Abstract
Polycyclic aromatic hydrocarbons (PAHs), organochlorine pesticides (OCPs) and phenolic compounds (PCs) are persistent organic compounds. Contamination of these potentially toxic organic pollutants in soils and sediments is most studied environmental compartments. In recent past, studies were carried out on PAHs, OCPs and PCs in various soils and sediments in India. But, this is the first study on these pollutants in soils and sediments from an urbanized river flood plain area in Delhi, India. During 2018, a total of fifty-four samples including twenty-seven each of soil and sediment were collected and analyzed for thirteen priority PAHs, four OCPs and six PCs. The detected concentration of ∑PAHs, ∑OCPs and ∑PCs in soils ranged between 473 and 1132, 13 and 41, and 639 and 2112 µg/kg, respectively, while their concentrations in sediments ranged between 1685 and 4010, 4.2 and 47, and 553 and 20,983 µg/kg, respectively. PAHs with 4-aromatic rings were the dominant compounds, accounting for 51 and 76% of total PAHs in soils and sediments, respectively. The contribution of seven carcinogen PAHs (7CPAHs) in soils and sediments accounted for 43% and 61%, respectively, to ∑PAHs. Among OCPs, p, p’-DDT was the dominant compound in soils, while α-HCH was found to be dominated in sediments. The concentrations of ∑CPs (chlorophenols) were dominated over ∑NPs (nitrophenols) in both the matrices. Various diagnostic tools were applied for the identification of their possible sources in soil and sediments. The observed concentrations of PAHs, OCPs and PCs were more or less comparable with the recently reports from various locations around the world including India. Soil quality guidelines and consensus-based sediment quality guidelines were applied for the assessment of ecotoxicological health effect.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soils and sediments play important role in the distribution of organic pollutants (OCs) in the environment, particularly in tropical climates (Wilcke 2000). Contaminations of OCs in soil and sediments are affected by the characteristic properties of OCs, including water solubility, and contents and properties of the organic matter in the soil and sediments due to the affinity of OCs for organic matter (Li et al. 2018; Persson et al. 2007). With respect to their quantity and bulky holding capacity on organic matter, soil and sediments are considered as sink and re-emission sources of the OCs through volatilization, degradation and leaching (Aleksandra et al. 2019; Gough 1991). Worldwide, soil and sediments were the most studied environmental compartment for OCs including PAHs (Ghosh & Maiti 2019; Zhou et al. 2017; Goswami et al. 2016; Kumar et al. 2014a, b; Wang et al. 2008; Wilcke 2000), OCPs (Chakraborty et al. 2016; Kumar et al. 2016, 2014a; Wang et al. 2008) and PCs (Yahaya et al. 2019; Kumar et al. 2017, 2016, 2014c; Olujimi et al. 2010; Marianna 2004). The major sources of these OCs in soils and sediments are atmospheric transport depositions and anthropogenic activities (Wang et al. 2009; O’Driscoll et al. 2014; Li et al. 2018). Due to their toxicological properties and environmental concern, some compounds of PAHs, OCPs and PCs have been classified as priority pollutants by various international agencies (WHO 1989; EC 2001; USEPA 2015).
PAHs are released to the environment mainly from petroleum products (petrogenic sources) and combustion activities including incomplete combustion of coal, petroleum products and biomass (pyrogenic sources) (ATSDR 1995). PAHs emissions are correlated with thermal energy generation and consumption for rapid growth of population, urbanization and industrialization (Hafner et al. 2005). Released PAHs in atmosphere partitioned into particulate and gaseous phase, which then transported and deposited on the vegetation, water bodies and soil (Wang et al. 2009). PAHs are known to genotoxic effects including induced mutation through formation of DNA adduct and causes to developing carcinogenesis (ATSDR 1995). USEPA’s carcinogenicity risk assessment verification endeavor work group (ATSDR 1995) and WHO’s the International agency for research on cancer (IARC) (IARC 2010) classified carcinogenicity of priority PAHs. Benzo(a)pyrene (BaP) is classified as human carcinogen (group I), dibenzo(a,h)anthracene (DBA) as probable carcinogens (group 2A), and benzo(a)anthracene (BaA), chrysene (CHR), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), naphthalene (NPT) and indeno(1,2,3-cd)pyrene (Ind) classified as possible carcinogens (group 2B) (IARC 2010; ATSDR 1995).
OCPs including DDTs and HCHs are low-cost pesticides and have been widely used in public health, agriculture and industrial activities (ATSDR 2005, 2008). As isomers of DDT and HCH have been listed as persistent organic pollutants (POPs) under Stockholm Convention, their use had been prohibited or restricted in several countries, but these are still produced and used in many countries (UNEP 2017). Government of India banned the use of DDT and HCH in agriculture and restricted for use in public health programs (PPQS 2019; UNEP 2017). Their long-range atmospheric transport (LRAT) tendency makes them globally distributed in all the environmental compartments including regions far from their release sources, such as the Arctic regions (Becker et al. 2009). DDTs and HCHs are known for their properties of persistence, bio-accumulative, causative to wide range of chronic and acute toxicity (ATSDR 2005, 2008).
The PCs and their derivatives have been used in the production of pesticides, pharmaceutical products, wood preservatives and industrial applications (WHO 1989). They enter into environment through various anthropogenic sources including industrial activities or by domestic sewage, and their sorption to organic matter leads to persistent contamination in soils and sediments (WHO 1989; Santana et al. 2009). Some PCs originate from the transformation of pesticides (Marianna 2004) and vehicular emissions (Michalowicz and Duda 2007). Cholorophenols and nitrophenols show toxicity, mutagenicity and carcinogenicity, endocrine disrupters and vasodilator activities (Michalowicz and Duda 2007; Olujimi et al. 2010).
During past recent years, various soils and sediments were accessed in India for PAHs (Ghosh & Maiti 2019; Mitra et al. 2019; Khuman et al., 2018; Goswami et al. 2016; Kumar et al. 2015a, b, c), OCPs (Mitra et al. 2019; Kumar et al. 2018, 2016, 2014a, 2014b; Chakraborty et al. 2016; Hashmi & Menon 2015) and PCs (Kumar et al. 2018, 2016, 2014c). However, the literature for these POPs in soil and sediment from flood plains is not available in India. This is probably first report on these pollutants in soils and sediments from an urbanized river flood plains in India. During the present study, selected compounds of priority PAHs, OCPs and PCs were measured in soils and sediments from flood plain area of Yamuna River in Delhi, India. Their possible sources were identified using various diagnostic tools, and ecotoxicological health effects were evaluated using stipulated guidelines (CCME 2010; Buckman 2008; WDNR 2003).
Materials and methods
Solvents, chemicals and standards
HPLC-grade solvents & water, and analytical grade chemicals procured from Fisher Scientific (India) and Sigma-Aldrich (USA). Standard solutions of individual PAHs [fluorene (FLE), phenanthrene (PHE), anthracene (ANT), fluoranthene (FLT), pyrene (PYR), benzo(a)anthracene (BaA), chrysene (CHR), benzo(b)fluoranthene (BbF), benzo(k)fluoranthene (BkF), Benzo(a)pyrene (BaP), benzo(g,h,i)perylene (BghiP), dibenzo(a,h)anthracene (DBA) and indeno(1,2,3-cd)pyrene (Ind)], OCPs (α-HCH, γ-HCH, p,p’-DDT and p,p’-DDE) and PCs [2-nitrophenol (2-NP), 2,4-dinitrophenol (2,4-DNP), 2-chlorophenol (2-CP), 2,4-dichlorophenol (2,4-DCP), 4-chloro-3-methylphenol (4-C,3-MP) and pentachlorophenol (PCP)] were purchased from AccuStandard (USA) and Supelco (Sigma-Aldrich, USA).
Study area and sampling
Soil and sediment samples were collected from flood area of Yamuna River in Delhi. Delhi (28.38°N and 77.13°E), the national capital of India, is a business center in the northern India which lies on the banks of the Yamuna River. Yamuna River receives most of the wastewater generated in Delhi through major and minor drains in 22 km stretch. Delhi with 1483 km2 area and 16.78 million of total population (DES 2016) generates 4155 MLD (million liters per day) of waste water. Out of generated waste water, treatment facility is available for 2693.7 MLD (Sulabh 2019). The transportation is predominantly road based with total numbers of registered vehicles of 9.7 million including two wheelers (62.90%), cars, & jeeps (30.77%) and commercial vehicles including auto rickshaw, taxes, buses and goods vehicles (6.33%) (DES 2016). Delhi has gasoline-based power plants and waste to energy plants for the generation of 2253 MW electricity (DERC 2017). There are numbers of designated industrial areas with various activities. The climate of Delhi is tropical semiarid with ambient temperature of 4–5ºC during months of December–January in winter season and 40–45ºC during months of May–June in summer. Generally, winds are north–western while south–eastern winds are prevalent during monsoon season. With annual average of ~ 714 mm, region received approximately 80% of rainfall during monsoon season (CPCB 2016).
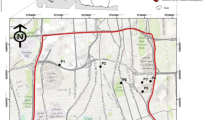
A total of fifty-four samples including twenty-seven each of soil and sediment were collected during June 2018 from three locations in Yamuna flood plains (~97 km2) (Fig. 1). One location (SV) is on upstream of River flood plain area to the Delhi. Second location (GC) is ~ 10–12 km downstream of first location in the middle of River flood plain area. Third location (OK) is ~ 10–12 km downstream of second location. Approximately 200 g of sub-surface (0–10 cm depth) soil and surficial sediment in triplicates was collected from three points in the radius of ~ 500 m of each location (nine samples from each location). Unwanted materials removed manually, and an aliquot of thoroughly mixed sample was taken into labeled pre-clean wide mouth amber glass containers. Containers with collected samples were preserved with ice and transported to the laboratory to store at 4–8 °C.
Sample extraction analysis and analytical quality control
The collected samples were air dried, homogenized and 1 mm size stored in amber glass bottles at 4 ± 2 °C for further processing. Samples for PAHs and OCPs were extracted three times with acetone–hexane (1:1 v/v) in ultrasonic bath. Pooled organic layer with pollutants passed through sodium sulfate on Whatman 41 filter paper and was concentrated to near 2 mL using a rotary evaporator (Eyela, Tokyo, Japan). Concentrated extracts were divided into two aliquots of ~1 mL each for clean-up for PAHs and OCPs separately following the USEPA method 3630C. For PCs, sample was extracted thrice with 0.1 M NaOH in methanol with ultrasonic bath, and pooled methanol extract was subjected to liquid–liquid extraction using dichloromethane at pH < 2. The organic phase was concentrated to near 5 mL, solvent exchanged to methanol and reduced to 1.0 mL for analysis (Kumar et al. 2014d).
Target individual PAHs were analyzed by HPLC (Agilent 1100, USA) equipped with diode array detector (DAD, λ = 254 nm), analytical column (LC-PAH Supelcosil™) and guard column (Eclipse XDB-C8). Gradient flow (1.0 mL min−1) of acetonitrile (60%) and water (40%) was used as mobile phase (Kumar et al. 2015a, b, c). The quantification of target OCPs was carried out using gas chromatograph (Perkin Elmer, Clarus 500, USA) equipped with an electron capture detector (ECD, 63Ni) and Elite-1 fused silica capillary column (Kumar et al. 2018). PCs were quantified by using HPLC equipped with DAD (λ = 280 nm), C18 analytical column (Ascentis®, Supelco, USA) and a guard column. Methanol with 0.15% o-phosphoric acid and water with 0.15% o-phosphoric acid were used in mobile phase with gradient flow @ 0.7 mL min−1 (Kumar et al. 2014d).
Strict required analytical quality control analysis was performed during determination of PAHs, OCPs and PCs. Quality control considerations were five-level calibration curves (r2, 0.999) of certified reference standards, calibration verification (sd, < 10%), method blanks in triplicate (analytes concentrations was < MDL ‘method detection limit’), duplicate analysis (sd, < 10%), accuracy and detection limits. The peak identification of the analytes was done by the accurate retention time of each individual standard. The mean of two measurements was used in calculations. The accuracy of method was assessed through matrix spiked recovery. The average recoveries for OCPs, PCs and PAH compounds ranged between 82 and 106% (± 11–16%), 51 and 95% (± 5–11%) and 94 and 100% (± 5–7%), respectively. Detection limits (DLs) were estimated using signal-to-noise ratio > 3:1 (s/n > 3) (WDNR 1994). The estimated DLs for PAHs, OCPs and PCs ranged between 0.01 and 0.12 µg/mL, 0.02 and 0.05 µg/mL and 3.3 and 18.6 µg/mL, respectively. Calibration range, linearity, DLs and recoveries are presented in Table S1.
Results and discussions
Distribution of PAHs
The descriptive concentrations of total PAHs and in soil and sediments are presented in Tables 1 and 2, respectively, and their individuals are depicted in Fig. 2. The mean of ∑PAHs was comparatively higher in sediments (3002 ± 956 µg/kg) than soils (785 ± 224 µg/kg). Average concentration of 3-ring to 6-ring PAHs in soils was 150 ± 85, 402 ± 65, 148 ± 33 and 85 ± 65 µg/kg, respectively, for 3-ring, 4-ring, 5-ring and 6-ring PAHs and accounted for 21%, 51%, 19% and 11%, respectively, to ∑PAHs. The dominant PAHs in soil were FLT (169 ± 26 µg/kg), CHR (107 ± 72 µg/kg), PHE (98 ± 76 µg/kg), PYR (73 ± 37 µg/kg), BbF (70 ± 38 µg/kg), BkF (66 ± 27 µg/kg) and BghiP (53 ± 51 µg/kg). The concentration of 3-ring, 4-ring, 5-ring and 6-ring PAHs in sediments ranges between 213 and 980 µg/kg, 1306 and 3330 µg/kg, 32 and 239 µg/kg and 29 and 125 µg/kg with the mean values of 522 ± 232, 2288 ± 757, 122 ± 72 and 69 ± 33 µg/kg, respectively, which accounted for 17%, 76%, 4% and 2%, respectively, to ∑PAHs. CHR, FLT, FLE and PYR were the dominant PAHs in sediment, and their average concentration was 1686 ± 794, 436 ± 424, 423 ± 174 and 152 ± 102 µg/kg, respectively (Fig. 2). The dominance of FLT, CHR, PYR and FLE indicated origin from combustion process (Khalili et al. 1995). Further, dominance of 4-ring PAHs over other homologs in both the matrices supported pyrogenic sources (Table 1). Dominance of FLT, PYR, BbF, BkF and CHR is reported for high-temperature combustion activities (Singh et al. 2012; Ravindra et al. 2008; Khalili et al. 1995).
Spatial distribution of ∑PAHs in soils at SV, GC and OK was 528 ± 49, 1031 ± 88 and 796 ± 5 µg/kg, respectively. However, in sediments, it was 1820 ± 125, 3567 ± 590 and 3618 ± 386 µg/kg at SV, GC and OK, respectively. The contribution of 4-ring PAHs (dominant homolog) in soils at SV, GC and OK was accounted for 64%, 45% and 51%, respectively, to ∑PAHs. However, their contribution in sediments at different locations was 77%, 73% and 79% to ∑PAHs at SV, GC and OK, respectively. FLT and CHR were the most dominant compound in soil and sediment, respectively. Average concentration of FLT in soil at SV, GC and OK was 183 ± 18 µg/kg, 188 ± 15 µg/kg and 136 ± 16 µg/kg, respectively (Tables 1 and 2). On the other hand, average concentration of CHR in sediments at SV, GC and OK was 824 ± 78, 2547 ± 483 and1687 ± 238 µg/kg, respectively (Tables S2 and S3). Dominance of FLT and CHR indicated combustion sources. Comparatively elevated levels of PAHs at GC can be characterized by its zone in the center of the city and influenced by major wastewater drains and vehicular emissions (Kumar et al. 2015a, 2018). OK is located at downstream of Delhi with cumulative sediment deposition through controlled water flow and wastewater discharges through two major drains. On the other hand, comparatively low levels at the SV may be due to its wide open area with low impact of direct discharges from anthropogenic activities.
The observed concentrations of PAHs in soil and sediments were compared with the recent similar other studies undertaken worldwide including India (Table S4) and found more or less comparable. The observed ∑PAHs in soils were lower than soils of Eastern India, Dhanbad and Delhi (India), Orlando & Thampa (USA), Pohang (South Korea), Kathmandu (Nepal), Shandong, Xian and Shengli (China), Vhembe and Cape Town (South Africa) and San Mateo Ixtatan (Guatemala), but, higher than Ghaziabad and Gwalior cities of India and Gyeonggi (South korea). Observed ∑PAHs in sediments (3002 ± 956 µg/kg) were lower than Cooum River (India), Ammer River (Germany), Daliao River (China), Cakung, Sunter & Ciliwung Rivers (Jakarta), Karoon River, (Iran) and Algoa River (South Africa), however, lower concentrations were reported from Hooghly River, Brahmaputra River, Ennore estuary and Adyar River in India, Tiber River (Italy), Xijiang River and Yinma River (China), Durban (South Africa), Cauca River (Columbia) and Huveaune River (France).
BaP toxicity equivalency (BaPTEQ) and toxic fraction of PAHs
BaP toxicity equivalency (BaPTEQ) represents the relative carcinogenic potential to the corresponding PAHs to BaP. Toxicity equivalency factors (TEFs) (Tsai et al. 2004) were used to estimate the BaPTEQ of the target PAHs relative to BaP (Table 3). The BaPTEQ was calculated by multiplying the concentration of individual PAHs with the corresponding TEF. Based on cancer risk to humans, the Canadian Government established safe level of BaPTEQ (600 µg kg−1) (CCME. 2010). Comparatively higher BaPTEQ was observed in sediments (range, 38–254 µg/kg, mean, 128 ± 78 µg/kg) than soils (range, 27–70 µg/kg, mean, 50 ± 16 µg/kg). ∑BaPTE in soils at SV, GC and OK was 26 ± 2 54 ± 14 and 53 ± 7 µg/Kg, respectively, while in sediments it was 41 ± 4, 206 ± 58 and 136 ± 5 µg/Kg, respectively. Seven PAHs (BaA, Ind, BaP, BbF, BkF, DBA, CHR) are suggested as carcinogenic PAHs (7CPAHs) (IARC 2010). Concentration of ∑7CPAHs in soils and sediments ranged between 168 and 457 µg/kg and 818 and 3270 µg/kg, with the mean value of 340 ± 122 µg/kg and 1837 ± 870 µg/kg, and their contributions were accounted for 43% and 61%, respectively, of ∑PAHs (Tables 1 and 2). However, BaPTEQ for ∑7CPAHs in soils and sediments was 44 ± 15 µg/kg (range, 23–68 µg/kg) and 126 ± 78 µg/kg (range, 126–78 µg/kg) and accounted for 98% and 99%, respectively, to ∑BaPTEQ. The major contributors to ∑BaPTEQ in soils were BaA, BbF, BkF, BaP and DBA, while, in sediments CHR, BaP and DBA were the major contributors. Sum BaPTEQ of major contributors in soil and sediments accounted for 89% and 94%, respectively, to ∑BaPTEQ (Table S5). These results prevail the importance of BaA, CHR, BbF, BkF, BaP and DBA in the carcinogenic potential for PAHs in the soils and sediments from flood plains in Delhi.
Possible sources of PAHs
Presences of different homologs of PAHs (PAHs with number of aromatic rings) and PAHs with different molecular weights within the environment are indicative of their possible origin sources (Kumar et al. 2014a, b, 2015a,b; Ray et al. 2013; Khaiwal et al. 2008; Khalili et al. 1995). During the present study, 4-rings PAHs were the dominant homolog, followed by 3-ring PAHs (Tables 1 and 2), which indicated mixed pyrogenic sources. These groups of PAHs can exist both within the vapor and particulate phase (Ray et al. 2013) and settle down in the vicinity of origin. The dominance of 3–4-rings PAHs has been reported from localized mixed sources coupled with atmospheric transport (Wang et al., 2009, 2017). PAHs with < 4 aromatic rings (low molecular weight-PAHs) are characterized by combustion of woods, grass and industrial oil, and petroleum products (Khalili et al. 1995; Wilcke 2007), while PAHs with ≥ 4 aromatic rings (high molecular weight-PAHs) are indicatives of pyrogenic activities at high temperature including coal combustion and vehicular emissions (Khalili et al. 1995; Marr et al. 1999). Generally, HMW-PAHs are likely to occur in closer proximity to emission sources, while LMW-PAHs transported to areas from the emission sources (Wang et al., 2009, 2017). Thus, homolog pattern of PAHs with different molecular weight shows local combustion sources coupled with atmospheric transported depositions to the study area (Ray et al. 2013; Singh et al. 2012).
Molecular diagnostic ratios (MDR) of selected PAHs concentrations including FLE/(FLE + PYR), ANT/(ANT + PHE), FLT/(FLT + PYR), BaA/(BaA + CHR), BbF/BkF, BaP/BghiP, BaP/(BaP + Chr) and Ind/(Ind + BghiP) were also used as tools for identification of possible sources of PAHs (Table 4). Observed average ratio of PAHs in soil and sediments was indicatives of various pyrogenic sources of PAHs emission, such as FLE/(FLE + PYR) (0.34–0.72) for petrol and diesel (Khaiwal et al. 2008), ANT/(ANT + PHE) (0.21–0.25) for petroleum & biomass combustions (Yunker et al. 2002), FLT/(FLT + PYR) (0.61–0.71) for biomass, coal combustion (Yunker et al. 2002), BaA/(BaA + CHR) (0.01–0.42) for petrogenic and combustion of petroleum and biomass (Yunker et al. 2002), BbF/BkF (1.35–1.50) for diesel engine and vehicular emissions (Dickhut et al. 2000), BaP/BghiP (0.47–2.89) for vehicular emissions and coal combustions (Simcik et al. 1999), BaP/(BaP + Chr) (0.05–0.18) for gasoline (Khalili et al. 1995) and Ind/(Ind + BghiP) (0.12–0.41) for petrogenic and petroleum combustions (Table 4). MDR analysis indicated mixed combustion activities including petrol, diesel, gasoline, biomass and coal combustions and vehicular emissions as the major sources. However, petrogenic sources can be associated with automobile workshops and accidental spillage. Correlation analysis was also performed as Pearson’s moment correlation coefficients, and significant correlation (p < 0.01) between the LMW-PAHs and HMW-PAHs was observed during the study (Table 5). Correlationship between FLE, PHE, ANT and CHR suggested biomass combustions sources, and correlation among BbF, BkF, BaP, DBA and Ind indicated emissions during the high temperature combustion process such as vehicles, industries and coal combustions (Marr et al. 1999). It is concluded that mixed pyrogenic sources of biomass and coal combustion and vehicular emissions could be the foremost significant sources of PAHs in study areas. Various researchers have reported similar PAHs sources from vehicles, diesel engines, coal, wood & biomass combustions and gasoline for Delhi region (Kumar et al. 2015a, b; Ray et al. 2013; Singh et al. 2012).
Distribution of organochlorine pesticides (HCHs and DDTs)
−Descriptive concentrations of ∑OCPs including ∑HCH and ∑DDT are presented in Tables 1 and 2, and their individual isomers are presented in Fig. 3. The ∑OCPs were comparatively higher in soils (25 ± 8.5 µg/kg) than sediments (21 ± 16 µg/kg). The observed concentrations of DDTs in soil were above (4.8–25 µg/kg) than HCHs (3.9–17 µg/kg). However, sediments contents of HCHs were above (2.5–29 µg/kg) than DDTs (0.41–18 µg/kg). Comparatively, higher concentration of ∑OCPs in soils was at GC (32 ± 10 µg/kg) than OK (22 ± 3 µg/kg) and SV (21 ± 8 µg/kg). Similar to soils, mean of ∑OCPs in sediments was also comparatively higher at GC (39 ± 8 µg/kg) than OK (20 ± 4 µg/kg) and SV (4.91 ± 0.72 µg/kg). Dominance of p,p’-DDT and γ-HCH was observed in both the matrices at all the locations (Fig. 3). Comparatively, α-HCH in soils was higher at OK, while γ-HCH, p,p’-DDT and p,p’-DDE were higher at GC (Table S2). However, in sediments concentration of α-HCH, γ-HCH, p,p’-DDT and p,p’-DDE was higher at GC than other locations (Table S3).
Observed concentrations of HCHs and DDTs were compared with similar other studies (Table S4). The HCHs are comparable with soils from Ghaziabad and Korba (India), Huangpi (China), Campanian (Italy), Indus (Pakistan) and KieuKy (Vietnam) and higher than Gwalior and Delhi (India), Nabeul (Tunisia) and Kathmandu, Pokhara, Birgunj (Nepal). On the other hand, HCHs in sediments were lower than Vasai, Sabarmati River, Cochin (India) and St. Lucia (South Africa) and higher than Hooghly River (India), Xijiang River and Jiulong River (China), Tiber River (Italy) and Ravi and Korang River (Pakistan). The concentrations of DDTs in soils were lower than Korba (India), Campanian (Italy), Nabeul (Tunisia), KieuKy (Vietnam), Kathmandu, Pokhara, Birgunj (Nepal), but higher than Huangpi (China), Delhi (India) and Indus (Pakistan). However, DDTs in sediments were higher than Tiber River (Italy) and lower than Brahamputra, Vasai, Sabarmati, Cochin (India) and River sediments of Ethopia, China, South Africa, Vietnam, Cameroon and Pakistan (Table S4).
Possible sources of OCPs
MDR and composition profiles were applied for identification of possible sources of HCH and DDT in soils and sediments. The α/γ-HCH ratio has been widely used to identify the possible sources of HCH in the environment (Pozo et al. 2017; Chakraborty et al. 2016; Kata et al. 2015; Kumar et al. 2016, 2014a; Becker et al. 2009). Dominance of α-HCH in soils and γ-HCH in sediments, and pooled average ratios of α/γ-HCH (0.35–1.08) (Table 6), indicated usage and input of technical HCH and lindane input in the study areas. Lower ratios of α/γ-HCH (≤ 1) were suggested for input of lindane formulation and higher ratios for technical HCH (Malaiyandi and Shah 1980). γ-HCH can transform into α-HCH in warm and humid tropical environment and higher intensity of solar ultraviolet radiations (Malaiyandi and Shah 1980). The Government of India had restricted the use of HCH for public health purposes and banned for use of in agriculture (UNEP 2011), while application of lindane has been exempted under protocol of Stockholm Convention, and subsequently, technical HCH was replaced with lindane for agricultural and pharmaceutical use in India (PPQS 2019; UNEP 2011). As p,p’-DDT in a tropical environment under aerobic conditions can transform to stable DDE metabolites (Aislabie et al. 1997), their ratio has been widely used for identification of the possible sources DDTs in the environment (Pokhrel et al. 2018; Pozo et al. 2017; Srirumali et al. 2015). Comparatively higher concentration of p,p’-DDT than p,p’-DDE and higher ratios (2.41–2.26) (Table 6) reflected recent contamination of DDTs. The higher ratio of (DDT/DDE) than one (> 1) suggests recent input of DDT, and lower ratio (< 1) indicates historical usage DDT (Tavares et al. 1999). DDT is produced in accordance with Stockholm Convention; however, the Government of India has banned the use of DDT in agriculture in 1989 and restricted for the control of vector-borne disease (UNEP 2011; PPQS 2019). Possible sources of HCHs and DDTs in Yamuna River flood plains are combined contamination from past and ongoing usage of HCH and DDT, coupled with the long-range atmospheric transport (LRAT) tendency of OCPs under tropical climatic. Similar sources have been reported by various researchers for these OCPs in different environmental compartments of India including Delhi region (Khuman and Chakraborty 2019; Mitra et al. 2019; Kumar et al. 2018; 2014a; Pozo et al. 2017; Chakraborty et al. 2016; Kata et al. 2015).
PCs in soils and sediments
The concentrations of total NPs, total CPs and their sums in soil and sediments are summarized in Tables 1 and 2, whereas distribution of individuals PCs is presented in Fig. 4. The mean concentration of NPs in sediments (569 ± 324 µg/kg) was above than soil (274 ± 313 µg/kg), while comparatively higher concentration of CPs was observed in soil (837 ± 279 µg/kg) than sediments (787 ± 431 µg/kg). Sum of NPs and CPs (∑PCs) in soils ranged between 639 and 2112 µg/kg (mean, 1111 ± 489 µg/kg), and their concentration in sediments was between 553 and 20,983 µg/kg (mean 1355 ± 640 µg/kg). 2,4-DCP (355 ± 265 µg/kg) was the dominant PCs in soils followed by 2-CP (291 ± 159 µg/kg) and 2-NP (210 ± 323 µg/kg), and in sediments 2,4-DNP (426 ± 275 µg/kg) was dominant, followed by 4-C,3-MP (345 ± 282 µg/kg), 2,4-DCP (255 ± 171 µg/kg) and 2-CP (248 ± 164 µg/kg) (Fig. 4). The mean of ∑PCs in soils and sediment was in increasing order at GC > OK > SV sampling locations. The average of ∑PCs in soils at SV, GC and OK was 938 ± 178, 1315 ± 804 and 1080 ± 413 µg/kg, respectively, and in sediment it was 608 ± 41, 1826 ± 311 and 1663 ± 469 µg/kg, respectively (Tables 1 and 2). The concentration of NPs and CPs was variable with sampling locations. Namely, NPs were comparatively higher at GC, and CPs were higher at OK. Among NPs, 2-NP (444 ± 542 µg/kg) and 2,4-DNP (720 ± 139 µg/kg) in soil and sediment were dominant at GC. However, 2,4-DCP (585 ± 397 µg/kg) and 4-C,3-MP (558 ± 200 µg/kg) were prominent CPs in both matrices at OK (Table S2 & Table S3).
Yamuna River receives majority of waste water generated in Delhi and is considered to be the primary source for contamination in the flood plains (DES 2016). Waste water including industrial & municipal, agricultural activities, application of pesticides and pharmaceuticals is the reported major sources of PCs in the environment (Zhou et al. 2017). Depositions of vehicular emissions (Michalowicz and Duda 2007) are may be the other possible sources of PCs, because Delhi is the national capital of India with large numbers of vehicles. Further, NPs are formed in the environment by reactions of phenol with nitrite ions and hydroxyl radical under the influence of solar UV irradiation (Patnaik and Khoury, 2004). However, sources of CPs include biotransformation of fungicides (2.4-dichlorophenoxyacetic acid) and reductive dechlorination of PCP under anaerobic conditions to the lower molecular weight CPs (Debadatta and Rajdeep, 2012; Persson et al. 2007; Laine and Jorgensen, 1996). PCP and its derivatives have been used worldwide, mainly in herbicides, biocides, pesticides and wood preservatives (McLellan et al. 2007). Sources of 4-C-3-MP can be associated with its use as an antiseptic and emissions during chlorination in water treatment plants (Yahaya et al. 2019; Zhou et al. 2017; Marianna 2004). The Pearson’s moment correlation analysis shows a significant correlation (p < 0.01) between γ-HCH, 2,4-DNP and 2-CP. Another significant correlation was also observed between p,p’-DDT, p,p’-DDE, 2-CP and PCP, and between 2-NP, 2-CP and PCP (Table 7). These correlations among OCP and PCs compounds indicated common sources of these pollutants in flood plains areas of Delhi. It has been reported that the PCs are used in the production of pesticides, and their transformation leads to environmental contaminations (Santana et al. 2009; Marianna 2004; WHO 1989). The observed concentrations of NPs were comparable with soils from India, but lower than sediments from China. CPs were comparable with soils from Germany and India, but lower than China, Sweden and South Korea (Table S7).
Health hazard of PAHs, OCPs and PCs
Stipulated soil quality guidelines (SQGs) and consensus-based sediment quality guidelines (CBSQGs) (Buckman 2008; WDNR 2003) were used for prediction of ecotoxicological health aspects of PAHs, OCPs and PCs in soil and sediments (Table S8). In comparison with guidelines, the concentrations of HCHs and DDTs in soils and sediments were lower than SQGs and CBSQGs, and classified (Wang et al., 2008)) as low polluted soil with HCHs and DDTs. Lower levels of PAHs than SQGs suggested low ecotoxicological health effect, and exceeded concentration of CHR in sediment at GC and OK locations may pose adverse effects on benthic biota, while other PAHs were lower than guidelines for soil and sediments, suggested low toxicological effects. BaP TPE (benzo(a)pyrene total potency equivalent), the cancer potency relative to BaP for all carcinogenic PAHs (BaA, BghiP, Ind, BaP, BbF + BkF, DBA, CHR), was estimated for soils (CCME 2010). Based on incremental lifetime cancer risk (ILCR) of 10−6–10−5, due to exposure from PAHs in soil, the Canadian government has stipulated 0.6–5.3 mg/kg as SQGDH (soil quality guideline for direct contact) (CCME 2010). The estimated BaP TPE (range, 0.02 –0.07 mg/Kg; mean, 0.04 mg/Kg) was lower than human health-based SQGDH. Pollution level of PAHs in soils was assessed as per anticipated classification by Maliszewska-Kordybach (1996), and soils were found to be weakly polluted at different locations. Further, the Nemerow composite index (NCI) was also used to assess soil quality (Kumar et al. 2014b) and observed NCI ranged between 0.02 and 0.19 with mean of 0.06, suggested safe category of PAHs pollution level. The observed levels of most of PCs were lower than the soil quality guidelines, except that 2,4-DNP and 2-CP were marginally above than guideline values (Buckman 2008), which may pose a health hazard to exposed biota. As no sediment quality guidelines are available for all PCs, PCP in sediment was found to be lower than CBSQGs (WDNR 2003). Intervention values (IVs) for PCs based on serious risk concentrations (SRC) were also applied for their ecotoxicological risk assessment during in this study. The observed levels of individual PCs were lower than the IVs of 2-CP, 2,4-DCP and PCP (Buckman 2008), indicated low risk due to PCs.
Conclusion
PAHs, OCPs and PCs were measured in soil and sediments from urbanized flood plain area for assessment of their possible sources and ecotoxicological health effects. Most of the target compounds were lower than recommended environmental guidelines suggested low ecotoxicological health effect. 3-ring and 4-ring PAHs were the major group of PAHs in soils and sediment. Composition profile, molecular ratio and correlation analysis of PAHs suggested mixed pyrogenic sources from diesel & petrol engines and fossil fuel and biomass combustion as the significant contributor of PAHs. Dominance of p,p’-DDT and γ-HCH, and isomeric ratio of between HCH and DDT compounds suggested past and recent input of HCHs and DDTs. The dominant PCs in soils and sediments were 2,4-DCP and 2,4-DNP, followed by 2-CP and 4-C,3-MP, respectively. Waste water and atmospheric reactions are the possible sources of PCs. Comparatively, higher contamination of pollutants in soils and sediments at GC than other location can be characterized by its position and influence of wastewater discharges through various drains.
References
Aleksandra, U.-J., Smreczak, B., & Agnieszka K—P. . (2019). Soil organic matter composition as a factor affecting the accumulation of polycyclic aromatic hydrocarbons. Journal of Soils and Sediments, 19, 1890–1900.
Aislabie, J. M., Richards, N. K., & Boul, H. L. (1997). Microbial degradation of DDT and its residues-a review. New Zealand J Agricultural Research, 40, 269–282.
ATSDR (Agency for Toxic Substances and Disease Registry). 1995. Toxicological profile for polycyclic aromatic hydrocarbons (PAHs). US Department of Health and Human Services, Public Health Service. Atlanta, GA. Accessed 10.01.2020. http://www.atdsr.cdc.gov/toxpro.les/phs69.
ATSDR. 2005. Toxicological profile for hexachlorocyclohexanes. Accessed 20.01.2020. http://www.atsdr.cdc.gov/toxprofiles/index.asp.
ATSDR. 2008. Toxicological profile for DDT, DDE, and DDD. Accessed 20.01.2020. http://www.atsdr.cdc.gov/toxprofiles/index.asp.
Becker, B., Halshall, C. J., Tych, W., Kallenborn, R., Schiabch, M., & Mano, S. (2009). Changing sources and environmental factors reduce the rates of decline of organochlorine pesticides in the Arctic atmosphere. Atmospheric Chemistry and Physics Discussion, 9, 515–540.
Buckman, M.F. 2008. NOAA screening quick reference tables (SQuiRTs). NOAA OR&R REPORT 08–1, Seattle Washington, Office of the Response and Restoration Division, National Oceanography and Atmospheric Administration, 34 pages.
CCME. 2010. Canadian soil quality guidelines for the protection of environmental and human health: Carcinogenic and other PAHs. In: Canadian environmental quality guidelines, 1999, 200 Winnipeg, Canada, http://www.ccme.ca.
Chakraborty, P., Khuman, S. N., Selvaraj, S., Sampath, S., Devi, N. L., Bang, J. J., & Katsoyiannis, A. (2016). Polychlorinated biphenyls and organochlorine pesticides in River Brahmaputra from the outer Himalayan Range and River Hooghly emptying into the Bay of Bengal: Occurrence, sources and ecotoxicological risk assessment. Environmental Pollution, 219, 998–1006.
Debadatta, D., & Rajdeep, K. (2012). Biodegradation of phenols by various indigenous microorganisms. Journal of Environmental Research Division, 7, 1–7.
DERC (Delhi Electricity Regulatory Commission). 2017. Annual Report 2016–2017. Accessed on 30.01.2020 at http://www.derc.gov.in/
DES (Directorate of Economics and Statistics). 2016. Statistical Abstract of Delhi, 2016. Government of National Capital Territory of Delhi, Delhi, India. Accessed 30.01.2020 at http://des.delhigovt.nic.in/wps/wcm/connect/DOIT_DES/des/home/.
Dickhut, R. M., Canuel, E. A., Gustafson, K. E., Liu, K., Arzayus, K. M., Walker, S. E., et al. (2000). Automotive sources of carcinogenic polycyclic aromatic hydrocarbons associated with particulate matter in the Chesapeake Bay Region. Environmental Science Technology, 34, 4635–4640.
EC (European Community). 2001. The list of priority substances in the field of water policy and amending directive, Council directive 2455/2001/ECC. Official Journal L331, 1–5.
Goswami, P., Ohura, T., Guruge, K. S., Yoshioka, M., Yamanaka, N., Akiba, M., & Munuswamy, N. (2016). Spatio-temporal distribution, source, and genotoxic potential of polycyclic aromatic hydrocarbons in estuarine and riverine sediments from southern India. Ecotoxicol Environmental Safety, 130, 113–123.
Ghosh SP & Maiti SK. 2019. Evaluation of PAHs concentration and cancer risk assessment on human health in a roadside soil: A case study. Human and Ecol Risk Assess: An International Journal. DOI: https://doi.org/10.1080/10807039.2018.1551052
Hafner, W. D., Carlson, D. L., & Hites, R. A. (2005). Influence of local human population on atmospheric polycyclic aromatic hydrocarbon concentrations. Environmental Science & Technology, 39, 7374–7379.
IARC (International Agency for Research on Cancer). 2010. IARC Monographs on the evaluation of carcinogenic risks to humans. Vol. 92, Some Non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. Lyone, France, https://monographs.iarc.fr/monographs-available/ Accessed 10.01.2020
Kata, M., Rao, S. R., & Mohan, K. R. (2015). Spatial distribution, ecological risk evaluation and potential sources of organochlorine pesticides from soils in India. Environmental Earth Sciences, 74, 4031–4038.
Khalili, N. R., Scheff, P. A., & Holsen, T. M. (1995). PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmospheric Environmental, 29, 533–542.
Khaiwal, R., Ranjeet Sokhi, R., & Grieken, R. V. (2008). Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmospheric Environment, 42(13), 2895–2921.
Kumar, B., Verma, V. K., Mishra, M., Sanjay, K. S., Sharma, C. S., & Akolkar, A. B. (2014a). Persistent organic pollutants in residential soils of North India and assessment of human health hazard and risks. Toxicol Environmental Chemistry, 96(2), 255–272.
Kumar, B., Verma, V. K., Kumar, S., & Sharma, C. S. (2014b). Polycyclic aromatic hydrocarbons in residential soils from an Indian city near power plants area and assessment of health risk for human population. Polycyclic Aromatic Compounds, 34(3), 191–213.
Kumar, B., Tyagi, J., Verma, V. K., Sharma, C. S., & Akolkar, A. B. (2014c). Distribution of eleven priority phenolic compounds in soils from mixed landuse and assessment of health hazard for human population. Advances in Applied Science Research, 5(2), 125–132.
Kumar, B., Verma, V. K., Sharma, C. S., & Akolkar, A. B. (2014d). Quick and easy method for determination of priority phenolic compounds in water and wastewater. Journal of Xenobiotics, 4(1), 46–52.
Kumar, B., Verma, V. K., Sharma, C. S., & Akolkar, A. B. (2015a). Priority Polycyclic Aromatic Hydrocarbons (PAHs): Distribution, Possible Sources and Toxicity Equivalency in Urban Drains. Polycyclic Aromatic Compounds, 36(4), 342–363.
Kumar, B., Verma, V. K., Tyagi, J., Sharma, C. S., & Akolkar, A. B. (2015b). Health Risk Due to Sixteen PAHs in Residential Street Soils from Industrial Region, Ghaziabad, Uttar Pradesh, India. Journal of Environment Protection and Sustainable Development, 1(2), 101–106.
Kumar, B., Verma, V. K., Sharma, C. S., & Akolkar, A. B. (2015c). Estimation of toxicity equivalency and probabilistic health risk on lifetime daily intake of polycyclic aromatic hydrocarbons from urban residential soils. Human and Ecol Risk Assess: An International Journal, 21(2), 434–444.
Kumar, B., Verma, V. K., Mishra, M., Tyagi, J., Sharma, C. S., & Akolkar, A. B. (2016). Quantification of nitrophenols, chlorophenols and hexachlorocyclohexanes in agricultural soils in the vicinity of industrial area for the assessment of human health hazard and risk. Human Ecol Risk Assess: An Int J, 22(1), 39–49.
Kumar, B., Verma, V. K., Rai, P., Kumar, S., & Sharma, C. S. (2017). Distribution and ecotoxicological risk assessment of priority phenols in river bank sediments. International Journal of Geology Earth & Environment Sciences, 7(2), 63–69.
Kumar, B., Mishra, M., Verma, V. K., Rai, P., & Kumar, S. (2018). Organochlorines in urban soils from Central India: probabilistic health hazard and risk implications to human population. Environmental Geochemistry and Health, 40, 2465–2480.
Khuman, S. N., & Chakraborty, P. (2019). Air-water exchange of pesticidal persistent organic pollutants in the lower stretch of the transboundary river Ganga, India. Chemosphere, 233, 966–974.
Khuman, S. N., Chakraborty, P., Cincinelli, A., Snow, D., & Kumar, B. (2018). Polycyclic aromatic hydrocarbons in surface waters and riverine sediments of the Hooghly and Brahmaputra Rivers in the Eastern and Northeastern India. Science and Total Environment, 636, 751–760.
Laine, M., & Jorgensen, K. (1996). Straw compost and bioremediated soil as inocula for the bioremedation of chlorophenols –contaminated soil. Applied Environment and Microbiology, 54, 1507.
Li, G., Sun, G. X., Ren, Y., Lu, X. S., & Zhu, Y. G. (2018). Urban soil and human health: a review. European Journal of Soil Science. https://doi.org/10.1111/ejss.12518.
Marr, L. C., Kirchstetter, T. W., & Harley, R. A. (1999). Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environmental Science Technology, 33, 3091–3099.
Marianna, C. (2004). Sources and transformations of chlorophenols in the natural environment. Science of the Total Environment, 322, 21–39.
Malaiyandi, M., & Shah, S. (1980). Evidence of photoisomerization of hexachlorocyclohexane isomers in the ecosphere. Journal of Environmental Science Health, 887–910(A19), 887–910.
McLellan, I., Carvalho, M., PereiraC, S., Hursthouse, A., Morrison, C., Tatner, P., et al. (2007). The environmental behaviour of polychlorinated phenols and its relevance to cork forest ecosystems: a review. Journal Environmental Monitor, 9, 1055–1063.
Mitra, S., Corsolini, S., Pozo, K., Audy, O., Sarkar, S. K., & Biswas, J. K. (2019). Characterization, source identification and risk associated with polyaromatic and chlorinated organic contaminants (PAHs, PCBs, PCBzs and OCPs) in the surface sediments of Hooghly estuary, India. Chemosphere, 221, 154–165.
Maliszewska-Kordybach, B. (1996). Polycyclic aromatic hydrocarbons in agricultural soils in Poland: preliminary proposals for criteria to evaluate the level of soil contamination. Applied Geochemical, 11, 121–127.
O’Driscoll, K., Mayer, B., Su, J., & Mathis, M. (2014). The effects of global climate change on the cycling and processes of persistent organic pollutants (POPs) in the North Sea. Ocean Science, 10, 397–409.
Olujimi, O. O., Fatoki, O. S., Odendaal, J. P., & Okonkwo, J. O. (2010). Endocrine disrupting chemicals (phenol and phthalates) in the South African environment: A need for more monitoring. Water SA, 36(5), 671–682.
Patnaik, P., & Khoury, J. (2004). Reaction of phenol with nitrite ion: pathways of formation of nitrophenols in environmental waters. Water Research, 38, 206.
Persson, Y., Lundstedt, S., Berg, L., & Tysklind, M. (2007). Levels of chlorinated compounds (CPs, PCPPs, PCDEs, PCDFs and PCDDs) in soils at contaminated sawmill sites in Sweden. Chemosphere, 66, 234–242.
Pozo, K., Sarkar, S. K., Estellano, V. H., Mitra, S., Audi, O., Kukucka, P., et al. (2017). Passive air sampling of persistent organic pollutants (POPs) and emerging compounds in Kolkata megacity and rural mangrove wetland Sundarban in India: An approach to regional monitoring. Chemosphere, 168, 1430–1438.
PPQS (Plant Protection Quarantine & Storage). (2019). Directorate of Plant Protection Quarantine & Storage. Central Insecticides Board & Registration Committee. Lists of pesticides banned, refused registration and restricted. http://ppqs.gov.in/ Accessed 30.01.2020.
Ray, S., Khillare, P. S., & Kim, K. H. (2013). Profiles, carcinogenic potencies, sources and association of black carbon and polycyclic aromatic hydrocarbons in size-fractionated urban and forest soils of Delhi, India. International Journal of Environmental Engineering and Management, 4(6), 581–584.
Santana, C. M., Ferrera, Z. S., Padron, M. E. T., & Rodrigues, J. J. S. (2009). Methodologies for the extraction of phenolic compounds from environmental samples: new approaches. Molecules, 14, 298–320.
Simcik, M. F., Eisenreich, S. J., & Lioy, P. J. (1999). Source apportionment and source/sink relationship of PAHs in the coastal atmosphere of Chicago and Lake Michigan. Atmospheric Environment, 33, 5071–5079.
Singh, D. P., Gadi, R., & Mandal, T. K. (2012). Levels, sources, and toxic potential of polycyclic aromatic hydrocarbons in urban soil of Delhi, India. Human Ecology Risk Assess: An International Journal, 18(2), 393–411.
Sulabh, 2019. National status of waste water generation & treatment. Sulabh Bhawan, New Delhi-110045. Accessed 03.04.2020, http://www.sulabhenvis.nic.in/Database/STST_wastewater_2090.aspx.
Tavares, T. M., Beretta, M., & Costa, M. C. (1999). Ratio of DDT/DDE in the all Saints Bay, Brazil, and its use in environmental management. Chemosphere, 38, 1445–1452.
Tsai, P. J., Shih, T. S., Chen, H. L., et al. (2004). Assessing and predicting the exposures of polycyclic aromatic hydrocarbons (PAHs) and their carcinogenic potencies from vehicle engine exhausts to highway toll station workers. Atmospheric Environmental, 38, 333–343.
USEPA (United States Environmental Protection Agency). 2015. Appendix A to 40 CFR, Part 423–126 Priority Pollutants. Accessed 30.01.2020, https://www.gpo.gov/fdsys/pkg/CFR-2018-title40-vol31/pdf/CFR-2018-title40-vol31-part423-appA.pdf.
UNEP (United Nations Environmental Programme). 2011. National implementation plans Stockholm Convention on persistent organic pollutants (POPs). Accessed 21 March 2019, http://chm.pops.int/Implementation/NIPs/NIPTransmission/tabid/253/Default.aspx.
UNEP (United Nations Environmental Programme). 2017. Revised Texts and Annexes. http://www.pops.int/Home/tabid/2121/Default.aspx. Accessed 15.02.2020
Wang, X., Wang, D., Qin, X., & Xu, X. (2008). Residues of organochlorine pesticides in surface soils from college school yards in Beijing, China. Journal Environmental Science, 20, 1090–1096.
Wang, Z., Ma, X., Na, G., Lin, Z., Ding, Q., & Yao, Z. (2009). Correlations between physicochemical properties of PAHs and their distribution in soil, moss and reindeer dung at Ny-Ålesund of the Arctic. Environmental Pollution, 157, 3132–3136.
Wang, C., Wu, S., Zhou, S., Shi, Y., & Song, J. (2017). Characteristics and source identification of polycyclic aromatic hydrocarbons (PAHs) in urban soils: A review. Pedosphere, 27(1), 17–26.
WDNR (Wisconsin Department of Natural Resources). 2003. Consensus-based sediment quality guidelines (CB-SQGs) recommendations for use and application interim guidance. Accessed 15.01.2020, https://dnr.wi.gov/files/PDF/pubs/rr/RR088.pdf.
WHO (World Health Organization). (1989). Environmental health criteria 93. Chlorophenols other than pentachlorophenol. Geneva.
Wilcke, W. (2000). Polycyclic aromatic hydrocarbons (PAHs) in soil–a review. Journal of Plant Nutrition Soil Science, 163, 229–243.
Yahaya, A., Okoh, O. O., Agunbiade, F. O., & Okoh, A. L. (2019). Occurrence of phenolic derivatives in Buffalo River of eastern Cape South Africa: exposure risk evaluation. Ecototocol Environment and Safety, 171, 887–893.
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, H. R., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33, 489–515.
Zhou, M., Zhang, J., & Sun, C. (2017). Occurrence ecological and human health risk and seasonal variations of phenolic compounds in surface water and sediments of a potential polluted river basin in China. International Journal Environmental Research Public Health, 14(10), 1–14.
Acknowledgements
The authors are grateful to competent authorities of Central Pollution Control Board for providing the necessary facilities to conduct study. Piyush, VK and AT are also thankful to CPCB for permission to carried out study for postgraduate dissertations. The views expressed in this paper are those of authors and do not necessarily reflect the organization’s.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, B., Verma, V.K., Mishra, M. et al. Assessment of persistent organic pollutants in soil and sediments from an urbanized flood plain area. Environ Geochem Health 43, 3375–3392 (2021). https://doi.org/10.1007/s10653-021-00839-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-021-00839-9