Abstract
Indoor dust samples were collected from 42 microenvironments of residential buildings (RB, 15 samples), official buildings (OB, 10 samples), laboratory rooms (LR, 7 samples), and school classroom (SCR, 10 samples) in Bushehr, whereby the concentration of zinc, copper, lead, cadmium, nickel, and chromium was studied. The results of this study indicated that the mean concentrations of Zn, Cu, Pb, Cd, Cr, and Ni in the indoor dust samples were 567.18, 186.09, 209.01, 5.31, 143.20, and 57.09 mg/kg in RB, 1077.34, 539.67, 274.89, 8.12, 155.30, and 92.55 mg/kg in OB, 246.40, 149.56, 127.2, 1.96, 43.45, and 91.09 mg/kg in LR and 271.43, 189.84, 164.44, 3.06, 124.20, and 70.09 mg/kg in SCR. The results of principal cluster analysis showed that the heavy metals in indoor environments were mostly originated from smoking tobacco and cigarette, traffic sources, old building materials, and building paint colors. The results of this study also revealed that the concentration of heavy metals in indoor dust had a negative and significant relationship (P value < 0.05 in most cases) with rate of ventilation, and a positive and significant relationship with smoking inside buildings (P value < 0.05 in most cases). The bioaccessibility for zinc, copper, lead, cadmium, nickel, and chromium was 69.12, 40.08, 43.33, 79.81, 31.10, and 6.31%, respectively, in indoor dust. Further, risk assessment showed that the risk values of carcinogenicity and non-carcinogenicity resulting from heavy metals inside the studied microenvironments had exceeded the recommended safe limit by EPA. In terms of potential ecological risks, it was found that heavy metals in these microenvironments have exceeded the hazardous ecological levels presented by different indices and can have considerable negative ecological effects. Thus, it is essential that further and better studies and monitoring be performed on these environments, and suitable control recommendations and solutions should be regulated for this public health threat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The people spend a considerable portion of their time (adults around 88% of the day and children around 71–79% of the day) in indoor environments including houses, offices, classes, laboratories, etc. (Okorie et al. 2012; Rostami et al. 2019). The activities that are performed in buildings including cooking, doing household chores and sweeping the house, painting walls, and usage of heating systems may play a significant role in the production of indoor pollutants (Kurt-Karakus 2012; Fazlzadeh et al. 2012; Naddafi et al. 2019a). In addition, penetration of air containing particulate matters (PM) from the outside environment and entry of the soil from the outside through the shoes of the residents are two other major ways of transmission of pollution to buildings (Hunt et al. 2006; Naddafi et al. 2019b). Therefore, the mineral pollutants present in the soil and PM such as heavy metals enter the household dust. Accordingly, the indoor dust can act as a sink for different pollutants (such as heavy metals), indicating their accumulation and characterization in indoor environments. According to the study by Lidia (Morawska 2004), the particles that exist in indoor dust are very variable in terms of shape and size such that 58% of particulates have a size range of 44–149 µm, and 6–35% lie within the range of 30–60 µm, which are small enough for penetration into the human body through inhalation or ingestion. Indoor dust causes exposure of residents to pollution in two ways: (1) transmission of pollutants into the body alongside airborne particles (Layton and Beamer 2009) and (2) exposure because of ingestion of indoor dust (Roberts et al. 2009). Therefore, indoor dust is a growing public health concern, which has attracted the attention of many researchers to study the exposure of different individuals to this mixture especially the elderly, infants, and sensitive groups such as pregnant women and cardiovascular patients.
As mentioned earlier, indoor dust is a complex and heterogeneous compound of organic and inorganic particles which can absorb and accumulate different heavy metals in it (Wang et al. 2016). Heavy metals constitute a group of pollutants which have attracted the attention of academia, research centers, state organizations, and others worldwide due to the risks they pose to the public health. Metals such as lead, cadmium, mercury, zinc, and chromium have been known as agents that are harmful for the human health, environment, and animals due to their stability in the environment (Zheng et al. 2010; Karbasdehi et al. 2016; Jafari et al. 2018). Some of these metals are mutagenic and carcinogen for humans. They are also associated with different diseases such as cardiovascular disease, nervous system, hematological and bone diseases, objective failure, gum bleeding, and tremor (Rashed 2008; Sun et al. 2013). Concerning the maximum brain growth and differentiation of children in their young ages, neonates and infants are specifically at risk of damage caused by exposure and toxicity with heavy metals (Bellinger 1995; Habibollahi et al. 2019). In addition, the absorption of heavy metals through digestive system and sensitivity of hemoglobin to these metals are far greater in children than in adults (Li et al. 2012). Therefore, profound understanding of the concentration, sources, and properties of heavy metal pollution in indoor dust can be helpful in developing managerial solution to protect the health of residents.
Unlike developed countries, due to absence or failure to implement environmental health policies, living and work environments in developing countries are not healthy. In addition, these countries lack a proper learning environment for children and therefore students as well as teachers are often exposed to different types of pollution inside educational environments. Bushehr is one of the industrial cities in the south of Iran located in the Persian Gulf northern coastline. The main industries in this city include petrochemical industries as well as oil and gas refineries, cement and stone crushing factories, atomic power plant, machineries, pharmaceuticals, food packaging industries, etc. In addition, due to the economic growth of this city, the rate of growth in the number of vehicles is also high in this city (based on an Internet search). Further, there is a large number of urban construction sites (such as residential, urban, administrative, and transportation) in the center of this city which have created large amounts of environmental pollutants. This city is also exposed to Middle Eastern Dust (MED), where the mean concentration of particulate matters in this city has been 143.7 µg/m3, and 3.5% of mortality in this city is attributed to the particulate matters present in the air of this city. Therefore, air pollution in this city is a very important issue threatening the health of Bushehrian citizens. Nevertheless, so far no study has been performed on the indoor air pollution in this city. Accordingly, the present study as the first study on the heavy metals in the indoor air in this city was designed and performed with the following objectives: (1) determining the concentration of heavy metals including lead, cadmium, chromium, nickel, copper, and zinc in the indoor particles of different buildings (residential buildings, work offices, laboratories, and school classrooms) of Bushehr city, (2) determining the enrichment factor of these metals and their potential sources, (3) investigating the effect of properties of buildings and their factors on the concentration of heavy metals, (4) determining the bioaccessibility of the chosen metals, and (5) assessing the health risk of exposure to heavy metals through ingestion, inhalation, and skin contact.
Materials and methods
Study area and sample collection
The present study was conducted in Bushehr Town (geographical coordinates: 28.9234° N and 50.8203° E, an average annual temperature of 25 °C and mean annual precipitation of 180 mm) located in the south of Iran and northern part of the Persian Gulf. In recent decades, different gas and petrochemical industries have been established with a set of light and heavy industries as well as supporting facility such as factories and warehouses in this town. This phenomenon has caused the development of various environmental problems in this region. In addition, as the capital of energy of Iran, Bushehr is a very important region for employment, which attracts people from different areas of Iran to this town; thus, marginalization has increased in this area. As a result, urban activities such as the production of urban wastes and their un-controlled discharge into environments, discharge of urban wastewaters, etc., have diminished the quality of environments in this region.
Totally, 42 samples of indoor dust sedimented inside different buildings in Bushehr city were collected which included residential buildings (RB, 15 samples), laboratory rooms (LR, 7 samples), official buildings (OB, 10 samples), and school classrooms (SCR, 10 samples) from May to July 2016. Map of the study area and the information associated with each of the buildings are provided in Fig. 1 and Table1, respectively. Before initiating the sampling, first the necessary explanations were given to persuade the house owners, managers of schools, and laboratories as well as offices to acquire the permission for sampling their indoor dust. Once they were persuaded, the sampling operation was initiated after receiving informed consent from them. For each of the buildings, background information including ventilation rate, number of residents, number of electric appliances, presence of smokers, the orientation of the building, the time past the last painting, and other information were collected using a researcher-made questionnaire. The samples were collected via vacuum cleaner from the mentioned regions, and all samples were sealed in polyethylene bags, labeled, and then transferred to laboratory. The weight of each sample was about 10 g. In the laboratory, the dust samples were placed inside a desiccator for 24 h, screened via a polystyrene screen with 1 mm mesh, and then dried at 24 °C. With this mesh size, it was ensured that all ranges of dust particles could pass through its pores, while small gravels, debris, wood pieces, etc., would be trapped by the sieve, whereby the final product was a homogeneous compound. The samples were placed inside plastic tubes, sealed with parafilm, and kept inside a fridge until device analysis.
Digestion of samples and analysis of heavy metals
To analyze the heavy metals, 1-g aliquot of dry dust was weighed and dissolved in 50 ml HCl 1 N. Then, it was exposed to 90 °C for 24 h in order to be evaporated and dried. Next, 30 ml of a mixture of nitric acid, perchloric acid, and hydrofluoric acid (with 3:2:1 v/v) was added to them, and eventually, it was exposed to 90 °C and fully digested (this process of digestion was replicated three times for each sample). The final solution of digestion was first concentrated and then diluted with 50 ml of deionized water and then passed through polycarbonate filters with a pore size of 0.4 micron. The digest samples were finally analyzed by atomic absorption spectrophotometry (AAS, GF 5000, Australia) to determine the concentration of zinc, copper, lead, cadmium, chromium, and nickel. For quality control and quality assurance, the devices were calibrated on a daily basis by calibration standards. A method blank, a spark blank, a matrix spike, and replication of samples were performed for each batch of samples. The extent of recovery for six metals in spiked blanks was obtained within the range of 74.3–112% with a SD of less than 10%, for 6 metals in the matrix spike samples within the range of 83.5–114.2% with SD of around 10.7%. Very trace amounts were observed for some metals in the method blank samples. These values were properly deducted from the values read in the samples. The limits of quantification (LOQs) of method for zinc, copper, lead, cadmium, chromium, and nickel were obtained as 0.29, 0.041, 0.033, 0.02, 0.032, and 0.025 µg/g, respectively.
To evaluate the oral bioaccessibility, physiologically based extraction test (PBET) was conducted through stimulating the chemical conditions of the human digestive system. To perform this test, the method presented by Moreda-Pinero (Moreda-Piñeiro et al. 2011) as well as Cheng et al. (Kang et al. 2012) was adopted with minor modification. The entire digestion processing of this test was performed under absolute darkness conditions and simulated against human stomach. Briefly, 0.5 g of the dust samples was taken and weighed and added to a 100-ml polypropylene tube containing 50 ml of gastric juice (containing 2 g/L of pepsin in 0.15 M NaCl acidified with HCl up to pH = 1.8). Next, under darkness conditions, it was shaken at 37 °C for 2.5 h at 120 rpm. Thereafter, it was centrifuged at 3000 r/min for 10 min and eventually filtered via a Whatman filter paper 5 C and 0.45-micron syringe filter. The substances remaining in the reaction tube were re-added to 50 ml of intestinal juice (containing 2 g/L of pancreatin, 2 g/L of amylase, and 5 g/L of bile salts in 0.15 M NaCl, pH = 6.8) and shaken under darkness conditions at 37 °C for 4 h with 100 rpm. Next, after dropwise addition of concentrated hydrochloric acid, it was centrifuged for 5 min at 10,000 r/min and then filtered by Whatman filter paper % c and 0.45-micron syringe filter. Eventually, the resulting solutions were kept at − 4 °C until analysis of heavy metals by atomic absorption spectrophotometer (AAS) (GF 5000, Australia).
After determining the concentration, the bioaccessibility percentage of each mental is calculated by Eq. (1):
where the numerator represents the concentration of metals in the stomach and intestine as obtained by physiologically based extraction test, while the denominator represents the total concentration of metals (Kang et al. 2011).
Determining the possible sources of heavy metals
Detection of congeners of different pollutants in environmental matrices including indoor dust can be used to determine the possible sources of pollution. In this regard, principal component analysis (PCA) was used as a statistical method to determine the possible sources of heavy metals in the dusts taken from the indoor environments of Bushehr buildings. This mathematical technique analyzes the relationship between observational variables through reducing the dimensions of original datasets and decreasing their components. In our previous study, we used it for determining the possible sources of polychlorinated biphenyls (PCBs) in coastal sediments of Asalouyeh (Arfaeinia et al. 2019). In addition, different researchers have employed PCA technique to analyze the levels of different pollutants including asbestos fibers, PAHs, etc. (Li et al. 2012). In this study, varimax normalized rotation was used to maximize the values of loading factors and minimize the ambiguity for each component with regard to every rotated principal component. In order to validate the obtained results by PCA analysis, the number of samples should be (n > 30 + ((V + 3))/2); n is the number of samples and V represents the number of variables. Therefore, for this analysis, the results of all samples were combined with each other.
Alongside PCA analysis, enrichment factor was used to differentiate a human source from a natural source and to assess the extent of effect of human activities (Lin et al. 2011). This factor is calculated by Eq. (2):
where Cn is the concentration of heavy metal of interest in the indoor dust samples, Cref shows the concentration of reference element in indoor dust samples, Bn denotes the background levels of the intended heavy metal, and Bref is the background values of the reference element.
In this study, manganese was a conservative element and used as the reference element (Han et al. 2006). Note that in this study, background values of metals presented by Zhang Cheng (Cheng et al. 2018) were used, which were 82.1, 29.7, 28.9, 0.08, 73.7, and 31.1 mg/kg for zinc, copper, lead, cadmium, chromium, and nickel, respectively.
Pollution load index
To evaluate the contamination degree of heavy metals on the environment, two indices called ecological risk index (ERI) and geo-accumulation (I-geo) index were used. I-geo is calculated by the following equation:
where Cs denotes the calculated values and Bn represents the background values. Factor 1.5 is applied to control the changes in Bn values resulting from the environment. The different classifications of this index are provided in Table2 (Arfaeinia et al. 2019; Hakanson 1980). ERI also integrates factors of the potential ecological risk of each metal together and relates their ecological and environmental effects with their toxicity. This index is calculated by the following equation:
Where, \(C_{f}^{i}\) is the contamination factor of each metal, \(C_{0 - 1}^{i}\) represents the concentration of each metal in the sediment samples, \(C_{n}^{i}\) denotes the background value, \(Er^{i}\) is the ecological risk potential of each metal, and \(Tr^{i}\) is the toxic response coefficient. In this study, \(Tr^{i}\) values calculated by Hakanson (Hakanson 1980) were used, which were 1, 5, 5, 30, 2, and 3 for zinc, copper, lead, cadmium, chromium, and nickel, respectively. The interpretive classification of ERI index is provided in Table2.
Determining the degree of pollution by a given heavy metal requires that the pollutant metal concentration be compared with an unpolluted reference material (geochemical background). Such reference material should be an unpolluted or pristine substance that is comparable with the studied samples. In this study, the local reference material was considered by the concentrations of different heavy metals observed in the indoor dust of rural area (unindustrialized areas). The background levels of heavy metals were Zn (68.51 mg/kg), Cu (7.35 mg/kg), Pb (0.57 mg/kg), Cd (0.51 mg/kg), Cr (8.84 mg/kg), and Ni (15.5 mg/kg).
Estimating the daily intakes of heavy metals and assessing the health effects
There are three main ways for exposure of individuals (adults and children) to the heavy metals present in indoor dust: (1) through ingesting dust, (2) through inhaling resuspended particles through the mouth and nose, and (3) receiving through absorption of heavy metals from the dust attached to the skin. The dust with a diameter less than 63-microns can be easily resuspended in the air, and the smaller the size of particles, the longer they will remain suspended in the air, and therefore, they have a greater tendency to be absorbed by humans through inhalation (Rostami et al. 2019). Accordingly, in all equations, estimation of exposure is based on the assumption that indoor dusts have the same particle size distribution. The average daily exposure dose (ADD, mg.kg−1.day−1) of metals associated with indoor dust was calculated individually for the three mentioned ways using Eqs. (5–7):
Note that the daily exposure dose through inhalation and skin contact was calculated based on the total concentration of heavy metals (acidic digestion), but for the ingestion pathway, it was calculated based on oral bioaccessible concentrations.
For the carcinogenic metal (chromium), the lifetime average daily doses (LADDs) were calculated, and the extent of their carcinogenicity through the three mentioned paths was estimated using Eqs. (8–10):
In these equations, exposure-point upper confident limit content (CUCL) was employed to obtain the reasonable maximum exposure, which is the upper limit of the 95% confidence interval. Since the concentration of most elements present in the indoor dust samples had an almost non-normal distribution in the present study, the 95% upper confidence limit (UCL) was calculated through an approach called “adjusted central limit theorem (CLT)” as an Eq. (11):
In this equation, n is number of samples, α stands for the probability of error Type I (false positive); Zα represents the (1 − α) th quantile of the standard normal distribution. In 95% confidence level, Zα = 1.645.
Although the approach was developed for large data sets with a normal distribution, the theorem does not indicate how many samples are adequate for normality to hold. Nevertheless, when the sample size is moderate or small, generally the means will not have normal distribution though the non-normality is intensified by the distribution skewness. Hence, it can be potentially applied for moderate or small size data with abnormal distribution.
The hazard quotient was used to calculate the extent of non-carcinogenic effects of heavy metals associated with indoor dust. The hazard index refers to the sum of HQs for different materials or different paths. These values are calculated by Eqs. (12–15):
In these equations, RfD represents the homologous reference dose and SF is the homologous slope factor. In this research, RfD and SF values for chromium were taken from the values presented by EPA (29).
Hexavalent chromium (Cr(VI)) is far more toxic than trivalent chromium (Cr(III)). In this study, the toxicity of Cr(VI) was used for stating the worst situation of chromium, while RfD and SF of hexavalent chromium were assumed for the total chromium. The RfD and SF values used in this study are provided in Table 3. If HI value is less than one, no considerable risk of non-carcinogenic effects threatens individuals exposed to indoor dust in Bushehr city. However, values greater than 1 for HI indicate that there is the possibility of incidence of non-carcinogenic effects for the exposed individuals, whereby the risks grow with elevation of the HI value. Concerning the carcinogenic metals, the CR values between 1 × 10–6 and 1 × 10–4 represent acceptable or tolerable risk range, values greater than 1 × 10–4 are unacceptable, and values less than 1 × 10–6 have no carcinogenic effects.
Statistical analyses
Descriptive statistics and other analyses were performed by SPSS Statistical Package version 22.0 (SPSS Inc.). For assessing normality of data, Shapiro–Wilk test was used. Using this test, it was observed that the data obtained for most of heavy metals had non-normal distribution (P value < 0.001). Therefore, non-normal distribution of concentration data for heavy metals was considered for these indoor environments. The kurtosis and skewness parameters were calculated with regard to data distribution for heavy metals. Since the obtained data had non-normal distribution, to compare the difference between the data obtained for different indoor environments (residential, laboratories, offices, and classes), Kruskal–Wallis test was used.
Results and discussion
Heavy metals in the dust of indoor microenvironments
The descriptive statistics of concentration obtained for six metals of zinc, copper, cadmium, chromium, and nickel in the dust collected from four indoor environments of RB, OB, SCR, and LR are provided in Table 4. As can be seen, for some metals in the dust samples, high standard deviations are observed in Table 4, suggesting high heterogeneity of the analyzed samples. In addition, this table also indicates that the metals were detected in all of the collected samples, suggesting that heavy metals are among the very common pollutants in the studied site. It can also be stated that indoor dust is a source of accumulation of heavy metals (Olujimi et al. 2015; Górka-Kostrubiec 2015). The ΣMetal lies within the range of 1088–1476.01 with the mean of 1175.88 mg/kg in residential buildings, 1449–3180.26 with the mean of 2160.87 mg/kg in official buildings, 581.91–779.85 with the mean of 668.76 mg/kg in the laboratory, and 721.87–941.84 with the mean of 832.06 mg/kg in the classroom.
The statistical analysis also showed that the level of ΣMetal in the different microenvironments was in the order of OB > RB > SCR > LR. This order can be attributed to the number of items present in the room. Typically, official buildings and residential houses have a high density of electric appliances in comparison with the classroom and laboratories. Similarly, concerning classroom, although there are no appliances other than projector and computer, these classes are always equipped with numerous chairs and desks. The lower levels of heavy metals in the dust collected from laboratories can also be attributed to the fact that laboratories are typically equipped with powerful fume hoods which perform suitable ventilation, thereby easily expelling out heavy metals alongside small dust particles from the laboratory (Sun et al. 2018). In addition, the rate of entrance and exit of clients to and from official buildings is higher; the soil attached to the shoes of these clients is transferred to the offices, thereby creating large amounts of dust inside these environments (Hunt et al. 2006).
The analysis of composition (profiles of congeners) and concentrations of individual metals can help in detecting the source, fate, and transference of metals in different environmental matrices.
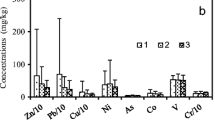
Heavy metal levels in each microenvironment followed the sequence: residential buildings Zn > Pb > Cu > Cr > Cd > Ni, office buildings Zn > Cu > Pb > Cr > Ni > Cd, laboratory rooms Zn > Cu > Pb > Ni > Cr > Cd, and school class rooms Zn > Cu > Pb > Cr > Ni > Cd. The results of this study indicated that the mean concentrations of Zn, Cu, Pb, Cd, Cr, and Ni in the indoor dust samples were 567.18, 186.09, 209.01, 5.31, 143.20, and 57.09 mg/kg in RB, 1077.34, 539.67, 274.89, 8.12, 155.30, and 92.55 mg/kg in OB, 246.40, 149.56, 127.2, 1.96, 43.45, and 91.09 mg/kg in LR and 271.43, 189.84, 164.44, 3.06, 124.20, and 70.09 mg/kg in SCR. Accordingly, these metals have been different due to the heterogeneity of samples and because of several variables which cannot be controlled in the sampling stage between the sampled microenvironments and between the samples. These differences can be explained with a high degree of variability of metals Table 4 regardless of the distinctive human activities in each section. The presence of lead, zinc, and copper is very noteworthy in all of the four indoor environments. All of these heavy metals are associated with human activities including building constructions, building paintings, rubber, and brakes as well as traffic of vehicles, lubrication, industrial colors, combustion of products as well as consumption fuels and cooking (Martínez and Poleto 2014). The high concentration recorded for Pb can be due to human activities including building painting, smoking, and penetration into indoor environments in response to emission of vehicles and secondary industrial combustion processes (Kylander et al. 2003). Copper is a heavy metal which due to its numerous physical properties has a wide range of applications in the production of home appliances including kitchen equipment, TV antenna, the body of electric equipment, etc., and usually accumulates in soil and dust in response to human activities (Barcan and Kovnatsky 1998). As zinc is used in the production of brass alloy, kitchen dishes, body of refrigerator, brake pads, oil tank, gasoline tank, and cylinder head gasket, it may originate from mechanical abrasion of household equipment as well as vehicle pieces, thereby entering the indoor environment (Al-Khashman 2004).
The enrichment factor was also calculated for all of the metals in the four indoor microenvironments. The average values of enrichment factor (EF) for heavy metals in indoor dust were ranked in the order of Cd > Cu > Pb > Zn > Ni > Cr.
The mean EF for cadmium (48.54), copper (23.62), lead (19.48), and zinc (11.43) has been larger than 5, suggesting that the indoor dusts of Bushehr have been enriched considerably (29). Unlike these metals, the EF obtained for chromium was 1.76, suggesting that this metal has mostly originated from natural sources (Han et al. 2006). It is also observed that the EF of cadmium (48.54) is far larger than 10. Therefore, it can be deduced that the concentration of this metal in the environmental matrices is heavily influenced by human activities (Han et al. 2006).
Sources and factors influencing heavy metal concentrations
Principal components analysis (PCA) was used as a statistical technique to determine the possible sources of heavy metals in the indoor dusts taken from different microenvironment in Bushehr City. To simplify the obtained results, three principal components were extracted accounting for 39.27, 27.43, and 19.21% of the total variance, respectively. The results of this analysis are presented in Fig. 2. The first component (C1) contains zinc, copper, and nickel which shows that this group of heavy metals may have a common source. The second component (C2) has also been associated with lead and cadmium which may have had a common source. Eventually, the third component (C3) has been related to nickel. Alongside PCA, the relationship between the concentration of different heavy metals was analyzed by a simple least-square regression model, with its results reported in Table 5. As can be seen in this table, most metals have a significant relationship with each other. It is seen that the metal pairs of Zn–Ni, Zn–Cu, Ni–Cu, and Cd–Pb are significantly interrelated with each other (P value < 0.01). All of these results suggest that these metal pairs emit into the environment from common sources. In this table, the highest correlations were found for the Zn–Cu metal pairs (r > 0.9).
Based on statistical analyses (PCA and regression model) and concerning the EF values obtained for heavy metals, we can determine the possible sources of these metals. The profile of heavy metals of factor 1 in PCA matches the characteristics of emission of zinc, copper, and nickel from sources including packaging waste, clothing accessories, stainless steel products, ceramics, electronic products, mechanical abrasion of electric pieces, vehicle pieces, and metal pieces used in household appliances as well as in other indoor environments (Chen and Lu 2018; Safari et al. 2018). Generally, factors such as the high temperature and humidity of Bushehr also cause facilitation of corrosion processes of metal pieces of buildings (doors and windows) which mostly contain Zn, Cu, and Ni alloys, eventually causing release of these metals to the environment and their accumulation in the dusts of this city. Concerning the factor 2 (lead and cadmium), it is also notable that these metals originate from sources such as ornamental materials, batteries, smoking cigarettes, and tobacco in indoor environments, the paint used for painting building walls, erosion and corrosion of rubber of vehicles, and the gases emitted by vehicles (Chandra Mouli et al. 2006; Yuen et al. 2012; Haghnazari et al. 2018). Therefore, factor 2 seems to originate from smoking cigarettes and tobacco, traffic sources, and latex color additives. Furthermore, according to Fig. 2 and Table 5, it is observed that chromium alone lies in Factor 3 and has no significant relationship with other metals. It was also observed that the EF of this metal (1.88) was close to 1, implying natural sources. Therefore, it can be concluded that chromium metal mostly originates from natural sources (Yongming et al. 2006).
From among the variables with possible effect on the concentration of heavy metals in indoor dust, ventilation rate, number of electronic appliances, number of inhabitants, smoking, the orientation of the building and the time from last painting were chosen and analyzed further. The effect of these variables was tested by Kruskal–Wallis nonparametric test, with its results presented in Table 6. As can be seen in this table, the ventilation rate has had a significant and negative relationship (P value < 0.05 in most cases) with the heavy metals and dust. In other words, with the increase in the ventilation rate, the concentration of heavy metals associated with indoor dust has diminished. These observations suggest that the ventilation rate is a key and influential factor for the concentration levels of pollutants in the indoor air (Sun et al. 2018). It was also observed that there is a positive and somehow significant (P value < 0.05 in some cases) relationship between the number of residents in these buildings and the concentration of heavy metals. This can be attributed to entrance of soil attached to footwear of residents into the buildings (as one of the two main ways of entrance of mineral pollutants) (Hunt et al. 2006). With regard to density of electric appliances inside buildings, Table 6 shows that there is a positive (but not very significant) relationship between this variable and the concentration of heavy metals. Similar results have been observed by Jianquang Sun et al. (Sun et al. 2018) with regard to the effect of density of electric appliances on the indoor air quality. Previous studies have also reported that smoking cigarettes can also be involved in the emission of heavy metals in indoor environments (Rasmussen et al. 2013). Therefore, in this study, the variable of “smoking” was also tested. In Table 6, it is observed that there is a significant and positive relationship between smoking inside buildings and the concentration of zinc (P value < 0.01), lead (P value < 0.05), and cadmium (P value < 0.05). These observations can be attributed to the contents of heavy metals in cigarette. These metals can be released into indoor dusts as suspended particles or soot from the cigarette (Pourkhabbaz and Pourkhabbaz, 2012). Therefore, smoking can be considered as one of the major sources of production of heavy metals in indoor dusts. With regard to the orientation of buildings, it was also observed that there is a positive and mild relationship between the orientation of buildings in the concentration of some of the studied heavy metals. In Iran, the wind direction is usually from the west to the east (Pourkhabbaz and Pourkhabbaz, 2012), thereby introducing large amounts of suspended particles from the outside air into the buildings with a westward orientation. Therefore, the factor of “the orientation of billings” can also be introduced as a factor increasing the indoor dust and in turn elevating the concentration of heavy metals present in the air. In addition, it was observed that with prolongation of time from the last painting of buildings, the level of heavy metals presents in indoor dust increased. The paints used for painting buildings in Iran are usually white latex paint (according to Internet search). This paint contains large amounts of lead carbonate (PbCO3) and lithopone (66% BaSO4 and 34% ZnSO4). Over time, the paints used for painting the walls of buildings undergo flaking, thereby entering the indoor dust as particles (Jaradat et al. 2004).
Bioaccessibility of heavy metals and assessing the risk resulting from them
The bioaccessibility results can determine how much of metals that have entered the body together with the dust is absorbed in the intestine and stomach. The results related to the PBET test showed that the bioaccessibility for zinc, copper, lead, cadmium, nickel, and chromium was 69.12, 40.08, 43.33, 79.81, 31.10, and 6.31% in the indoor dust, respectively. The results of our study were well congruent with the findings reported by previous studies, and a large difference was observed between the bioaccessibility of different metals. This difference can be attributed to the fact that different forms of these metals can occur in the stomach system (Morman et al. 2009). The results of bioaccessibility of metals were employed for determining the average daily exposure dose through ingestion and assessing the risk caused by uptake of heavy metals through this route.
The HQ values and the carcinogenicity risk of heavy metals are provided in Tables 7, 8, 9, and HI values are reported in Table 10. Among the carcinogenic metals, only chromium was analyzed and its carcinogenicity risk was assessed through three routes of exposure including ingestion, inhalation, and skin contact. As can be seen in Table 7, 8, 9, 10, the carcinogenic risk values for this metal for adults and children living in RB through ingestion, inhalation, and skin contact have been 1.2 × 10–4, 3.1 × 10–4, 7.1 × 10–9, 1.2 × 10–9, 4.5 × 10–4, and 9.3 × 10–5, respectively. In SCR, these values were 7.9 × 10–5, 7.4 × 10–10, and 7.4 × 10–5, respectively, for children and 6.5 × 10–5, 6.7 × 10–19, and 5.3 × 10–4 for adults. The risk of carcinogenicity for chromium for adults in OR through the three mentioned routes was 7.2 × 10–4, 8.1 × 10–3, and 5.3 × 10–5, respectively. Eventually, for adults in LR, they were 4.1 × 10–5, 5.1 × 10–11, and 1.5 × 10–5. As can be observed, the sum of carcinogenicity risk in the microenvironments RB and OB has been larger than 1*10–4, which is unacceptable limit. However, in SCR and LR, this value has been between 1*10–6 and 1*10–4, which is acceptable (Risk 1989). Similarly, for the risk of non-carcinogenicity, the total HI calculated for children in the microenvironments RB and SCR was 2.42 and 1.35, respectively. The total HI for adults in the microenvironments RB, OB, LR, and SCR was 2, 1.12, 1.96, and 1.12, respectively, as in Table 10. As can be seen, the non-carcinogenicity risk has exceeded the safe limit (HI = 1) recommended by EPA across all of the chosen microenvironments (Huang et al. 2018). As can be observed in Tables 7, 8, 9, ingestion is the main route of exposure to carcinogenicity risk of heavy metals in children, while for adults the main route of exposure is dermal contact. Since in the risk assessment, a lighter body weight and higher dust ingestion rates are considered for children, these findings are not surprising. Similar results have been reported by Kurt-Karakus for indoor dust in Istanbul city (Kurt-Karakus 2012). Based on ingestion + inhalation exposure pathway, none of them had HI larger than 1 for either children or adults (except for lead for children in RB). This level of lead in RB was calculated as 1.81 for children. Exposure to high doses of lead can lead to neurological disorders and impaired development of children (Ferreira-Baptista and Miguel 2005). In addition, cadmium is also a toxic cumulative metal whose target organ is kidneys (Burbure et al. 2003), though in the present study, the HI of this metal did not exceed the safe limit (HI = 1).
Pollution load index and evaluating ecotoxicological effects
In the present study, two indices called ecological risk index (ERI) and geo-accumulation (I-geo) were used. The results calculated for I-geo and ELR are provided in Tables 11 and 12, respectively. As seen in Table 11, based on I-geo index, RBs laid in the class of “moderately polluted” in terms of zinc, and nickel, in “moderately–highly polluted” with copper and cadmium, in “highly polluted” with chromium and “extremely polluted” in terms of lead. OBs also stood in the “extremely polluted” class in terms of copper and lead, and in “highly polluted” with zinc, cadmium, and chromium. Fortunately, except for Pb in the LRs which lies in “moderately–highly polluted” class in the rest of cases, LRs were in the “unpolluted” or “unpolluted to moderately polluted” classes. Moreover, except for Pb and Cd in the SCRs which lies in “highly polluted” class and Zn which lies in “highly to extremely polluted” class, in the rest of cases, SCRs were in the “unpolluted” or “unpolluted to moderately polluted” classes. The obtained ELR values for RB, OB, LR, and SCR microenvironments are provided in Table 12. As can be seen, the ecological risk of Pb for all microenvironments was classified as “very high.” The ecological risk of Cd for OB and RB was classified as “considerable.” The ecological risk of Cu for OB, RB, and SCR was classified as “moderately.” Fortunately, in the rest of metals, studied microenvironments were in the “low” ecological risk class. In a study conducted in Villavicencio in Colombia, the ecological risk potential of heavy metals present in dust street collected from a commercial region was determined as considerable (Trujillo-González et al. 2016). In another study performed in Tehran, this index was very high across all of the evaluated parts, though the population of the city is over 10 million people and different industrial activities exist there (Saeedi et al. 2012). The findings of this research suggest that the indoor dusts of Bushehr Town are heavily contaminated with heavy metals and can have significant negative effects. Overall, since these pollutants may have a long-term effect in indoor air, they have the potential of threatening human health (Romero-Romero et al. 2017). Therefore, it needs special attention by policy makers and engineers of the environment to protect the indoor air in this region.
Conclusion
Although the present study was the first to examine the concentration, bioaccessibility, and to assess the risk of exposure to heavy metals in the indoor air of residential buildings, official buildings, laboratory rooms, and school classrooms in Bushehr city, it offered valuable results. The results indicated that the concentration of heavy metals was considerably high in the indoor air of the microenvironments of Bushehr such that it can pose serious threats to the health of both children and adult residents. Results of principal cluster analysis indicated that the heavy metals in the indoor environments have mostly originated from cigarette and tobacco smoking, traffic sources, old construction materials, and building paints. In addition, a positive and significant relationship was observed between smoking inside the buildings and the concentration of zinc, lead, and cadmium. In this study, the bioaccessibility of metals was also tested. It was found that the bioaccessibility of different metals was significantly different with each other, which can be due to the fact that various forms of heavy metals may occur in the stomach and digestive system. Furthermore, the risk assessment resulting from exposure to heavy metals in indoor dust indicated that the carcinogenic and non-carcinogenic risk values resulting from heavy metals in the studied microenvironments had exceeded the safe limit recommended by EPA. In terms of potential ecological risks, it was observed that heavy metals in these microenvironments have exceeded the hazardous ecological levels presented by different indices and can have considerable negative ecological effects. Therefore, it is essential that better and further studies and monitoring are performed on these environments and suitable control solutions and recommendations be developed for this public health threat.
References
USEPA. (1989). Risk assessment guidance for superfund. Vol. I: Human health evaluation manual (Part A). EPA/540/1-89/002.
Al-Khashman, O. A. (2004). Heavy metal distribution in dust, street dust and soils from the work place in Karak Industrial Estate Jordan. Atmospheric Environment, 38(39), 6803–6812.
Arfaeinia, H., Dobaradaran, S., Moradi, M., Pasalari, H., Mehrizi, E. A., Taghizadeh, F., et al. (2019). The effect of land use configurations on concentration, spatial distribution, and ecological risk of heavy metals in coastal sediments of northern part along the Persian Gulf. Science of the Total Environment, 653, 783–791.
Barcan, V., & Kovnatsky, E. (1998). Soil surface geochemical anomaly around the copper-nickel metallurgical smelter. Water, Air, and Soil Pollution, 103(1–4), 197–218.
Bellinger, D. (1995). Neuropsychologic function in children exposed to environmental lead. Epidemiology. pp. 101–3.
Burbure, Cd, Buchet, J.-P., Bernard, A., Leroyer, A., Nisse, C., Haguenoer, J.-M., et al. (2003). Biomarkers of renal effects in children and adults with low environmental exposure to heavy metals. Journal of Toxicology and Environmental Health Part A, 66(9), 783–798.
Chandra Mouli, P., Venkata Mohan, S., Balaram, V., Praveen Kumar, M., & Jayarama, R. S. (2006). A study on trace elemental composition of atmospheric aerosols at a semi-arid urban site using ICP-MS technique. Atmospheric Environment, 40(1), 136–146.
Chen, X., & Lu, X. (2018). Contamination characteristics and source apportionment of heavy metals in topsoil from an area in Xi’an city, China. Ecotoxicology and Environmental Safety, 151, 153–160.
Cheng, Z., Chen, L.-J., Li, H.-H., Lin, J.-Q., Yang, Z.-B., Yang, Y.-X., et al. (2018). Characteristics and health risk assessment of heavy metals exposure via household dust from urban area in Chengdu. China. Science of The Total Environment, 619, 621–629.
Fazlzadeh DM, Rostami R, Zarei A, Feizizadeh M, Mahdavi M, Mohammadi A, et al. (2012). A survey of 24 hour variations of BTEX concentration in the ambient air of Tehran.
Ferreira-Baptista, L., & De Miguel, E. (2005). Geochemistry and risk assessment of street dust in Luanda, Angola: A tropical urban environment. Atmospheric Environment, 39(25), 4501–4512.
Górka-Kostrubiec, B. (2015). The magnetic properties of indoor dust fractions as markers of air pollution inside buildings. Building and Environment, 90, 186–195.
Habibollahi, M. H., Karimyan, K., Arfaeinia, H., Mirzaei, N., Safari, Y., Akramipour, R., et al. (2019). Extraction and determination of heavy metals in soil and vegetables irrigated with treated municipal wastewater using new mode of dispersive liquid–liquid microextraction based on the solidified deep eutectic solvent followed by GFAAS. Journal of the Science of Food and Agriculture, 99(2), 656–665.
Haghnazari, L., Mirzaei, N., Arfaeinia, H., Karimyan, K., Sharafi, H., & Fattahi, N. (2018). Speciation of As()/As(V) and Total Inorganic Arsenic in Biological Fluids Using New Mode of Liquid-Phase Microextraction and Electrothermal Atomic Absorption Spectrometry. Biological Trace Element Research, 183(1), 173–181.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control. A sedimentological approach. Water research, 14(8), 975–1001.
Han, Y., Cao, J., & Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi'an, Central China. Science of the Total Environment, 355(1–3), 176–186.
Huang, H., Jiang, Y., Xu, X., & Cao, X. (2018). In vitro bioaccessibility and health risk assessment of heavy metals in atmospheric particulate matters from three different functional areas of Shanghai China. Science of The Total Environment, 610–611, 546–554.
Hunt, A., Johnson, D. L., & Griffith, D. A. (2006). Mass transfer of soil indoors by track-in on footwear. Science of the Total Environment, 370(2–3), 360–371.
Jafari, A. J., Kermani, M., Kalantary, R. R., & Arfaeinia, H. (2018). The effect of traffic on levels, distribution and chemical partitioning of harmful metals in the street dust and surface soil from urban areas of Tehran, Iran. Environmental Earth Sciences, 77(2), 38.
Jaradat, Q. M., Momani, K. A., Jbarah, A.-A. Q., & Massadeh, A. (2004). Inorganic analysis of dust fall and office dust in an industrial area of Jordan. Environmental Research, 96(2), 139–144.
Kang, Y., Cheung, K. C., & Wong, M. H. (2011). Mutagenicity, genotoxicity and carcinogenic risk assessment of indoor dust from three major cities around the Pearl River Delta. Environment International, 37(3), 637–643.
Kang, Y., Man, Y. B., Cheung, K. C., & Wong, M. H. (2012). Risk assessment of human exposure to bioaccessible phthalate esters via indoor dust around the Pearl River Delta. Environmental Science and Technology, 46(15), 8422–8430.
Karbasdehi, V. N., Dobaradaran, S., Nabipour, I., Arfaeinia, H., Mirahmadi, R., & Keshtkar, M. (2016). Data on metal contents (As, Ag, Sr, Sn, Sb, and Mo) in sediments and shells of Trachycardium lacunosum in the northern part of the Persian Gulf. Data in Brief, 8, 966–971.
Kurt-Karakus, P. B. (2012). Determination of heavy metals in indoor dust from Istanbul, Turkey: Estimation of the health risk. Environment International, 50, 47–55.
Kylander, M. E., Rauch, S., Morrison, G. M., & Andam, K. (2003). Impact of automobile emissions on the levels of platinum and lead in Accra Ghana. Journal of Environmental Monitoring, 5(1), 91–95.
Layton, D. W., & Beamer, P. I. (2009). Migration of contaminated soil and airborne particulates to indoor dust. Environmental Science and Technology., 43(21), 8199–8205.
Li, B., Feng, C., Li, X., Chen, Y., Niu, J., & Shen, Z. (2012). Spatial distribution and source apportionment of PAHs in surficial sediments of the Yangtze Estuary, China. Marine Pollution Bulletin, 64(3), 636–643.
Li, X., Poon, C-s, & Liu, P. S. (2001). Heavy metal contamination of urban soils and street dusts in Hong Kong. Applied Geochemistry, 16(11–12), 1361–1368.
Lin, Y.-P., Cheng, B.-Y., Chu, H.-J., Chang, T.-K., & Yu, H.-L. (2011). Assessing how heavy metal pollution and human activity are related by using logistic regression and kriging methods. Geoderma, 163(3–4), 275–282.
Martínez, L. L. G., & Poleto, C. (2014). Assessment of diffuse pollution associated with metals in urban sediments using the geoaccumulation index (I geo). Journal of Soils and Sediments, 14(7), 1251–1257.
Morawska, L. (2004). Indoor particles, combustion products and fibres. Air Pollution (pp. 117–147). Berlin: Springer.
Moreda-Piñeiro, J., Moreda-Piñeiro, A., Romarís-Hortas, V., Moscoso-Pérez, C., López-Mahía, P., Muniategui-Lorenzo, S., et al. (2011). In-vivo and in-vitro testing to assess the bioaccessibility and the bioavailability of arsenic, selenium and mercury species in food samples. TrAC Trends in Analytical Chemistry, 30(2), 324–345.
Morman, S. A., Plumlee, G. S., & Smith, D. B. (2009). Application of in vitro extraction studies to evaluate element bioaccessibility in soils from a transect across the United States and Canada. Applied Geochemistry, 24(8), 1454–1463.
Naddafi, K., Nabizadeh, R., Rostami, R., Ghaffari, H. R., & Fazlzadeh, M. (2019a). Formaldehyde and acetaldehyde in the indoor air of waterpipe cafés: Measuring exposures and assessing health effects. Building and Environment, 165, 106392.
Naddafi, K., Nabizadeh, R., Rostamy, R., Kalan, M. E., Hassanvand, M. S., & Fazlzadeh, M. (2019b). Indoor air quality in waterpipe cafés: exposure level to particulate matter. Environmental Science and Pollution Research, 26(26), 26605–26616.
Okorie, A., Entwistle, J., & Dean, J. R. (2012). Estimation of daily intake of potentially toxic elements from urban street dust and the role of oral bioaccessibility testing. Chemosphere, 86(5), 460–467.
Olujimi, O., Steiner, O., & Goessler, W. (2015). Pollution indexing and health risk assessments of trace elements in indoor dusts from classrooms, living rooms and offices in Ogun State, Nigeria. Journal of African Earth Sciences, 101, 396–404.
Pourkhabbaz, A., & Pourkhabbaz, H. (2012). Investigation of toxic metals in the tobacco of different Iranian cigarette brands and related health issues. Iranian journal of basic medical sciences, 15(1), 636.
Rashed, M. N. (2008). Total and extractable heavy metals in indoor, outdoor and street dust from Aswan City, Egypt. CLEAN—Soil, Air, Water, 36(10–11), 850–857.
Rasmussen, P. E., Levesque, C., Chénier, M., Gardner, H. D., Jones-Otazo, H., & Petrovic, S. (2013). Canadian House Dust Study: Population-based concentrations, loads and loading rates of arsenic, cadmium, chromium, copper, nickel, lead, and zinc inside urban homes. Science of The Total Environment, 443, 520–529.
EPA U. Regional Screening Level (RSL) (2014) Summary Table (TR= 1E-6, HQ= 1). United States Environmental Protection Agency Washington (DC), 2014.
Roberts, J. W., Wallace, L. A., Camann, D. E., Dickey, P., Gilbert, S. G., & Lewis, R. G. et al. (2009). Monitoring and reducing exposure of infants to pollutants in house dust. In Reviews of Environmental Contamination and Toxicology (Vol. 201, pp. 1–39). Berlin: Springer.
Romero-Romero, S., Herrero, L., Fernández, M., Gómara, B., & Acuña, J. L. (2017). Biomagnification of persistent organic pollutants in a deep-sea, temperate food web. Science of the Total Environment, 605–606, 589–597.
Rostami, R., Zarei, A., Saranjam, B., Ghaffari, H. R., Hazrati, S., Poureshg, Y., et al. (2019). Exposure and risk assessment of PAHs in indoor air of waterpipe cafés in Ardebil Iran. Building and Environment, 155, 47–57.
Saeedi, M., Li, L. Y., & Salmanzadeh, M. (2012). Heavy metals and polycyclic aromatic hydrocarbons: Pollution and ecological risk assessment in street dust of Tehran. Journal of Hazardous Materials, 227, 9–17.
Safari, Y., Karimaei, M., Sharafi, K., Arfaeinia, H., Moradi, M., & Fattahi, N. (2018). Persistent sample circulation microextraction combined with graphite furnace atomic absorption spectroscopy for trace determination of heavy metals in fish species marketed in Kermanshah, Iran, and human health risk assessment. Journal of the Science of Food and Agriculture, 98(8), 2915–2924.
Sun, G., Li, Z., Bi, X., Chen, Y., Lu, S., & Yuan, X. (2013). Distribution, sources and health risk assessment of mercury in kindergarten dust. Atmospheric Environment, 73, 169–176.
Sun, J., Xu, Y., Zhou, H., Zhang, A., & Qi, H. (2018). Levels, occurrence and human exposure to novel brominated flame retardants (NBFRs) and Dechlorane Plus (DP) in dust from different indoor environments in Hangzhou China. Science of The Total Environment, 631, 1212–1220.
Trujillo-González, J. M., Torres-Mora, M. A., Keesstra, S., Brevik, E. C., & Jiménez-Ballesta, R. (2016). Heavy metal accumulation related to population density in road dust samples taken from urban sites under different land uses. Science of the Total Environment, 553, 636–642.
Wang, J., Li, S., Cui, X., Li, H., Qian, X., Wang, C., et al. (2016). Bioaccessibility, sources and health risk assessment of trace metals in urban park dust in Nanjing, Southeast China. Ecotoxicology and Environmental Safety, 128, 161–170.
Yongming, H., Peixuan, D., Junji, C., & Posmentier, E. S. (2006). Multivariate analysis of heavy metal contamination in urban dusts of Xi'an, Central China. Science of The Total Environment, 355(1), 176–186.
Yuen, J. Q., Olin, P. H., Lim, H. S., Benner, S. G., Sutherland, R. A., & Ziegler, A. D. (2012). Accumulation of potentially toxic elements in road deposited sediments in residential and light industrial neighborhoods of Singapore. Journal of Environmental Management, 101, 151–163.
Zheng, N., Liu, J., Wang, Q., & Liang, Z. (2010). Heavy metals exposure of children from stairway and sidewalk dust in the smelting district, northeast of China. Atmospheric Environment, 44(27), 3239–3245.
Acknowledgement
This research work was financial supported by Bushehr University of Medical Sciences (Grant No. 2636), and we gratefully acknowledge them.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hashemi, S.E., Fazlzadeh, M., Ahmadi, E. et al. Occurrence, potential sources, in vitro bioaccessibility and health risk assessment of heavy metal in indoor dust from different microenvironment of Bushehr, Iran. Environ Geochem Health 42, 3641–3658 (2020). https://doi.org/10.1007/s10653-020-00598-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00598-z