Abstract
The bioavailability and toxicity of zinc to aquatic life depend on dissolved organic matter (DOM), such as Suwannee River Fulvic Acid (SRFA), which plays an important role in the speciation of zinc. This study examined reactions of SRFA with zinc at different concentrations from pH 3.0 to 9.0, and competitive binding of calcium/magnesium and zinc to SRFA at pH 6.0, using in situ absorbance. Interactions of Zn2+ with SRFA chromophores were evidenced by the emergence of features in Zn-differential spectra. Among all Zn2+–SRFA systems, dominant peaks, located at 235, 275 and 385 nm, and the highest intensity at 235 nm indicated the replacement of protons by the bound Zn2+. The Zn2+ binding with SRFA could be quantified by calculating the changes of the slopes of Zn-differential log-transformed absorbance in the wavelength range of 350–400 nm (denoted as DS350–400) and by comparing the experimental data with predictions using the Non-Ideal Competitive Adsorption (NICA–Donnan) model. DS350–400 was correlated well with the bound Zn2+ concentrations predicted by NICA–Donnan model with or without Ca2+ or Mg2+. Ca2+ and Mg2+ only affect intensity of the Zn-differential and Zn-differential log-transformed absorbance, not shape. In situ absorbance can be used to gain further information about Men+–DOM interactions in the presence of various metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dissolved organic matter (DOM) affects the speciation of zinc in natural waters (Mueller et al. 2012) and plays an important role in protecting aquatic organisms against toxicity of excessive zinc (Heijerick and Janssen 2000, Schamphelaere et al. 2005). The composition of DOM relies on sources of organic matter, ionic strength, pH and major cation in water (Leenheer and Croué 2003). Current techniques for quantifying DOM–metals interactions are very diverse, such as ion exchange technique (IET) (Fortin et al. 2010), ion-selective electrodes (ISEs) (Valilue et al. 2016), cathodic stripping voltammetry (Wasag and Grabarczyk 2016) and Donnan membrane technique (Jones et al. 2016). Although these methods have been used to determine free metal ion concentrations in natural waters, the performance of many assays remains inadequate for the complexity of analytical matrices and very low ambient metal concentrations.
Absorbance spectroscopy has been used widely to test the reactivity between DOM and free metal ions. It was proved to be high sensitive to the variation of the composition of DOM and could track trace metal ion concentrations without any sample pre-concentration (Yan et al. 2013; Gao et al. 2015a, b). However, the absorbance spectroscopy of DOM–metals is featureless. An alternative in situ method, differential absorbance spectroscopy (DAS) (Dryer et al. 2008), was proved to be useful for revealing interpretable features associated with deprotonation of DOM molecules and the reaction with metal ions. In addition, Windermere Humic Aqueous Model (WHAM) and the Non-Ideal Competitive Adsorption (NICA)–Donnan model can predict concentrations of free metal ions to DOM binding in natural waters (Dudal and Gérard 2004).
In this study, we used in situ absorbance spectroscopy to track interactions between DOM and Zn2+ per se at a wide range of pHs, as well as Zn2+ in the presence of different concentrations of Ca2+/Mg2+ at pH 6.0. These background ions affect various heavy metals binding on SRFA. The spectroscopic data were processed and compared with the predicted data of NICA–Donnan model, which allowed the quantitation of metal binding on SRFA.
Materials and methods
Reagents and chemicals
All chemicals used are reagent grade, unless mentioned specifically. All solutions were prepared using Milli-Q water (18.2 MΩ cm−1, Millipore Corp., MA, USA). Suwannee River Fulvic Acid (SRFA) (standard number 1S101F) was acquired from the International Humic Substances Society (IHSS). SRFA is deemed to be representative of a wide range of DOM types in the environment (Maurice 2015). The dissolved organic carbon (DOC) concentration of SRFA was 5.0 mg L−1 as DOC. The concentrations of residual metal cations in the SRFA sample were negligible (Kuhn et al. 2015). Ionic strength of SRFA solutions (0.01 mol L−1) was regulated by adding NaClO4 background electrolyte. Stock solutions (Zn2+, Ca2+ and Mg2+) were prepared using ZnCl2, CaCl2·2H2O and MgCl2·6H2O salts from Aladdin reagent Shanghai Co. Ltd. (Aldrich Chemical Company, Shanghai, China).
Titrations
We conducted Zn2+ titrations according to the procedures for different metal ions previously described (Yan and Korshin 2004; Yan et al. 2013, 2015; Gao et al. 2015a, b). A certain amount of Zn2+ stock solution was added into a series of 40 mL-jars SRFA at pH 5.0–9.0, and a series of 40 mL-jars SRFA with or without 0.00025 and 0.0025 mol L−1 Ca2+ or 0.0004 and 0.004 mol L−1 Mg2+ at ionic strength 0.01 mol L−1, pH 6.0.
Total Zn2+ concentrations were varied from 0 to 10−6 mol L−1, which was below its precipitation level determined using Visual MINTEQ. We analyzed SRFA–metal complexation using the NICA–Donnan model (Benedetti et al. 1996; Kinniburgh et al. 1999; Milne et al. 2003). Complexation constants from Visual MINTEQ database used in the calculations are marked in Table S1. We adjusted the pH of the solutions by adding small amounts of HClO4 or NaOH. Also, we added 0.01 mol L−1 MES buffer to the solutions with low buffering capacity (e.g., at pH 6.0). This buffer does not affect the ionic strength of SRFA solutions or absorbing light above ca. 230 nm (And and Rorabacher 1999). After adding metal stock solution, followed by mixing and standing for 30 min, absorbance spectra of aliquots from solutions with various metal concentrations were conducted on a Perkin-Elmer Lambda 2550 UV–Vis spectrophotometer with a 5-cm cell at wavelengths from 200 to 800 nm.
Absorbance data processing
Data processing of SRFA absorbance spectra was done as described in previous studies (Yan and Korshin 2004; Yan et al. 2013, 2016; Gao et al. 2015a, b). The differential linear spectra and differential log-transformed spectra were calculated using Eqs. (1), (2), respectively (Dryer et al. 2008; Gao et al. 2015a, b):
In these formulas, A λ,i is DOM absorbance measured at the wavelength λ for any selected condition (i), and A λ,ref is DOM absorbance measured at the wavelength λ for an applicable reference (ref, e.g., zero total metal or zinc concentration, DOC is the concentration of organic carbon (mg/L), and length is the cell length (in cm). The slopes and differential slopes of log-transformed absorbance spectra of DOM were calculated as follows:
In the above equations, S 350–400 is the slope of the linear correlation that fit the log-transformed DOM absorbance spectra in the range between 350 and 400 nm. S 350–400,i and S 350–400,ref are the spectral slopes determined for any selected experimental condition and applicable reference (ref, e.g., zero total metal or zinc concentration), respectively. The prefix D denotes the differential between any selected experimental condition and the applicable reference against which that differential is calculated.
Results and discussion
Effect of Zn2+ binding on SRFA chromophores
As illustrated in Fig. 1a for SRFA at pH 9.0 and ionic strength 0.01 mol L−1, the zero-order absorbance spectra of SRFA have no conspicuous features and the intensity of them decreases near exponentially with the observation wavelength. By increasing zinc concentration, subtle but consistent changes in zero-order absorbance spectra were detected, which was consistent with previous studies of metal–SRFA interactions (Yan and Korshin 2004; Yan et al. 2013; Gao et al. 2015a, b). To gain more information of the interactions of Zn2+–SRFA, we calculated DOC-normalized differential spectra (DAS) using Eq. 1, presented in Fig. 1b. Three distinct bands with peaks at ca. 235, 275 and 385 nm were identified, and the strongest was at ca. 235 nm. The shape change of absorbance spectra was stable when zinc concentration increased. As previously described (Dryer et al. 2008), the band at ca. 280 nm was likely associated with the deprotonation of carboxylic groups in SRFA, while the bands at ca. 244 nm and in the 300–390 nm region reflected the deprotonation of phenolic group in SRFA.
Absorbance spectra of SRFA recorded at various concentrations of zinc at pH 9.0 and ionic strength 0.01 mol L−1. a Zero-order spectra; b DOC-normalized differential spectra; c log-transformed spectra; and d differential log-transformed spectra at a constant DOC concentration 5.0 mg L−1 with cell length 5 cm and ref. without zinc
Figure 1c demonstrates that the logarithms of absorbance of Zn2+–SRFA decreased linearly with the wavelength. However, the log-transformed spectra were made of several regions (e.g., <250, 250–400 and >400 nm) with slightly different slopes.
Figure 1d shows that the slopes of the log-transformed spectra have consistent changes at various zinc concentrations, and the slope of log-transformed absorbance of SRFA in wavelengths 350–400 nm would be the most sensitive range to the change of Zn2+ concentrations. Thence, the absolute slope values of log-transformed spectra in this wavelength range and their changes (denoted as S 350–400 and DS350–400, respectively) were used to quantify the binding of Zn2+–SRFA.
The slope changes of log-transformed spectra in 350–400 nm (DS350–400) calculated for Zn2+–SRFA at pH values ranging from 5.0 to 9.0 and an ionic strength at 0.01 mol L−1 are shown in Fig. 2.
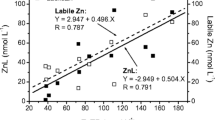
The datasets of DS350–400 values in comparison with the concentrations of Zn2+–SRFA complexes estimated using the NICA–Donnan model are shown in Fig. 3. These calculations used the complexation constants from Visual MINTEQ database (Supporting Information Table S1). For all examined pHs, DS350–400 values were linearly correlated with the modeled concentrations of SRFA-bound Zn2+.
The competitive binding of Zn2+ and Ca2+ by SRFA was examined at pH 6.0. Background Ca2+ concentrations were set at 0.0, 0.00025 and 0.0025 mol L−1, respectively. Differential spectra for this system were calculated and compared with the reference conditions with the same pH, ionic strength and the selected background Ca2+ concentration in the absence of Zn2+. The differential spectra of the Ca2+–Zn2+–SRFA system for the three Ca2+ background levels and various total Zn2+ concentrations are shown in Fig. 4.
No obvious change in the shape of the Zn2+-differential spectra was observed at various Ca2+ levels and the intensity of Zn2+-differential spectra decreased in the presence of Ca2+, which were similar to previous study (Gao et al. 2015a, b). This indicates that competing Ca2+ reduced the amount of SRFA sites available for Zn2+ binding. When comparing the intensity data of Zn2+-differential spectra for different background concentrations of Ca2+, the conspicuous site of the Zn2+–SRFA absorbance with 0.00025 mol L−1 Ca2+ was different from others. A hypothesis was made that low Ca2+ concentration would be more influential. Further researches were needed here.
Similar results of SRFA spectra in the increasing concentration of Zn2+ and Ca2+ are shown in Fig. S2, which proved that the slope of log-transformed absorbance of Zn2+–Ca2+–SRFA in the wavelengths range of 350–400 nm is most sensitive. The DS350–400 was calculated at varying total zinc concentrations in three background levels of Ca2+ to illuminate the occurrence and contributions of these alternative mechanisms, as shown in Fig. 5.
This figure shows that DS350–400 increases gradually with total Zn2+ concentrations, but this effect is suppressed by background Ca2+.
The relationships between the changes of the spectral slope in the range of 350–400 nm and the Zn2+ concentrations bound to SRFA, simulated by the NICA–Donnan model, at three different Ca2+ concentrations, are shown in Fig. 6. These calculations used the complexation constants from Visual MINTEQ database (Supporting Information Table S1).
Correlation between DS350–400 and the amount of SRFA-bound Zn2+ is predicted by the NICA–Donnan model for three different background Ca2+ levels at pH 6.0 and ionic strength 0.01 mol L−1.
It demonstrates that for all examined background Ca2+ levels, DS350–400 values measured in a wide range of Zn2+ concentrations are linearly correlated with the modeled concentrations of SRFA-bound Zn2+ irrespective of the background Ca2+ concentrations. Additionally, this figure also demonstrates that the DS350–400 values did not form a simple dataset when compared with the sum of molar concentrations of Zn2+–SRFA and Ca2+–SRFA complexes ([Zn2+–SRFA] + [Ca2–SRFA]) in solutions, rather than α[Zn2+–SRFA] +β [Ca2+–SRFA] (α, β represent the account of the respective differential spectra).
Effect of Mg2+ on Zn2–SRFA interaction
The competitive binding of Zn2+ and Mg2+ by SRFA was examined at the same condition as in Ca2+–Zn2+–SRFA systems except background Mg2+ levels at 0.0004 and 0.004 mol L−1. Differential spectra for this system were calculated versus the reference conditions at the same pH, same ionic strength, no Zn2+, and either selected background Mg2+ concentration. The differential spectra of Mg2+–Zn2+–SRFA are shown in Fig. 7.
In comparison with the data in Fig. 4a, the intensity of Zn2+-differential spectra in the presence of Mg2+ was also suppressed; however, the effect of Mg2+ on the spectral intensity of Zn2+–SRFA was less than Ca2+. This indicates that Mg2+ would also compete with binding sites available for Zn2+, the nature of these sites remained the same irrespective of the background Mg2+ concentrations, but differential spectra of Zn2+–SRFA have few obvious features in the presence of Mg2+. The DS350–400 of Zn2+–Mg2+–SRFA at various Zn2+ concentrations and different Mg2+ concentration is displayed in Fig. 8.
This figure evidences that DS350–400 increases gradually with total Zn2+ concentrations in the presence of various Mg2+, and the effect was suppressed similarly by 0.0004 mol L−1 Mg2+ or 0.004 mol L−1 Mg2.
The relationships between DS350–400 and concentrations of Zn2+ bound onto SRFA from the NICA–Donnan model in the presence of Mg2+ are shown in Fig. 9.
It manifests that DS350–400 values in a wide range of Zn2+ concentrations are linearly correlated with the predicted Zn2+ concentrations, bound onto SRFA, irrespective of the background Mg2+ concentrations. Additionally, the DS350–400 values are formed by datasets compared with the sum of molar concentrations of α[Zn2+–SRFA] + β [Mg2+–SRFA] (α, β represent the account of the respective differential spectra).
Conclusions
The differential spectra of Zn2+–SRFA had consistent changes and performed metal-specific features, which reflected the nature and amount of interactions between Zn2+ and SRFA. The features in differential spectra were ascribed to carboxylic and phenolic functional groups in SRFA and caused by the replacement of the SRFA-bound protons by Zn2+.
The differences in the effects of Zn2+ and Ca2+/Mg2+ on SRFA chromophores allowed quantitating the competition between Zn2+ and Ca2+/Mg2+ binding on SRFA sites in Zn2+–Ca2+/Mg2+–SRFA system, which could be explained well by examining the intensity and shapes of differential spectra of SRFA. The features of differential spectra of Zn2+–Mg2+–SRFA system are not obvious. However, significant differences of the response were observed in Zn2+ differential spectra in the presence of 0.00025 mol L−1 Ca2+, which require further investigation.
The binding of Zn2+ onto SRFA is not significantly affected by Mg2+ (e.g., 0.0005 or 0.005 mol L−1) at pH 6.0. The competition of Zn2+/Ca2+/Mg2+ binding by SRFA can be quantified by calculating the DS350–400 and predicted by the NICA–Donnan model. This study demonstrated the applicability of the NICA–Donnan model in elucidating interactions of the SRFA with Zn2+ at a wide range of pHs, and different concentrations of Ca2+ and Mg2+ by in situ absorbance. NICA–Donnan model can be used to gain further information about Men+–DOM interactions in the presence of various metals at different pHs and ionic strengths
References
And, A. K., & Rorabacher, D. B. (1999). Noncomplexing tertiary amines as “better” buffers covering the range of pH 3–11. Temperature dependence of their acid dissociation constants. Analytical Chemistry, 71(15), 3140–3144.
Benedetti, M. F., Riemsdijk, W. H. V., Koopal, L. K., Kinniburgh, D. G., Gooddy, D. C., & Milne, C. J. (1996). Metal ion binding by natural organic matter: From the model to the field. Geochimica et Cosmochimica Acta, 60(14), 2503–2513.
Dryer, D. J., Korshin, G. V., & Fabbricino, M. (2008). In situ examination of the protonation behavior of fulvic acids using differential absorbance spectroscopy. Environmental Science and Technology, 42(17), 6644–6649.
Dudal, Y., & Gérard, F. (2004). Accounting for natural organic matter in aqueous chemical equilibrium models: A review of the theories and applications. Earth-Science Reviews, 66(3–4), 199–216.
Fortin, C., Couillard, Y., Vigneault, B., Vigneault, B., & Campbell, P. G. C. (2010). Determination of free Cd, Cu and Zn concentrations in lake waters by in situ diffusion followed by column equilibration ion-exchange. Aquatic Geochemistry, 16(16), 151–172.
Gao, Y. (2015). Effects of dissolved organic matter (DOM) on metal release from solid phases typical for corrosion processes and characterization of interactions between DOM and metal cations by in situ spectroscopic methods. ProQuest Dissertations & Theses, Ann Arbor, MI, USA, pp. 129.
Gao, Y., Yan, M. Q., & Korshin, G. V. (2015a). Effects of calcium on the chromophores of dissolved organic matter and their interactions with copper. Water Research, 81, 47–53.
Gao, Y., Yan, M. Q., & Korshin, G. V. (2015b). Effects of ionic strength on the chromophores of dissolved organic matter. Environmental Science and Technology, 49(10), 5905–5912.
Heijerick, D. G., & Janssen, C. R. (2000). Influence of water quality characteristics on the bioavailability and toxicity of zinc for three freshwater invertebrates. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 126(Suppl 1), 67.
Jones, A. M., Xue, Y., Kinsela, A. S., Wilcken, K. M., & Collins, R. N. (2016). Donnan membrane speciation of Al, Fe, trace metals and REEs in coastal lowland acid sulfate soil-impacted drainage waters. Science of the Total Environment, 547, 104–113.
Kinniburgh, D. G., Riemsdijk, W. H. V., Koopal, L. K., Borkovec, M., Benedetti, M. F., & Avena, M. J. (1999). Ion binding to natural organic matter: Competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 151(1), 147–166.
Kuhn, K. M., Neubauer, E., Hofmann, T., Von Der, K. F., Aiken, G. R., & Maurice, P. A. (2015). Concentrations and distributions of metals associated with dissolved organic matter from the Suwannee River. Environmental Engineering Science, 32, 54–65.
Leenheer, J. A., & Croué, J. P. (2003). Characterizing aquatic dissolved organic matter. Environmental Science and Technology, 37(1), 18A–26A.
Maurice, P. A. (2015). Special issue introduction: Dissolved organic matter from the Suwannee River (GA, USA). Environmental Engineering Science, 32(1), 1–3.
Milne, C. J., Kinniburgh, D. G., van Riemsdijk, W. H., & Tipping, E. (2003). Generic NICA-Donnan model parameters for metal-ion binding by humic substances. Environmental Science and Technology, 37(5), 958–971.
Mueller, K. K., Lofts, S., Fortin, C., & Campbell, Peter G. C. (2012). Trace metal speciation predictions in natural aquatic systems: incorporation of dissolved organic matter (DOM) spectroscopic quality. Environmental Chemistry, 9(4), 356–368.
Schamphelaere, K. A. C. D., Unamuno, V. I. R., Tack, F. M. G., Vanderdeelen, J., & Janssen, C. R. (2005). Reverse osmosis sampling does not affect the protective effect of dissolved organic matter on copper and zinc toxicity to freshwater organisms. Chemosphere, 58(5), 653–658.
Valilue, Z., Vardin, M. T., & Kalhor, E. G. (2016). Design and construction of ion-selective electrode based on a new Schiff base and its application in determination of copper (II) ions. Indian Journal of Chemistry Section A-Inorganic Bio-Inorganic Physical Theoretical & Analytical Chemistry, 55(1), 51–56.
Wasąg, J., & Grabarczyk, M. (2016). Adsorptive stripping voltammetry of In (III)) in the presence of cupferron using an in situ plated bismuth film electrode. Analytical Methods, 8(17), 3605–3612.
Yan, M. Q., & Korshin, G. V. (2004). Comparative examination of effects of binding of different metals on chromophores of dissolved organic matter. Environmental Science and Technology, 48, 3177–3185.
Yan, M. Q., Lu, Y. J., Gao, Y., Benedetti, M. F., & Korshin, G. V. (2015). In-situ investigation of interactions between magnesium ion and natural organic matter. Environmental Science and Technology, 49(14), 8323–8329.
Yan, M. Q., Ma, J., & Ji, G. (2016). Examination of effects of Cu(II) and Cr(III) on Al(III) binding by dissolved organic matter using absorbance spectroscopy. Water Research, 93, 84–90.
Yan, M. Q., Wang, D., Korshin, G. V., & Benedetti, M. F. (2013). Quantifying metal ions binding onto dissolved organic matter using log-transformed absorbance spectra. Water Research, 47(7), 2603–2611.
Acknowledgements
The authors acknowledge financial support from the Shenzhen Science and Technology Program (Grant JCYJ20160308103848156).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10653_2017_1_MOESM1_ESM.docx
Ca2+ and Mg2+ only affect intensity of the Zn-differential and Zn-differential log-transformed absorbance (DOCX 10312 kb)
Rights and permissions
About this article
Cite this article
Zhang, T., Wang, T. & Lu, Y. Study of the interactions of dissolved organic matter with zinc ion and the impact of competitive metal ions (Ca2+ and Mg2+) by in situ absorbance. Environ Geochem Health 40, 1979–1986 (2018). https://doi.org/10.1007/s10653-017-0001-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-017-0001-z