similar values of 13C isotope in the studied urinary stone samples. Uric acid stones showed higher 13C isotope values compared to Whewellite and calcium oxalate/hydroxyapatite, while Krouse et al. (1987) showed that uric acid stones exhibited lower values of 13C isotope than calcium stones did. The amount of 13C isotope depletion in kidney stones in the study area is probably related to eating habits. Foods of C4 plant origin such as corn and millet are used in smaller quantities by the residents of the study area. Additionally, the statistics prepared by the patients indicate the consumption of more protein substances like meat in their eating habits. The combination of C and O isotopes of human and animal hard tissues depends largely on their eating habits. In human tissues, carbon isotope ratios can vary individually and geographically due to different eating habits and patterns (Ayliffe et al., 2004; Cerling et al., 2006; Nardoto et al., 2006). The trend of changing carbon isotope ratios in eating habits belongs to plants that undergo photosynthetic pathways C4, C3 or CAM. As Minagawa (1992) indicated, C3 plants have an average 13C isotope of approximately − 25‰, while the plant C4 on average 13C shows approximately − 11‰ (Table 9). Terrestrial and marine animal resources have a carbon isotope ratio of approximately − 15 and − 18‰ (Table 9) (Chandrajith et al., 2019). The oxygen isotope ratio probably indicates the isotope composition of the ingested water as well as the food. A linear relationship was found between drinking water consumption and hard tissues of the human body observed in previous studies (Krouse et al., 1987; Levinson et al., 1987; Longinelli, 1984). Since the patients selected for this study were from different climatic regions in the study area, it is expected that there will be wider variations in 18O values. Further research is needed to investigate the relationship between eating habits and kidney stones’ isotopic composition.
Conclusion
According to mineralogical studies conducted using X-ray diffraction analysis, urinary stones in Lorestan Province are in 5 groups of calcium oxalate, urate, cystine, phosphate and mixed stones. SEM images clearly confirm the results of this analysis. The results of ICP-MS showed that the Ca had the highest abundance compared to other elements in urinary stones in the study area. This is owing to the role of Ca in most of the basic functions in cell metabolism. The results of chemical analysis show that the major oxides of urinary stones, which have been collected in Lorestan Province, contain P2O5 and CaO. Other existing oxides are MgO, SO3, N2O and K2O. Comparison of the results of this study to some parts of Iran such as Khuzestan and Fars provinces as well as regions of Sri Lanka, Jordan, Czech Republic and Italy shows the major elements such as Ca, Mg and K, in the studied urinary stones compared to others regions which have lower concentrations. Calculation of the correlation coefficients for major oxides and trace elements shows that P2O5 and CaO are not significantly associated with each other, while MgO is positively correlated with P2O5. The positive correlation between Na2O and P2O5 is due to the affinity of the alkali metal for phosphate. A positive correlation between Na and Ca indicates that Na substitution for Ca is due to the similarity of the ionic radius in the crystal structure. In addition, the positive correlation between Mg and Pb indicates the tendency of Mg to enter the structure of phosphate minerals. The average isotopes 13C and 16O are − 33.71 and − 20.57, respectively. The reduced trace element content of 13C isotope compared to urinary stones elsewhere indicates the role of nutrients in stone formation. Kidney stones can be to some extent used for forensic research, since they provide information about human exposure to trace elements. This study demonstrated that the distribution and presence of elements in urinary stones were not related only to geochemical factors, but they included environmental conditions, mineralogy and biology.
Data availability
Not applicable.
References
Abboud, I. A. (2008). Mineralogy and chemistry of urinary stones: patients from North Jordan. Environmental Geochemistry Health, 30(5), 445–463. https://doi.org/10.1007/s10653-007-9128-7
Abboud, I. A. (2008). Analyzing correlation coefficients of the concentrations of trace elements in urinary stones. Jordan Journal of Earth Environmental Science, 1(2), 73–80.
Abboud, I. A. (2008). Concentration effect of trace metals in Jordanian patients of urinary calculi. Environmental Geochemistry and Health, 30(1), 11–20. https://doi.org/10.1007/s10653-007-9103-3
Afaj, A. H., & Sultan, M. A. (2005). Mineralogical composition of the urinary stones from different provinces in Iraq. Scientific World Journal, 5, 24–38. https://doi.org/10.1100/tsw.2005.2
Akoudad, S., Szklo, M., McAdams, M. A., Fulop, T., Anderson, C. A. M., Coresh, J., & Kottgen, A. (2010). Correlates of kidney stone disease differ by race in a multi-ethnic middle-aged population: The ARIC study. Preventive Medicine, 51(5), 416–420. https://doi.org/10.1016/j.ypmed.2010.08.011
Al-Eisa, A. A., Al-Hunayyan, A., & Gupta, R. (2002). Pediatric urolithiasis in Kuwait. International Urology and Nephrology, 33(1), 3–6. https://doi.org/10.1023/a:1014419830292
Ancharov, A. I., Nizovskii, A. I., Gridnev, S. A., Feofilov, I. V., & Vichkanov, A. N. (2005). An attempt of in vivo X-ray diffraction analysis of kidney stones with the use of synchrotron radiation". Nuclear Instruments Methods Physics Research, 543(1), 302–305. https://doi.org/10.1016/j.nima.2005.01.245
Atakan, I. H., Kaplan, M., Seren, G., Aktoz, T., Gül, H., & Inci, O. (2007). Serum, urinary and stone zinc, iron, magnesium and copper levels in idiopathic calcium oxalate stone patients. International Urology and Nephrology, 39(2), 351–356. https://doi.org/10.1007/s11255-006-9050-4
Atan, L., Andreoni, C., Ortiz, V., Silva, E. K., Pitta, R., Atan, F., & Srougi, M. (2005). High kidney stone risk in men working in steel industry at hot temperatures. Urology, 65(5), 858–861. https://doi.org/10.1016/j.urology.2004.11.048
Athanasiadou, D., Godelitsas, A., Sokaras, D., Karydas, A. G., Dotsika, E., & Xanthos, S. (2017). New Insights into the chemical and isotopic composition of human body biominerals II: COM kidney stones from Greece. International Archives Urology and Complication, 3, 1–20. https://doi.org/10.23937/2469-5742/1510020
Ayliffe, L. K., Cerling, T. E., Robinson, T., West, A. G., Sponheimer, M., & Passey, B. H. (2004). Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet. Oecologia, 139(1), 11–22. https://doi.org/10.1007/s00442-003-1479-x
Basiri, A., Shakhssalim, N., Ghahestani, S. M., & Basiri, H. (2010). The demographic profile of urolithiasis in Iran: a nationwide epidemiologic study. International Urology and Nephrology, 42(1), 119–126. https://doi.org/10.1007/s11255-009-9588-z
Basiri, A., Shakhssalim, N., Khoshdel, A. R., Ghahestani, S. M., & Basiri, H. (2010). The demographic profile of urolithiasis in Iran: a nationwide epidemiologic study. International Urology Nephrology, 42(1), 119–126. https://doi.org/10.1007/s11255-009-9588-z
Bazin, D., Chevallier, P., Matzen, G., Jungers, P., & Daudon, M. (2007). Heavy elements in urinary stones. Urological Research., 35(4), 179–184. https://doi.org/10.1007/s00240-0070099-z
Becker, G. (2007). Uric acid stones. Nephrology, 12, S21–S25.
Bellizzi, V., Nicola De, L., Minutolo, R., Russo, D., Cianciaruso, B., & Andreucci, M. (1998). Effects of water hardness on urinary risk factors for kidney stones in patients with idiopathic nephrolithiasis. Nephron, 81, 66–70. https://doi.org/10.1159/000046301
Cerling, T. E., Wittemyer, G., Rasmussen, H. B., Vollrath, F., Cerling, C. E., & Robinson, T. J. (2006). Stable isotopes in elephant hair document migration patterns and diet changes. Proceedings of the National Academy of Sciences of the United States of America, 103(2), 371–373. https://doi.org/10.1073/pnas.0509606102
Chandrajith, R., Wijewardana, G., Dissanayake, C. B., & Abeygunasekara, A. (2006). Biomineralogy of human urinary calculi (kidney stones) from some geographic regions of Sri Lanka. Environmental Geochemistry and Health, 28(4), 393–399. https://doi.org/10.1007/s10653-006-9048-y
Chandrajith, R., Weerasingha, A., Premaratne, K. M., Gamage, D., Abeygunasekera, A. M., Joachimski, M. M., & Senaratne, A. (2019). Mineralogical, compositional and isotope characterization of human kidney stones (urolithiasis) in a Sri Lankan population. Environmental Geochemistry and Health, 41(5), 1881–1894. https://doi.org/10.1007/s10653-018-0237-2
Daudon, M., Bader, C. A., & Jungers, P. (1993). Urinary calculi: Review of classification methods and correlations with etiology. Scanning Microscopy, 7(3), 1081–1106.
Daudon, M., Bouzidi, H., & Bazin, D. (2010). Composition and morphology of phosphate stones and their relation with etiology. Urological Research, 38(6), 459–468. https://doi.org/10.1007/s00240-010-0320-3
Deeming, S., & Weber, C. (1977). Evaluation of hair analysis for determination of zinc status using rats. American Journal of Clinical Nutrition, 30(12), 2047–2052. https://doi.org/10.1093/ajcn/30.12.2047
Delfan, B., Kazemeini, H., & Bahmani, M. (2015). Identifying effective medicinal plants for cold in Lorestan province, West of Iran. Journal of Evidence-based Complementary Alternative Medicine, 20(3), 173–179. https://doi.org/10.1177/2F2156587214568458
Frackowiaka, A., Skibinski, P., Gawe, W., Zaczynska, E., Czarny, A., & Gancarz, R. (2010). Synthesis of glycoside derivatives of hydroxyanthraquinone with ability to dissolve and inhibit formation of crystals of calcium oxalate Potential compounds in kidney stone therapy. European Journal of Medicinal Chemistry., 45(3), 1001–1007. https://doi.org/10.1016/j.ejmech.2009.11.042
Gholami, M., Mirzaei, S., & Jomehzadeh, A. (2011). Gamma background radiation measurement in Lorestan province. Iran International Journal of Radiation Research, 9(2), 89–93.
Giannossi, M. L., & Summa, V. (2013). An observation on the composition of urinary calculi: environmental influence. Medical Geochemistry (pp. 67–90). Dordrecht: Springer. https://doi.org/10.1007/978-94-007-4372-4_5
Giannossi, M. L., Mongelli, G., Tateo, F., & Summa, V. (2012). Mineralogical and morphological investigation of kidney stones of a Mediterranean region (Basilicata, Italy). Journal X-Ray Science Technology, 20(2), 175–186. https://doi.org/10.3233/XST-2012-0327
Giannossi, M. L., Summa, V., & Mongelli, G. (2012). Trace element investigations in urinary stones: A preliminary pilot case in Basilicata (Southern Italy). Journal of Trace Elements in Medicine and Biology, 27(2), 91–97. https://doi.org/10.1016/j.jtemb.2012.09.004
Golovanova, O., Palchik, N., & Maksimova, N. A. (2006). Comparative characterization of the microelement composition of kidney stones from patients in the Novosibirsk and Omsk Regions. Chemical for sustainable development., 15(1), 55–61.
Gupta, N. P., & Kesarwani, P. (2002). Current approaches in the medical management of urolithiasis: A review article. Indian Journal of Urology, 19(1), 20.
Heiri, O., Lotter, A. F., & Lemcke, G. (2001). Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. Journal of Paleolimnology, 25, 101–110. https://doi.org/10.1023/A:1008119611481
Hesse, A. (2009). Urinary stones. In F. Lang (Ed.), Encyclopedia of molecular mechanisms of disease (pp. 2144–2147). Springer.
Hesse, A., & Sanders, G. (1988). Atlas of infrared spectra for the analysis of urinary concrements. Georg Thieme.
Hesse, A., Berg, W., Schneider, H. J., & Hienzsch, E. (1976). A contribution to the formation mechanism of calcium oxalate urinary calculi. Urological Research, 4(4), 157–160.
Hesse, A., Tiselius, H. G., & Jahnen, A. (2002).Urinary stones–diagnosis. Treatment, and prevention of recurrence (2nd ed, pp. 228). Basel: Karger.
Hoefs, J., & Armbruster, T. (1978). 13C/12C-Verha¨ltnisse in menschlichen Harnkonkrementen. Naturwissenschaften, 65(11), 586–589.
Holmes, P., James, K. A. F., & Levy, L. S. (2001). Is low-level environmental mercury exposure of concern to human health? Science of the Total Environment, 408, 171–182. https://doi.org/10.1016/j.scitotenv.2009.09.043
Joost, J., & Tessadri, R. (1986). Trace element investigations in kidney stone patients. European Urology, 13(4), 264–270.
Kerr, A., & Laing, M. (1992). Mineralogical Studies of human urinary cal cull from Natal. Environmental Geochemistry and Health, 14, 19–25. https://doi.org/10.1007/BF01783622
Keshavarzi, B., Yavarashayeri, N., Irani, D., Moore, F., Zarasvandi, A., & Salari, M. (2015). Trace elements in urinary stones: A preliminary investigation in Fars Province. Iran. Environmental Geochemistry and Health, 37(2), 377–389. https://doi.org/10.1007/s10653-014-9654-z
Keshavarzi, B., Yavar Ashayeri, N., Moore, F., Irani, D., Asadi, S., Zarasvandi, A., & Salari, M. (2016). Mineralogical composition of urinary stones and their frequency in patients: Relationship to gender and age. Minerals, 6(4), 131.
Knoll, T. (2010). Epidemiology, pathogenesis, and pathophysiology of urolithiasis. European Urology Supplements, 1, 802–806.
Kohri, K., Garside, J., & Blacklock, N. (1988). The role of magnesium in calcium oxalate urolithiasis. British Journal of Urology, 61(2), 107–115. https://doi.org/10.1111/j.1464-410x.1988.tb05057.x
Kohri, K., Kodama, M., Ishikawa, Y., Katayama, Y., Takada, M., Katoh, Y., Kataoka, K., Iguchi, M., & Kurita, T. (1989). Magnesium-to-calcium ratio in tap water, and its relationship to geological features and the incidence of calcium-containing urinary stones. Journal of Urology, 142(5), 1272–1275. https://doi.org/10.1016/s0022-5347(17)39054-7
Krouse, H. R., & Levinson, A. A. (1984). Geographical trends of carbon and sulphur isotope abundances in human kidney stones. Geochimica Cosmochimica Acta, 48(1), 187–191. https://doi.org/10.1016/0016-7037(84)90360-0
Krouse, H. R., Levinson, A. A., Piggott, D., & Ueda, A. (1987). Further stable isotope investigations of human urinary stones: Comparison with other body components. Applied Geochemistry, 2(2), 205–211.
Kuta, J., Machat, J., Benova, D., Cervenka, R., & Koristkova, T. (2012). Urinary calculia-typical source of information on mercury in human biomonitoring. Open Chemistry., 10(5), 1475–1483. https://doi.org/10.2478/s11532-012-0063-9
Levinson, A. A., Luz, B., & Kolodny, Y. (1987). Variations in oxygen isotopic compositions of human teeth and urinary stones. Applied Geochemistry, 2(4), 367–371. https://doi.org/10.1016/0883-2927(87)90021-7
Longinelli, A. (1984). Oxygen isotopes in mammal bone phosphate: A new tool for paleohydrological and paleoclimatological research? Geochimica Et Cosmochimica Acta, 48(2), 385–390. https://doi.org/10.1016/0016-7037(84)90259-X
Lowenstam, H. A., & Margulis, L. (1980). Calcium regulation and the appearance of calcareous skeletons in the fossil record. In M. Omori (Ed.), The mechanisms of biomineralization in animals and plants (pp. 289–300). WatabeN(eds) Tokai University Press.
Lowenstam, H. A., & Weiner, S. (1989). On Biomineralization. Oxford University Press.
McGrath, K. M. (2001). Probing material formation in the presence of organic and biological molecules. Advanced Material, 13(12), 989–992.
Meyer, J. L., & Angino, E. E. (1977). The role of trace metals in calcium urolithiasis. Investigative Urology, 14(5), 347–350.
Minagawa, M. (1992). Reconstruction of human diet from r13C and r15N in contemporary Japanese hair: A stochastic method for estimating multi-source contribution by double isotopic tracers. Applied Geochemistry, 7(2), 145–158.
Mohamed Farook, N. A., Mozhiyras, P., & Nalini, R. (2006). Inhibition of mineralization of urinary stone forming minerals by medicinal plants. European Journal of Chemistry, 12, 182–185. https://doi.org/10.1007/BF02867665
Munoz, J. A., & Valiente, M. (2005). Effects of trace metals on the inhibition of calcium oxalate crystallization. Urological Research, 33(4), 267–272. https://doi.org/10.1007/s00240-005-0468-4
Muñoz, J. A., López-Mesas, M., & Valiente, M. (2010). Development and validation of a simple determination of urine metabolites (oxalate, citrate, uric acid and creatinine) by capillary zone electrophoresis. Talanta, 81(1–2), 392–397.
Nardoto, G. B., Silva, S., Kendall, C., Ehleringer, J. R., Chesson, L. A., & Ferraz, E. S. B. (2006). Geographical patterns of human diet derived from stable isotope analysis of fingernails. American Journal of Physical Anthropology, 131(1), 137–146. https://doi.org/10.1002/ajpa.20409
Paolo, C., Thomas, D., & Yigal, E. (2013). Medical Geochemistry. Geological Materials and Health, 1, 7–12. https://doi.org/10.1007/978-94-007-4372-4
Perk, H., Ahmet Serel, T., Kosar, A., Deniz, N., & Sayin, A. (2002). Analysis of the trace element contents of inner nucleus and outer crust parts of urinary calculi. Urologia Internationalis, 68(4), 286–290. https://doi.org/10.1159/000058452
Qaader, D. S., Yousif, S. Y., & Mahdi, L. K. (2006). Prevalence and etiology of urinary stones in hospitalized patients in Baghdad. Eastern Mediterranean Health Journal, 12, 853–861.
Robertson, W. G., & Peacock, M. (1980). The cause of idiopathic calcium stone disease: Hypercalciuria or hyperoxaluria?. Nephron, 26(3), 105–110.
Romero, V., Akpinar, H., & Assimos, D. G. (2010). Kidney stones: A global picture of prevalence, incidence, and associated risk factors. Reviews in Urology, 12(2–3), e86.
Safarinejad, M. R. (2007). Adult urolithiasis in a population-based study in Iran: prevalence, incidence, and associated risk factors. Urological Research, 35(2), 73–82. https://doi.org/10.1007/s00240-007-0084-6
Schwartz, B. F., Schenkman, N. S., Bruce, J. E., Leslie, S. W., & Stoller, M. L. (2002). Calcium nephrolithiasis: effect of water hardness on urinary electrolytes. Urology, 60, 23–30. https://doi.org/10.1016/s0090-4295(02)01631-x
Sekkoum, K., Cheriti, A., Taleb, S., & Belboukhari, N. (2011). FTIR spectroscopic study of human urinary stones from El Bayadh district (Algeria). Arabian Journal of Chemistry, 9(3), 330–334. https://doi.org/10.1016/j.arabjc.2011.10.010
Siener, R., Jahnen, A., & Hesse, A. (2004). Influence of a mineral water rich in calcium, magnesium and bicarbonate on urine composition and the risk of calcium oxalate crystallization. European Journal of Clinical Nutrition., 58(270), 276. https://doi.org/10.1038/sj.ejcn.1601778
Simkiss, K., & Wilbur, K. M. (1989). Biomineralization: cell biology and mineral deposition (p. 92101). CA: San Diego.
Singh, V. K., Rai, A. K., Rai, P. K., & Jindal, P. K. (2009). Cross-sectional study of kidney stones by laser-induced breakdown spectroscopy. Laser Medicine Science, 24(749), 759. https://doi.org/10.1007/s10103-008-0635-2
Sobhi, N. (2006). The mineralogy and chemistry of urinary stones from the Arabian Gulf. Internet Site, 7, 11–17.
Sood, A., Qualls, C., & Schuyler, M. (2010). Leptin, adiponectin, and asthma: findings from a population-based cohort study. Annals of Allergy, Asthma & Immunology, 104(4), 355.
Talwar, I. M., Yagyik, Y., & Lal, N. (1991). Thermally stimulated polarization studies of kidney stones. Biomaterials, 12, 518–520. https://doi.org/10.1016/0142-9612(91)90152-z
Touryan, L. A., Lochhead, M. J., Marquardt, B. J., & Vogel, V. (2004). Sequential switch of biomineral crystal morphology using trivalent ions. Nature Materials, 3(4), 239–243. https://doi.org/10.1038/nmat1096
Wijayarathna, K. S. N., & Abeygunasekera, A. M. (2013). Pathogenesis of nephrolithiasis. The Journal of Urology, 31(3), 28–32. https://doi.org/10.1016/j.juro.2012.11.069
Zarasvandi, A., Heidari, M., Sadeghi, M., & Mousapoor, E. (2013). Major and trace element composition of urinary stones, Khuzestan province, Southwest Iran. Journal of Geochemical Exploration, 131, 52–58. https://doi.org/10.1016/j.gexplo.2012.08.014
Zarasvandi, A., Carranza, E. J. M., Heidari, M., & Mousapour, E. (2014). Environmental factors of urinary stones mineralogy, Khouzestan Province Iran. Journal of African Earth Sciences, 97, 368–376. https://doi.org/10.1016/j.jafrearsci.2014.04.031
Funding
No financial support has received to conduct the current study.
Author information
Authors and Affiliations
Contributions
Seadat Aghajari contributed to data acquisition, data curation and formal analysis, Ahad Nazarpour was involved in conceptualization, writing—original draft, and validation, and Sima Sabzalipour and Rozbahani contributed to data curation, formal analysis and validation.
Corresponding author
Ethics declarations
Conflicts of interest
There is no conflict of interest regarding this research and its dissemination.
Animal research
Animal was not used in this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aghajari, S., Sabzalipour, S., Nazarpour, A. et al. Mineralogy, geochemistry, 13C and 16O isotopic characteristics of urinary stones in Iran, a case study of Lorestan Province. Environ Geochem Health 43, 5157–5176 (2021). https://doi.org/10.1007/s10653-021-00986-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00986-z