Abstract
To investigate the distribution of mercury (Hg) in Futian mangrove wetland, surface sediments from land to sea were collected, including Kandelia obovata, Avicennia marina, Sonneratia caseolaris, and mud flat. The ecological risks of Hg in sediments were also assessed. The results showed that mangrove forests acidified sediments and promoted the accumulation of salinity and organic matter in sediments. Hg concentrations in both mangrove forests (154.7–218.4 ng g−1) and mud flat sediments (226.3–251.9 ng g−1) surpassed the background level (71.0 ng g−1). Furthermore, Hg concentrations in sediments decreased gradually from sea to land at all depth. From the bottom to the top layer sediment, Hg concentration decreased gradually in the sediments near land, while it kept vertically stable in the coastal area, indicating its pollution may mainly come from the coastal area rather than the land to some extent. Although the mean values of geo-accumulation indexes revealed uncontaminated to moderately contaminated levels, the mean values of potential ecological risk coefficients revealed considerable ecological risk of Hg to the environment, deserving further attention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangrove forests thrive widely in the intertidal zones on most tropical and subtropical coasts (Conrad et al. 2017). As one of the most productive ecosystems in the world, mangroves not only provide wood, food, and medicines for human beings (Walters et al. 2008), but also stabilize fine sediments, prevent coastal erosion, and maintain water quality and biodiversity (Arrivabene et al. 2016). Moreover, the mangrove sediments have a large capacity to retain heavy metals and reduce the potentially toxicological effects due to the characteristics of fine-grained texture, low redox condition as well as enrich of sulfide and organic matters (Chai et al. 2015; Gordeeva et al. 2017). Mercury (Hg) is widely considered to be one of the most toxic heavy metals due to its strong biological toxicity and complex migration process among ecosphere, atmosphere, hydrosphere, and biosphere (Luo et al. 2012; Jardine et al. 2013; Li et al. 2016a). It has been reported that Hg has no known essential biological function (Chakraborty et al. 2015), but elevated Hg levels in human bodies can lead to serious damage on health, resulting in neurological, immunological, and nephrological disorders, etc. (Cornelis et al. 2005; Zahir et al. 2005; Dhivert et al. 2016). At present, more and more researches are conducted to investigate the distribution, speciation, and transportation of Hg in mangrove sediments, where surroundings were heavily affected by human activities (Kehrig et al. 2012; Machado et al. 2016; Haris et al. 2017).
Futian mangrove forest in Shenzhen Bay is the only mangrove located within the large cities of China, which is deeply influenced by human activities. Many studies found that with the rapid urbanization and industrialization of Hong Kong, Shenzhen, and other coastal cities of Pearl River Estuary (PRE) since the early 1990s, a large amount of industrial wastewater has been discharged into the Shenzhen Bay (Huang et al. 2003; Zuo et al. 2008; Li et al. 2012; Chan et al. 2016), and abundant heavy metals have been deposited in Futian mangrove sediments simultaneously (Tam and Wong. 2000; Zan et al. 2002; Tang et al. 2016). Nowadays, some studies have investigated Hg and methyl Hg, as well as Hg flux in Futian mangrove forest sediments with low sampling density (Ding et al. 2009, 2010a; Li et al. 2016a). In the sediment of Futian mangrove forest, the spatial distributions of heavy metals (Cu, Cr, Cd, Ni, and As) varied in different mangrove communities from land to sea (Li et al. 2016b). Therefore, the distance from land and the type of mangrove communities may also act as an important factor in affecting Hg accumulation in sediments (Bayen 2012). However, no systematic and specialized research is available on the distribution and assessment of Hg in surface sediments of Futian mangrove forest with high sedimentation rate (Li et al. 2012). The data are also basic in making the long-term management and conservation policies. Therefore, the objectives of this study were to (1) determine the distribution of Hg and physicochemical properties in surface sediments of different mangrove communities and mud flat from land to sea and (2) assess the current potential ecological risk of Hg.
Materials and methods
Study area
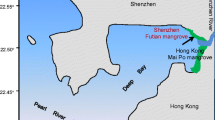
The study was carried out in the Futian Mangrove National Natural Reserve (22°32′N, 114°03′E, Fig. 1), located in the northeast coast of Shenzhen Bay, Guangdong province, China. The Futian Mangrove National Reserve covers an area of 367.64 ha distributed along 9 km of the coast. The mean annual temperature is 22 °C, the mean annual precipitation is 1927 mm mostly between May and September, and the tides in Shenzhen Bay are semidiurnal, with an average range of 1.9 m. There are three rivers flow into the mangrove reserve directly, including Shenzhen river, Xinzhou river, and Fengtang river (Zan et al. 2002). The mangrove forest communities distribute with a 100–400-m width from land to sea, including Kandelia obovata, Avicennia marina, Sonneratia caseolaris, and mud flat, respectively (Xie et al. 2010).
Sample collection and sediment analysis
According to sedimentation rate (1.38 cm a−1) in Futian mangrove forest (Li et al. 2012), the top 0–20 cm depth of sediments would encompass the deposited sediments after 2000s. In December 2015, three mangrove communities and mud flat were chosen in Futian mangrove forest, China. In each of these four swamps, three sampling sites were distributed along the coastline. In each sampling site, three surface sediments (0–20 cm depth) were collected with a 10-cm-diameter PVC tube which was washed by deionized water after soaking by dilute nitric acid. Then, each sediment core was sliced into 5-cm layers. Three subsamples at the same depth interval were collected to mix a composite sample in a plastic tray. Thus, there are 48 sediment samples in total (Fig. 1). All sediment samples were immediately sealed with plastic bags and transported back to the laboratory on the same day.
The sediment samples were air-dried, powdered, and sieved through 0.25 mm sieve. The sediment pH and salinity were determined in deionized water using mass ratios of 1:5 (sediment to water) (Li et al. 2016a). The total organic carbon (TOC) was determined by multi N/C 3100 analyzer (Jena, Germany). The particle size was analyzed by a particle size analyzer (Mastersizer 3000, Malvern, UK). The bulk sediment samples were separated into fine particles (clay + silt) (< 63 μm) and coarse particles (> 63 μm) (Parthasarathi et al. 2015). To determine Hg concentrations in sediment, 0.1 g of sediment samples was subjected to Milestone ETHO microwave digestion in a mixture of 9 ml nitric acid (HNO3), 3 ml hydrofluoric acid (HF), and 1 ml hydrochloric acid (HCl). The Hg concentration in extracting solution was analyzed by using manual atomic fluorescence spectrophotometer (Brooks Rand Labs-Model III, America).
Ecological risk analysis
The geo-accumulation index (Igeo) was implied to calculate the enrichment of Hg. The Igeo was introduced by Müller and can effectively explain the heavy metal pollution level by anthropogenic activities (Müller 1969) The Igeo is defined as: \( I_{\text{geo}} = \log_{2} (C_{n} / 1.5B_{n} ) \), where Cn is Hg concentration in sediments and Bn is the background concentration of Hg. The constant factor 1.5 is the background matrix correction factor due to lithogenic effects. The Igeo result can be interpreted into seven classes (Table 1).
The potential ecological risk coefficient (\( E_{r}^{i} \)) was chosen to evaluate the ecological risk of Hg. The method was proposed by Hakanson (1980), and it combined the content and the biological toxicity comprehensively. The \( E_{r}^{i} \) of Hg can be defined as: \( E_{r}^{i } \) = Cn/Bn*Tn, where Cn is Hg concentration in sediments; Bn is the background concentration of Hg; and the Tn is the biological toxicological coefficients of Hg (Tn = 40). The degree of ecological risk can be categorized as follows: \( E_{r}^{i} \) < 40, low risk; 40 ≤ \( E_{r}^{i} \) < 80, moderate risk; 80 ≤ \( E_{r}^{i} \) < 160, considerable risk; 160 ≤ \( E_{r}^{i} \) < 320, high risk; \( E_{r}^{i} \) ≥ 320, very high risk.
Statistical analysis
The data were expressed as mean ± standard deviation. Data statistics and analysis were conducted with Excel 2013. The Spearman correlation analysis and two-way ANOVA were performed at 5% level by SPSS 16.0 statistics package (SPSS Inc., Chicago, IL, USA).
Results and discussion
Sediments characteristics
Sediment, the primary compound of mangrove ecosystem, could accumulate various contaminants from incoming tidal waters and freshwater sources (Marchand et al. 2016; Zhang et al. 2018). The physicochemical properties of the sediments have been proved to be important in affecting Hg accumulation, formation, and transportation (Machado et al. 2002; Shi et al. 2010; Zhou et al. 2010). In Fig. 2, the pH in sediments varied from 5.8 to 6.6 in mangrove forest, slightly lower than that in mud flat (6.5–6.8). These results are consistent with the previous studies (Zhou et al.2010; Li et al. 2016c), suggesting that mangrove forests could acidify sediment due to microbial decomposition of mangrove litter and oxidation of FeS2 and FeS (Tam and Wong. 2000). The salinity in all sediments ranged from 3.7 to 6.2‰, and the salinity in K. obovata and A. marina sediments was much higher than those in mud flat, which may be related to their strong ability of salt accumulation (Wang and Lin 2003). TOC content in sediments significantly decreased from land to sea. TOC content in the sediments of S. caseolaris and A. marina slightly increased in the surface layer, which may be derived from the decomposition of litter and the metabolism of algae on the surface sediments (Zhou et al. 2010; Li et al. 2016a; Machado et al. 2016). In particular, TOC content (5.2–6.3%) in K. obovata sediment is significantly higher than most of the mangrove wetlands in China (1.2–3.8%) (Ding et al. 2010b). The large fluctuation of salinity and the high TOC content in K. obovata sediments may relate to that K. obovata is close to land and significantly affected by the input of domestic sewage (mainly from Fengtang river) and runoff from land (Zhang et al. 2000; Liu et al. 2014). The sediments in the study area mainly consisted of fine particles (< 63 um), with strong heterogeneity, and no significant changes from land to sea have been observed, indicating the similar hydrologic condition and depositional environment (Zan et al. 2002). It is noteworthy that the content of fine particles in the S. caseolaris sediments gradually decreased from the bottom to the top layers, which was possibly related to the baffle effect of roots and trunks. The high density respiration roots could effectively reduce the hydrologic movement of tides (Liu et al. 2008), which facilitate the deposition of larger particulates in tides. The result of two-way ANOVA showed that sediment pH and salinity were significantly affected by mangrove community and sediment depth; sediment TOC content was significantly affected by mangrove community and its interaction with sediment depth (P < 0.001) (Table 2).
Hg accumulation in surface sediments
In Table 3, Hg concentrations in mangrove forests and mud flat sediments were 154.7–218.4 and 226.3–251.9 ng g−1, respectively, which were higher than the background value of 71 ng g−1 (Ding et al. 2009). The higher Hg concentration was consistent with previous studies (Ding et al. 2009; He et al. 2015; Li et al. 2016a), which may be related to the input of industrial wastewater from neighboring regions since 2000s. And, it was higher than many mangrove wetlands, such as Shankou, Beilun estuary, and Yunxiao in China (Ding et al. 2009), as well as Port Klang in Malaysia (Haris et al. 2017) and Sundarban in India (Kwokal et al. 2012). Furthermore, Hg concentration in this study was lower than Fugong, Quanzhou bay (Ding et al. 2009) in China and Guanabara in Brazil where the environments were strongly influenced by anthropogenic activities (Machado et al. 2002). Compared with the guideline values, Hg concentrations were close to the threshold effect concentration (TEC), but much lower than the probable effect concentration (PEC), indicating low threat of Hg to the organisms in mangrove ecosystem.
Hg concentrations in mud flat were higher than that in mangrove communities and decreased gradually from sea to land at all the same depths (mud flat > S. caseolaris > A. marina > K. obovata) (Table 3, Fig. 3). From the bottom to the top layers of sediment, Hg concentration in sediments decreased gradually in K. obovata and A. marina sediments which are close to the land, while stable trends of Hg concentrations were detected in S. caseolaris and mud flat sediments near the coastal area (Fig. 3). Similar conclusions have also been reported by Li et al. (2015) that the concentrations of Cu, Pb, and Zn in surface sediments (10–20 cm) were slightly higher than the surface sediments (0–10 cm) in Futian mangrove forest near the land. One possible explanation may be that a series of strict environmental protection measures have been carried out, including introducing sewage treatment facilities and relocation of polluting industries from Shenzhen since 2000s (Li et al. 2016c). In addition, the results of two-way ANOVA indicated that the concentrations of Hg were significantly affected by different mangrove communities (P < 0.001) (Table 2).
With higher organic matter and fine particulates content, sediment in mangrove forest could retain more heavy metals compared to mud flat (Zhou et al. 2010; Liu et al. 2010). However, mangrove plants have relatively low uptake of Hg, with most Hg deposited in sediments (Machado et al. 2016). In Table 4, Hg concentration in sediments was negatively related to TOC (r = – 0.611, P < 0.01), indicating that the sources for Hg and organic matter may be different. In particular, the higher Hg concentration in mud flat sediments indicated its pollution sources coming from marine coastal environment. Although the terrigenous industrial contaminants have been alleviated due to the implementation of environmental protection policies after 2000s (Machado et al. 2002), the marine Hg pollution mainly from the PRE was not controlled and managed effectively. Since 1980s, huge amounts of heavy metal contaminants have been discharged into the PRE, such as 8293 t heavy metal input coming from direct fluvial transport by the Pearl River in 2003 (Li et al. 2007). The Shenzhen Bay received tremendous amount of PRE water containing abundant fine particles, which is a major carrier for transporting trace metals from the upstream source area to the coastal zone in Pearl River Mouth Basin (Ip et al. 2005; Ye et al. 2012; Zhao et al. 2017). Moreover, Shenzhen Bay is a semi-closed bay with poor hydrodynamic conditions, especially in the mangrove areas near the shore (Tang et al. 2016), resulting in the absorption of Hg by the fine particles and gradual deposition from sea to land. In addition, the correlation among Hg concentration and fine particles was not significant (Table 4), in agreement with previous studies (Bravo et al. 2011; Haris et al. 2017), indicating that fine particles were not an important factor in affecting Hg accumulation in sediment.
Ecological risk of mercury pollution in sediment
To determine the effects of anthropogenic activities on Hg accumulation in sediment, Igeo was applied to facilitate the interpretation of sediment quality. Taking the strong biotoxicity of Hg into account, \( E_{r}^{i} \) was also used to evaluate the possible biological effects of Hg. In the present study, the background concentration of Hg (71.0 ng g−1) was referenced to the study by Ding et al. (2009). Results showed that the distribution of Igeo and \( E_{r}^{i} \) of Hg in sediments was similar to the corresponding concentrations (Fig. 4), with gradually decreased trends from sea to land. Furthermore, reduced trend was also detected from the bottom to the top layers in the sediments of K. obovata and A. marina marshes. These results may be related to that the anthropogenic metal emission from land has declined as a result of strict environmental policies like improving wastewater treatment facilities and moving polluting industries in the surrounding areas away. The values of Igeo in all sediments changed from 0.5 to 1.2 with a mean of 0.9, meaning that the pollution level caused by Hg in sediments ranged from uncontaminated to moderately contaminated. Furthermore, the values of \( E_{r}^{i} \) varied between 87.1 and 141.9 with a mean of 114.8, suggesting that mercury posed considerable ecological risk to the environment (Fig. 4). Compared with \( E_{r}^{i} \) values of other heavy metals in Futian mangrove sediments, the \( E_{r}^{i} \) value of Hg is lower than Cd (\( E_{r}^{i} \) ≥ 320), but much higher than that of Cu, Pb, Zn, and Cr (\( E_{r}^{i} \) ≤ 40) (Li et al. 2015), which might be the cause for concern.
Conclusions
In this study, sediment in K. obovata, A. marina, S. caseolaris, and mud flat (from land to sea) was analyzed for distribution and ecological risk of Hg in surface sediments of Futian mangrove forest, China. Mangrove forest reduced pH and promoted salinity and TOC content in sediment, compared to mud flat. Totally, Hg concentration in all sediments ranged from 154.7 to 251.9 ng g−1. In the horizontal direction, Hg concentrations reduced from sea to land (from mud flat to K. obovata). In the vertical direction, Hg concentration was stable at sea side (mudflat) and decreased at land side (K. obovata) from the bottom to the top layer of sediment. Thus, Hg pollutants may mainly come from the marine coastal environment rather than land. The geo-accumulation index (Igeo) and potential ecological risk coefficient (\( E_{r}^{i} \)) revealed that Futian mangrove sediments were polluted by Hg to some extent. Further study should be conducted on the speciation of inorganic Hg and methyl Hg and the bioavailability and toxicity of Hg to organisms.
References
Arrivabene, H. P., Campos, C. Q., Souza, I. D. C., Wunderlin, D. A., Milanez, C. R. D., & Machado, S. R. (2016). Differential bioaccumulation and translocation patterns in three mangrove plants experimentally exposed to iron. Consequences for environmental sensing. Environmental Pollution, 215, 302–313.
Bayen, S. (2012). Occurrence, bioavailability and toxic effects of trace metals and organic contaminants in mangrove ecosystems: A review. Environment International, 48, 84–101.
Bravo, A. G., Bouchet, S., Amouroux, D., Poté, J., & Dominik, J. (2011). Distribution of mercury and organic matter in particle-size classes in sediments contaminated by a waste water treatment plant: Vidy Bay, Lake Geneva, Switzerland. Journal of Environmental Monitoring, 13(4), 974–982.
Chai, M. W., Shen, X. X., Li, R. L., & Qiu, G. Y. (2015). The risk assessment of heavy metals in Futian mangrove forest sediment in Shenzhen Bay (South China) based on SEM–AVS analysis. Marine Pollution Bulletin, 97(1–2), 431–439.
Chakraborty, P., Sarkar, A., Vudamala, K., Naik, R., & Nath, B. N. (2015). Organic matter—A key factor in controlling mercury distribution in estuarine sediment. Marine Chemistry, 173, 302–309.
Chan, A. K. Y., Xu, W. Z., Liu, X. S., Cheung, S. G., & Shin, P. K. S. (2016). Sediment characteristics and benthic ecological status in contrasting marine environments of subtropical Hong Kong. Marine Pollution Bulletin, 103, 360–370.
Conrad, S. R., Santos, I. R., Brown, D. R., Sanders, L. M., van Santen, M. L., & Sanders, C. J. (2017). Mangrove sediments reveal records of development during the previous century (Coffs Creek estuary, Australia). Marine Pollution Bulletin, 122, 441–445.
Cornelis, R., Caruso, J., Crews, H., & Heumann, K. (2005). Handbook of elemental speciation II: Species in the environment, food, medicine and occupational health. Chichester: Wiley.
Dhivert, E., Grosbois, C., Courtin-Nomade, A., Bourrain, X., & Desmet, M. (2016). Dynamics of metallic contaminants at a basin scale-Spatial and temporal reconstruction from four sediments cores (Loire fluvial system, France). Science of the Total Environment, 541, 1504–1515.
Ding, Z. H., Liu, J. L., Li, L. Q., Lin, H. N., & Wu, H. (2010a). Distribution of Hg in mangrove plants and correlation with Hg speciation in sediments. Environmental Science, 31(9), 2234–2239. (in Chinese with English abstract).
Ding, Z. H., Liu, J. L., Li, L. Q., Lin, H. N., Wu, H., & Hu, Z. Z. (2009). Distribution and speciation of mercury in surficial sediments from main mangrove wetlands in China. Marine Pollution Bulletin, 58(9), 1319–1325.
Ding, Z. H., Wu, H., Liu, Y., Yuan, Y. T., & Zhang, L. (2010b). Preliminary study on the distribution and impact factors of methylmercury in surficial sediments from main mangrove wetlands of China. Environmental Science, 31(8), 1701–1707. (in Chinese with English abstract).
Gordeeva, O. N., Belogolova, G. A., & Pastukhov, M. V. (2017). Mercury speciation and mobility in soils of industrial areas in the Baikal region, Southern Siberia. Russia. Environmental Earth Sciences, 76(16), 558.
Hakanson, L. (1980). An ecological risk index for aquatic pollution control-a sedimentological approach. Water Research, 14(80), 975–1001.
Haris, H., Aris, A. Z., & Mokhtar, M. B. (2017). Mercury and methylmercury distribution in the intertidal surface sediment of a heavily anthropogenically impacted saltwater-mangrove-sediment interplay zone. Chemosphere, 166, 323–333.
He, B., Li, R. L., Chai, M. W., Qiu, G. Y., & Shen, X. X. (2015). Distribution and speciation of mercury (Hg) in Futian Mangrove wetland Shenzhen Bay. Ecology and Environmental Sciences, 24(3), 469–475. (in Chinese with English abstract).
Huang, X. P., Li, X. D., Yue, W. Z., Huang, L. M., & Li, Y. X. (2003). Accumulation of Heavy Metals in the Sediments of Shenzhen Bay, South China. Environmental Science, 24(4), 144–149. (in Chinese with English abstract).
Ip, C. C., Li, X. D., Zhang, G., Wai, O. W., & Li, Y. S. (2005). Trace metal distribution in sediments of the Pearl River Estuary and the surrounding coastal area, South China. Environmental Pollution, 138(3), 494–504.
Jardine, T. D., Kidd, K. A., & Driscoll, N. O. (2013). Food web analysis reveals effects of pH on mercury bioaccumulation at multiple trophic levels in streams. Aquatic Toxicology, 132–133, 46–52.
Kehrig, H. A., Pinto, F. N., Moreira, I., & Malm, O. (2012). Heavy metals and methylmercury in a tropical coastal estuary and a mangrove in brazil. Organic Geochemistry, 34(5), 661–669.
Kwokal, Z., Sarkar, S. K., & C-Bilinski, S. F. S. (2012). Mercury concentration in sediment cores from Sundarban mangrove wetland, India. Journal of Soil Contamination, 21(4), 525–544.
Li, R. L., Chai, M. W., Guo, M., & Qiu, G. Y. (2016a). Sediment accumulation and mercury (Hg) flux in Avicennia marina forest of Deep Bay, China. Estuarine Coastal and Shelf Science, 177, 41–46.
Li, R., Chai, M., & Qiu, G. Y. (2016b). Distribution, fraction, and ecological assessment of heavy metals in sediment-plant system in mangrove forest, South China Sea. PLoS One, 11(1), e147308.
Li, R. L., Chai, M. W., Qiu, Q. Y., & He, B. (2012). Mercury and Copper accumulation during last fifty years and their potential ecological risk assessment in sediment of mangrove wetland of Shenzhen, China. Environmental Science, 33(12), 4276–4283. (in Chinese with English abstract).
Li, R. Y., Li, R. L., Chai, M. W., Shen, X. X., Xu, H. L., & Qiu, G. Y. (2015). Heavy metal contamination and ecological risk in Futian mangrove forest sediment in Shenzhen Bay, South China. Marine Pollution Bulletion, 101(1), 448–456.
Li, Q., Wu, Z., Chu, B., Zhang, N., & Cai, S. (2007). Heavy metals in coastal wetland sediments of the Pearl River Estuary, China. Environmental Pollution, 149(2), 158–164.
Li, R. L., Xu, H., Chai, M. W., & Qiu, G. Y. (2016c). Distribution and accumulation of mercury and copper in mangrove sediments in Shenzhen, the world’s most rapid urbanized city. Environmental Monitoring and Assessment, 188(2), 87.
Liu, J. L., Li, L. Q., Lin, H. N., Wu, H., & Ding, Z. H. (2008). Characters of gain size of sediments from mangrove wetlands of China. Journal of Xiamen University (Natural Science), 47(6), 891–893. (in Chinese with English abstract).
Liu, Y., Wei, J., Huang, X. F., Peng, Y. S., & Xu, J. R. (2014). Composition and contents of organic acids in root exudates of mangrove Aegiceras corniculatum and Kandelia candel. Chinese Journal of Applied and Environmental Biology, 20(5), 850–855. (in Chinese with English abstract).
Liu, J. C., Yan, C. L., Kate, L. S., Zhang, R. F., & Lu, H. L. (2010). The distribution of acid-volatile sulfide and simultaneously extracted metals in sediments from a mangrove forest and adjacent mudflat in Zhangjiang Estuary, China. Marine Pollution Bulletin, 60(8), 1209–1216.
Luo, W., Wang, T., Jiao, W., Hu, W., Naile, J. E., Khim, J. S., et al. (2012). Mercury in coastal watersheds along the Chinese Northern Bohai and Yellow Seas. Journal of Hazardous Materials, 215–216, 199–207.
Machado, W., Moscatelli, M., Rezende, L. G., & Lacerda, L. D. (2002). Mercury, zinc, and copper accumulation in mangrove sediments surrounding a large landfill in southeast Brazil. Environmental Pollution, 120(2), 455–461.
Machado, W., Sanders, C. J., Santos, I. R., Sanders, L. M., Silva-Filho, E. V., & Luiz-Silva, W. (2016). Mercury dilution by autochthonous organic matter in a fertilized mangrove wetland. Environmental Pollution, 213, 30–35.
Marchand, C., Fernandez, J. M., & Moreton, B. (2016). Trace metal geochemistry in mangrove sediments and their transfer to mangrove plants (New Caledonia). Science of the Total Environment, 562, 216–227.
Müller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2(108), 108–118.
Parthasarathi, C., Arindam, S., Krushna, V., & Bejugam, N. N. (2015). Organic matter-A key factor in controlling mercury distribution in estuarine sediment. Marine Chemistry, 173, 302–309.
Shi, J. B., Ip, C. C. M., Zhang, G., Jiang, G. B., & Li, X. D. (2010). Mercury profiles in sediments of the Pearl River Estuary and the surrounding coastal area of South China. Environmental Pollution, 158(5), 1974–1979.
Tam, N. F., & Wong, Y. S. (2000). Spatial variation of heavy metals in surface sediments of Hong Kong mangrove swamps. Environmental Pollution, 110(2), 195–205.
Tang, T. J., Han, L., Peng, Y., Chen, C. X., & Xie, L. S. (2016). Pollution and prevention countermeasure for water environment of Shenzhen Bay. Environmental Science and Management, 41(2), 43–45. (in Chinese with English abstract).
Vane, C. H., Harrison, I., Kim, A. W., Moss-Hayes, V., Vickers, B. P., & Hong, K. (2009). Organic and metal contamination in surface mangrove sediments of south china. Marine Pollution Bulletin, 58(1), 134–144.
Walters, B. B., Rönnbäck, P., Kovacs, J. M., Crona, B., Hussain, S. A., Badola, R., et al. (2008). Ethnobiology, socio-economics and management of mangrove forests: A review. Aquatic Botany, 89(2), 220–236.
Wang, W. Q., & Lin, P. (2003). Element distribution in mangroves and salt tolerant mechanism. Scientia Silvae Sinicae, 39(4), 30–36. (in Chinese with English abstract).
Xie, H. W., Wen, B., Guo, Y., Shi, Y. Z., & Wu, Y. H. (2010). Community characteristics and distribution of metal elements in mangroves in Futian of Shenzhen, China. Guihaia, 30(1), 64–69. (in Chinese with English abstract).
Ye, F., Huang, X., Zhang, D., Tian, L., & Zeng, Y. (2012). Distribution of heavy metals in sediments of the Pearl River Estuary, Southern China: Implications for sources and historical changes. Journal of Environmental Sciences, 24(4), 579–588.
Zahir, F., Rizwi, S. J., Haq, S. K., & Khan, R. H. (2005). Low dose mercury toxicity and human health. Environmental Toxicology and Pharmacology, 20, 351–360.
Zan, Q. J., Wang, Y. J., & Wang, B. S. (2002). Accumulation and cycle of heavy metal in Sonneratia apetala and S. caseolaris mangrove community at Futian of Shenzhen, China. Environmental Science, 23(4), 81–88. (in Chinese with English abstract).
Zhang, J. H., Li, W., & Pan, N. M. (2000). Absorption, accumulation and cycle of several heavy metals by mangrove. Yunnan Environmental Science, 19(S), 53–56. (in Chinese with English abstract).
Zhang, J., Li, H., Zhou, Y., Li, H., & You, J. (2018). Bioavailability and soil-to-crop transfer of heavy metals in farmland soils: A case study in the Pearl River Delta, South China. Environmental Pollution, 235, 710–719.
Zhao, G., Ye, S., Yuan, H., Ding, X., & Wang, J. (2017). Surface sediment properties and heavy metal pollution assessment in the Pearl River Estuary, China. Environmental Science and Pollution Research, 24(3), 2966–2979.
Zhou, Y., Zhao, B., Peng, Y., & Chen, G. (2010). Influence of mangrove reforestation on heavy metal accumulation and speciation in intertidal sediments. Marine Pollution Bulletin, 60(8), 1319–1324.
Zuo, P., Wang, Y. P., Cheng, J., & Min, F. Y. (2008). Distribution characteristics of heavy metals in surface sediments and core sediments of the Shenzhen Bay in Guangdong Province, China. Acta Oceanologica Sinica, 30(4), 71–79. (in Chinese with English abstract).
Acknowledgements
This work was financially supported by the Program of Science and Technology of Shenzhen (JCYJ20160330095549229, KQJSCX20160226110414), and the Program of Assessment and Restoration of Mangrove Geiwei of Shenzhen Bay.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niu, Z., Cao, Y., Zhao, W. et al. Distribution and assessment of mercury (Hg) in surface sediments of Futian mangrove forest, China. Environ Geochem Health 41, 125–134 (2019). https://doi.org/10.1007/s10653-018-0151-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-018-0151-7