Abstract
The health risks of metal exposure due to the high consumption of fish were assessed for a riverine population living on the Caribbean coast of Colombia. The concentrations of metals (Cd, Cr, Cu, Hg, Ni, Pb and Zn) in the edible tissues of fish were determined and used for risk assessment. The daily fish consumption of residents (n = 95) was as high as 283, 366 and 469 g/day in children (CH), women of childbearing age (WCHA) and the remaining population groups (RP), respectively. The estimation of the potential risk (HQ) indicated that there was no health risk from most of the metals, because they did not exceed their related reference doses, with values of HQ < 1. Although the concentrations of Pb and Hg were not particularly high in fish (<0.2 µg/g), their possible health effects for vulnerable groups are of great concern due to the extremely high fish intake. The Pb intake for all groups was higher than the lower confidence limit of the benchmark dose for nephrotoxicity and neurodevelopmental effects in children. The weekly intake of methylmercury was also elevated, with values approximately 3, 2 and 1.5 times the provisional tolerable weekly intake for CH, WCHA and RP, respectively. Moreover, higher Hg levels were found in top predators, whereas maximum levels for other metals were found in bottom-feeding fish. This study highlights that an accurate data of daily intake, a continuous monitoring of metals in fish and their related fish consumption advisories to protect subsistence fishing communities are recommended in a local and worldwide context.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The intake of fish provides a healthy source of energy, high-quality protein, fat-soluble vitamins and essential minerals (Pieniak et al. 2010; Castaño et al. 2015). In addition, health benefits, including reduced risks of coronary heart diseases, diabetes and hypertension, and positive contributions to foetal growth and development, are related to the presence of omega-3 polyunsaturated fatty acids in fish (Mozaffarian and Wu 2011; Swanson et al. 2012; Wenstrom 2014; Christensen et al. 2015; EFSA 2015). The numerous health benefits provided by fish may be compromised by the presence of toxic metals, which can have harmful effects on the human body if consumed in high quantities. Hence, contamination in fish has become a topic of global concern, not only because of the threat to the fish themselves, but also due to the health risks for frequent human consumers (Bosch et al. 2015; Rahman et al. 2012; Olmedo et al. 2013; Yu et al. 2014; Rose et al. 2015; Perello et al. 2015). Metals, such as copper (Cu) and zinc (Zn), are essential metals since they play important roles in biological systems; however, Cu can be toxic when present in excess, with the most noticeable chronic effect being liver damage (de Romaña et al. 2011). Whereas intoxication by excessive Zn exposure is rare, Zn deficiency is widespread and has a detrimental impact on growth, neuronal development and immunity, and in severe cases its consequences are lethal (Plum et al. 2010). Chromium (Cr) is an essential nutrient required for sugar and fat metabolism, and the health hazards associated with its exposure are dependent on its oxidation state, which ranges from the low toxicity of the metal form to the high toxicity of the hexavalent form [Cr(VI)] (Velma et al. 2009) which can exhibit genotoxic effects (Nickens et al. 2010). Other metals such as lead (Pb), cadmium (Cd), nickel (Ni) and mercury (Hg) are toxic, even in trace amounts. Pb mainly affects the central nervous system, kidney and blood, and prolonged exposure can result in coma, mental retardation and even death (Al-Busaidi et al. 2011; Bello et al. 2016). The chronic toxicity of Cd causes a reduction in renal and reproductive functions, high blood pressure, tumours and liver dysfunction (Satarug et al. 2010; Zaza et al. 2015). Ni exposure causes lung inflammation, fibrosis, emphysema and tumours (Forti et al. 2011; Buekers et al. 2015). Finally, Hg exposure can lead to neurological deficits and reduced cognitive ability in foetuses (Oken et al. 2008; Choi et al. 2014), and in adults it can cause paraesthesia, numbness of the hands and feet, coordination difficulties, ischaemic cerebrovascular accidents, dementia and depression (Kondo 2000). Almost all of the Hg that accumulates in fish tissue is in its most toxic form, methylmercury (MeHg), which can cause harmful effects to human health (Diez 2009; Nakamura et al. 2014).
The rapid development of industry, agriculture, urbanisation and population growth has led to an increase in pollution of aquatic ecosystems by the discharge of metals. In Colombia, chemical pollution has been detected in close proximity to the major cities of the Magdalena basin, and effluents discharged by industries on the Colombian coast arise predominantly from Cartagena and Barranquilla, and, to a lesser degree, Puerto Bolivar, Riohacha, Santa Marta, Tolú, Coveñas and Turbo (UNEP 2006; Invemar 2001). The Mallorquín swamp (Magdalena basin) is the only coastal lagoon in the Department of Atlántico with estuarine features included in the Ramsar List (Decree 3888, Ministry of Environment, Housing and Territorial Development 2009). The Convention on Wetlands, called the Ramsar Convention, is an intergovernmental treaty that provides the framework for national action and international cooperation for the conservation and wise use of wetlands and their resources. The Mallorquín swamp provides a habitat for food, shelter and reproduction of some species, thus ensuring the survival and growth of the larvae of crustaceans, molluscs, fish and other organisms. Unfortunately, the Mallorquín swamp is being degraded by chemical pollution discharged by industrial area of Barranquilla, the accumulation of contaminants in mangrove forests, and the high sediment loads in the Magdalena River causing increased sedimentation (UNEP 2006). Therefore, this swamp is not exempt from the environmental problems that plague the water bodies of coastal areas, mainly caused by the accumulation of metal in sediments (Pedraza 2009; Franco and León-Luna 2010) or in fish species such as Mugil incilis and Eugerres plumieri (García and Luque 2008; Sierra-Gutiérrez 2003); however, there is no information about its health potential risk to consumers. This issue merits greater attention because the Mallorquín swamp is a great source of fishing resources, being a habitat for a total of 36 species, including 22 fish families. In this area, there are two riverine communities with about 20,000 riverbank dwellers, and more than 400 people are directly dependent on the fish species of the swamp (Arrieta and De la Rosa 2003).
In this study, the concentrations of essential and toxic metals, including Cd, Cr, Cu, Hg, Ni, Pb and Zn, were determined in some fish species of commercial interest from the Mallorquín swamp. Analysis was performed in edible muscle tissue with the aim to assess the accumulation of metals in the most consumed fish species in order to determine a human health risk assessment of metal contamination through the consumption of fish.
Materials and methods
Study area
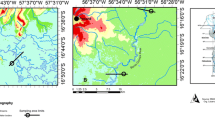
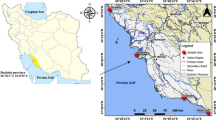
The Mallorquín swamp is located in the centre of the northern coast of Colombia, in the Atlántico department (74°52′00″W and 11°05′00″N) (Fig. 1). It is part of the 2250 km2 of the northern flood alluvial plain of the Magdalena River (Galvis et al. 1992). Its shape is irregular and imprecise, with an area of approximately 2400 ha. It is bordered to the north by the Caribbean Sea; to the south by the Circunvalar Avenue; to the east by the western breakwater of the navigable channel of the Magdalena River; and to the west by the mouth of the Grande Stream, which provides large volumes of water to the swamp in the rainy season (Cormagdalena-Cra-Uninorte 1998). The Mallorquín swamp is subject to a considerable anthropic pressure, especially from domestic and industrial wastewaters, and a variety of pollutant substances carried by the Magdalena River, Arroyo León and the ancient landfill of Barranquilla (Franco and León-Luna 2010). Petrol refineries, distilleries, food processing and packing industries (e.g. meat, chicken, shrimp and fish), pulp and paper manufacturers and chemical industries (organic and inorganic), and contaminated river sediments are some of the main sources of water pollution.
Collection and analysis of samples
Five fish species (Mugil curema, Arius bonillai, Centropomus undecimalis, Lutjanus griseus and Eugerres plumieri) were selected, taking into account their trophic relationship in the ecosystem, as they are the most consumed by the settlers of the Mallorquín swamp and are present in the fishery throughout the year. Through two sampling campaigns carried out in August 2011 and February 2012, we found that local fishermen caught a total of 56 specimens (Table 1). The fish were individually packaged in labelled polyethylene bags, refrigerated and transported to the laboratory, where length and weight were measured. Following the procedure described by UNEP/IOC/IAEA/FAO (1990), the pectoral fin of the left side next to the skin was removed, and with a knife, a portion of 3 cm in width was cut out.
Subsequently, the total mercury (THg) concentration was determined by atomic absorption spectrophotometry with cold vapour (CV-AAS) (Thermo Scientific model iCE series 3500) prior to the acid digestion of the samples (0.5 g wet weight, ww) (hereafter, all concentrations are reported as wet weight unless otherwise indicated) with H2SO4/HNO3 (2:1) for 2 h at 100 °C (Sadiq et al. 1991). The analyses of the other metals (Cu, Pb, Cd, Zn, Cr and Ni) were performed by atomic absorption spectrophotometry using a graphite furnace (Thermo Scientific model iCE series 3500) prior to the acid digestion of the sample (0.5 g ww) with HNO3/HCl (3:1) for 3 h at 95 °C (Hülya and Erhan 2007). The analytical quality control of the method was evaluated in triplicate with certified reference material (CRM) IAEA 407. The different metal concentration in the CRM was found in good agreement with the certified value, and the recovery percentage from 91 to 98%. The limits of detection for the different metals were: 0.010 µg/g for Cd, 0.04 µg/g for Cr, 0.05 µg/g for Cu, 0.014 µg/g for Hg, 0.014 µg/g for Ni, 0.011 µg/g for Pb and 0.016 µg/g for Zn.
Human health risk assessment
A questionnaire-based dietary survey was conducted with inhabitants of the Mallorquín swamp by randomly selecting and surveying 95 healthy people from the general population. All the participants were local residents, and dietary data for the relevant year were collected during detailed face-to-face interviews. Thereafter, the participants were asked about a number of aspects related to fish consumption habits (i.e. number of meals per week, total amount and type of fish consumed per week, and meal size). The sampling population was divided into three population groups with the following features: children, CH (1–15 years old), women of childbearing age, WCHA (16–45 years old), and the rest of the population, RP.
The potential human health risk assessment was conducted by considering the estimated daily intake (EDI) (µg/kg bw/day), calculated using the following equation:
where C m is the mean metal concentration in edible muscle tissue (µg/g), DI is the intake of fish consumed per day (g/day), and BW is the mean body weight (bw) of the participants (kg).
Risk may be characterised using a hazard quotient (HQ). This is the ratio of the EDI of a chemical to a reference dose (RfD, µg/kg bw/day) defined as the maximum tolerable daily intake of a specific metal that does not result in any deleterious health effects:
There would be no obvious hazard if the value of HQ was less than 1. If HQ > 1, then the EDI of a particular metal exceeds the RfD, indicating that there is a potential risk associated with that metal. The RfDs used in this study are given in Table 2.
According to the USEPA (2000), the maximum allowable fish consumption rate (CRlim, in g/day) of contaminated fish considering the noncarcinogenic effect of a contaminant can be calculated as follows:
The USEPA calculation of the CRlim considering the noncarcinogenic effects of multiple contaminants in single or multiple fish species is as follows:
where P j is the proportion of a given species j in the diet (unitless); C mj is the concentration of contaminant m in species j (mg/kg); and P m is the proportion by weight of the chemical in the diet (unitless).
In the case of Hg/MeHg, exposure of consumers and related health risks are usually expressed as provisional tolerable daily intake (PTDI) or weekly intake (PTWI), a reference value established by the joint FAO/WHO Expert Committee on Food Additives (JECFA 2015). The PTWI is the amount of a substance that can be consumed weekly over an entire lifetime without appreciable risk to health and is an end-point used for food contaminants. Its value represents permissible human weekly exposure, protecting the most susceptible part of the population. In the case of MeHg, the developing foetus is considered to be the most sensitive subgroup, and neurodevelopment the most sensitive outcome (UNEP 2008).
Because most of the Hg in fish is MeHg and most (greater than 95%) of the MeHg ingested through fish consumption is readily absorbed into the body through the gastrointestinal tract, exposure to MeHg (or intake) can be estimated if information is available on the following: (a) types (i.e. species) and amounts (such as frequency and serving size) of fish ingested per unit time (such as day or week); (b) THg concentrations in the species of fish ingested; and (c) the BW of persons (or groups) consuming the fish (UNEP 2008). Using the above information, the assessment of the risk associated with Hg intake was estimated by the weekly intake of MeHg (WIMeHg), per kilogram of body weight of the person exposed (μg/kg bw/week), using the equation described by UNEP (2008):
where WFC (i.e. 7 × DI) is the weekly fish consumption (in g).
Furthermore, the permissible safety level (MeHgPSL), which is the allowable concentration of MeHg in fish consumed by humans, was calculated using the following equation (JECFA 2006):
Similarly, we estimated the maximum amount of fish, per person, that can be consumed weekly (MFW) without producing harmful health effects, using the following equation:
Statistical analysis
The results of the analysis for each species are presented as the mean ± standard deviation of the samples analysed. The information was subjected to an exploratory analysis using the Kolmogorov–Smirnov test to demonstrate normality. The differences between fish species were tested for significance using nonparametric tests (Mann–Whitney U and Kruskal–Wallis test). In order to establish the association between variables, we used the Pearson’s linear correlation analysis, and previous dataset log-transformation. The statistical analysis was developed with the SPSS 10.5, establishing a significance level of p ≤ 0.05.
Results and discussion
Table 1 shows the types of fish species caught during the study in the Mallorquín swamp. Three of the species are carnivorous (A. bonillai, C. undecimalis and L. griseus), representing 57% of the samples collected. Thirty-six per cent of the specimens showed detrivore habits (M. curema), and 7% of the individuals showed euryphagous habits (E. plumieri). M. curema presented the highest average total length, and A. bonillai showed the greatest average weight. E. plumieri demonstrated the lowest averages for both total length and weight.
Metal concentrations in the analysed fish species
The metal concentrations present in the five species analysed are presented in Fig. 2. The seven elements (Hg, Cu, Pb, Cd, Zn, Cr and Ni) were detected in the muscle tissue of all fish species. Among these metals, Zn exhibited the highest average concentration (12.1 µg/g). The highest concentrations of Zn, Cr and Pb were evidenced in M. curema while the highest levels of Cu and Hg were recorded in E. plumieri. Moreover, significant differences (p < 0.05) were obtained between fish species for Zn, Cr, Ni and Hg, whereas no significant differences were found for the rest of metals.
On the other hand, Cr and Hg were the only metals to follow a clear trophic pattern (Fig. 3). The levels of Cr in M. curema were three and ten times higher than in A. bonillai and the rest of fish species, respectively. The concentration of Cr (Fig. 3b) decreased from detrivore to euryphagous habits (i.e. decreased towards higher trophic levels), which suggested no biomagnification. Many studies (Neff 2002; Asante et al. 2010; Ikemoto et al. 2008; Zhang et al. 2013; Hao et al. 2013) have found that Cr concentrations decrease in organisms higher up a food chain. It is well established that toxicity, mobility and bioavailability of Cr depend on its chemical speciation (Velma et al. 2009). Hexavalent chromium [Cr(VI)] is toxic, mutagenic, carcinogenic and more mobile than its reduced trivalent form [Cr(III)]. Considering the relatively greater mobility of Cr(VI), it can easily pass through cell membranes where, under normal physiological conditions, it can be reduced to short-lived intermediates Cr(V) and Cr(IV), the end-product Cr(III), and can generate free radicals that can damage DNA. It is usually assumed that the reduction to insoluble Cr(III) renders Cr less soluble and less toxic (Cheung and Gu 2007). Since Cr(III) cannot enter the cell, it can be removed from the organism; hence, Cr is not considered likely to biomagnify in the aquatic food chain.
With the exception of E. plumieri, THg increased with trophic level (Fig. 3a). A comparison between Hg levels of the individual fish species produced the following order: M. curema > A. bonillai > C. undecimalis > L. griseus > E. plumieri. The relative trophic level for the analysed species is based on generalised knowledge of the species (www.fishbase.com). M. curema feed on microscopic or filamentous algae and small juveniles of planktonic organisms, while A. bonillai, C. undecimalis and L. griseus are piscivorous species, feeding on a combination of fish and crayfish. Finally, E. plumieri feeds on aquatic insects, crustaceans, micro-bivalves and detritus occurring in sediment. Several studies reported positive correlations between trophic levels and THg in various food webs, suggesting that Hg was biomagnified in organisms at higher trophic levels (Ikemoto et al. 2008; Tadiso et al. 2011). The statistical significant differences (p < 0.05) found between E. plumieri and M. curema, and the highest concentrations of Hg in E. plumieri, a demersal fish species feeding mainly by grubbing in sediments, are probably related to Hg content in surface sediments, as described previously (Carrasco et al. 2011).
In our study, the concentrations of Cr (0.3–3.41 µg/g dry weight (dw)), Ni (0.15–1 µg/g dw) and Pb (0.66–2.03 µg/g dw) were slightly lower than those reported previously in Mugil incilis from the Mallorquín swamp (Invemar 2005). Similarly, Cd levels (0.06–0.16 µg/g dw) were also lower than those detected by Sierra-Gutiérrez (2003). However, we found higher contents of Cu (0.09–0.80 µg/g dw) and Zn (13.88–21.36 µg/g dw) than those previously reported for M. incilis in this area (Cu: 0.09–0.94 µg/g dw and Zn: 13.88–27.88 µg/g dw) (Sierra-Gutiérrez 2003; Invemar 2005).
Potential risk estimation
The assessment tool indicated that, of the 95 people surveyed, only 2% did not consume fish. Fish was consumed once a week by 18% of respondents, from 2 to 3 times a week by 41%, from 4 to 5 times a week by 13%, and heavy consumption was reported by 26% of respondents (≥6 times a week).
According to the questionnaire, the fish species preferred for consumption by the entire population were: M. curema (62%), followed by E. plumieri (20%), A. bonillai (6.5%), C. undecimalis and L. griseus (2%), and other species (7.5%) such as Prochilodus magdalenae (Bocachico) and Caranx hippos (Jurel). In the present study, the potential health risk associated with each of the metals was calculated assuming body weight averages of 37.4 kg for CH, 68.6 kg for WCHA and 73.3 kg for RP, and a daily fish intake of 282.8 g in CH, 366.1 g for WCHA and 468.8 g in RP (Table 3). The calculations were performed using Eqs. (1)–(5). The RfDs used for the different metals were those established by the JECFA (2015), the United States Environmental Protection Agency (EPA) (UNEP 2008) and the Agency for Toxic Substances and Disease Registry (ASTDR) (2000) (Table 2). In 2011, based on the results of a meta-analysis of epidemiological data, the JECFA re-evaluated Pb and estimated that the previously established PTWI of 25 μg/kg bw could no longer be considered health protective and it was withdrawn. Therefore, the Benchmark Dose Lower Confidence Limit (BMDL) (EFSA 2010) was used for our calculations. Because of the unavailable reference values for total Cr, calculations were performed using the individual RfDs for Cr(III) and Cr(VI) (USEPA 2016). For MeHg, a PTWI of 1.6 μg/kg bw/week (0.23 μg/kg bw/day) based on JECFA was used (JECFA 2006) for CH and WCHA groups, and 3.2 μg/kg bw/week for RP group.
The values of estimated daily intake, consumption rate and hazard quotient, as well as MFW, for Hg are given in Tables 2 and 3.
The EDI values calculated for Cd, Cu, Ni and Zn were below the tolerable intake reference levels established by JECFA and USEPA, and therefore did not pose a threat to human health. The assessment of the risk associated with Pb and Cr intakes was, however, more disturbing. As shown in Fig. 4, of all the metals analysed, Cr presented the greatest potential risk to consumers, since its HQ exceeded one (HQ = 2.2).
For the other metals, the HQ approach suggested that no human health risk was linked to the Cu and Ni concentrations in fish from the region (HQ ≤ 0.10), while there was a low Cd and Zn risk, particularly in children (HQ = 0.39 and HQ = 0.32, respectively). Cd has no biological functions and adversely affects many organs and tissues, including the kidney (by inducing renal tubular dysfunction, proteinuria and chronic kidney failure), the heart (aortic and coronary atherosclerosis), the lung (fibrosis) and the central nervous system (producing neurological disorders, such as hyperactivity and learning problems, in children) (Houston 2007). Although Zn levels in the analysed fish species were much higher than the other metals, according to the HQ values there was no evidence of a high human health risk. In fact, this metal is a cofactor for several important enzymes, including those involved in RNA and DNA synthesis, and also plays an important role in stabilising the structure of a large number of proteins, including signalling enzymes at all levels of cellular signal transduction (Chasapis et al. 2012). However, Zn has a tendency to be bio-accumulated over its lifetime in the fatty tissues and muscles of aquatic organisms, including fish, thus affecting their reproductive physiology (Rahman et al. 2012).
Risk assessment of Pb exposure
It is important to note that Pb has no biological function, and thus, even at low concentrations could generate potential adverse health effects such as nephrotoxicity and neurotoxicity (Garcia-Leston et al. 2010). In 2010, the PTDI value of 3.57 μg/kg bw per day determined by the WHO (1986) was withdrawn by EFSA (2010) and replaced with three Benchmark Dose Lower Confidence Limits (BMDLs) to assess the effects of lead exposure in humans: BMDL10 for nephrotoxicity at 0.63 μg/kg bw/day, BMDL01 for cardiovascular effects at 1.5 μg/kg bw/day and BMDL01 for developmental neurotoxicity (which would apply to foetuses and infants) at 0.5 μg/kg bw/day. According to our calculations, the mean Pb intakes for the CH (1.08 μg/kg bw/day), WCHA (0.76 μg/kg bw/day) and RP (0.91 μg/kg bw/day) groups were below the BMDL for heart disease, but the EDI values were above the BMDLs for renal effects in the whole studied population and neurodevelopmental effects in children.
Risk assessment of Cr exposure
For Cr, the values of HQ in the three groups ranged from 1.6 to 2.2, and elicited a high concern for potential health effects, especially for children (HQ = 2.2). In fact, the average daily fish intake for the CH group was 283 g/day, which is higher than the CRlim (Table 3).
Chromium is considered an essential naturally occurring element, where only trivalent [Cr(III)] and hexavalent [Cr(VI)] forms are of biological significance. Although both forms are harmful, Cr(VI) is considered the most toxic form. Hence, the International Agency for Research in Cancer (IARC) has classified Cr(VI) under class 1 as a compound which is known to be a human carcinogen based on sufficient evidence of carcinogenicity in humans (IARC 1990). However, small amounts of Cr(III) are believed to be essential for human health since it is an essential dietary nutrient; however, it can be toxic in large doses. Cr(III) is required to potentiate insulin and for normal glucose metabolism. In fact, Cr(III) deficiency has been associated with cardiovascular disease, impaired fertility, impaired glucose tolerance and maturity-onset diabetes (WHO 1996). There is also evidence that Cr(III) can induce DNA damage (Rudolf and Cervinka 2009), cytotoxicity (Shrivastava et al. 2005) or sister chromatid exchanges (Cohen et al. 1993), and it has been classified by the IARC under Group 3, “unclassifiable as to carcinogenicity in humans” (IARC 1990). Several studies in humans and animals show that hexavalent inorganic chromium compounds are better absorbed than trivalent inorganic forms, which are very poorly absorbed through the skin, lung and gastrointestinal tract (Dayan and Payne 2001). Cr(VI) enters cells more readily than Cr(III) compounds and is ultimately reduced to Cr(III). The reduction of Cr(VI) could be considered to serve as a detoxification process, and once absorbed and retained in biological tissue chromium compounds seems to exist as Cr(III) (DeFlora and Wetterhahn 1989; Dayan and Payne 2001). The HQ calculations presented here are based on the individuals RfDs for both Cr(III) and Cr(VI) (Table 2). According to the assessment of the HQ for Cr, there are possible risks to human health from consuming the evaluated fish species if the chemical form is Cr(VI) (Fig. 4). Nevertheless, the EFSA Panel on Contaminants in the Food Chain (CONTAM Panel) (EFSA 2014) considers all reported analytical results in food as Cr(III). This assumption was based on the fact that oxidation of Cr(III) to Cr(VI) would not be favoured in such reducing medium as food. However, it should be noted that if even a small proportion of total chromium in food was in the form of Cr(VI), it could contribute substantially to Cr(VI) exposure (EFSA 2014).
Risk assessment of Hg exposure
In 2001, the EPA adopted a revised RfD for MeHg of 0.1 μg/kg bw/day based on an analysis by the National Research Council (NRC) of the studies from the Seychelles Islands (Grandjean et al. 1997), the Faroe Islands (Myers et al. 2003) and New Zealand (Crump et al. 1998). This RfD was based on neurological developmental effects in children associated with in utero exposure to MeHg from the maternal diet, and it is related to a maternal hair Hg concentration of 1.0 μg/g (UNEP 2008). In 2006, the JECFA established a PTWI for MeHg at 1.6 μg/kg bw/week for women of childbearing age and young children, but allows a higher ingestion rate (3.2 μg/kg bw/week) for members of the general population. The JECFA confirmed that the PTWI of 1.6 μg/kg bw week was based on the most sensitive toxicological endpoint (developmental neurotoxicity) in the most susceptible species (humans). Intakes of up to twice the PTWI would not pose a risk of neurotoxicity to adults, except potentially for women of childbearing age because of the effects on the embryo and foetus. Differences between the EPA and JECFA reference values are mainly due to differences in conversion from maternal hair and blood levels. The EPA used an uncertainty factor of 10, slightly higher than the factor of 6.4 used by the JECFA (Mergler et al. 2007). It should be noted that calculations in the present study were performed with the PTWI adopted by the JECFA.
The values for WFC (i.e. the amount of fish consumed weekly) ranged from 1980 to 3282 g/week and are above the MFW in all cases (Table 3). Indeed, WFC values for CH, WCHA and RP were 3.3, 2.3 and 1.4 fold higher than MFW, respectively. Likewise, results indicate that WIMeHg values were approximately 3, 2 and 1.4 times the PTWI. Accordingly, since these values are above the reference values, this provides evidence for a potential health risk for all the population groups, with the risk increasing in the following order: RP < WCHA < CH. Therefore, according to UNEP guidelines for identifying populations at risk from Hg exposure (UNEP 2008), when the WIMeHg exceeds the PTWI, it is necessary to refine the estimate based on the MeHg concentrations in fish. Thus, in the studied area, regulatory measures and public education regarding the risks from Hg in fish should be considered. These measures should take into account the amount of fish consumed and the levels of Hg in the fish. Consequently, establishing strategies to address the consumption of fish species at low trophic levels (e.g. E. plumieri) might decrease Hg exposure and protect the foetuses of pregnant women. Although Hg values in E. plumieri are the highest, their frequency of consumption is low (2%) and thus there is little concern about their consumption. On the other hand, M. curema was the most frequently consumed fish in all the groups (62%), but since its Hg content is low, its intake should be healthier for fish consumers. Unfortunately, this species also showed the highest Cr levels (1.32 μg/g), suggesting it should be discarded from the diet of the inhabitants of the swamp. Therefore, the general population, and especially sensitive population groups, such as pregnant women and young children, they should be recommended against eating the high levels they are already eating. Reducing the amount of fish ingested per week will reduce the possible health risks of consumers. The FDA and EPA issued a joint consumer advisory about Hg in fish and shellfish for women who might become pregnant; women who are pregnant; nursing mothers; and young children. One of the key points of the advisory states that individuals in these groups should eat up to 12 oz (340 g or two average meals) a week of a variety of fish and shellfish that are lower in Hg. It should be noted that children living in the Mallorquín swamp eat about 280 g per day, which represent about six times the levels recommended by the joint advisory.
Conclusion
The results obtained in the current study revealed that the highest concentrations of metals in the muscle tissue of the analysed fish species were recorded for Zn and the lowest for Cd. The fish species M. curema accumulated significant (p < 0.05) highest levels of Zn, Cr and Pb. On the other hand, with respect to the health risk due to metal exposure from fish intake, the estimation of the potential risk (HQ) indicated that there was no health risk for most of the metals (e.g. Cd, Cu, Cr, Ni and Zn) because they do not exceed their related RfDs, with values of HQ < 1. Under the assumption that all chromium in fish is Cr(III), the mean dietary exposure across all groups was well below the PTDI and therefore does not raise concerns for public health. However, if even a small proportion of total chromium in fish is in the form of Cr(VI), it could cause deleterious health risks. Therefore, for future studies in other geographical areas, in order to improve the risk assessment, there is a need to obtain data on the content of Cr(III) and Cr(VI) in fish. On the other hand, although the concentrations of Pb and Hg were not particularly high in fish muscle, their risk is of great concern due to the extremely high fish intake by all the groups, especially for vulnerable groups such as children and women of childbearing age.
Despite contaminants, fish is rich in many important nutrients and offers genuine health benefits, but unfortunately, subsistence fish-eating communities, because of their consumption habits, cannot replace with other nutrition sources. For riverine populations around the world, fishing is a source of income and their diet is based almost exclusively on fish; therefore, restricting fish consumption would not be desirable. Nevertheless, our study shows that extremely high daily intake results in a high risk for Hg and Pb, and points out an urgent need to limit the consumption of large amounts of fish. Thus, in other geographical areas worldwide, where traditional communities are settled, an accurate estimation of the intake for risk assessment evaluation is mandatory. Our results suggest that fish meals consumed and serving sizes are the key for evaluating risk, even at low levels of metals. Moreover, the findings of our survey underscore the important role of government agencies and international organizations to recommended maximum amounts of fish (no matter what type of fish is) that riverine populations should consume. It has been well established that due to the process of biomagnification, fish near the top of aquatic food chains are more likely to have high levels of Hg than fish lower in food chains; nevertheless, this study shows that for other metals such as Cr and Pb, maximum levels were found in bottom-feeding fish. Therefore, recommend consumption of fish in the bottom of the trophic chain may be appropriate to reduce the risk of Hg, but may not be suitable for other metals. In a worldwide context, it is necessary a continuous upgrading on international food safety and quality standards on fish; however, in a local context, in order to protect riverine populations, a periodical monitoring of metals (not only Hg) and associated fish consumption advisories should be mandatory.
References
Agency for Toxic Substances and Disease Registry (ATSDR). (2000). Summary of public health chrome. Atlanta, GA: Department of Health and Human Services in the US, Public Health Service.
Al-Busaidi, M., Yesudhason, P., Al-Mughairi, S., Al-Rahbi, W. A. K., Al-Harthy, K. S., Al-Mazrooei, N. A., et al. (2011). Toxic metals in commercial marine fish in Oman with reference to national and international standards. Chemosphere, 85, 67–73.
Arrieta, L., & De la Rosa, J. (2003). Structure of fish community in the Cienaga de Mallorquín, Colombian Caribbean. Boletin de Investigaciones Marinas y Costeras, 32, 231–242.
Asante, K. A., Agusa, T., Kubota, R., Mochizuki, H., Ramu, K., Nishida, S., et al. (2010). Trace elements and stable isotope ratios (δ13C and δ15 N) in fish from deep-waters of the Sulu Sea and the Celebes Sea. Marine Pollution Bulletin, 60, 1560–1570.
Bello, O., Naidu, R., Rahman, M. M., Liu, Y., & Dong, Z. (2016). Lead concentration in the blood of the general population living near a lead–zinc mine site, Nigeria: Exposure pathways. Science of the Total Environment, 542, 908–914.
Bosch, A. C., O’Neill, B., Sigge, G. O., Kerwath, S. E., & Hoffman, L. C. (2015). Heavy metals in marine fish meat and consumer health: A review. Journal of the Science of Food and Agriculture,. doi:10.1002/jsfa.7360.
Buekers, J., De Brouwere, K., Lefebvre, W., Willems, H., Vandenbroele, M., Van Sprang, P., et al. (2015). Assessment of human exposure to environmental sources of nickel in Europe: Inhalation exposure. Science of the Total Environment, 521, 359–371.
Carrasco, L., Benejam, L., Bayona, J. M., & Díez, S. (2011). Methylmercury levels and bioaccumulation in the aquatic food web of a highly mercury-contaminated reservoir. Environmental International, 37, 1213–1218.
Castaño, A., Cutanda, F., Esteban, M., Pärt, P., Navarro, C., Gómez, S., et al. (2015). Fish consumption patterns and hair mercury levels in children and their mothers in 17 EU countries. Environmental Research, 141, 58–68.
Chasapis, C., Loutsidou, A., Spiliopoulou, C., & Stefanidou, M. (2012). Zinc and human health: An update. Archives of Toxicology, 86, 521–534.
Cheung, K. H., & Gu, J. D. (2007). Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: A review. International Biodeterioration and Biodegradation, 59, 8–15.
Choi, A. L., Mogensen, U. B., Bjerve, K. S., Debes, F., Weihe, P., Grandjean, P., et al. (2014). Negative confounding by essential fatty acids in methylmercury neurotoxicity associations. Neurotoxicology and Teratology, 42, 85–92.
Christensen, K. Y., Thompson, B. A., Werner, M., Malecki, K., Imm, P., & Anderson, H. A. (2015). Levels of nutrients in relation to fish consumption among older male anglers in Wisconsin. Environmental Research, 142, 542–548.
Cohen, M. D., Kargacin, B., Klein, C. B., & Costa, M. (1993). Mechanisms of chromium carcinogenicity and toxicity. Critical Revews in Toxicology, 23, 255–281.
Cormagdalena-Cra-Uninorte. (1998). Feasibility study for the recovery of the Mallorquín swamp. Executive report, Corporación Autónoma Regional del Atlántico, Barranquilla, p. 254.
Crump, K. S., Kjellstrom, T., Shipp, A. M., Silvers, A., & Stewart, A. (1998). Influence of prenatal mercury exposure upon scholastic and psychological test performance: Benchmark analysis of a New Zealand cohort. Risk Analysis, 18, 701–713.
Dayan, D. A., & Paine, A. J. (2001). Mechanisms of chromium toxicity, carcinogenicity and allergenicity: Review of the literature from 1985 to 2000. Human and Experimental Toxicology, 20, 439.
Decree Number 3888. (2009). Wetlands of international interest: Ministry of Environment, Housing and Territorial Development. Republic of Colombia, p. 8.
DeFlora, S., & Wetterhahn, K. E. (1989). Mechanisms of chromium metabolism and genotoxicity. Life Chemistry Reports, 7, 169–244.
de Romaña, D.L., Olivares, M., Uauy, R., & Araya, M. (2011). Risks and benefits of copper in light of new insights of copper homeostasis. Journal of Trace Elements in Medicine and Biology, 25, 3–13.
Diez, S. (2009). Human health effects of methylmercury exposure. In Whitacre, D. M. (Ed.) Reviews of environmental contamination and toxicology (vol. 198, pp. 111–132).
EFSA. (2010). European food safety authority panel on contaminants in the food chain (CONTAM); scientific opinion on lead in food. EFSA Journal, 8, 1570–1720.
EFSA. (2014). Scientific opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSA Journal, 12, 3595.
EFSA. (2015). Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA Journal, 13, 3982. doi:10.2903/j.efsa.2015.3982.
Forti, E., Salovaara, S., Cetin, Y., Bulgheroni, A., Pfaller, R. W., & Prieto, P. (2011). In vitro evaluation of the toxicity induced by nickel soluble and particulate forms in human airway epithelial cells. Toxicology in Vitro, 25, 454–461.
Franco, A. J., & León-Luna, I. M. (2010). Geoquímica y concentraciones de metales pesados en un organismo de interés comercial (Corbula caribaea. D’orbigny, 1842) en la zona submareal superficial de la Ciénaga de Mallorquín-Atlántico. Boletín Científico CIOH No. 28, ISSN 0120-0542, Cartagena de Indias, Colombia, pp. 69–83. http://www.cioh.org.co/dev/publicaciones/resumboletin/28/cioh_bc_r2804.html. Accessed Nov 2016.
Galvis, O., Téllez. S., & Lora, A. (1992). Contribution to the knowledge of the environmental characteristics of the Mallorquín swamp. VIII Semin. Nac. Cien. Tecnol. Mar BCC, Bogotá, vol. 1, pp. 483–489.
García, G., & Luque, M. (2008). Analysis of heavy metals (Cd, Cr, Fe, Pb and Zn) in the muscle tissue of Eugerres plumieri and Mugil incilis from the Mallorquín swamp, Atlántico. Degree work. “Universidad del Atlántico, Barranquilla.
Garcia-Leston, J., Mendez, J., Pasaro, E., & Laffon, B. (2010). Genotoxic effects of lead: An updated review. Environmental International, 36, 623–636.
Grandjean, P., Weihe, P., White, R. F., Debes, F., Araki, S., Murata, K., et al. (1997). Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicology and Teratology, 19, 417–428.
Hao, Y., Chen, L., Zhang, X., Zhang, D., Zhang, X., Yu, Y., et al. (2013). Trace elements in fish from Taihu Lake, China: Levels, associated risks, and trophic transfer. Ecotoxicology and Environmental Safety, 90, 89–97.
Houston, M. (2007). The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infraction. Alternative Therapies in Health and Medicine, 13, 128–133.
Hülya, K., & Erhan, Ü. (2007). Heavy metal concentrations in water, sediment, fish and some benthic organisms from Tigris river, Turkey. Environmental Monitoring and Assessment, 131, 323–337.
IARC monographs on the evaluation of carcinogenic risks to humans: chromium, nickel and welding, vol. 49, International Agency for Cancer Research, Lyons, France, 1990.
Ikemoto, T., Tu, N., Okuda, N., Iwata, A., Omori, K., Tanabe, S., et al. (2008). Biomagnification of trace elements in the aquatic food web in the Mekong Delta, South Vietnam using stable carbon and nitrogen isotope analysis. Archives of Environmental Contamination and Toxicology, 54, 504–515.
Invemar. (2001). Report of the state of marine and coastal environments in Colombia: 2000. Series of general documents, Santa Marta, pp. 138.
Invemar. (2005). Technical report about the first monitor of heavy metals in water, sediments and organisms of the Mallorquín swamp, Department of the Atlántico.
JECFA. (2006). Safety evaluation of certain food additives and contaminants. 67th Joint FAO/WHO Expert Committee on Food Additives, WHO, Rome.
JECFA. (2015). Joint food and agriculture organization/world health organization expert committee on food additives. Summary and conclusions of the meetings of the joint FAO/WHO Expert Committee on food additives. http://apps.who.int/food-additives-contaminants-jecfa-database/Search.aspx. Accessed Nov 2016.
Kondo, K. (2000). Congenital Minamata disease: Warnings from Japan’s experience. Journal of Child Neurology, 15, 458–464.
Mergler, D., Anderson, H. A., Chan, L. H. M., Mahaffey, K. R., Murray, M., Sakamoto, M., et al. (2007). Methylmercury exposure and health effects in humans: A worldwide concern. Ambio, 36(1), 3–11.
Mozaffarian, D., & Wu, J. (2011). Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. Journal of the American College of Cardiology, 20, 2047–2067.
Myers, G. J., Davidson, P. W., Cox, C., Shamlaye, C. F., Palumbo, D., Cernichiari, E., et al. (2003). Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet, 361, 1686–1692.
Nakamura, M., Hachiya, N., Murata, K. Y., Nakanishi, I., Kondo, T., Yasutake, A., et al. (2014). Methylmercury exposure and neurological outcomes in Taiji residents accustomed to consuming whale meat. Environment International, 68, 25–32.
Neff, J. M. (2002). Chromium in the ocean. In J. M. Neff (Ed.), Bioaccumulation in marine organisms (pp. 131–143). Amsterdam: Elsevier.
Nickens, K. P., Patierno, S. R., & Ceryak, S. (2010). Chromium genotoxicity: a double-edged sword. Chemico-Biological Interactions, 188, 276–288.
Oken, E., Radesky, J., Wright, R., Bellinger, D., Amarasiriwardena, C., & Kleinman, P. (2008). Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. American Journal of Epidemiology, 167, 1171–1181.
Olmedo, P., Hernandez, A. F., Pla, A., Femia, P., Navas-Acien, A., & Gil, F. (2013). Determination of essential elements (copper, manganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury-selenium balance. Food and Chemical Toxicology, 62, 299–307.
Pedraza, L., (2009). Analysis of heavy metals (Zn, Cu, Cd, Fe, Cr) in the Clam Tivela mactroides (Born 1778) of the Mallorquín swamp, Department of the Atlántico. Degree work. “Universidad del Atlántico, Barranquilla.
Perello, G., Vicente, E., Castell, V., Llobet, J. M., Nadal, M., & Domingo, J. L. (2015). Dietary intake of trace elements by the population of Catalonia (Spain): results from a total diet study. Food Additives and Contaminants Part A-Chemistry Analysis Control Exposure and Risk Assessment, 32, 748–755.
Pieniak, Z., Verbeke, W., & Scholderer, J. (2010). Health-related beliefs and consumer knowledge as determinants of fish consumption. Journal of Human Nutrition and Dietetics, 5, 480–488.
Plum, L. M., Rink, L., & Haase, H. (2010). The essential toxin: Impact of zinc on human health. International Journal of Environmental Research and Public Health, 7, 1342–1365. doi:10.3390/ijerph7041342.
Rahman, M., Molla, A., Saha, N., & Rahman, A. (2012). Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chemistry, 134, 1847–1854.
Rose, M., Fernandes, A., Mortimer, D., & Baskaran, C. (2015). Contamination of fish in UK fresh water systems: risk assessment for human consumption. Chemosphere, 122, 183–189.
Rudolf, E., & Cervinka, M. (2009). Trivalent chromium activates Rac-1 and Src and induces switch in the cell death mode in human dermal fibroblasts. Toxicological Letters, 188, 236–242.
Sadiq, M., Zaidi, T., & Al-Mohana, M. (1991). Sample weight and digestion temperature as critical factors in mercury determination in fish. Bulletin of Environmental Contamination and Toxicology, 47, 335–341.
Satarug, S., Garett, S. H., Sens, M. A., & Sens, D. A. (2010). Cadmium, environmental exposure, and health outcomes. Environmental Health Perspectives, 118, 182–190.
Shrivastava, H. Y., Ravikumar, T., Shanmugasundaram, N., Babu, M., & Nair, B. U. (2005). Cytotoxicity studies of chromium(III) complexes on human dermal fibroblasts. Free Radical Biology and Medicine, 38, 58–69.
Sierra-Gutiérrez, F. D. (2003). Contenido de metales pesados (Cobre, Cadmio y Zinc) en la lisa (Mugil incilis) en la Ciénaga de Mallorquín (Atlántico). Degree work. Uni-Atlántico (In Spanish).
Swanson, D., Block, R., & Mousa, S. (2012). Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Advances in Nutrition, 1, 1–7.
Tadiso, T., Borgstrom, R., & Rosseland, B. (2011). Mercury concentrations are low in commercial fish species of Lake Ziway, Ethiopia, but stable isotope data indicated biomagnification. Ecotoxicology and Environmental Safety, 74, 953–959.
UNEP. (2006). Isaza, C. F. A., Sierra-Correa, P. C., Bernal-Velasquez, M., Londoño, L. M., & Troncoso, W. Caribbean Sea/Colombia and Venezuela, Caribbean Sea/Central America and Mexico, GIWA Regional assessment 3b, 3c. University of Kalmar, Kalmar.
UNEP. (2008). Guidance for identifying populations at risk from mercury exposure, Geneva, Switzerland. http://www.who.int/foodsafety/publications/chem/mercuryexposure.pdf. Accessed Nov 2016.
UNEP/IOC/IAEA/FAO. (1990). Contaminant monitoring programmes using marine organisms: Quality Assurance and Good Laboratory Practice. Reference Methods for Marine Pollution Studies N°57.
USEPA. (2000). Guidance for assessing chemical contaminant data for use in fish advisories. Risk assessment and fish consumption limits, EPA 823-B-00-008 (3rd edn., Vol. 2, p. 383). Washington, DC: USEPA Office of Water. https://www.epa.gov/sites/production/files/2015-06/documents/volume2.pdf. Accessed Nov 2016.
USEPA. (2016). Environmental Protection Agency, integrated risk information system. CRC. http://www.epa.gov/iris/. Accessed January 2016.
Velma, V., Vutukuru, S. S., & Tchounwou, P. B. (2009). Ecotoxicology of hexavalent chromium in freshwater fish: A critical review. Reviews on Environmental Health, 24, 129–145.
Wenstrom, K. D. (2014). The FDA’s new advice on fish: It’s complicated. American Journal of Obstetrics and Gynecology, 211, 475–478.
WHO. (1986). Lead (evaluation of health risk to infants and children). World Health Organization. http://www.inchem.org/documents/jecfa/jecmono/v21je16.htm. Accessed January 2016.
WHO. (1996). World Health Organization. Trace elements in human nutrition and health. Geneva: WHO Library Cataloguing in Publication Data.
Yu, Y. X., Wang, X. X., Yang, D., Lei, B. L., Zhang, X. L., & Zhang, X. Y. (2014). Evaluation of human health risks posed by carcinogenic and non-carcinogenic multiple contaminants associated with consumption of fish from Taihu Lake, China. Food and Chemical Toxicology, 69, 86–93.
Zaza, S., Balogh, K., Palmery, M., Pastorelli, A., & Stacchini, P. (2015). Human exposure in Italy to lead, cadmium and mercury through fish and seafood product consumption from Eastern Central Atlantic Fishing Area. Journal of Food Composition and Analysis, 40, 148–153.
Zhang, X., Xu, Q., Man, S., Zeng, X., Yu, Y., Pang, Y., et al. (2013). Tissue concentrations, bioaccumulation, and biomagnifications of synthetic musks in freshwater fish from Taihu Lake, China. Environmental Science and Pollution Research, 20, 311–322.
Acknowledgements
The authors are grateful to the Laboratory of Toxicology and Environmental Management of the Universidad de Córdoba by their collaboration to analyse the concentrations of the metals in the studied species. We appreciate the anonymous reviewers for their many insightful comments and suggestions to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuentes-Gandara, F., Pinedo-Hernández, J., Marrugo-Negrete, J. et al. Human health impacts of exposure to metals through extreme consumption of fish from the Colombian Caribbean Sea. Environ Geochem Health 40, 229–242 (2018). https://doi.org/10.1007/s10653-016-9896-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-016-9896-z