Abstract
This study developed a method to build relationships between chemical fractionations of heavy metals in soils and their accumulations in rice and estimate the respective contribution of each geochemical speciation in the soils from the Yangtze River Delta, China. In contaminated areas, residue and humic acid-bound fractions in soils were the main phases for most heavy metals. The mobility of heavy metals was in this following order: Cd > Pb ≈ Zn > Ni > As ≈ Cr > Hg. Transfer factors calculated by the ratios of specific fractionations of heavy metals in the soil–rice system were used to assess the capability of different metal speciation transfer from soil to rice. The carbonate and Fe/Mn oxyhydroxides bound phase had significant positive correlations with total metal concentrations in rice. Hg uptake by rice might be related to the exchangeable and carbonate-bound fractions of soil Hg. Results of PCA analysis of transfer factors estimated that the labile fractions (i.e. water soluble, exchangeable and carbonate bound) contributed more than 40% of the heavy metal accumulations in rice. Effect of organic matter and residue fraction on metals transfer was estimated to be ~ 25 to ~ 30% while contribution of humic acid and Fe/Mn oxyhydroxides-bound fractions was estimated to be ~ 20 to ~ 30%. Modified risk assessment code (mRAC) and ecological contamination index (ECI) confirmed that the soil samples were polluted by heavy metals. Soil Cd contributed more than 80% of mRAC. Contrarily, the main contributors to ECI were identified as As, Hg, Pb and Zn. The average values of total target hazard quotient (TTHQ) and Risktotal were above 1 and 10–4 respectively, implying people living in the study area were exposed to both non-carcinogenic and carcinogenic risk. As and Pb were the main contributor to high TTHQ value while As, Cd and Cr in rice contributed mostly to Risktotal value. Spatial changes of ecological risk indexes and human health risk indexes showed that the samples with high TTHQ values distributed in the area with high values of mRAC. Likewise, the area with high ECI values and with high carcinogenic risk overlapped.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the severe environmental issues, heavy metal pollutions in soil and biological adverse effect were widely concerned (Alloway, 2013). Heavy metals in soil could cause not only ecological issues (Nannipieri et al., 1990), but also human health problems (Kim et al., 2015; Satarug & Moore, 2004). Previous researches have reported that heavy metals such as Cd, Pb and Cr could cause cardiovascular systems and nervous system disorder, renal failure and increased carcinogenic risk (Bandara et al., 2008; IARC, 2004; Phan et al., 2019; Yang et al., 2004; Zhang et al., 2015). The pollution status and ecological effect of heavy metals are commonly reflected by bioavailability/bioaccessibility or biotoxicity (Shahid et al., 2017). Since the labile fraction of heavy metals could enter plants or animals easily, ecological effect of heavy metals is closely related to mobility and speciation of heavy metals (Kabata-Pendias & Pendias, 2001; Landner & Reuther, 2005).
Mobilization of heavy metals which is decided by speciation can influence their bioavailability or bioaccessibility directly (Dean, 2010; Landner & Reuther, 2005; Shahid et al., 2017). Generally speaking, it is recognized that the labile fraction determined by sequential extraction can enter bioorganic issue easily (Dean, 2010; Landner & Reuther, 2005). Therefore, distribution and geochemical speciation of heavy metals in soil could aid in characterizing the transfer of heavy metals and assessment of ecological risk (Reeder et al., 2006). To better understand mobility and ecological effect of heavy metals, sequential extraction procedures have been used broadly using chemicals or chelating resin, which take into account effects of soil properties fully. The speciation, distribution and mobilization of heavy metals in soil could be identified well (Gabarrón et al., 2019; Moral et al., 2005; Pardo et al., 2011; Wen et al., 2020a). According to research by Tessier et al. (Tessier et al., 1979), the speciation of metals is classified as water-soluble, exchangeable, carbonate-bound, oxide-bound, organic matter-bound (including weak organic and strong organic matters) and residual fractions basically. Water-soluble, exchangeable even acid-soluble (carbonate-bound) parts of heavy metals in soil or sediment are often identified as labile fraction, which can be extracted by water, weak acid, EDTA-NH4OAc, or chelate resin like DGT film (Abd Aziz et al., 2019; Meers et al., 2007; Peng et al., 2019; Ure, 1996). However, different extraction procedures and chemical extractants may lead to discrepancy in labile proportion of heavy metals in soils. (Luo et al., 2019; Sumalatha et al., 2019; van der Ent et al., 2019; Wen et al., 2020b).
Although labile fractions of heavy metals are considered as the bioavailable fraction. Non-labile parts of heavy metals also can contribute to metals accumulation in plant or human potentially (Hutchinson et al., 2000; Liu et al., 2017; van der Ent et al., 2019). Most researches focused on influence of the labile fraction of heavy metals in soil on bioavailability or bioaccessibility (Alan & Kara, 2019; Nolan et al., 2005; Onyatta & Huang, 1999; Tang et al., 2006). However, there are few researches that have discussed the contribution of different heavy metals speciation in soil to plants, especially acid-soluble, oxide-bound, organic matter-bound fractions.
In addition, speciation analysis could give an insight about ecological risk of heavy metals. Since 1960s, various quality indexes have been developed and used to assess potential threat of heavy metals to agroecosystem and human health (Adriano, 2001; Hakanson, 1980; Li et al., 2014; Müller, 1969). For instance, the assessment indexes such as ecological risk index (RI) and modified hazard quotient (mHQ), which take toxicity and biological effect into account, were developed and applied (Benson et al., 2018b; Saeedi & Jamshidi-Zanjani, 2015). These methods could give overall assessments for heavy metals pollution and ecological effect in soil or sediment. Considering mobility of heavy metals, indexes of risk assessment code (RAC) and modified RAC (mRAC) were developed using labile fraction of heavy metals, which also reflect potential of mobilization of heavy metals in various environmental media (Gusiatin & Kulikowska, 2014; Saeedi & Jamshidi-Zanjani, 2015).
To further investigate and quantitatively estimate the transfer and contribution of different chemical forms of heavy metals in soil to rice, geochemical speciation of heavy metals in soil and metals in rice were analysed, and contribution of forms of heavy metals in soil to rice was calculated and estimated using a statistical method in the present study. Transfer of heavy metals in soil to rice could be characterized. In addition, ecological risk assessment based on speciation analysis and health risk assessment was applied to estimate the potential risk of heavy metals in the agroecosystem in the Yangtze River Delta.
Materials and methods
Study area and sampling
The study area located at the junction of Wuxi and Suzhou, Jiangsu Province, China, which had been identified as contaminated by heavy metals, through a geochemical soil survey that measured 103,000 surface soils covering the entire province (Liao et al., 2007). Surface soil samples and paired rice grain samples were collected from 36 sampling sites (Figure S1). The sampling density was 1 sample/4 km2 in “highly contaminated area”, 1 sample/16 km2 in “medium/slightly contaminated area” and 1 sample/32 km2 in “uncontaminated area”, according the classification by Liao et al. (2007). Systematic sampling scheme was designed. Surface soils (0–20 cm depth) were composited from four subsamples randomly within 50 m of the site using a plastic shovel and then stored in plastic bags and taken to the laboratory to store at room temperature (~ 25 °C). The rice grain samples were collected synchronously during the pre-harvest drainage period and sealed in plastic bags and taken back to laboratory.
Chemical analysis
Rice
The raw paddy rice ears were dried at 25 °C for at least 5 days. The husks were removed from the rice grains, and the brown rice grains were ground into fine powder using a mortar at room temperature. A proportion (0.5 g) of the powdered rice grain samples (> 200 mesh) were put into a plastic tube after being oven dried (at 50 °C for 1 day). The rice grain samples were digested using a mixture of HCl–HNO3–HClO4–HF as described in Mao et al. (2019).
Soil
The soil samples were air-dried for 72 h and then passed through a 100-mesh sieve to remove pebbles and debris. Approximately 1.0 g of dried soil samples was ground to fine power (< 0.075 mm) and digested with a mixture of HCl–HNO3–HClO4–HF (Mao et al., 2019).
Arsenic and Hg were analysed on an XGY-1011A Atmospheric Fluorescence Spectrometer (AFS). The concentrations of Cd, Cr, Ni, Pb and Zn in digestion solutions were measured using a Thermo Fisher Scientific X Series Inductively Coupled Plasma-Mass Spectrometer (ICP-MS).
Every 10th sample was analysed in duplicate for comparison purposes. Relative deviations of replicate measurements were < 10% accuracy range. The reagent blanks and certificated standards were included in each batch of the samples, to verify the accuracy and precision of the chemical analysis. Certificated reference soil (GSS-2, GSS-4 and GSS-6) and rice grain (GSB-1) were checked every 10 samples and were in good agreement with their certificated concentrations.
Sequential extraction procedures
Geochemical speciation of heavy metals in soil was analysed though sequential extraction procedure modified from Tessier et al. (1979). The modified extraction method classified metal speciation into 7 chemical forms through procedure designed by China Geological Survey (CGS, 2005):
-
1.
Fraction A (water-soluble fraction) 2.5 g of soil samples was mixed with 25 mL deionized water and shaken for 30 min with continuous agitation with ultrasonic at 25 °C;
-
2.
Fraction B (exchangeable fraction) The residue from extraction A was mixed with 25 mL MgCl2 solution (1 mol/L, pH = 7.0 ± 0.2), shaken and continuous agitation with ultrasonic at 25 °C for 30 min.
-
3.
Fraction C (carbonates-bound fraction) The residue from extraction B was mixed with 25 mL 1 mol/L (NaOAc), pH = 5.0 ± 0.2 of NaOAc/HOAc solution, shaken and continuous agitation with ultrasonic at 25 °C for 1 h.
-
4.
Fraction D (humic acid-bound fraction) The residue from extraction C was mixed with 50 mL Na4PO7·10H2O solution with 0.1 mol/L, pH = 10.0 ± 0.2.
-
5.
Fraction E (Fe/Mn oxyhydroxides-bound fraction) The residue from step D was extracted with 50 mL 0.25 mol/L NH2OH·HCl + 0.25 mol/L HCl solution, and shaken with ultrasonic for1 h at 25 °C.
-
6.
Fraction F (organic matter-bound fraction) The residue from step E was mixed with 3 mL HNO3 (0.02 mol/L), 5 mL H2O2 (30%, pH = 2.0 ± 0.2, adjusted using 1:1 HNO3), shaken for 1.5 h at 83 ± 3 °C, then added 3 mL H2O2 (30%, pH = 2.0 ± 0.2, adjusted using 1:1 HNO3) and extracted for 70 min at 83 ± 3 °C, when temperature of mixture dropped to 25 °C, added 2.5 mL NH4AcO (3.2 mol/L)–HNO3 (3.2 mol/L) solution, and then diluted to 25 mL and stay overnight or 10 h.
-
7.
Fraction residue The residue from step F was dissolved with 5 mL concentrated HCl + HNO3 + HClO4 (1:1:1) and 5 mL HF (1.15 g/mL) on hot plate; then remove HF using 5 mL HCl (1:1 with H2O).
The residue of each step in extraction procedure was washed with Milli-Q water for 10 min prior to next step. The average recoveries for sequential extractions were 91.3 ± 9.1%, 90.7 ± 8.3%, 93.5 ± 6.1%, 89.7 ± 10.1%, 93.4 ± 6.9%, 92.2 ± 8.3%, 91.1 ± 8.6% for As, Cd, Cr, Hg, Ni, Pb, and Zn, respectively, which confirmed the robustness the analysis of geochemical speciation used in this study.
Statistical analysis
For statistical analyses, all concentration data of oven dry-weight soil or rice grain samples were presented in mg kg−1. The transfer factor (TF) calculated by ratio of heavy metals in rice to soil was used to express the transfer of heavy metals from soil to rice as shown below:
where TFtotal represent the transfer factor of heavy metal; ci represent the metal concentration in rice grain; Ctotal represent total concentration of heavy metal in soil; Cwatersoluble, Cexchangeable, Ccarbonate, Chumic, CFe/Mn, Corganic, Cresidue represent the concentration of heavy metal extracted in each step of the sequential extraction procedures, respectively; TFwatersoluble, TFexchangeable, TFcarbonate, TFhumic, TFFe/Mn, TForganic, TFresidue represent the transfer factor for water-soluble, exchangeable, carbonate-bound, humic acid-bound, Fe/Mn oxyhydroxides-bound, organic matter-bound and residue fraction, respectively.
Data statistical analysis, principal component analysis and multiple linear regression were performed using SPSS software package (SPSS Inc. version 24). The spatial distributions of the metal concentrations, ecological and health risk indexes were generated using Arc GIS (ESRI, Inc. version 10.2).
Ecological risk assessment
Ecological risk assessment of heavy metals in soil was applied to understand and estimate potential risk based on mobility and total concentrations of heavy metals.
The prevalent index for metal mobility is risk assessment code (RAC), which is calculated through labile fraction of heavy metal (water-soluble, exchangeable and carbonates-bound fractions) and total metal concentration in soil. Based on this index, modified risk assessment code (mRAC) takes ecological toxic effect into account (Saeedi & Jamshidi-Zanjani, 2015).
where Cwater-soluble represents water-soluble fraction of heavy metal in soil; Cexchangeable represents exchangeable fraction of heavy metal in soil; Ccarbonate represents carbonate-bound fraction of heavy metal in soil; Ctotal represent total concentration of heavy metal in soil.
where RACi is RAC value of the ith metal; Tri is toxic factor of ith metal according to Hakanson (1980); and n is the number of metals. Cr was thought to have similar geochemical behaviour to Ni; therefore, Tr value of Cr is the same as Ni (Long et al., 1995; Saeedi & Jamshidi-Zanjani, 2015). The criterion for mRAC proposed by Saeedi and Jamshidi-Zanjani (2015) is: mRAC < 1, no potential adverse effect; 1 ≤ mRAC < 10, low potential adverse effect; 10 ≤ mRAC < 30, medium potential adverse effect; 30 ≤ mRAC < 50, high potential adverse effect; mRAC ≥ 50, very high potential adverse effect.
Total concentrations of soil metals are often used to assess their potential ecological risk. Considering the ecotoxic effect, TEL (threshold effect level), PEL (probable effect level), SEL (severe effect level), the indexes of modified hazard quotient (mHQ) and ecological contamination index (ECI) were used to assess potential risk of heavy metals in soil in this study (Benson et al., 2018b). As described by Benson et al. (2018b), ECI is derived from mHQ values and principal component analysis, and mHQ was estimated using the following formula:
where mHQi is the mHQ value of the ith metal and TELi, PELi and SELi have been reported in researches before (Table S1, MacDonald et al., 2000; Smith et al., 1996). The criteria for mHQ are: mHQ < 0.5, nil to very low severity of contamination; 0.5 ≤ mHQ < 1.0, very low severity of contamination; 1.0 ≤ mHQ < 1.5, low severity of contamination; 1.5 ≤ mHQ < 2.0, moderate severity of contamination; 2.0 ≤ mHQ < 2.5, considerable severity of contamination; 2.5 ≤ mHQ < 3.0, high severity of contamination; 3.0 ≤ mHQ < 3.5, very high severity of contamination; 3.5 < mHQ, extreme severity of contamination.
The proposed equation for ECI calculation is expressed as:
where Ej is the derived eigenvalue of heavy metal concentrations in PCA analysis. The same Ej were used when heavy metals were explained by the same principal component. The criteria for ECI are proposed as: ECI < 2, uncontaminated; 2 ≤ ECI < 3, uncontaminated to slightly contaminated; 3 ≤ ECI < 4, slightly to moderately contaminated; 4 ≤ ECI < 5, moderately to considerably contaminated; 5 ≤ ECI < 6, considerably to highly contaminated; 6 ≤ ECI < 7, highly contaminated; 7 < ECI, extremely contaminated.
Health risk assessment
In the present study, the average daily intake (ADI) was proposed to assess human health risk, and both carcinogenic and non-carcinogenic risk for each metal in rice grain based on ADI (mg/kg-day) were calculated by the following equation (U.S. EPA, 2011):
where ci represent the metal concentration in rice grain (mg kg−1); IR represents the daily ingestion of rice (mg day−1); EF represents the exposure frequency (day year−1); ED represent the exposure duration (year); BW represents the individual body weight (kg); and AT represents the overall exposed time period (day), which can be calculated using AT = ED × 365 (days). To estimate ADI, the exposure duration was 72 years and average individual body weight was considered as 61.75 kg for adults (NHFPC, 2015). The average daily ingestion of rice was estimated as 400.8 g day−1 (Pan et al., 2007; Zhao et al., 2013).
The targeted hazard quotient (THQ) was usually utilized to estimate the non-carcinogenic hazard risk. The THQ is defined as the quotient of the chronic daily intake, or the dose divided by the toxicity threshold value, which is referred to as the reference dose (RfD) of a specific chemical. The targeted hazard quotient (THQi) of a specific single chemical i was determined as:
where ADIi represent the average daily intake of the ith metal; RfDi represent the reference dose of the ith metal (mg kg·day−1) via oral intake.
In this study, the total targeted hazard quotient (TTHQ) was used to assess the total non-carcinogenic risk, attributed to heavy metal exposure. The TTHQ is the summation of calculated THQ of each heavy metal, which calculated using Eq. (7).
If the TTHQ < 1, this indicates that the heavy metal exposure does not lead to evident adverse health effects.
The carcinogenic risk (Riski) can be estimated by the probability of an individual developing cancer, as shown in Eq. (8), and total risk (Risktotal) can be estimated by the summation of the carcinogenic risk caused by all the heavy metal exposure, as calculated by Eq. (9).
Riski represent the probability of an individual developing cancer over a lifetime; SFi represent the carcinogenicity slope factor of the ith heavy metal (kg day·mg−1); Risktotal is the total sum of risk of heavy metals. In this study, the SF values were selected as 1.5 kg day·mg−1 for As (U.S. EPA, 2011), 15 and 0.0085 kg day·mg−1 for Cd and Pb respectively, from the California OEHHA Toxicity Criteria Database. The criteria for risk are: Risk > 1 × 10–4, unacceptable; 1 × 10–6 < Risk < 1 × 10–4, acceptable or tolerable; Risk < 1 × 10–6, no significant carcinogenic effects (U.S. EPA, 2001).
Results and discussion
Heavy metals accumulation characteristics in soil and rice
The concentrations of heavy metals in rice and soil samples from the 36 sites are shown in Table 1. The heavy metals in rice were variable. Zinc had the highest level in rice grain with a range of 16.9–33.5 mg/kg. However, Cd, Ni and Hg had relative higher coefficient of variations (CV), which were above 50%, and these results indicated that some samples might be polluted by anthropogenic activities. Compared with the Food Safety National Standard (GB2762-2017) (NHCNMPA, 2017), 5–10% of the samples exceeded the maximum levels of As, Cd, Cr and Hg in rice. The heavy metal concentrations in rice were comparable to those reported in samples collected from the Yangtze River Delta (Mao et al., 2019). In comparison with other contaminated areas in Asian countries such as Vietnam, the As and Cd levels in soils and plants in our study area were much lower (Hoang et al., 2019; Ko et al., 2020).
In general, the reported heavy metal concentrations in soils and rice grains from Jiangsu province (Li et al., 2017) were comparable to those in the present study. However, results obtained in this study (Table 1) showed that soil samples had elevated levels of Hg and Pb with higher coefficient of variations (CV > 50%), indicating that anthropogenic imports of Hg and Pb were high. About 88.89% of soil samples had Hg concentration higher than the limitation of soil quality standard (MEE, 2018). In addition, some samples were also contaminated by Cd (5 samples in 36), Pb (8 samples in 36) and Zn (3 samples in 36). In this study, soil Hg contents were higher than those reported in the Yangtze River Delta (Mao et al., 2019) and global average level of heavy metal (McLaughlin et al., 1999).
As the spatial distributions of soil and rice metals shown in Fig. 1, the hotspots with high concentrations of heavy metals in soil were highlighted. Soil samples in the central part of the study area were polluted by all metals, indicating the same sources for these metals. Through the comparison of metal distributions of rice grains and soils, results showed that only area with high rice and soil Cd, Hg and Ni levels overlapped (Fig. 1), indicating that soil Cd, Hg and Ni contents greatly influenced the accumulation of these metals in rice. The different spatial distribution patterns of As, Cr, Pb and Zn between soil and rice reflected that the different geochemical behaviours and accumulation characteristics may be affected by speciation of these metals (Huang et al., 2018).
Geochemical speciation of heavy metals in soil
To better understand the distribution characteristics of heavy metals in soil and rice, geochemical speciation of the heavy metals was analysed (Fig. 2). Speciation of heavy metals in soil is thought to be helpful to explore the geochemical behaviour of heavy metals in soil–rice system (Huang et al., 2018; Kabata-Pendias & Pendias, 2001). The results showed that residue and humic acid-bound fraction seemed to be the main forms for most heavy metals (Fig. 2). Percentage of average residue fraction for all heavy metals were in the order of Hg, Cr > Ni > Zn > Pb > As > Cd. This result is similar to the researches before (Dong et al., 2019; Pardo et al., 2011), which reported that residue fraction, humic acid-bound fraction and organic matter-bound fraction were the dominant fractions for these metals. Cd was different from other heavy metals, with higher percentage of exchangeable fraction, which is about 50% of total soil Cd. Traditionally, water-soluble, exchangeable fraction and carbonate-bound fraction are considered as the labile and easily available parts in soil (Kabata-Pendias & Pendias, 2001; Luo et al., 2012). As reported in researches before, the labile part of soil Cd could reach to 70–80% of the total content (Chavez et al., 2016; Khaokaew et al., 2011; Sun et al., 2007). On the other hand, humic acid-bound fraction for As exceeded 50% of total soil As. In previous researches, humic acid-bound and Fe/Mn oxyhydroxides-bound fractions were dominant in soil As, which showed the influence of organic matter and redox condition on soil As bioavailability (Fu et al., 2011; Williams et al., 2011). Except for As, humic acid-bound fraction for other heavy metals just varied around 15–22% of their total concentrations, which is similar to the results reported due to possible presence of metals-organic/humic acid complex (Dong et al., 2019; Moral et al., 2005; Pardo et al., 2011). Further, Fe/Mn oxyhydroxides-bound fractions for As, Ni, Pb and Zn fell into range from ~ 10 to ~ 25% of total soil metals concentrations, which indicated that pH change could release metals from this form to more labile forms (Muhammad et al., 2012; Sun et al., 2007). Han et al. (2020) reported that the exchangeable fraction of Cd and Zn in paddy soils was decreased and Fe/Mn oxyhydroxides-bound fraction was increased by using limestone amendments, which elevated the pH value of soil–porewater environment. Generally speaking, based on the proportion of water-soluble, exchangeable and carbonate-bound fractions, the mobility of heavy metals was in the order: Cd > Pb, Zn > Ni > As, Cr > Hg.
Geochemical speciation influences heavy metals uptake by rice
In this study, the transfer factors of heavy metals from soil to rice were expressed as TFtotal, while the transfer factors of metals from each geochemical speciation in soil to their contents in rice were identified as TFwatersoluble for water-soluble fraction, TFexchangeable for exchangeable, TFcarbonate for carbonate-bound fraction, TFhumic humic acid-bound fraction, TFFe/Mn for Fe/Mn oxyhydroxides-bound fraction, TForganic for organic matter-bound fraction and TFresidue for residue fraction. The correlations between heavy metals in rice and transfer factor of metals from soil, each geochemical form to rice, are presented in Table S2. It is found that except for Hg, other metals in rice had significant correlations with their corresponding transfer factors from soil to rice. In addition, grain Cd, Cr and Ni had relative high correlation coefficients (r > 0.950, p < 0.01). The similar results were also reported in researches before (Mao et al., 2019; Williams et al., 2007). This indicated that heavy metals transfer from soil to rice could be mainly affected by intake process of rice. For all geochemical forms, only TFcarbonate and TFFe/Mn had significant (p < 0.01) correlations with heavy metals in rice (Table S2). TForganic for Ni and Pb highly correlated with Ni and Pb levels in rice, which showed the possible effect of organic matter on intake of Ni and Pb. For instance, organic matter can supply organic chemicals to soils solution that act as chelates and thus enhance metal availability to rice (Zeng et al., 2011). Significant correlations between rice Cd and transfer factors of all geochemical forms of soil Cd seemed to suggest the high mobility and bioavailability of soil Cd (Dean, 2010). Because of the high mobility of Cd as discussed above, different forms of soil Cd could move and supply each other to reach chemical equilibrium (Khaokaew et al., 2011; Mohamed et al., 2010; Zhang et al., 2012). Meanwhile, Hg intake by rice might be related to the exchangeable and carbonate-bound fractions of soil Hg.
In a previously reported research on soil metal contamination in Turkey (Alan & Kara, 2019), PCA was used to analyse the correlations between plant and different geochemical forms. It is useful to discuss the effect of speciation of heavy metals in soil on their accumulation and transfer in plant quantitatively. However, further discussion and the contribution of each form of heavy metals in soil to their accumulation or transfer in plant tissues were not provided. Principal component analysis (PCA) was applied to identify the differences between transfer factor of seven geochemical forms of soil heavy metals in this study. Transfer factors of each form of heavy metals from soil to rice were used to characterize the effect of each form on accumulation of heavy metals in rice. The results of PCA after varimax rotation including percentage of variance and loadings of selective principal components (PCs) are shown in Table S3. In this study, selective principal components explained more than 95% of variance for each metal. In addition, according to the loadings of transfer factors of each form on principal components, relative contributions of each forms to accumulations of heavy metals in rice were discussed (Fig. 3). Different numbers of selective principal components were obtained for each heavy metal. Seven and six PCs were obtained to explain the contributions of geochemical behaviours of each form for As and Pb accumulation in rice, respectively. Meanwhile, four PCs were selected for Cr, Ni and Zn, whereas only three PCs explained the geochemical behaviour of Cd. Further, the accumulation process of Hg in rice was characterized by the fifth PCs.
Contribution of geochemical speciation of soil metals to accumulation of rice metals based on percentage of variance explained by principal components in PCA analyses for transfer factors of geochemical forms of heavy metals to rice. Fraction A: water soluble; Fraction B: exchangeable; Fraction C: carbonate-bound; Fraction D: humic acid-bound; Fraction E: Fe/Mn oxyhydroxides-bound; Fraction F: organic matter-bound
It was found that Fe/Mn oxyhydroxides-bound fraction contributed the highest effect on rice As (18.29%), illustrating that the soil redox change caused by Fe/Mn oxyhydroxides has impacts on both mobility and bioavailability of soil As (Marin et al., 1993; Rahman & Naidu, 2009). Fe and Mn are redox sensitive ions in the paddy field environment; for instance, soils may contain various FeOOH minerals such as ferrihydrite, goethite and hematite that played an important role in determining the As(III) and As(V) mobility (Kumarathilaka et al., 2018). Mn oxide minerals also have the ability to influence the As mobility in paddy soils, by oxidizing As(III) to As(V) or by adsorbing As(V). Therefore, Fe/Mn oxyhydroxides-bound fraction is the most significant speciation that can influence As transformation in soil–rice system while the labile parts including water-soluble (15.78%), exchangeable (15.67%) and carbonate-bound (13.29%) fractions contributed more than 40% of rice As. Although humic acid-bound As was about 50% of soil total As. The humic acid-bound fraction only contributed 9.93% to rice As, which may be resulted from As sorption and mobilization by humic acid in soil (Thanabalasingam & Pickering, 1986; Wang & Mulligan, 2009). Except for Hg, the geochemical behaviour of Fe/Mn oxyhydroxides on metals accumulation in rice was explained by PC1 in all PCA analyses, indicating that soil redox change could be a main factor of geochemical activity of soil metals (Bourg & Loch, 1995). In fact, soil redox condition change could cause heavy metals to mobilize to plant or to other more stable speciation, such as clay minerals or organic matters to reach a chemical equilibrium (Bourg & Loch, 1995; Tan et al., 2005). Furthermore, through the PCA analyses for As, Cd, Cr, Ni and Pb, PC1 reflected the contribution of Fe/Mn oxyhydroxides to the transfer of metals from soil to rice.
Water-soluble and carbonate-bound fractions of soil Cd contributed 32.11% of rice Cd. According to the loading of transfer factors of each forms in Table S3, exchangeable Cd would take at least one quarter of PC1 contribution (> ~ 10%). This meant that the labile part of soil Cd contributed more than 40% to rice Cd as well as As. Likewise, it could be inferred that labile part of all soil metals contributed over 40% to the accumulation of rice metals (Fig. 3). Moreover, the contribution of labile soil Hg just reached 40.69%. Similar to Fe/Mn oxyhydroxides-bound fraction, the organic matter-bound fractions for Cd, Cr, Ni, Pb and Zn were also explained by PC1 in these analyses. This result was similar to the study reported before (Alan & Kara, 2019), in which it was found that plant Cd, Cr, Pb and Zn had the same PCA loading as Fe/Mn oxyhydroxides and organic matter. From PCA analyses results, it can be inferred that effect of organic matter or residue fraction on transfer of metals ranged from ~ 10 to ~ 15% (Fig. 3). As immobilized fraction, organic matter and residue part of metals were thought to have a total ~ 25–30% of effect of metals transfer from soil to rice. This meant that contribution of humic acid and Fe/Mn oxyhydroxides-bound fraction could be estimated from ~ 20 to 30%. Generally, it can be concluded that labile fraction (water soluble, exchangeable and carbonate bound) had more than 40% of contribution to heavy metals accumulation in rice, although proportion of labile fraction was different for all metals. Furthermore, the contributions of organic matter-bound and residue fractions could be the result of chemical equilibrium caused by translocation of other forms of heavy metals.
Ecological risk assessment and human health risk assessment of heavy metals
It is important to make the ecological risk assessment of heavy metals in soil for primary investigation on potential threat of heavy metals to agroecosystem. In this study, two methods were used to evaluate ecological risk: one is modified risk assessment code (mRAC) and the other is ecological contamination index (ECI). The most important difference between them is that mRAC emphasizes on ecological risk of labile part of heavy metals in soil, whereas ECI is derived from modified hazard quotient (mHQ) takes into account ecotoxic effect based on total soil metals.
Modified risk assessment code
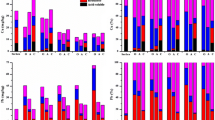
According to Eqs. 1 and 2, mRAC values of heavy metals in soil were calculated and are shown in Table S4 and Fig. 4a, b. According to mRAC criteria, all heavy metals in soil had no to medium potential adverse effect on ecology except Cd. The mRAC values of Cd in soils were ranked as “high effect” to very high effect.” This is resulted from the high proportion of labile fractions of soil Cd with amount of water-soluble, exchangeable and carbonate-bound fraction above 60% (Fig. 3). Cd was thought to have posed the biggest ecological risk in this study area. However, the mRAC values of soil As, Cr and Hg for most samples ranged from 1 to 10, which means a low effect on ecological risk.
As discussed above, labile fractions of soil As, Cr and Hg were lower than Ni, Pb and Zn. Therefore, mRAC values of As, Cr and Hg were identified as “low effect” for most samples. On the other hand, although labile parts of soil Pb and Zn were little higher than Ni, mRAC of Ni was higher than Pb and Zn (Fig. 4a). This difference could be resulted from the toxic factors (Tr) selected indicating toxic effect. Meanwhile, mRAC values of Ni, Pb and Zn fell in the range between 10 and 30, showing a “medium adverse effect” on ecological risk. From Fig. 4b, the average value of mRAC for soil samples was 21.72, which indicated that heavy metals had the medium potential adverse effect on soil in this study. Cd contributed more than 80% of total mRAC of soil samples. In addition, the contribution of heavy metal to mRAC followed the order: Cd > Hg > As > Pb > Ni > Cr > Zn, which was different from the order of proportion of labile part of heavy metals in soil (Fig. 3) due to different Tr values.
Ecological contamination index
Index mHQ and ECI based on total concentration of heavy metals in soil fully considered ecotoxic effects using TEL, PEL and SEL. The result of mHQ for specific heavy metal showed that Cd was very low severity of contamination (Table S4 and Fig. 4c). This was totally different from mRAC, since mHQ calculated from low level to the high for soil heavy metals. Considering the ecotoxic effect in all scenarios, mHQ is more comprehensive than mRAC (Benson et al., 2018a). The contamination levels of As, Cr, Ni, Pb and Zn for most samples were ranked from low severity to considerable severity. In addition, the mHQ values of As, Cr and Ni ranged from 1.5 to 2.0 for more than 80% of samples (Fig. 4c), which means As, Cr and Ni were moderately contaminated while more than 35% and 80% of samples were considered as low severity of contamination by Pb and Zn, respectively. The mHQ value of most soil Hg was above 2.0, indicating that soil samples were polluted by Hg severely. Proportion of both considerably severe and high severe contaminated samples were about 20%. Moreover, extremely contaminated soil samples by Hg accounted for 35% of total samples (Fig. 4c). It can be inferred that the order of mHQ values of soil metals is: Hg > Cr > Ni > As > Pb > Zn > Cd. Based on mHQ values and PCA analysis, ECI was calculated with mean value of 34.23, which showed soil samples were extremely contaminated by heavy metals. As shown in Fig. 4d, Hg contributed 31.50% to the ECI value, and the contribution of both As and Pb was 16.54%. Because mHQ of soil Hg was the highest. It can be seen that contribution of heavy metals to ECI followed the order: Hg > As = Pb > Zn > Cr > Ni > Cd. The results of contamination level assessment were different between mRAC and ECI. As discussed above, mRAC based on the labile part of heavy metals mainly depended on Cd. Because only 5 in 36 samples had Cd level exceeding the standard, mRAC was classified as “medium potential adverse effect” in this study. While due to high Hg levels in most soil samples (32 in 36 samples) over standard limit, ECI value indicated that soil samples were extremely contaminated.
Non-carcinogenic risk
To further investigate the potential risk to human health, targeted hazard quotient (THQ) and carcinogenic risk (Risk) were applied to evaluate the non-carcinogenic and carcinogenic health risk of heavy metals through the ingestion pathways of rice, respectively. The results of non-carcinogenic risk (THQ and TTHQ) and carcinogenic risk (Risk) assessment for adults are displayed in Table S5 and Fig. 5. According to the EPA standard (U.S. EPA, 2011), THQ values of As and Pb for all samples were above 1, which means people in our study area would be exposed to As and Pb (Fig. 5a). Although Cd, Cr and Hg in some samples exceeded the standard limits (GB2762-2017), rice Cd, Cr, Hg, Ni and Zn did not have adverse effect on human health. As and Pb contributed 35.66% and 47.88% of TTHQ, respectively, which resulted in TTHQ > 1 with mean value of 8.83 (Fig. 5b). However, each of the rest metals only contributed below 6%. The contribution of heavy metals to TTHQ followed the order: Pb > As > Zn > Hg > Cd > Ni > Cr (Fig. 5b), which is consistent with the metal contribution sequence to ECI. Contribution of Pb, As, Zn and Hg was higher than Cd, Cr and Ni for both ECI and TTHQ. Although proportion of labile part of Cd was more than 60%, rice Cd did not pose significant non-carcinogenic risk to human health (Fig. 5a,b). As discussed above, labile fraction of soil metals could contribute about 40% to rice metals. Therefore, the contribution of rice Cd to TTHQ was below 5%.
Carcinogenic risk
Results of the carcinogenic risk analysis are shown in Table S5 and Fig. 5. The carcinogenic risk of As, Cd and Cr exposure to rice was at unacceptably high levels of 141 per 100,000, 475 per 100,000 and 214 per 100,000 respectively, according to the criteria established by the EPA (U.S. EPA, 2011). The carcinogenic risk in the study area is even higher than that in Northwestern Thailand, where 20–60 in 100,000 residents over a lifetime of 75 years could suffer from cancer as a result of daily rice consumption (Chanpiwat et al., 2019). In addition, the THQ of rice Pb fell into the acceptable level (Fig. 5c). With mean value at 8.32 × 10–3, more than 50% of total cancer risk (Risktotal) was attributed to Cd (Fig. 5d) while contribution of Cr and As was 25.74% and 17.03%, respectively. It can be found that rice As posed significant non-carcinogenic and carcinogenic risk to human health. Nevertheless, carcinogenic risk rather than non-carcinogenic risk was mainly affected by Cd and Cr.
The spatial change of ecological risk and human health risk indexes is displayed in Fig. 6. It can be seen that high values of mRAC and TTHQ are distributed in the northwest and southeast of the study area. Although Cd contributed more than 80% of mRAC, spatial change of soil Cd was different from mRAC. Further, more than 80% of TTHQ value depended on rice As and Pb. Therefore, the TTHQ change coincided with the spatial change of As and Pb in Fig. 1. Likewise, the spatial distribution of ECI value is similar to Risk (Fig. 6). Because the area with high concentrations of soil As, Hg, Pb and Zn overlapped, rice Cd and Cr were main reason for change of Risk value. This could be the results of transfer character reported before (Mao et al., 2019). Low concentration of rice metals could be detected when it grown in the corresponding soil samples with high concentration of soil metals, which made the distribution differences between mRAC and TTHQ, ECI and Risk.
Conclusion
For heavy metals in rice and soil samples in the study area, soil Hg in more than 80% of samples exceeded national standard limit. Similar patterns were observed between spatial distributions of rice and soil Cd, Hg, Pb and Zn concentrations, which means Cd, Hg, Pb and Zn could come from the same sources with high levels in the same area. The result is in accordance with the PCA result of soil As, Hg, Pb and Zn. In the present study, the soil contamination in urban and suburban areas is much more serious than in rural areas, because the discharge intensity of potential sources, i.e. atmospheric deposition, solid wastes and industrial effluents, is much larger.
Geochemical speciation analysis showed that residue and humic acid-bound fractions were the main forms for most heavy metals. More than 50% of soil As was bound to humic acid. Proportion of water-soluble, exchangeable and carbonate-bound fractions of Cd breached over 50%. The mobility of heavy metals was in the order: Cd > Pb, Zn > Ni > As, Cr > Hg.
Transfer factor-related analysis indicated that carbonate-bound and Fe/Mn oxyhydroxides-bound forms had significant correlations with heavy metals in rice. Significant correlations were observed between rice Cd and transfer factors of all geochemical forms of soil Cd. Hg intake by rice might be related to the exchangeable and carbonate-bound fractions of soil Hg. Based on loading of transfer factors of each metal form, it can be concluded that labile fraction contributed more than 40% of heavy metal accumulations in rice. Moreover, effect of organic matter and residue fraction on transfer of metals was estimated to be ~ 25 to ~ 30%, which may be the result of chemical equilibrium caused by mobilization of heavy metals.
Modified risk assessment code (mRAC) and ecological contamination index (ECI) showed soil samples were exposed to pollution by heavy metal. Based on labile part of heavy metals, Cd contributed more than 80% of mRAC. Contrarily, the main contributors to ECI were identified as As, Hg, Pb and Zn, which were explained by same PC in PCA of soil metals. Average TTHQ and Risktotal values of heavy metals through oral ingestion of rice were above 1 and 10–4, respectively, implying people living in the study area were exposed to both unacceptable level of non-carcinogenic risk and carcinogenic risk caused by local produced rice consumption. As and Pb were the main contributors to TTHQ, while Risktotal depended on As, Cd and Cr. Spatial changes of ecological risk indexes and human health risk indexes showed that samples with high TTHQ values distributed in the area with high values of mRAC. In this study area, the safety of rice products should be given some serious consideration, engaging in the production of rice plants should be forbidden and corresponding remedial strategies should be formulated. Further, selectively cultivating low As, Cd, Pb-accumulating crops can be used to avoid ecological risks of heavy metals and to realize the safe usability of contaminate farmland.
Data availability
The authors do not have permission to share data.
References
Abd Aziz, A., Lee, B.-T., Han, H.-J., & Kim, K.-W. (2019). Assessment of the stabilization of heavy metal contaminants in soils using chemical leaching and an earthworm bioassay. Environmental Geochemistry and Health, 41, 447–460.
Adriano, D. (2001). Trace elements in terrestrial environments: Biogeochemistry, bioavailability, and risks of metals. Springer.
Alan, M., & Kara, D. (2019). Assessment of sequential extraction methods for the prediction of bioavailability of elements in plants grown on agricultural soils near to boron mines in Turkey. Talanta, 200, 41–50.
Alloway, B. J. (2013). Heavy metals in soils. In B. J. Alloway (Ed.), Sources of heavy metals and metalloids in soils (pp. 11–50). Springer.
Bandara, J., Senevirathna, D., Dasanayake, D., Herath, V., Bandara, J., Abeysekara, T., & Rajapaksha, K. H. (2008). Chronic renal failure among farm families in cascade irrigation systems in Sri Lanka associated with elevated dietary cadmium levels in rice and freshwater fish (Tilapia). Environmental Geochemistry and Health, 30, 465–478.
Benson, N. U., Adedapo, A. E., Fred-Ahmadu, O. H., Williams, A. B., Udosen, E. D., Ayejuyo, O. O., & Olajire, A. A. (2018a). New ecological risk indices for evaluating heavy metals contamination in aquatic sediment: A case study of the Gulf of Guinea. Regional Studies in Marine Science, 18, 44–56.
Benson, N. U., Adedapo, A. E., Fred-Ahmadu, O. H., Williams, A. B., Udosen, E. D., Ayejuyo, O. O., & Olajire, A. A. (2018). A new method for assessment of sediment-associated contamination risks using multivariate statistical approach. MethodsX, 5, 268–276.
Bourg, A. C. M., & Loch, J. P. G. (1995). mobilization of heavy metals as affected by pH and redox conditions. In W. Salomons & W. M. Stigliani (Eds.), biogeodynamics of pollutants in soils and sediments: risk assessment of delayed and non-linear responses (pp. 87–102). Springer.
Chanpiwat, P., Hensawang, S., Suwatvitayakorn, P., & Ponsin, M. (2019). Risk assessment of bioaccessible arsenic and cadmium exposure through rice consumption in local residents of themae tao sub-district, northwestern Thailand. Environmental Geochemistry and Health, 41, 343–356.
Chavez, E., He, Z. L., Stoffella, P. J., Mylavarapu, R. S., Li, Y. C., & Baligar, V. C. (2016). Chemical speciation of cadmium: An approach to evaluate plant-available cadmium in ecuadorian soils under cacao production. Chemosphere, 150, 57–62.
CGS. (2005). Geological survey technical standard. China Geological Survey.
Dean, J. R. (2010). Heavy metal bioavailability and bioaccessibility in soil. In S. P. Cummings (Ed.), Bioremediation: Methods and protocols (pp. 15–36). Humana Press.
Dong, H., Feng, L., Qin, Y., & Luo, M. (2019). Comparison of different sequential extraction procedures for mercury fractionation in polluted soils. Environmental Science and Pollution Research, 26, 9955–9965.
Fu, Y., Chen, M., Bi, X., He, Y., Ren, L., Xiang, W., Qiao, S., Yan, S., Li, Z., & Ma, Z. (2011). Occurrence of arsenic in brown rice and its relationship to soil properties from Hainan Island, China. Environmental Pollution, 159, 1757–1762.
Gabarrón, M., Zornoza, R., Martínez-Martínez, S., Muñoz, V. A., Faz, Á., & Acosta, J. A. (2019). Effect of land use and soil properties in the feasibility of two sequential extraction procedures for metals fractionation. Chemosphere, 218, 266–272.
Gusiatin, Z. M., & Kulikowska, D. (2014). The usability of the IR, RAC and MRI indices of heavy metal distribution to assess the environmental quality of sewage sludge composts. Waste Management, 34, 1227–1236.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control: A sedimentological approach. Water Research, 14, 975–1001.
Han, H. J., Lee, J. U., Ko, M. S., & Kim, K. W. (2020). Comparison of five extraction methods for evaluating cadmium and zinc immobilization in soil. Environmental Geochemistry and Health, 42, 4203–4212.
Hoang, H. T., & Baeumle, R. (2019). Complex hydrochemical characteristics of the middle-upper pleistocene aquifer in soc trang province, southern vietnam. Environmental Geochemistry and Health, 41, 325–341.
Huang, Q., Yu, Y., Wan, Y., Wang, Q., Luo, Z., Qiao, Y., Dechun, S., & Li, H. (2018). Effects of continuous fertilization on bioavailability and fractionation of cadmium in soil and its uptake by rice (Oryza sativa L.). Journal of Environmental Management, 215, 13–21.
Hutchinson, J. J., Young, S. D., McGrath, S. P., West, H. M., Black, C. R., & Baker, A. J. M. (2000). Determining uptake of ‘non-labile’ soil cadmium by thlaspi caerulescens using isotopic dilution techniques. New Phytologist, 146, 453–460.
IARC. (2004). IARC monographs on the evaluation of carcinogenic risks to humans. some drinking-water disinfectants and contaminants, including Arsenic, Lyon. France.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants. CRC.
Khaokaew, S., Chaney, R. L., Landrot, G., Ginder-Vogel, M., & Sparks, D. L. (2011). Speciation and release kinetics of cadmium in an alkaline paddy soil under various flooding periods and draining conditions. Environmental Science & Technology, 45, 4249–4255.
Kim, H. S., Kim, Y. J., & Seo, Y. R. (2015). An overview of carcinogenic heavy metal: Molecular toxicity mechanism and prevention. Journal of Cancer Prevention, 20, 232–240.
Ko, M. S., Nguyen, T. H., Kim, Y. G., Linh, B. M., & Kim, K. W. (2020). Assessment and source identification of As and Cd contamination in soil and plants in the vicinity of the Nui Phao Mine, Vietnam. Environmental Geochemistry and Health, 42, 4193–4201.
Kumarathilaka, P., Seneweera, S., Meharg, A., & Bundschuh, J. (2018). Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors: A review. Water Research, 140, 403–414.
Landner, L., & Reuther, R. (2005). Speciation, mobility and bioavailability of metals in the environment, metals in society and in the environment: A critical review of current knowledge on fluxes, speciation, bioavailability and risk for adverse effects of copper, chromium, nickel and zinc (pp. 139–274). Springer.
Li, T., Chang, Q., Yuan, X., Li, J., Ayoko, G. A., Frost, R. L., Chen, H., Zhang, X., Song, Y., & Song, W. (2017). Cadmium transfer from contaminated soils to the human body through rice consumption in southern Jiangsu Province, China. Environmental Science: Processes & Impacts, 19, 843–850.
Li, Z., Ma, Z., van der Kuijp, T. J., Yuan, Z., & Huang, L. (2014). A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Science of The Total Environment, 468–469, 843–853.
Liao, Q., Evans, L. J., Gu, X., Fan, D., Jin, Y., & Wang, H. (2007). A regional geochemical survey of soils in Jiangsu Province, China: Preliminary assessment of soil fertility and soil contamination. Geoderma, 142, 18–28.
Liu, Y., Bello, O., Rahman, M. M., Dong, Z., Islam, S., & Naidu, R. (2017). Investigating the relationship between lead speciation and bioaccessibility of mining impacted soils and dusts. Environmental Science and Pollution Research, 24, 17056–17067.
Long, E. R., Macdonald, D. D., Smith, S. L., & Calder, F. D. (1995). Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environmental Management, 19, 81–97.
Luo, P., Xiao, X., Han, X., Ma, Y., Sun, X., Jiang, J., & Wang, H. (2019). Application of different single extraction procedures for assessing the bioavailability of heavy metal(loid)s in soils from overlapped areas of farmland and coal resources. Environmental Science and Pollution Research, 26, 14932–14942.
Luo, X., Yu, S., & Li, X. (2012). The mobility, bioavailability, and human bioaccessibility of trace metals in urban soils of Hong Kong. Applied Geochemistry, 27, 995–1004.
MacDonald, D. D., Ingersoll, C. G., & Berger, T. A. (2000). Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Archives of Environmental Contamination and Toxicology, 39, 20–31.
Mao, C., Song, Y., Chen, L., Ji, J., Li, J., Yuan, X., Yang, Z., Ayoko, G. A., Frost, R. L., & Theiss, F. (2019). Human health risks of heavy metals in paddy rice based on transfer characteristics of heavy metals from soil to rice. Catena, 175, 339–348.
Marin, A. R., Masscheleyn, P. H., & Patrick, W. H. (1993). Soil redox-pH stability of arsenci species and its influence on arsenic uptake by rice. Plant & Soil, 152, 245–253.
McLaughlin, M. J., Parker, D. R., & Clarke, J. M. (1999). Metals and micronutrients: Food safety issues. Field Crops Research, 60, 143–163.
Meers, E., Du Laing, G., Unamuno, V., Ruttens, A., Vangronsveld, J., Tack, F. M. G., et al. (2007). Comparison of cadmium extractability from soils by commonly used single extraction protocols. Geoderma, 141, 247–259.
MEE. (2018). Soil environmental quality risk control standard for soil contmination of agricultural land. Ministry of Ecology and Environment PRC.
Mohamed, I., Ahamadou, B., Li, M., Gong, C., Cai, P., Liang, W., et al. (2010). Fractionation of copper and cadmium and their binding with soil organic matter in a contaminated soil amended with organic materials. Journal of Soils and Sediments, 10, 973–982.
Moral, R., Gilkes, R. J., & JordÁn, M. M. (2005). Distribution of heavy metals in calcareous and non-calcareous soils in Spain. Water, Air, and Soil Pollution, 162, 127–142.
Muhammad, I., Puschenreiter, M., & Wenzel, W. W. (2012). Cadmium and Zn availability as affected by pH manipulation and its assessment by soil extraction, DGT and indicator plants. Science of The Total Environment, 416, 490–500.
Müller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. The Journal of Geology, 2, 108–118.
Nannipieri, P., Grego, S., & Ceccanti, B. (1990). Ecological significance of the biological activity in soil. Soil Biochemistry, 6, 293–355.
NHCNMPA. (2017). Maximum levels of contaminants in food, GB2762, Beijing.
NHFPC. (2015). 2014 report on Chinese resident’s chronic disease and nutrition. http://en.nhfpc.gov.cn/2015-06/15/c_45788.htm. National Health and Family Planning Commission.
Nolan, A. L., Zhang, H., & McLaughlin, M. J. (2005). Prediction of zinc, cadmium, lead, and copper availability to wheat in contaminated soils using chemical speciation, diffusive gradients in thin films, extraction, and isotopic dilution techniques. Journal of Environmental Quality, 34, 496–507.
Onyatta, J. O., & Huang, P. M. (1999). Chemical speciation and bioavailability index of cadmium for selected tropical soils in Kenya. Geoderma, 91, 87–101.
Pan, X., Yuan, B., Shi, Z., & Dai, Y. (2007). Investigation on dietary status among rural and urban residents in Jiangsu. Jiangsu Journal of Preventive Medicine (China), 18, 6–9.
Pardo, F., Jordán, M. M., Sanfeliu, T., & Pina, S. (2011). Distribution of Cd, Ni, Cr, and Pb in smended soils from licante Province (SE, Spain). Water, Air, & Soil Pollution, 217, 535–543.
Peng, Y., Shen, Y., Ge, M., Pan, Z., Chen, W., & Gong, B. (2019). Efficient extraction of heavy metals from collagens by sulfonated polystyrene nanospheres. Food Chemistry, 275, 377–384.
Phan, K., Phan, S., Se, S., Sieng, H., Huoy, L., & Kim, K. W. (2019). Assessment of water quality and trace metal contaminations in Mondolkiri province in the northeastern part of Cambodia. Environmental Geochemistry & Health, 41, 401–409.
Rahman, F., & Naidu, R. (2009). The influence of arsenic speciation (As-III & As-v) and concentration on the growth, uptake and translocation of arsenic in vegetable crops (silverbeet and amaranth). greenhouse study. Environmental Geochemistry and Health, 31, 115–124.
Reeder, R. J., Schoonen, M. A. A., & Lanzirotti, A. (2006). Metal speciation and its role in bioaccessibility and bioavailability. Reviews in Mineralogy and Geochemistry, 64, 59–113.
Saeedi, M., & Jamshidi-Zanjani, A. (2015). Development of a new aggregative index to assess potential effect of metals pollution in aquatic sediments. Ecological Indicators, 58, 235–243.
Satarug, S., & Moore, M. R. (2004). Adverse health effects of chronic exposure to low-level cadmium in foodstuffs and cigarette smoke. Environmental Health Perspectives, 112, 1099–1103.
Shahid, M., Dumat, C., Khalid, S., Niazi, N.K., & Antunes, P.M.C. (2017). Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. In: Pim de Voogt (Ed.), Reviews of environmental contamination and toxicology 241. Springer, pp. 73-137.
Smith, S. L., MacDonald, D. D., Keenleyside, K. A., Ingersoll, C. G., & Jay, F. L. (1996). A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. Journal of Great Lakes Research, 22, 624–638.
Sumalatha, J., Naveen, B. P., & Malik, R. K. (2019). Toxic metals removal from industrial sludge by using different leaching solutions. Journal of the Institution of Engineers (India) Series A, 100, 337–335.
Sun, L., Chen, S., Chao, L., & Sun, T. (2007). Effects of flooding on changes in Eh, pH and speciation of cadmium and lead in contaminated soil. Bulletin of Environmental Contamination and Toxicology, 79, 514–518.
Tan, W., Liu, F., Feng, X., Huang, Q., & Li, X. (2005). Adsorption and redox reactions of heavy metals on Fe–Mn nodules from Chinese soils. Journal of Colloid and Interface Science, 284, 600–605.
Tang, X., Zhu, Y., Cui, Y., Duan, J., & Tang, L. (2006). The effect of ageing on the bioaccessibility and fractionation of cadmium in some typical soils of China. Environment International, 32, 682–689.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the sepeciation of particulate trace-metals. Analytical Chemistry, 51, 844–851.
Thanabalasingam, P., & Pickering, W. F. (1986). Arsenic sorption by humic acids. Environmental Pollution Series B, Chemical and Physical, 12, 233–246.
Ure, A. M. (1996). Single extraction schemes for soil analysis and related applications. Science of the Total Environment, 178, 3–10.
U.S. EPA. (2001). Risk assessment guidance for superfund: Volume III—Part A, process for conducting probabilistic risk assessment, Washington DC.
U.S. EPA. (2011). Exposure factors handbook: 2011 Edition, Washington, DC.
Van der Ent, A., Nkrumah, P. N., Tibbett, M., & Echevarria, G. (2019). Evaluating soil extraction methods for chemical characterization of ultramafic soils in Kinabalu Park (Malaysia). Journal of Geochemical Exploration, 196, 235–246.
Wang, S., & Mulligan, C. N. (2009). Enhanced mobilization of arsenic and heavy metals from mine tailings by humic acid. Chemosphere, 74, 274–279.
Wen, Y., Li, W., Yang, Z., Zhang, Q., & Ji, J. (2020a). Enrichment and source identification of Cd and other heavy metals in soils with high geochemical background in the karst region, Southwestern China. Chemosphere, 245, 125620.
Wen, Y., Li, W., Yang, Z., Zhuo, X., Guan, D.-X., Song, Y., et al. (2020b). Evaluation of various approaches to predict cadmium bioavailability to rice grown in soils with high geochemical background in the karst region, Southwestern China. Environmental Pollution, 258, 113645.
Williams, P. N., Villada, A., Deacon, C., Raab, A., Figuerola, J., Green, A. J., et al. (2007). Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environmental Science & Technology, 41, 6854–6859.
Williams, P. N., Zhang, H., Davison, W., Meharg, A. A., Hossain, M., Norton, G. J., et al. (2011). Organic matter-solid phase interactions are critical for predicting arsenic release and plant uptake in Bangladesh paddy soils. Environmental Science & Technology, 45, 6080–6087.
Yang, Q., Shu, W., Qiu, J., Wang, H., & Lan, C. (2004). Lead in paddy soils and rice plants Lechang and its potential health risk around lead/zinc Mine, Guangdong, China. Environment International, 30, 883–889.
Zeng, F., Ali, S., Zhang, H., Ouyang, Y., Qiu, B., Wu, F., et al. (2011). The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environmental Pollution, 159, 84–91.
Zhang, C., Ge, Y., Yao, H., Chen, X., & Hu, M. (2012). Iron oxidation-reduction and its impacts on cadmium bioavailability in paddy soils: A review. Frontiers of Environmental Science & Engineering, 6, 509–517.
Zhang, L., Mo, Z., Qin, J., Li, Q., Wei, Y., Ma, S., et al. (2015). Change of water sources reduces health risks from heavy metals via ingestion of water, soil, and rice in a riverine area, South China. Science of The Total Environment, 530–531, 163–170.
Zhao, M., Wang, C., Li, T., Yi, N., He, X., Wu, H., et al. (2013). Acute risk assessment of cumulative dietary exposure to organophosphorus pesticide among people in Jiangsu province. Journal of Hygiene Research, 42, 844–848.
Funding
This work was supported by grants from the project of Monitoring Technology and Forewarning of Heavy Metal Pollution in Typical Agricultural Land of Jiangsu Province (Grant No. KJXM2016039), and the project of Ecological and Geological Environment Monitoring of Land (Agricultural Land) in Jiangsu Province, and the project of Comprehensive Geological Survey of Modern Demonstration Area in Southern Jiangsu, the National Natural Science Foundation of China (Grant 41673095, 41907141) and the Fundamental Research Funds for the Central Universities (Grant No. Swu 118203).
Author information
Authors and Affiliations
Contributions
Yuanyuan Wang contributed to investigation, methodology, visualization and manuscript writing. Weiwei Xu contributed to conceptualization, data validation and project administration. Jizhou Li contributed to data analysis and interpretation. Yinxian Song contributed to research concept, experiment design, and critical revision of the article. Ming Hua contributed to collection and assembly of data. Wenbo Li contributed to sample collecting and analysing. Yubo Wen contributed to critical revision of the article. Tianyuan Li contributed to critical revision of the article. Xinxing He contributed to sample collecting and analysing.
Corresponding authors
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this article.
Consent for publication
The authors confirms that the work described has not been published before; that it is not under consideration for publication elsewhere; that its publication has been approved by all co-authors, if any; that its publication has been approved (tacitly or explicitly) by the responsible authorities at the institution where the work is carried out.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Xu, W., Li, J. et al. Assessing the fractionation and bioavailability of heavy metals in soil–rice system and the associated health risk. Environ Geochem Health 44, 301–318 (2022). https://doi.org/10.1007/s10653-021-00876-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00876-4