Abstract
Soil-bound polycyclic aromatic hydrocarbons (PAHs) in farmland are critical to human health. The level, composition, source, and cancer risk of sixteen PAHs in agricultural soil from Ningde, China, were investigated. The results indicated that the total concentrations of 16 PAHs ranged from 77.3 to 1188 ng g−1, with a mean value of 406 ng g−1. Five-ring PAHs were found to have the highest concentrations (148 ± 133 ng g−1), followed by four-ring (120 ± 101 ng g−1), three-ring (61.9 ± 54.2 ng g−1), six-ring (44.6 ± 61.0 ng g−1), and two-ring (31.3 ± 31.0 ng g−1). Employing positive matrix factorization (PMF), four PAH sources including biomass burning (36.3%), coal combustion (35.5%), traffic emissions (16.4%), and coke source (11.8%) were identified. Incremental lifetime cancer risk (ILCR) results showed that ILCR values ranged from 7.1 × 10−4 to 1.1 × 10−3, which will cause moderate-to-high cancer risk to human health mainly via the soil ingestion and dermal contact exposure pathways. The source-oriented results indicated that coal combustion (32.7%), traffic emission (34.3%), and biomass burning (32.4%) had similar contributions to the total cancer risk. Therefore, more attention should be paid to these pyrolysis-originated sources to protect humanity from the health risk of PAHs.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polycyclic aromatic hydrocarbons (PAHs), as typical persistent organic pollutants (POPs), have aroused much attention due to their persistence, long-range transportation, and resist degradation properties. Therefore, PAHs are found in various environmental mediums such as soil (Xing et al. 2011; Yang et al. 2016), sediment (Tolosa et al. 2004; Zhang et al. 2012), water (Zheng et al. 2016), and air (Khairy and Lohmann 2013; Zhuo et al. 2017). PAHs in the environment are mainly from natural processes (i.e., forest fire and volcano eruption) and anthropogenic activities (i.e., vehicle emissions, combustion of organic matters, and industrial processes). Airborne particulates originated from pyrolysis processes are transported in the atmosphere and ultimately dry–wet deposit in the upper layer of soil (Desalme et al. 2013).
As an important sink of pollutants, soil PAHs have attracted much attention due to their bio-accumulative, carcinogenic and mutagenic potencies and may allow the entry of PAHs into food chain (Peng et al. 2011; Xing et al. 2011; Yang et al. 2012; Man et al. 2013; Jia et al. 2017). Increasing PAH concentrations in soil resulted from human activities have caused scientific concern as these elevated concentrations in the soil will pose a higher risk to human health (Man et al. 2013). The widely used method to assess the human health associated with PAHs is incremental lifetime cancer risk (ILCR). This method estimates the incremental probability that an individual will develop cancer during his lifetime based on BaP toxic equivalent concentration. However, this method cannot distinguish the risk of different PAH sources, which exhibited different toxicities (Li et al. 2014; Lang et al. 2015). Recently, a new receptor model-incremental lifetime cancer risk method was developed to quantify the carcinogenic risks associated with sources of particle-bound PAHs (Liu et al. 2015). The study showed that the amount of human exposure to soil-bound PAHs was higher than through air or water (Menzie et al. 1992). Thus, it is important to figure out the PAHs in the soil. However, previous studies mainly concerned about PAH concentrations (Tang et al. 2005; Peng et al. 2013), compositions (Jiang et al. 2009), distribution (Liu et al. 2010; Wang et al. 2010), sources (Dai et al. 2008; Chen et al. 2013), and health risk (Man et al. 2013; Yang et al. 2016) in urban soil, while researches focused on PAHs in agricultural soil are limited (Duan et al. 2015; Tong et al. 2018) and thus need more attention due to its significance in human health.
Therefore, in this study, agricultural soil samples were collected and analyzed to (1) investigate the levels, compositions, and relationship between PAHs and organic matters, (2) to attribute the PAH sources employing positive matrix factorization (PMF) model and (3) to assess the soil-bound PAHs health risk to human and source-oriented risk using ILCR and PMF method.
Materials and methods
Sample collection
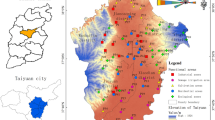
A total of sixty-two (forty from paddy land and twenty from vegetable land) topsoil samples (0–20 cm) were collected from the arable soils in the study area during November 2009. The locations of the sampling sites are shown in Fig. 1. At each location, four subsamples were collected from 50 m × 50 m grid and thoroughly mixed to form a composite sample with a pre-cleaned stainless steel scoop. All samples were wrapped in aluminum foil, sealed in polythene zipped bags and then transported to the laboratory. Soil samples were air-dried at room temperature, sieved through a 1-mm steel sieve, and then stored at −4 °C until analysis.
Chemical analysis
The detailed PAHs analysis procedure was described in previous study (Xing et al. 2011). Briefly, ten grams of air-dried soil spiked with 5 µL (200 µg mL−1) surrogates (mixture of five deuterated PAHs: naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12) was Soxhlet-extracted with 120–140 mL dichloromethane (DCM) for 24 h. Activated copper granules were added to the collection flask to remove elemental sulfur. Extracts were firstly solvent-exchanged to n-hexane and further concentrated to 2–3 mL by rotary evaporation. The deactivated alumina/silica (v/v = 1:2) gel column was used to clean the extracts, and PAHs were eluted with 30 mL DCM/hexane (2/3, v/v). The cleaned extracts were volume-reduced to 0.2 mL under a gentle nitrogen stream. Hexamethylbenzene (1000 ng) was added as an internal standard before the instrumental analysis.
Sixteen USEPA priority PAHs: naphthalene (Nap), acenaphthylene (Acy), acenaphthene (Ace), fluorene (Flu), phenanthrene (Phe), anthracene (Ant), fluoranthene (Fla), pyrene (Pyr), benzo[a]anthracene (BaA), chrysene (Chr), benzo[b]fluoranthene (BbF), benzo[k]fluoranthene (BkF), benzo[a]pyrene (BaP), indeno[1,2,3-cd] pyrene (IcdP), dibenzo [a, h] anthracene (DBA), and benzo [g, h, i] perylene (BghiP) were measured using GC–MS (Agilent 6890-5975). Samples (1 µL) were injected in splitless mode, separated by a DB-5 capillary column (30 m × 0.25 mm i. d × 0.25 µm film thickness) under the carrier gas (helium) at a constant flow of 1.5 mL min−1, and programmed temperature: initially at 60 °C for 5 min, increased to 290 °C, at a rate of 3 °C min−1 and held for 40 min. The PAH fractions were detected by mass detector equipped with electron impact (EI) source and operated in selected ion monitoring (SIM) mode. The target compounds were identified by comparison of retention times and mass spectra match to their corresponding standards.
The total organic compound (TOC) was measured with a TOC (liquiTOC, Elementar, Germany) by conventional standard procedures (Qu et al. 2015). Briefly, about 3 g of the freeze-dried soil was treated with 1 mol L−1 HCl for 24 h to remove inorganic carbon thoroughly. And then, it was dried overnight at 85 °C until the weight ceased to change. Fifty milligrams of the treated soil was used to determine TOC.
Quality assurance and quality control
The procedural blanks, spiked blanks, and duplicate samples were analyzed in every 10 samples for quality assurance and quality control. The average surrogate recoveries were 67.4 ± 12.5%, 75.4 ± 17.2%, 82.4 ± 14.5%, 86.1 ± 15.6%, and 96.2 ± 12.4% for naphthalene-d8, acenaphthene-d10, phenanthrene-d10, chrysene-d12, and perylene-d12, respectively. Limit of detection (LOD) was calculated as three times of the signal-to-noise (S/N) level in blank samples. The LOD for the target PAHs ranged from 0.07 to 0.58 ng g−1 dry weight. Standard PAHs solution was analyzed for daily instrument correction of the calibration, and the deviation was less than ± 15%. All reported values were recovery corrected and expressed on a dry weight basis.
PMF model description
PMF model is a recommended receptor model by the USEPA to source apportionment of pollutants, and it has been widely used in soil-bound PAH source apportionment (Wang et al. 2013, 2015a, b; Zhao et al. 2014). Briefly, the PMF decomposes a matrix of i × j dimension (X) into two matrices: source contribution matrix (G) and source profile matrix (F):
where xij is the concentration of jth PAH species measured in ith receptor sample; gik represents the contribution of the kth source to the ith sample; fkj stands for the mass fraction of the jth compound in the kth source, and eij is the residual for each sample/species. The target of PMF model is to calculate the minimum of the objective function Q considering the residuals (eij) and uncertainty (uij):
There are two types of uncertainty: sample-specified and equation-based. In this study, the equation-based uncertainty was introduced into PMF calculation (Table S1). If the concentration is greater than method detection limit (MDL), the uncertainty is calculated according to the following equation:
If the concentration is less than MDL, the uncertainty is estimated using the following equation:
The USEPA PMF (5.0) model was employed in this study. Nap and Ace were excluded from calculation due to their bad correlations with the total PAH concentrations. Acy, BkF, IcdP, and BghiP were categorized into weak due to their scaled residuals out of − 3 ~ + 3. The rest species were grouped into strong. The factor number was tested from 2 to 6, and each base model was run with a random seed for 20 iterations. The optimum factor number was co-determined by Q value, observed/predicted concentrations, bootstrap results, and the physical meaning of factor compositions (Bressi et al. 2014). Finally, four factors were considered as the optimal solution, which showed a significant correlation between observed and predicted PAH concentrations (r2 = 0.90, p < 0.01).
The uncertainties and error estimation of four-factor solution were estimated using bootstrap (BS), displacement of factor elements (DISP), and BS-DISP method. In BS run, base and boot factors were matched with mapping over 95%, which indicated BS uncertainties can be interpreted and the number of factors may be appropriate (US EPA 2014). The BS-DISP results showed that 92% of BS case was accepted and there were 5 swaps occurred in DISP. Therefore, the F-peak (0.2) value was employed to eliminate the rotation ambiguity, and almost all species in each factor were within the 5th–95th percentile of bootstrap. Finally, the F-peak = 0.2 result was reported.
Human cancer risk assessment model
We evaluated the potential cancer risk imposed on farmers or workers as a result of being in contact with contaminated soil. The pathways of human exposure to PAHs through soil are three main categories: (a) direct ingestion of substrate particles; (b) dermal absorption of pollutants in particles adhered to exposed skin; and (c) inhalation of re-suspended particles emitted from soil through the mouth and nose (Man et al. 2013). The ILCR through each of the three pathways within the scope of the study has been calculated according to the following equations adapted from the USEPA standard models (Peng et al. 2011; Qu et al. 2015):
where Csoil is the sum of toxic equivalent concentration to BaP in soil (ng g−1); CSF is the carcinogenic slope factor (mg kg−1 d−1)−1; BW is the average body weight (kg); IRsoil is the ingestion rate of soil (mg d−1); EF is the exposure frequency (d yr−1); ED is the exposure duration (yr); AT is the average life span (d); SA is the surface area of the skin that contacts the soil (cm2 d−1); AF is the skin adherence factor for soil (mg cm−2); ABS is the dermal absorption factor (chemical specific); IRair is the inhalation rate (m3 d−1); PEF is the particle emission factor (m3 kg−1). The carcinogenic slope factor (CSF) based on the cancer-causing ability of BaP was addressed as 7.3, 25, and 3.85 (mg kg−1 d−1)−1 for ingestion, dermal contact, and inhalation, respectively (Peng et al. 2011). The other parameters for evaluating human cancer risk are listed in Table 1. The total risk was estimated as the sum of individual risk of above three exposure pathways. Qualitative descriptions of possible cancer risks are as follows: very low when the estimated ILCR is less than 10−6; low from 10−6 to 10−4, moderate from 10−4 to 10−3, high from 10−3 to 10−1, and very high when the value is greater than 10−1 (Man et al. 2013).
Results and discussion
Levels and compositions of PAHs
The concentrations of PAHs are shown in Table 2. The difference of total concentrations of PAHs (Σ16PAHs) in vegetable soils (489 ± 372 ng g−1) and paddy (360 ± 240 ng·g−1) soils was not significant (p > 0.05). According to soil classification polluted by PAHs (Maliszewska-Kordybach 1996), the soil in this study was classified into non-contaminated to contaminated. However, the sum of ten PAHs in this study was beyond the Dutch target value (1000 ng g−1) and intervention value (4000 ng g−1) (VROM 2000). Besides, the sum of seven carcinogenic PAHs toxic equivalent concentrations to BaP was beyond the Canadian environmental quality guidelines for agricultural and industrial usage (5300 ng g−1, CCME 2008) as well.
The average concentrations of Σ16PAHs (406 ng·g−1) in this study were higher than those in agricultural soils from Huanghuai plain (130 ng·g−1, Yang et al. 2012), Henan (24.4 ng g−1, Feng et al. 2017), Xinzhou (202 ng g−1, Zhao et al. 2014), and Shanghai (365 ng·g−1, Jia et al. 2017), but were remarkably lower than those from Chengdu (3234 ng g−1, Xing et al. 2011), Beijing (1228 ng·g−1, Peng et al. 2011), Nanjing (3330 ng·g−1, Wang et al. 2015a, b), and Changzhi (917 ng·g−1, Liu et al. 2017). Generally, PAH concentrations in urban soil are higher than that in rural or agricultural soil (Man et al. 2013; Wang et al. 2015a, b). In this study, the average concentrations of PAHs were indeed lower than that measured in urban soil from Beijing (1228 ng g−1, Peng et al. 2013), Nanjing (3330 ng g−1, Wang et al. 2015a, b), Dhanbad (3488 ng g−1, Suman et al. 2016), and Kumasi (1398 ng g−1, Bortey-Sam et al. 2014).
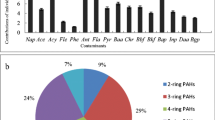
As shown in Fig. 2, the five-ring PAHs including BbF, BkF, BaP, and DBA had highest concentrations (148 ± 133 ng g−1), followed by four-ring (120 ± 101 ng g−1), three-ring (61.9 ± 54.2 ng g−1), six-ring (44.6 ± 61.0 ng g−1), and two-ring (31.3 ± 31.0 ng g−1). To be more specific, Chr, BbF, BkF, and BaP were considered to be the predominant compounds by their contributions being 19.0, 10.7, 10.8, and 11.7%, respectively, to the total PAHs. High molecular weight (HMW) PAHs are found to be the dominant compounds in urban soil (Peng et al. 2011; Wang et al. 2015a, b), while the percentages of low molecular weight (LMW) PAHs are greater than 50% in agricultural soil (Zhao et al. 2014; Chai et al. 2017). In this study, the HMW PAHs accounted for about 41.7% of the total PAHs, which had the similar PAH compositions profile in previous studies concerning PAHs in agricultural soil (Yang et al. 2012; Zhao et al. 2014; Liu et al. 2016).
Correlation analysis
The correlations among the individual PAHs can be performed to give an insight into the origins of these compounds (Yang et al. 2012). Correlation coefficient matrix (Table 3) among the individual PAHs showed that most of the compounds, especially the four-, five-, six-ring PAHs, were significantly positively correlated with each other. However, low molecular weight PAHs, such as Nap, Acy, and Ace, did not show significant positive correlations among these compounds and with the HMW group. This was due to different sources of these two PAH groups. The vapor pressure of semi-volatile organic compound (SVOC), such as PAHs, is one of the reasons to most properties for soil–air partitioning of SVOC. Thus, PAHs vapor pressure determined the compounds vapor–particle partitioning, which means that the available fraction is to undergo long-range atmospheric transport and phase transfer into the soil (Agarwal et al. 2009). Low molecular weight PAHs exhibit high atmospheric mobility, making them easier to be transported from far away locations by atmospheric transportation (Chung et al. 2007).
TOC in soil is considered as a key factor influencing the sorption, sequestration, and fate of PAHs in soil (Jiang et al. 2009; Duan et al. 2015). The contents of TOC in our study varied from 0.65 to 7.46%, with a mean value of 2.23%. As shown in Table 3, TOC was significantly correlated with a part of individual and total PAHs (p < 0.05), such as Acy, Flu, and Σ16PAHs. However, we cannot observe the higher correlations characterized by r value (r > 0.8). Lack of correlation between TOC and PAHs was interpreted as the result of non-equilibrium adsorption (Jiang et al. 2009; Wang et al. 2013). Compared with HMW PAHs, LMW PAHs are more likely subjected to soil–air exchange due to their high atmospheric mobility property, and thus result in the high correlations with TOC over time (Wang et al. 2013). The difference of correlation between TOC and LMW PAHs (r = 0.33, p < 0.01) and HMW PAHs (r = 035, p < 0.01) was not significant (Table 3). This fact reflected that these compounds had a possible trend of approaching the adsorption equilibrium status with TOC in soils, and were influenced simultaneously by other complex factors, such as pH, clay content, temperature, and organic matter composition. Additionally, it might be interpreted by the influences of the new introduction of organic fertilizers on soil-bound PAHs (Huang et al. 2006).
Source identification
Isomer ratio
Molecular indices based on isomer ratios of selected individual PAH have been established to distinguish petrogenic and pyrogenic sources of PAHs (Jiang et al. 2009; Xing et al. 2011; Yang et al. 2012). Fla/(Fla + Pyr) and IcdP/(IcdP + BghiP) have been used to identify the potential PAH emission sources in published studies (Jiang et al. 2009; Peng et al. 2011). The principle of molecular indices as identifying signatures has been described in previous papers (Yunker et al. 2002; Agarwal et al. 2009). The cross-plot for molecular indices of selected PAHs is presented in Fig. 3.
The values of Fla/(Fla + Pyr) in almost all samples were greater than 0.5, indicating that the pyrogenic source of PAHs originated from coal/biomass combustion. The result was consistent with some research from other regions, such as Delhi (Agarwal et al. 2009), Poland (Maliszewska-Kordybach et al. 2009), and Huanghuai plain (Yang et al. 2012), which speculated that coal/biomass combustion was the dominant sources of PAHs in agricultural soil. Although the IcdP/(IcdP + BghiP) ratios in some samples were less than 0.5, suggesting that the PAHs were derived from petrogenic or pyrogenic sources, the IcdP/(IcdP + BghiP) ratios for the great majority of soils above 0.5 were indicative of the typical attribution of coal/biomass combustion. Overall, pyrogenic sources, mainly coal/biomass combustion, were the main sources of PAHs in Ningde agricultural soils. This fact was in good agreement with the statistical results reported by the national bureau of statistics of China, which showed that coal was the major energy source and taken up about 76.7% of China’s energy (NBSC 2009).
Positive matrix factorization (PMF)
Four factors were extracted, and their source compositions are shown in Fig. 4. Factor 1 was responded for 35.5% of the total PAHs and heavily loaded on BbF, Chr, Pyr, BaP, and Fla. The source profile of this factor was similar to the emissions from coal combustion (Li et al. 2003; Chen et al. 2005). Therefore, factor 1 represented the coal combustion. Factor 2 was predominated by IcdP, DBA, and BghiP. These high molecular weight PAHs are from high-temperature combustion processes (Mai et al. 2001, 2003) such as diesel combustion and emission (Simcik et al. 1999). Therefore, factor 2 was labeled as traffic emission, and this factor accounted for 16.4% of the total PAHs. Factor 3 was mainly loaded on Acy, Flu, Phe, Fla, and Pyr. Flu and Phe are from coke ovens (Simcik et al. 1999), and Flu is the dominant compound in the coke oven molecular signature (Khalili et al. 1995). Thus, factor 3 was identified as coke source and explained 11.8% of the total PAHs. Factor 4 was dominated by Flu, Phe, Ant, BaA, and BbF. Flu, Ant, and Fla are considered to be the tracers of wood/grass burning (Khalili et al. 1995; Jenkins et al. 1996; Ou et al. 2010). Thus, factor 4 was attributed to biomass burning and explained 36.3% of the total PAHs.
The estimated source contributions identified by PMF are shown in Fig. 5a. Coal combustion and biomass burning totally accounted for about 71.8% of the total PAHs in agricultural soil from Ningde. Research on PAH sources in urban soil found that vehicle emission was the most contributor to the total PAHs (Wang et al. 2013; Suman et al. 2016; Cao et al. 2017). However, studies concerning PAH sources in agricultural soil indicated that biomass burning and coal combustion contribute most to the mass concentrations (Yang et al. 2012; Zhao et al. 2014). The agreement between diagnosis ratios of PAHs and PMF model to source identification indicated that the PAH sources were well allocated in this study.
Health risk assessment
The cumulative probabilities of ILCR via three different exposure pathways are shown in Fig. 6a. At 95th percentile, the estimated total ILCR ranged from 7.1 × 10−4 to 1.1 × 10−3, which will cause moderate-to-high cancer risk. To be more specific, the average ILCR of soil ingestion, dermal contact, and inhalation was 3.3 × 10−4, 5.9 × 10−4, and 2.3 × 10−8, respectively, which suggested that cumulative risk (10−6 ~ 10−4) may be caused through ingestion and dermal pathways. These two exposure pathways were the main routines to the soil-bound PAHs, while the risk via the soil inhalation was negligible in the study area. The same results were also reported in other agricultural soil (Tong et al. 2018) and street dust (Gope et al. 2018).
To find out which sources contributed most to the cancer risk, the source-oriented risk assessment was apportioned by PMF model and ILCR mode as shown in Fig. 6b–d. The average soil-bound BaP toxic equivalent concentrations of sixteen PAHs attributed to the coal combustion, traffic emission, coke source, and biomass burning were 20.8 ± 31.2, 21.5 ± 30.0, 0.38 ± 0.46, and 20.3 ± 25.2 ng g−1, respectively. As shown in Fig. 5b, the percentage of source contributions to the estimated ILCR was much different from that to the total mass concentration of the 16 PAHs. Traffic emission accounting for 16.4% of the total PAH mass concentrations was found to contribute most to the total cancer risk (34.3%). The percentage contributions of coal combustion to the total mass concentrations (35.6%) and to the total cancer risk (32.7%) were similar (Fig. 5b). The similar percentage was found for biomass burning. Coke source contributing least to the mass contribution (11.8%) was also found accounted for least of the total cancer risk (0.6%). The source apportionment of cancer risk in different exposure scenarios was conducted and is shown in Fig. 6. Coal combustion had highest cancer risk exerted on human among the three exposure pathways. As discussed above, the traffic emission contributed most to the total cancer risk; however, it ranked second in source-oriented risk assessment. Coke source exhibited the lowest ILCR value among the four identified PAH sources in each exposure pathway.
The results clearly suggested that soil contamination prevention and controls should take source and exposure pathways into consideration, besides a control on the total mass concentration. The control priority should be given to combustion sources which emitted higher concentrations of carcinogenic PAHs. Special attention also should be paid to soil ingestion and the dermal contact associated with soil-bound PAHs cancer risk.
Conclusion
Sixteen USEPA priority PAHs were measured in 22 vegetable farmlands and 40 paddy soil samples from Ningde, China. The total concentrations of PAHs in these two types of soil did not show a significant difference, and the soil pollution level was classified into non-contaminated and contaminated. High molecular weight PAHs originated form combustion were found to be the dominant compounds in soil samples. The isomeric ratio of PAHs and PMF model also indicated that PAHs were mainly from biomass burning, coal combustion, traffic emission, and coke source. The health risk assessment was estimated by ILCR method and showed moderate-to-high cancer risk mainly due to the soil ingestion and dermal contact. Biomass burning, coal combustion, and traffic emission were found to have similar contributions to the total cancer risk. Therefore, special focus also should be paid to soil ingestion and dermal contact pathways and control the pyrolysis processes-originated PAHs into the soil.
References
Agarwal, T., Khillare, P. S., Shridhar, V., & Ray, S. (2009). Pattern, sources and toxic potential of PAHs in the agricultural soils of Delhi, India. Journal of Hazardous Materials, 163(2–3), 1033–1039. https://doi.org/10.1016/j.jhazmat.2008.07.058.
Bortey-Sam, N., Ikenaka, Y., Nakayama, S. M. M., Akoto, O., Yohannes, Y. B., Baidoo, E., et al. (2014). Occurrence, distribution, sources and toxic potential of polycyclic aromatic hydrocarbons (PAHs) in surface soils from the Kumasi Metropolis, Ghana. Science of the Total Environment, 496, 471–478. https://doi.org/10.1016/j.scitotenv.2014.07.071.
Bressi, M., Sciare, J., Ghersi, V., Mihalopoulos, N., Petit, J.-E., Nicolas, J. B., et al. (2014). Sources and geographical origins of fine aerosols in Paris (France). Atmospheric Chemistry and Physics, 14(16), 8813–8839. https://doi.org/10.5194/acp-14-8813-2014.
Cao, H., Chao, S., Qiao, L., Jiang, Y., Zeng, X., & Fan, X. (2017). Urbanization-related changes in soil PAHs and potential health risks of emission sources in a township in Southern Jiangsu, China. Science of the Total Environment, 575, 692–700. https://doi.org/10.1016/j.scitotenv.2016.09.106.
CCME. (2008). Canadian soil quality guidelines carcinogenic and other polycyclic aromatic hydrocarbons (PAHs). https://www.mendeley.com/research-papers/canadian-soil-quality-guidelines-carcinogenic-other-polycyclic-aromatic-hydrocarbons-pahs. Accessed 19 August 2017.
Chai, C., Cheng, Q., Wu, J., Zeng, L., Chen, Q., Zhu, X., et al. (2017). Contamination, source identification, and risk assessment of polycyclic aromatic hydrocarbons in the soils of vegetable greenhouses in Shandong, China. Ecotoxicology and Environmental Safety, 142, 181–188. https://doi.org/10.1016/j.ecoenv.2017.04.014.
Chen, M., Huang, P., & Chen, L. (2013). Polycyclic aromatic hydrocarbons in soils from Urumqi, China: Distribution, source contributions, and potential health risks. Environmental Monitoring and Assessment, 185(7), 5639–5651. https://doi.org/10.1007/s10661-012-2973-6.
Chen, Y. J., Sheng, G. Y., Bi, X. H., Feng, Y. L., Mai, B. X., & Fu, J. M. (2005). Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environmental Science and Technology, 39(6), 1861–1867. https://doi.org/10.1021/es0493650.
Chung, M. K., Hu, R., Cheung, K. C., & Wong, M. H. (2007). Pollutants in Hong Kong soils: Polycyclic aromatic hydrocarbons. Chemosphere, 67(3), 464–473. https://doi.org/10.1016/j.chemosphere.2006.09.062.
Dai, J., Li, S., Zhang, Y., Wang, R., & Yu, Y. (2008). Distributions, sources and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in topsoil at Ji’nan city, China. Environmental Monitoring and Assessment, 147(1–3), 317–326. https://doi.org/10.1007/s10661-007-0123-3.
Desalme, D., Binet, P., & Chiapusio, G. (2013). Challenges in tracing the fate and effects of atmospheric polycyclic aromatic hydrocarbon deposition in vascular plants. Environmental Science and Technology, 47(9), 3967–3981. https://doi.org/10.1021/es304964b.
Duan, Y., Shen, G., Tao, S., Hong, J., Chen, Y., Xue, M., et al. (2015). Characteristics of polycyclic aromatic hydrocarbons in agricultural soils at a typical coke production base in Shanxi, China. Chemosphere, 127, 64–69. https://doi.org/10.1016/j.chemosphere.2014.12.075.
Feng, J., Li, X., Zhao, J., & Sun, J. (2017). Distribution, transfer, and health risks of polycyclic aromatic hydrocarbons (PAHs) in soil-wheat systems of Henan Province, a typical agriculture province of China. Environmental Science and Pollution Research, 24(22), 18195–18203. https://doi.org/10.1007/s11356-017-9473-8.
Gope, M., Masto, R. E., George, J., & Balachandran, S. (2018). Exposure and cancer risk assessment of polycyclic aromatic hydrocarbons (PAHs) in the street dust of Asansol city, India. Sustainable Cities and Society, 38, 616–626. https://doi.org/10.1016/j.scs.2018.01.006.
Huang, H., Li, S., Li, X., Yao, J., Cao, W., Wang, M., & Liu, R. (2006). Analysis on the status of organic fertilizer and its development strategies in China. Soil & Fertilizer Sciences in China, 2006(1), 3–8 (in Chinese).
Jenkins, B. M., Jones, A. D., Turn, S. Q., & Williams, R. B. (1996). Emission factors for polycyclic aromatic hydrocarbons from biomass burning. Environmental Science and Technology, 30(8), 2462–2469. https://doi.org/10.1021/es950699m.
Jia, J., Bi, C., Guo, X., Wang, X., Zhou, X., & Chen, Z. (2017). Characteristics, identification, and potential risk of polycyclic aromatic hydrocarbons in road dusts and agricultural soils from industrial sites in Shanghai, China. Environmental Science and Pollution Research, 24(1), 605–615. https://doi.org/10.1007/s11356-016-7818-3.
Jiang, Y.-F., Wang, X.-T., Wang, F., Jia, Y., Wu, M.-H., Sheng, G.-Y., et al. (2009). Levels, composition profiles and sources of polycyclic aromatic hydrocarbons in urban soil of Shanghai, China. Chemosphere, 75(8), 1112–1118. https://doi.org/10.1016/j.chemosphere.2009.01.027.
Khairy, M. A., & Lohmann, R. (2013). Source apportionment and risk assessment of polycyclic aromatic hydrocarbons in the atmospheric environment of Alexandria, Egypt. Chemosphere, 91(7), 895–903. https://doi.org/10.1016/j.chemosphere.2013.02.018.
Khalili, N. R., Scheff, P. A., & Holsen, T. M. (1995). PAH source fingerprints for coke ovens, diesel and gasoline engines, highway tunnels, and wood combustion emissions. Atmospheric Environment, 29(4), 533–542. https://doi.org/10.1016/1352-2310(94)00275-P.
Lang, Y., Li, G., Wang, X., Peng, P., & Bai, J. (2015). Combination of Unmix and positive matrix factorization model identifying contributions to carcinogenicity and mutagenicity for polycyclic aromatic hydrocarbons sources in Liaohe delta reed wetland soils, China. Chemosphere, 120, 431–437. https://doi.org/10.1016/j.chemosphere.2014.08.048.
Li, A., Jang, J. K., & Scheff, P. A. (2003). Application of EPA CMB8.2 model for source apportionment of sediment PAHs in Lake Calumet, Chicago. Environmental Science and Technology, 37(13), 2958–2965. https://doi.org/10.1021/es026309v.
Li, G., Lang, Y. H., Yang, W., Peng, P., & Wang, X. M. (2014). Source contributions of PAHs and toxicity in reed wetland soils of Liaohe estuary using a CMB–TEQ method. Science of the Total Environment, 490, 199–204. https://doi.org/10.1016/j.scitotenv.2014.05.001.
Liu, Y., Chen, L., Zhao, J., Wei, Y., Pan, Z., Meng, X.-Z., et al. (2010). Polycyclic aromatic hydrocarbons in the surface soil of Shanghai, China: Concentrations, distribution and sources. Organic Geochemistry, 41(4), 355–362. https://doi.org/10.1016/j.orggeochem.2009.12.009.
Liu, G., Guo, W., Niu, J., An, X., & Zhao, L. (2017). Polycyclic aromatic hydrocarbons in agricultural soils around the industrial city of Changzhi, China: Characteristics, spatial distribution, hotspots, sources, and potential risks. Journal of Soils and Sediments, 17(1), 229–239. https://doi.org/10.1007/s11368-016-1490-6.
Liu, G., Niu, J., Guo, W., An, X., & Zhao, L. (2016). Ecological and health risk-based characterization of agricultural soils contaminated with polycyclic aromatic hydrocarbons in the vicinity of a chemical plant in China. Chemosphere, 163, 461–470. https://doi.org/10.1016/j.chemosphere.2016.08.056.
Liu, G. R., Peng, X., Wang, R. K., Tian, Y. Z., Shi, G. L., Wu, J. H., et al. (2015). A new receptor model-incremental lifetime cancer risk method to quantify the carcinogenic risks associated with sources of particle-bound polycyclic aromatic hydrocarbons from Chengdu in China. Journal of Hazardous Materials, 283, 462–468. https://doi.org/10.1016/j.jhazmat.2014.09.062.
Mai, B., Fu, J., Zhang, G., Lin, Z., Min, Y., Sheng, G., et al. (2001). Polycyclic aromatic hydrocarbons in sediments from the Pearl river and estuary, China: spatial and temporal distribution and sources. Applied Geochemistry, 16(11–12), 1429–1445. https://doi.org/10.1016/S0883-2927(01)00050-6.
Mai, B., Qi, S., Zeng, E. Y., Fu, J., Sheng, G., Peng, P., et al. (2003). Distribution of polycyclic aromatic hydrocarbons in the coastal region off Macao, China: Assessment of input sources and transport pathways using compositional analysis. Environmental Science and Technology, 37(21), 4855–4863. https://doi.org/10.1021/es034514k.
Maliszewska-Kordybach, B. (1996). Polycyclic aromatic hydrocarbons in agricultural soils in Poland: Preliminary proposals for criteria to evaluate the level of soil contamination. Applied Geochemistry, 11(1), 121–127. https://doi.org/10.1016/0883-2927(95)00076-3.
Maliszewska-Kordybach, B., Smreczak, B., & Klimkowicz-Pawlas, A. (2009). Concentrations, sources, and spatial distribution of individual polycyclic aromatic hydrocarbons (PAHs) in agricultural soils in the Eastern part of the EU: Poland as a case study. Science of the Total Environment, 407(12), 3746–3753. https://doi.org/10.1016/j.scitotenv.2009.01.010.
Man, Y. B., Kang, Y., Wang, H. S., Lau, W., Li, H., Sun, X. L., et al. (2013). Cancer risk assessments of Hong Kong soils contaminated by polycyclic aromatic hydrocarbons. Journal of Hazardous Materials, 261, 770–776. https://doi.org/10.1016/j.jhazmat.2012.11.067.
Menzie, C. A., Potocki, B. B., & Santodonato, J. (1992). Exposure to carcinogenic PAHs in the environment. Environmental Science and Technology, 26(7), 1278–1284. https://doi.org/10.1021/es00031a002.
NBSC. (2009). Statistical Yearbook of China 2009. National Bureau of Statistics of China.
Ou, D., Liu, M., Cheng, S., Hou, L., Xu, S., & Wang, L. (2010). Identification of the sources of polycyclic aromatic hydrocarbons based on molecular and isotopic characterization from the Yangtze estuarine and nearby coastal areas. Journal of Geographical Sciences, 20(2), 283–294. https://doi.org/10.1007/s11442-010-0283-x.
Peng, C., Chen, W. P., Liao, X. L., Wang, M. E., Ouyang, Z. Y., Jiao, W. T., et al. (2011). Polycyclic aromatic hydrocarbons in urban soils of Beijing: Status, sources, distribution and potential risk. Environmental Pollution, 159(3), 802–808. https://doi.org/10.1016/j.envpol.2010.11.003.
Peng, C., Ouyang, Z., Wang, M., Chen, W., Li, X., & Crittenden, J. C. (2013). Assessing the combined risks of PAHs and metals in urban soils by urbanization indicators. Environmental Pollution, 178, 426–432. https://doi.org/10.1016/j.envpol.2013.03.058.
Qu, C., Qi, S., Yang, D., Huang, H., Zhang, J., Chen, W., et al. (2015). Risk assessment and influence factors of organochlorine pesticides (OCPs) in agricultural soils of the hill region: A case study from Ningde, southeast China. Journal of Geochemical Exploration, 149, 43–51. https://doi.org/10.1016/j.gexplo.2014.11.002.
Simcik, M. F., Eisenreich, S. J., & Lioy, P. J. (1999). Source apportionment and source/sink relationships of PAHs in the coastal atmosphere of Chicago and Lake Michigan. Atmospheric Environment, 33(30), 5071–5079. https://doi.org/10.1016/S1352-2310(99)00233-2.
Suman, S., Sinha, A., & Tarafdar, A. (2016). Polycyclic aromatic hydrocarbons (PAHs) concentration levels, pattern, source identification and soil toxicity assessment in urban traffic soil of Dhanbad, India. Science of the Total Environment, 545–546, 353–360. https://doi.org/10.1016/j.scitotenv.2015.12.061.
Tang, L., Tang, X.-Y., Zhu, Y.-G., Zheng, M.-H., & Miao, Q.-L. (2005). Contamination of polycyclic aromatic hydrocarbons (PAHs) in urban soils in Beijing, China. Environment International, 31(6), 822–828. https://doi.org/10.1016/j.envint.2005.05.031.
Tolosa, I., de Mora, S., Sheikholeslami, M. R., Villeneuve, J.-P., Bartocci, J., & Cattini, C. (2004). Aliphatic and aromatic hydrocarbons in coastal caspian Sea sediments. Marine Pollution Bulletin, 48(1–2), 44–60. https://doi.org/10.1016/S0025-326X(03)00255-8.
Tong, R., Yang, X., Su, H., Pan, Y., Zhang, Q., Wang, J., et al. (2018). Levels, sources and probabilistic health risks of polycyclic aromatic hydrocarbons in the agricultural soils from sites neighboring suburban industries in Shanghai. Science of the Total Environment, 616–617, 1365–1373. https://doi.org/10.1016/j.scitotenv.2017.10.179.
US EPA. (2014). Positive matrix factorization model for environmental data analyses. Overviews and Factsheets. https://www.epa.gov/air-research/positive-matrix-factorization-model-environmental-data-analyses. Accessed 20 September 2016
VROM. (2000). Ministry of Housing, Spatial Planning and Environment, Circular on Target Values and Intervention Values for Soil Remediation, Spatial Planning and Environment. Netherlands: Ministry of Housing.
Wang, X., Chen, L., Wang, X., Lei, B., Sun, Y., Zhou, J., et al. (2015a). Occurrence, sources and health risk assessment of polycyclic aromatic hydrocarbons in urban (Pudong) and suburban soils from Shanghai in China. Chemosphere, 119, 1224–1232. https://doi.org/10.1016/j.chemosphere.2014.10.019.
Wang, W., Massey Simonich, S. L., Xue, M., Zhao, J., Zhang, N., Wang, R., et al. (2010). Concentrations, sources and spatial distribution of polycyclic aromatic hydrocarbons in soils from Beijing, Tianjin and surrounding areas, North China. Environmental Pollution, 158(5), 1245–1251. https://doi.org/10.1016/j.envpol.2010.01.021.
Wang, X. T., Miao, Y., Zhang, Y., Li, Y. C., Wu, M. H., & Yu, G. (2013). Polycyclic aromatic hydrocarbons (PAHs) in urban soils of the megacity Shanghai: Occurrence, source apportionment and potential human health risk. Science of the Total Environment, 447, 80–89. https://doi.org/10.1016/j.scitotenv.2012.12.086.
Wang, C., Wu, S., Zhou, S., Wang, H., Li, B., Chen, H., et al. (2015b). Polycyclic aromatic hydrocarbons in soils from urban to rural areas in Nanjing: Concentration, source, spatial distribution, and potential human health risk. Science of the Total Environment, 527–528, 375–383. https://doi.org/10.1016/j.scitotenv.2015.05.025.
Xing, X. L., Qi, S. H., Zhang, J. Q., Wu, C. X., Zhang, Y., Yang, D., et al. (2011). Spatial distribution and source diagnosis of polycyclic aromatic hydrocarbons in soils from Chengdu Economic Region, Sichuan Province, western China. Journal of Geochemical Exploration, 110(2), 146–154. https://doi.org/10.1016/j.gexplo.2011.05.001.
Yang, B., Xue, N., Zhou, L., Li, F., Cong, X., Han, B., et al. (2012). Risk assessment and sources of polycyclic aromatic hydrocarbons in agricultural soils of Huanghuai plain, China. Ecotoxicology and Environmental Safety, 84, 304–310. https://doi.org/10.1016/j.ecoenv.2012.07.027.
Yang, J., Yu, F., Yu, Y., Zhang, J., Wang, R., Srinivasulu, M., & Vasenev, V. I. (2016). Characterization, source apportionment, and risk assessment of polycyclic aromatic hydrocarbons in urban soil of Nanjing, China. Journal of Soils and Sediments. https://doi.org/10.1007/s11368-016-1585-0
Yunker, M. B., Macdonald, R. W., Vingarzan, R., Mitchell, R. H., Goyette, D., & Sylvestre, S. (2002). PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Organic Geochemistry, 33(4), 489–515. https://doi.org/10.1016/S0146-6380(02)00002-5.
Zhang, Y., Guo, C. S., Xu, J., Tian, Y. Z., Shi, G. L., & Feng, Y. C. (2012). Potential source contributions and risk assessment of PAHs in sediments from Taihu Lake, China: Comparison of three receptor models. Water Research, 46(9), 3065–3073. https://doi.org/10.1016/j.watres.2012.03.006.
Zhao, L., Hou, H., Shangguan, Y., Cheng, B., Xu, Y., Zhao, R., et al. (2014). Occurrence, sources, and potential human health risks of polycyclic aromatic hydrocarbons in agricultural soils of the coal production area surrounding Xinzhou, China. Ecotoxicology and Environmental Safety, 108, 120–128. https://doi.org/10.1016/j.ecoenv.2014.05.034.
Zheng, B., Wang, L., Lei, K., & Nan, B. (2016). Distribution and ecological risk assessment of polycyclic aromatic hydrocarbons in water, suspended particulate matter and sediment from Daliao River estuary and the adjacent area, China. Chemosphere, 149, 91–100. https://doi.org/10.1016/j.chemosphere.2016.01.039.
Zhuo, S., Shen, G., Zhu, Y., Du, W., Pan, X., Li, T., et al. (2017). Source-oriented risk assessment of inhalation exposure to ambient polycyclic aromatic hydrocarbons and contributions of non-priority isomers in urban Nanjing, a megacity located in Yangtze River Delta, China. Environmental Pollution, 224, 796–809. https://doi.org/10.1016/j.envpol.2017.01.039.
Acknowledgements
This study was finically supported by the National Natural Science Foundation of China (Nos. 41073070, 41473095, and 41773124). This study was also financially supported by the Open Research Fund of Joint Innovative Centre for pollution control and the resource utilization technology in mining area, Hubei Polytechnic University (NO. xt201302).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zheng, H., Qu, C., Zhang, J. et al. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soils from Ningde, China: levels, sources, and human health risk assessment. Environ Geochem Health 41, 907–919 (2019). https://doi.org/10.1007/s10653-018-0188-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0188-7