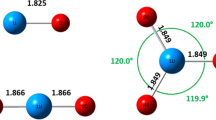
Abstract
Study of uranium interstitial compositions of non-stoichiometric oxides UO2+x (x ∈ 0.1–0.02) in gas and condense phases has been presented, using various soft-ionization mass spectrometric methods such as ESI-, APCI-, and MALDI-MS at a wide dynamic temperature gradient (∈ 25–300 °C). Linearly polarized vibrational spectroscopy has been utilized in order to assign unambiguously, the vibrational frequencies of uranium non-stoichiometric oxides. Experimental design has involved xUO2.66·yUO2.33, xUO2.66·yUO2.33/SiO2, xUO2.66·yUO2.33/SiO2 (NaOH) and SiO2/x′NaOH·y′UO2(NO3)2·6H2O, multicomponent systems (x = 1, y ∈ 0.1–1.0 and x′ = 1, y′ ∈ 0.1–0.6) as well as phase transitions UO2(NO3)2·6H2O → {U4O9(UO2.25)} → U3O7(UO2.33) → U3O8(UO2.66) → {UO3}, thus ensuring a maximal representativeness to real environmental conditions, where diverse chemical, geochemical and biochemical reactions, including complexation and sorption onto minerals have occurred. Experimental factors such as UV-irradiation, pH, temperature, concentration levels, solvent types and ion strength have been taken into consideration, too. As far as uranium speciation represents a challenging analytical task in terms of chemical identification diverse coordination species, mechanistic aspects relating incorporation of oxygen into UO 2+x form the shown full methods validation significantly impacts the field of environmental radioanalytical chemistry. UO2 is the most commonly used fuel in nuclear reactors around the globe; however, a large non-stoichiometric range ∈ UO1.65–UO2.25 has occurred due to radiolysis of water on UO2 surface yielding to H2O2, OH·, and more. Each of those compositions has different oxygen diffusion. And in this respect enormous effort has been concentrated to study the potential impact of hazardous radionuclide on the environment, encompassing from the reprocessing to the disposal stages of the fuel waste, including the waste itself, the processes in the waste containers, the clay around the containers, and geological processes. In a broader sense, thereby, this study contributes to field of environmental analysis highlighting the great ability of various soft-ionization MS methods, particularly, MALDI-MS one, for direct assay of complex multicomponent heterogeneous mixtures at fmol–attomol concentration ranges, along with it the great instrumental features allowing, not only meaningful quantitative, but also structural information of the analytes, thus making the method indispensable for environmental speciation of radionuclides, generally.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The environmental assessment of risk to the human health from toxic and radioactive contamination and control of radionuclides in nature is of primary concept and importance for the human well-being. The environmental protection strategies for monitoring the migration processes of radionuclides in the geosphere and long storage of nuclear waste have involved complex models of environmental processes and experimental chemical, geochemical, physical and biological factors (Stoliker et al. 2011; Ilton et al. 2012; Jung et al. 2012; Malaviya and Singh 2012; Chandrasekaran et al. 2011; Sundararajan et al. 2011; Mayer et al. 2013; Walton and Mitchell 2013; Qiu and Burns 2013; Hocking et al. 2013; Knope and Soderholm 2013). This complexity of the theoretical modeling and the experimental design of radionuclide migration in surface and groundwater; its speciation in wastewater, sorption onto soils, sediments and manure has been further complicated by the dual physical nature uranium between rare element and semiconductor as a result of the characteristics of 5f-electrons at an intermediate localization regime. The 5f-correlation energy and 5f-bandwidth are of same order of magnitude, causing an intermediate behavior of uranium compounds and delocalization electronic properties (Schoenes 1987; Wick and McDowell 1918; Kalkowski et al. 1987; Petiau et al. 1986; Denning 1992, 2007; Winkler and Gray 2012). Therefore, the migration and bioavailability of uranium in the nature, particularly, and actinides in a broad context, is governed by molecular reactions and experimental conditions in solid, aqueous and/or gas phases. But the largest part of studies have shown that the coordination chemistry of uranium is mainly resembled to peculiar to uranyl UO2 2+-ion, thus connecting the theoretical models to mechanisms, where uranium is immobilized under environmental oxidized conditions as a uranyl ion, considering, however, the fact that it often can be found as uranyl mineral schoepite (UO3·2H2O) in contaminated soils and ground water (Duff et al. 2002). Stable uranium ion in oxidation state 6+ may exists, also, through uranates which a UO3 form (Duff et al. 2002; Ivanova and Spiteller 2014). In chemically reductive area UVI has exhibited biotic reduction to UIV, which may precipitated depending on microbiological factors in the environment (Ray et al. 2011). The classical bulk reaction yielding to UIV has involved interaction with pyrite (FeS2), which is most common sulfide mineral. The uranium uptake has been associated with 2FeS2 + [UO2]2+ + 2H2O + 7O2 ⇒ 2Fe3+ + 4SO4 2− + [UO2](s) + 4H+ (Scott et al. 2007; Klinkhammer and Palmers 1991). The reductive precipitation of UVI → UIV, which usually coupled FeII → FeIII oxidation process has resulted to non-stoichiometric compositions. The last forms have been often observed under reductive precipitation coupled with oxidation process of sulfides to polysulfides and sulfur, as well (Scott et al. 2007). In spite of mineral type, uranium has exhibited own redox behavior, thus causing precipitation in the presence of carbonates, governed by a process (Klinkhammer and Palmers 1991; Clark et al. 1995; Altmaier et al. 2013):
The uraninite form ([UO2](c)) is therefore a resulting one by sulfate microbial mediated reduction in more soluble uranium(VI) carbonate complex [UO2(CO3)3]4− (Chappaz et al. 2010; Klinkhammer and Palmers 1991). Other forms of uranium in sediment such as [U3O7](s) and [U3O8](s) are result of following reactions (Chappaz et al. 2010):
The environmental speciation, thereby, has involved complex (bio)geochemical reactions in solution, sorption/desorption processes onto minerals, biological processes causing distribution of uranium various oxidation states and chemical compositions (Conradson et al. 2004; Malliakas et al. 2012; Lee et al. 2009; Chaumont et al. 2012; Park et al. 1990; Van Horn and Huang 2006). In parallel, the minerals themselves near to geosphere, when they are exposed to the effect of aerobic atmospheric conditions are oxidized and/or decomposed yielding different oxidative products, thus causing different uptake behavior of heavy metals in the environment, particularly reflecting in mind uranium and other radionuclides (Scott et al. 2007). In addition, there is still lack of knowledge about fixation mechanisms of uranium forms in the sediments (Chappaz et al. 2010). Furthermore, the mainly studied equilibrium sorption models onto environmental subsurfaces describing uranium transport, relevant to large number of mineralogical, hydrological and biogeochemical processes have shown, in fact, a non-equilibrium kinetic control of the sorption of uranium species onto mineral surfaces (Um et al. 2010; Guerra et al. 2010). The aqueous speciation depends mainly on carbon dioxide, uranium concentration levels, pH, pressure, and temperature (Conradson et al. 2004; Malliakas et al. 2012; Lee et al. 2009; Chaumont et al. 2012; Park et al. 1990; Van Horn and Huang 2006). For the purpose of nuclear waste management, knowledge of coordination ability of uranium including various phases, solvent effect causing different complexation behavior and formation of different coordination compounds with the inclusion of solvent molecules is great importance, too (Conradson et al. 2004; Malliakas et al. 2012; Lee et al. 2009; Chaumont et al. 2012; Park et al. 1990; Van Horn and Huang 2006). Therefore, from analytical viewpoint, uranium O-containing compositions as analytical objects are among most complex coordination system known. The elaboration of quantitative protocols, therefore, is a non-trivial research task, requiring reliable and highly representative to real systems, theoretical modeling and experimental design, encompassing environmental conditions and the complexity and nature, thus minimizing the risking of inaccuracy (He et al. 2010; Lotnik et al. 2001; Yusov and Shilov 2007; Kulyukhin and Kamenskaya 2010; Nagaishi et al. 1998; Gorshkov et al. 2001; Rios et al. 2012). In spite of numerous studies, which have devoted to uranium speciation, the error assessment has been mainly limited to determination of overall measurements error (He et al. 2010; Lotnik et al. 2001; Yusov and Shilov 2007; Kulyukhin and Kamenskaya 2010; Nagaishi et al. 1998; Gorshkov et al. 2001; Rios et al. 2012). The composition of uranium target sample may be significantly alerted, due to formation of non-stoichiometric oxides UO2+x both in condense and in gas-phase (x ∈ 0.1–0.02). Furthermore, the mobility of oxide atom is rapid and reversible process (He et al. 2010; Lotnik et al. 2001; Yusov and Shilov 2007; Kulyukhin and Kamenskaya 2010; Nagaishi et al. 1998; Gorshkov et al. 2001; Rios et al. 2012). The identification of mechanistic aspects of formation of non-stoichiometric oxides is a challenging research task. It, however, represents important step in uranium speciation. In this respect, the fluorescence spectroscopy has already found place as analytical method for environmental assessment. Because of electronic 1Σg → 2ΠU transitions within UV–VIS–NIR region of O–U–O unit of single type to vacant quasi-atomic orbitals of 5f shell have very characteristic spectroscopic profile (Meinrath et al. 2000; Batrakov and Puchkova 2003; Hubbard and Griffiths 1987). For environmental purposes, the representative results have been obtained modeling the natural systems. The ligands have been mainly hydroxide, carbonate or humic substances (Klinkhammer and Palmers 1991; Lesher et al. 2013). Metal–organic complexes generally is accepted that played important role in marine geochemistry of uranium and commonly the speciation in surface and ground water, where interactions with labile uranium complexes of organic ligands (mainly oxalate) with sediment–water interface occurred (Klinkhammer and Palmers 1991; Duff et al. 2002). The oxides and carbonates are other largely distributed inorganic soluble complexes, which sorb onto natural minerals (Duff et al. 2002). The co-precipitation is another geochemical process influencing the uranium migration and bioavailability, occurred in the presence of oxide minerals or mineral phases such as silicates (Duff et al. 2002). It is observed in aged uranium contained soils and subsurface environmental phases. Furthermore: (a) the precipitation of UVI → UIV depending on concentration of silicates in sediments may caused the formation of UIV–silica colloids (Dreissig et al. 2011). The U–O–Si bond formation has been observed in those forms, where the coordination environment has been found comparable to coffinite (USiO4); and (b) study of the uranium distribution in sediments in the environmental area near to nuclear waste storage has been shown that the uranium mobility curtailed via the precipitation of silicates and phosphates (Um et al. 2010). The uranophane ([Ca(UO2)2(SiO3OH)2(H2O)5]) and uranyl phosphate ([Ca(UO2)2(PO4)2(H2O)10–12]) forms have been determined (Um et al. 2010). To ensure a maximal representativeness toward the real geochemical processes with participation of uranium, the theoretical model and the experimental design reported involve study in the pH range ∈ 2–4, and 4–5, where mainly [UO2]2+ and [UO2(OH)]+ cations occurred. The latter species has been observed during the uranium driving across the sediment/water interface, here depending of the environmental conditions, the process [UO2]2+ + H2O ⇒ [UO2(OH)]+ + H+ (Chappaz et al. 2010; Sun et al. 2014; Gu et al. 2003). The concentration level has been μg L−1 (Meinrath et al. 2000; Batrakov and Puchkova 2003; Hubbard and Griffiths 1987). The real pH range, however, involves pH ∈ 4–7 overlapped with the pH one of UO2(OH)2, UO2(OH) −3 , UO2(OH) 2+2 species, observed at pH > 7. Furthermore in the presence of CO2, UO2(CO3) x−y ions are also formed. The uranium quantitation based usually on Laporte-forbidden internal 5f transitions, which have overall low intensities (εν ∈ 50–100 L mol−1 cm−1), limited the application of electronic absorption and fluorescence. The vibronic states are sensitive to the environment in the inner coordination sphere, thus effecting the 5f transitions in solutions, which for pH > 7 of multicomponent analyte environmental mixtures need extrapolations. For the low environmental analyte concentrations, the chemometric approximations of the complex spectroscopic profile increased the inaccuracy. Thus, there is no dough that mass spectrometry is an irreplaceable method for achievement of analytical trustworthy information. Its capability to obtain quantitative data for the analyte at concentration level of fg g−1; instrumental flexibility and variety of ionization techniques; including wide dynamic temperature regime; fast for operation and stage limited sample preparation steps, encompassing analysis in solutions, semi-liquid and solid state, makes mass spectrometry a method of choice for uranium speciation (Pajo et al. 2001; Steppert et al. 2012; Crawford et al. 2012; Rutkowski et al. 2011; Gresham et al. 2011; Jennings et al. 1989). Its applicability involves complex multicomponent homogeneous and heterogeneous environmental samples, including stages of the nuclear fuel cycle such as treatment of liquid wastes and/or dried fuel storage, where the assessment of uranium oxides reactivity ∈ 100–250 °C is of considerable interest (He et al. 2010; Lotnik et al. 2001; Yusov and Shilov 2007; Kulyukhin and Kamenskaya 2010; Nagaishi et al. 1998; Gorshkov et al. 2001; Rios et al. 2012). Notwithstanding that our continues research interest for methodological MS developments and analytical implementation has been focused on elaboration of analytical protocols for environmental assessment mainly of organic contaminations, foodstuffs monitoring, and drugs of abuse analysis (Sukul et al. 2010; Kusari et al. 2009; Banerjee et al. 2006; Smejkalova et al. 2006; Frimmel et al. 2002; Choi et al. 2012; Ivanova and Spiteller 2012a, b, c, 2014; Lamshoeft et al. 2011; Spiteller 1985, 1987, 2012; Spiteller and Saiz-Jimenez 1990), the herein presented study is focused on uranium speciation in gas and condense phase, utilizing mass spectrometry and vibrational spectroscopy. Nevertheless of the generally higher method detection limits of the vibrational spectroscopic methods, the characteristic vibrational profile ∈ 970–900 cm−1 of ν asO=U=O (ν3) stretching vibration (Best et al. 1988; Guimbretiere et al. 2012; Liang et al. 2005; Mueller et al. 2009; He et al. 2002; Ivanova and Spiteller 2014) enable the utilization of the method for quantitative purposes. The full method validation applicable to real environmental problematic. Because of the environmental analysis, modeling and experimental design of radionuclide speciation is complex. For this reason the presented analytical strategy encompassed all critical aspects related to the representativeness and recovery. As shown in the European Atomic Energy Community Treaty and report from the International Atomic Energy Agency, main contributions of the scientific research are limited to the uranium qualitative speciation in terms of contaminated soils, surface and groundwater, sediments, etc. highlighting the variety of possible forms, depending on the complex environmental factors, but rare the attention is focused on the quantitation (International Atomic Energy Agency 2004; Publications Office of the European Union 2011). The latter one encompassed mainly determination of isotopic actinide ratios through hard ionization MS methods. For the purpose of foodstuffs monitoring and control in Europa, the tolerable daily uranium intake of 0.6 µg kg−1 bw per day was established by World Health Organization. Since the research into biological impacts of uranium is a relatively new science as well as still have not direct understanding of biochemical mechanisms of its activity, the reported herein calibration and standardization are consistent with the overall method detection limits.
On the view on the above-stated facts then, the efforts in this paper are into the following directions, aiming to answer the open questions about the coordination ability of the uranium forms under environmental conditions, their chemical transformations in solution and in solid state as well as their surface behaviors onto SiO2, elucidating non-stoichiometric compositions of UO2+x such as xUO2.66·yUO2.33, xUO2.66·yUO2.33/SiO2, xUO2.66·yUO2.33/SiO2 (NaOH) and SiO2/x′NaOH·y′UO2(NO3)2·6H2O and the phase transitions occurred UO2(NO3)2·6H2O → {U4O9(UO2.25)} → U3O7(UO2.33) → U3O8(UO2.66) → {UO3}, focusing attention on molecular structure and the coordination chemistry of uranium form determining the thermodynamics of the ligand exchange processes in condense phase as well as the mechanistic aspects of the surface bonding, which appear highly sensitive toward the ligand environment from the inner coordination sphere around the uranium. In addition, the efforts are focused on examination of the relationship between molecular structure physical properties allowing from the, on the one hand, elaboration of precise analytical protocols both based on IR- and MALDI-MS methods. On the other hand, the crucial knowledge about the structure of the uranium complexes and their unambiguous distinguishing and assignment to corresponding form allow to model further highly realistic environmental processes with participation of those forms and thus to predict accurately their thermodynamic and kinetic parameters. In this context we should pointed out still here in the introduction section of the paper that in fact the absence of structural studies about the uranium complexes as well as coordination compounds with other actinides prevents the creation of a realistic molecular level models, to reliably express the real migration processes in the nature. Given that our study contributes to the fundamental understanding of the coordination behavior of uranium depending on a large scale of experimental conditions, allowing with treating the analytical quantitative determination of various uranium forms, having rather applied aspects to the problems of the environmental radioanalytical chemistry than to the phenomenology of the chemistry of f-elements, generally.
As it has been pointed out still in the abstract, the significance of the analytical objects studied herein concentrated in the fact that the UO2 is among the most commonly used fuel din the nuclear reactors; however, the radiolysis of the water causing formation of the mentioned H2O2, OH·, an relating species yielded to numerous non-stoichiometric compositions UO2±x , each of which have different diffusion properties, meaning that the study of those compositions allows to understand the oxygen migration (Moore et al. 2013; Yakub 2014; Berthinier et al. 2013; Perry 2015). Such studies have significant impact to many interdisciplinary fields such as environmental analytical chemistry, radioanalytical chemistry, (bio)geochemistry and the fourth.
Experimental
Physical methods and techniques for analysis
HPLC–ESI–MS/MS measurements were taken by TSQ 7000 instrument (Thermo Fisher Inc., Rockville, MD, USA). The mobile phases were 0.1 % v/v aqueous HCOOH (A), 0.1 % v/v HCOOH in CH3CN (B), 0.1 % v/v HCOOH in CH3CN:CH3OH (C), 0.01 % NaOH in CH3CN (D); 0.1 % NaOH/Na2CO3 in CH3CN:CH3OH (E). Electrospray ionization mass spectrometry was performed on a triple quadruple mass spectrometer (TSQ 7000 Thermo Electron, Dreieich, Germany) equipped with an ESI 2 source. The operation conditions were: capillary temperature 180 °C; sheath gas 60 psi, corona 4.5 μA and spray voltage 4.5 kV. Sample was dissolved in CH3CN (1 μg mL−1) and was injected in ion source by an autosampler (Surveyor) with a flow of pure CH3CN (0.2 μL min−1). Data processing was performed by Excalibur 1.4 software. A standard LTQ Orbitrap XL (Thermo Fisher Inc.) instrument was used for MALDI-MS measurements, using UV laser source at 337.2 nm. An overall mass range of m/z 100–1000 was scanned by Orbitrap analyzer. The ImageQuest 1.0.1 program package was used. The laser energy values were ∈ 11.3–26.7 μJ. The number of averaged laser shots lies ∈ 35–92, MALDI flow rate values were ∈ 25.0–30.0; the acquisition time was ∈ 21.3–122.5 min, the corresponding elapsed scan time range lies ∈ 20.4–1.12 s, respectively.
Chromatographic separation was performed with a Gynkotek (Germering, Germany) HPLC instrument, equipped with a preparative Kromasil 100 C18 column (250 × 20 mm, 7 μm; Eka Chemicals, Bohus, Sweden) and a UV detector set at 250 nm. The above shown mobile phases were used at a flow rate of 4 μL min−1. The HPLC analysis was performed on a Phenomenex (Torrance, CA, USA) RP-18 column (Jupiter 300, 150 × 2 mm, 3 μm) under same chromatographic conditions. The quantitative analysis was performed on a Shimadzu UFLC XR (Kyoto, Japan) instrument, equipped with an auto sampler, PDA, an on-line degasser and column thermostat. As stationary phase a Phenomenex Luna Phenyl-Hexyl column (150 mm × 3 mm i.d., 3 μm particle size) was used. The mobile phase consisted of 0.02 % (v/v) TFA in water (solvent F) and CH3CN:CH3OH 75:25 (v/v; solvent G). Separation was achieved by a gradient analysis starting with 55F–45G, increasing amount of solvent B in 30 min to 75 % and 30.1 min to 100 % G, stop time 40 min. For equilibration, a post time of 15 min was applied. Other parameters: flow rate 0.30 mL min−1, injection volume 5 μL, detection wavelength 280 nm; column temperature 35 °C.
The UV–VIS and fluorescence spectra were recorded on Tecan Safire Absorbance/Fluorescence XFluor 4 V 4.40 spectrophotometer.
The vibrational spectra were obtained on Bomem Michelson 100 and Thermo Nicolet 6700 FTIR spectrometers ∈ 400–400 cm−1, operating at resolution ±1 and 0.5 cm−1 as well as equipped with wire-grad polarizer. Polarized measurements were obtained in nematic host, using 200 scans per sample, according methodology already described (Koleva et al. 2008; Ivanova and Kolev 2012).
Sample preparation techniques for UV-MALDI-MS measurements
The UV-MALDI-MS matrix-analyte samples were prepared, using dried droplet technique (Ivanova and Spiteller 2012a). The 0.154 g of DHA (or DHB) or 0.164 g of CHCA matrix compounds were dissolved in 25 mL solvent mixtures A–E, which aliquots were measured under ESI-, APCI- and UV-MALDI-MS ionization methods. The solid-state samples for MALDI-MS measurements were dried on vacuum at r.t on sample targets.
Validation
The MS linear dynamic range were verified, analyzing ten standard solutions of UO2(NO3)2·6H2O and UF6 ∈ 1.05–100.05 ppq. Each standard was measured in triplicate. The UF6 solutions were used directly after their preparation. The period of time was ∈ 0–2 h. The intra-day precision and accuracy were determined at various replicated levels of standard solutions. The precision was calculated as relative SD, while the accuracy was obtained as the relative mean error. The data, in terms of intra-laboratory-variability, obtained at same laboratory, same instruments and same samples were evaluated. The recovery was performed by adding of known amounts of standard solutions to each of uranium compositions, followed by direct UV-MALDI-MS analysis. The obtained concentration limits of detection and quantitation were discussed in terms of the method and instrumental variables.
Analytes
The crystalline UO2(NO3)2·6H2O and SiO2 were Sigma-Aldrich product, used for direct assay without further purification. The U3O7 and U3O8 were obtained under heating of UO2(NO3)2·6H2O in a dynamic temperature range of 100–250 °C in aluminum corps and O2 flow. The oxidation process via road UO2(NO3)2·6H2O → U3O7(UO2.33) → U3O8(UO2.66) can be described qualitatively and quantitatively at the later stages, which rates were very temperature dependent. Thus, at temperature range ∈ 150–175 °C the UO2.33 was isolated, while ∈ 200–250 °C—UO2.66. The distinct crystallographic phase between UO2 → UO3 transition occurred (Hall et al. 1965; Allen and Tempest 1986) is controlled by spectroscopic methods, since the specific local properties of the microscopic sub-structures or clusters affect the vibrational characteristics of the analytes (Ivanova and Spiteller 2014). Portions of heated samples at different temperature dynamic ranges were measured and identified. The uranyl speciation on SiO2 was studied obtaining representative samples ∈ 25–250 °C with dynamic range of 10 °C, heating the mixtures of UO2(NO3)2·6H2O:SiO2 at molar ratios 1:1–1:20.
Computational methods
Statistical, mathematical and other logistic methods (chemometrics)
The experimental and theoretical spectroscopic patterns were processed by R4Cal Open Office STATISTICs for Windows 7 program package. Baseline correction and curve-fitting procedures were applied (Kelley 1999; Beebe et al. 1998; Madsen et al. 2004; Miller 2000; Massart et al. 1988). The statistical significance of each regression coefficient and the model fit was checked (resp. determined) by the use of t- and F-tests.
Results and discussion
Speaking of argumentation and structure of “Results and discussion” part of the paper, we refer to following important aspects of systematization of data obtained. Firstly, IR-spectroscopy and methods of mass spectrometry have significantly different instrumental characteristics, particularly pointing out method and instrumental detection limits. Contrastively to IR-spectroscopy, where concentration levels vary about at 1.4 %, attomol concentrations have been achieved employing MS-based methods. Given that, methodological contributions and quantitative protocols developed in this paper are presented and discussed by instrumental methods. There is no dough that mass spectrometry has irreplaceable place in the field of the environmental analytical chemistry exactly with its great instrumental features, which have already been said. However, IR-spectroscopy privies meaningful structural information in condense phase along with unique spectroscopic profile to each of analyte studied like MS isotope profile, so that, both of those methods are employed into material identification, furthermore, unambiguously. Moreover, the ability of IR-spectroscopy to assign various polymorph modifications of the matter in solid state enables to control and complement the methodological contributions and developments of sample preparation technique, particularly for MALDI-MS mass spectrometry, demonstrated herein, where the sample preparation technique allows obtaining of various polymorph, co-crystals even different crystalline structures, which can be easy identified, employing IR-spectroscopy, on the base on it well-known advantages such as above-mentioned ability for materials identification, available techniques for solution and solid-state analysis, easy, fast for operation and well-developed sample handing techniques, become routine approaches for qualitative and quantitative analysis including as well as low and easy instrumental cost and support. As far as methodological contributions to those methods, including instrumental elaborations and implementations along with sample preparation approaches has own weight to the field of analytical chemistry, different from elaborations and developments of quantitative protocols for concrete analytical problem, we have chosen to sub-divide the results obtained into two sub-sections to each method, dealing with methodological contributions and the quantitative analysis. The section methodological development has been presented as supporting information file due to the reasons stated above, concentrating the discussion on the quantitative IR-analysis carried out using those methodological contributions having main impact to the field of the environmental analysis of radionuclides.
Quantitative vibrational analysis: performance parameters
Multicomponent composition set of U3O8, U3O7, and UO3 in SiO2/xUO2.66·yUO2.33, SiO2/NaOH/xUO2.66·yUO2.33 and SiO2/x′NaOH·y′UO2(NO3)2·6H2O, where x = 1, y ∈ 0.1–1.0 and x′ = 1, y′ ∈ 0.1–0.6 are quantified, within the frame of a dynamic temperature range ∈ 100–250 °C (Table S1). The analysis carried out has utilized integral intensity ratios as a function of the elemental compositions in UO2.66 showing a mean value 953.60031, SD(yEr±) = 2.51267, and SE(yEr±) = 1.25633, respectively. The relative high standard deviation is explained with the overlapping profile of the spectroscopic patterns of those mixtures. The obtained linear regression approximation has resulted to A = −0.21735 ± 0.01044, B = 2.97708 ± 0.09287, r 2 = 0.98124, SD = 4.45349 and p = 0.01876, respectively. The analysis of UO3 in presence of NaOH has been extrapolated by a polynomial function A = −3.38407, B 1 = 207.91296, B 2 = −445.73148, B 3 = 270.09259 at a high level of confidence (r 2 = 0.9999, p < 0.0001). The deviation of linearity we have explained as a result of amounts of non-stoichiometric compositions to UO2(OH)NO3.
Mass spectrometric method development
The presented data utilized ESI-MS, APCI-MS, and MALDI-MS ionization techniques encompassed analytes from solution (ESI or APCI) or in situ solid state (MALDI) to gas phase. The processes allowed to examine environmental liquid or semi-liquid samples or solids which are of significant importance for the field of environmental radioanalytical chemistry. The capability of APCI-method to operate at large dynamic temperature range is also employed for analysis of phase transitions of uranium species. Nevertheless that, the one step fragmentation statement is accepted for assignment of MS ions in gas phase, multi-step fragmentation is rather common for complex MS patterns. Moreover, diversity of experimentally observed uranium species in the gas phase and its capability for overall formation of ionic species with different oxidation state or (U) x O +y ions may be explained in terms of multi-step processes. As MALDI-MS ionization/desorption mechanism is a complex from phenomenological viewpoint under laser irradiation (λ ex = 337.2 nm), the reduction in uranyl ions is assigned as previously proposed (Gorshkov et al. 2001). On the other site, the mobile phase compositions B–E used in the ESI-MS and APCI-MS measurements, included CH3CN as solvent component, while A contained only solvent water that possible formation of [UO2(H2O)5]2+ and [UO2(CH3CN)5]2+ dications is also evaluated (Chaumont et al. 2012; Kulyukhin and Kamenskaya 2010). As the quantitation by ESI-MS and MALDI-MS method is conceptually different, due to different, virtually continuous and pulsed ionization techniques, the sensitivity and reproducibility are discussed comprehensively. Furthermore, ionization and transfer efficiency, which have effect on sensitivity of MALDI-MS method and depended strongly of sample type, the volatility effect of uranium analytes onto analytical quantities is also discussed.
The MS spectra of standards UVIO2(NO3)2·6H2O and UVIF6 under different MS ionization methods are examined (Fig. S6). The employment of mobile phase A revealed for UVIO2(NO3)2·6H2O (t = 40 °C) MS peaks at m/z 810 (U3O6)+, 334, 318, 287, 270 and 254 of UO + n (n = 1–6) as well as peak at m/z 238 of U+ (Jennings et al. 1989; Downs and Gardner 1984; Beauchamp 1976; Compton 1977; Kalinin et al. 2002). The low-abundance peaks at m/z 349, 525, 541 and 557 are assigned to doubly charged [U2O13N]2+, [U3O21]2+, [U3O23]2+ and [U3O25]2+, ions. The proposed elemental compositions can be summarized as [U n O y ]2+ or [U n O y N z ]+ ones. These peaks provided direct evidence for multi-step fragmentation pathways and parallel reaction processes in the gas phase. The results are in accordance with already assumed process of stabilization of UIVUVIO7 subunit in U3O7. Moreover, they agreed well with the studied compositions at temperature range, involving uranium glass-state (Hall et al. 1965; Allen and Tempest 1986). The MS data using the mobile phases B, C and E (t = 25 °C) exhibited peaks at m/z 475 [C10H15N2O5U]+. Similar the data in solution, those in the gas-phase showed formation of [UO2(CH3CN)5]+ complex. However, the process is associated with UVI → UV redox mechanism. There are no experimental evidences for stable [UO2(H2O)5]2+ complexes in gas phase. The usage of mobile phase D caused observation of MS peak at m/z 287 of [UO2(OH)]+ ion (Chappaz et al. 2010; Sun et al. 2014; Gu et al. 2003). The increase in temperature up to range of ∈ 150–200 °C leads to observation of MS peaks at m/z 625, 574 and 634, which are assigned to [(UO2)2(OH)5]+, [(UO2)2(OH)2]+, and [(CH2O9U2)]+ cations (APCI-MS, mobile phase D, T = 300 °C). As has been previously reported (Downs and Gardner 1984; Beauchamp 1976; Compton 1977; Kalinin et al. 2002), the MS spectra of UF6 revealed the ions of [UF n ]+ type (n = 1–5), assigned to the peaks at m/z 257, 276, 295, 314 and 333. The expected doubly charged U2+, [UF2]2+, or [UF4]2+ does not quantitatively observed. The metastable peaks of [U2F10]+ and [U2F11]+ ions at m/z 666 and 685 are obtained as low-abundance signals at mobile phases A–C, and E (APCI-MS, t = 300 °C), respectively.
The MS spectra of compositions xUO2.66·yUO2.33, SiO2/xUO2.66·yUO2.33 and SiO2/NaOH/xUO2.66·yUO2.33 are depicted in Figs. 1, 2 and 3. By contrast MS spectra of standards, those of xUO2.66·yUO2.33 compositions at variety of x, y and T = 40 °C (mobile phases A–C and E) exhibited MS peak at m/z 1096 (U4O9 + or UO2.25), respectively. It is important to clarify the question, however, is crystalline and glass-like local structure assigned as U4O9 (Hall et al. 1965; Allen and Tempest 1986) correspond to the stricture in the gas phase. It is of importance for speciation of inorganic coordination compounds uranium with OH−, F−, NO3 −, SO4 2− or CO3 −, due to great reactivity of uranium in the gas phase and capability to form large number of stoichiometric and non-stoichiometric compounds. Same is valid for its reactivity in condense phase under experimental conditions (Ivanova and Spiteller 2014; Altmaier et al. 2013; Perry 2015).
Quantitative mass spectrometry: performance parameters
Since main efforts emphasized the great capability of mass spectrometry in its variety of ionization approaches, to provide precise analytical information for uranium speciation in environmental samples, in order to ensure a high reproducibility and acceptable accuracy toward real analytical objects (Perez-Bendito and Rubio 1999) as analytical standards, UVIO2(NO3)2·6H2O and UVIF6 salts are used. The choice based on their relatively poor and low-abundance mass spectrometric patterns ∈ m/z 810–200 under soft ionization MS conditions (Jennings et al. 1989; Downs and Gardner 1984; Beauchamp, 1976; Compton 1977; Kalinin et al. 2002). The second standard is involved to evaluate the data quality obtained by the first standard, because of the standards samples are especially important for speciation in the complex environmental matrixes. They must be as similar to the unknown sample as possible. The uranium speciation is very complex, involving migration kinetics and thermodynamics of the interstitial oxygen through the soil, water and air matrixes. Thus, the standardization procedure is a non-trivial one. The application of strictly stoichiometric uranium salts enables estimation of sampling bias, which appeared amount the most problematic component for assessment (Ivanova and Spiteller 2014. The UV-MALDI-MS measurements are taken employing the method of internal standard, where the high resolving power allowed to quantify the complex multicomponent mixtures. By contrast ESI-MS and APCI-MS method validation has been obtained, using low-abundance peak at m/z 283 (U+) common for both standards and those at m/z 270, 345 (UO2 +, U2O13N2 +, UVIO2(NO3)2·6H2O) and 276 (UF2 +, UF6), respectively.
Accuracy, precision, repeatability and reproducibility
The ultra-high resolving power UV-MALDI-MS data collection enable high quality of assess and measurements. The r 2 ∈ 0.99999–1 is obtained accounting for that the lowest boundary is only formally presented. The SD(yEr±) of three replicated measurements by MALDI-MS method of three sample with content SiO2/U2.33 × 0.6UO2.66 × xNaOH at x = 0.4, 0.8 and 1.2 is ∈ 0–5.77 × 10−6, while SD(yEr±) values—∈ 0–3.14 × 10−5, respectively (Table 2).
Concentration limits of detection and quantitation
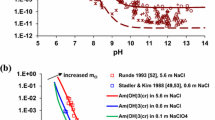
Concentration LODs (receptively LOQs) were defined as lowest determinable analyte concentration, statistically different from blank samples. Due to high complexity of environmental samples and the difficulty to obtain blanks from sources similar to given sample and in parallel without content of uranium analytes, the common practice to prepare the blanks as sole available choice, simulating the real environmental matrix, is carried out. The dynamic concentration range using as standard UO2(NO3)2·6H2O (Fig. 3; Table 3) revealed r 2 = 0.99985 and SD = 1.61 × 106. The data processing involved polynomial extrapolation of order 3 (ESI-MS, m/z 270). The r 2 = 0.99625 (SD = 1.30 × 107) is obtained by linear extrapolation ∈ 10.5–100.05 ppq concentration range. The concentration LODs of 0.08 ppq is achieved for all of analyte studied (LOQs, 0.16 ppq, Table 1).
Conclusion
In summary, firstly, we have reported in this paper uranium speciation of non-stoichiometric compositions and their adsorbed onto SiO2 surface, utilizing IR-spectroscopy and MALDI mass spectrometry, both qualitatively and qualitatively. The main methodological contributions to IR-spectroscopy consist of assignment of IR-patterns to the vibrational modes of non-stoichiometric compositions employing polarized spectroscopy. It, in fact, is a unique tool for an unambiguous experimental assignment of the vibrational modes to corresponding symmetry classes of molecular motions, thus allowing to study the corresponding chemical transformations, yielding to change of the molecular structure of the analyte along with corresponding polymorph modifications of the crystalline state of those non-stoichiometric compositions. The contribution to the quantitation procedure has determining the reliability level of IR-spectroscopy for determination of multicomponent mixtures of such as compositions. As it can be expected for a complex IR-profile, showing broad and overlapping IR-bands level of confidences decreases in r 2 = 0.98124. The methodological contributions to MALDI-MS have involved assignment of fragmentation patterns of each composition allowing their usage for qualitative purposes within the frame of MS-based spectrometric library along with the contribution to the quantitative analysis, developing a protocol for determination of analytes. Significant accuracy and level of confidence r 2 ∈ 0.99999–1 were obtained for SiO2/U2.33·0.6UO2·66xNaOH systems at x = 0.4, 0.8 and 1.2 (SD(yEr±) values ∈ 0–3.14 × 10−5), operating at concentration LODs 0.08 ppq. The theoretical modeling and experimental design is maximal representative to real environmental biogeochemical reactions of uranium sorption onto sediments. Because of marine geochemistry of uranium and its speciation in surface and groundwater with heterogeneous sediment–water interface is governed by reductive precipitation of UVI → UIV yielding usually non-stoichiometric uranium oxides. Such compositions have been often obtained under environmental conditions due to reductive precipitation of UVI coupled with oxidation process with participation of sulfides, thus giving corresponding polysulfides and sulfur. The co-precipitation affected the uranium migration and bioavailability, which, however, occurred in presence of oxide minerals or mineral phases such as silicates. Furthermore, precipitation of UVI → UIV depending on concentration of silicates in sediments caused formation UIV–silica colloids of coffinite type. In addition, the uranium distribution in sediments in environment near to nuclear waste storage is characterized with precipitation of silicates of uranophane type such ([Ca(UO2)2(SiO3OH)2(H2O)5]). Therefore, the analysis carried out is highly relevant to environmental radioanalytical and protection problematic.
Abbreviations
- APCI:
-
Atmospheric pressure chemical ionization (mass spectrometry)
- CHCA:
-
α-Cyano-4-hydroxycinnamic acid
- DHA:
-
2,4-Dihydroxy benzoic acid
- DHB:
-
2,5-Dihydroxy benzoic acid
- ESI:
-
Electrospray ionization (mass spectrometry)
- IR:
-
Infrared (spectroscopy)
- IR-LD:
-
Infrared spectroscopy with linear-dichroic irradiation
- LODs:
-
Limits of detection (concentration)
- LOQs:
-
Limit of quantitation (concentration)
- MALDI:
-
Matrix-assisted laser desorption/ionization (mass spectrometry)
- MS:
-
Mass spectrometry
- SD:
-
Standard deviation
- UV–VIS:
-
Ultraviolet–visible (spectroscopy)
References
Allen, G., & Tempest, P. (1986). Ordered defects in the oxides of uranium. In Proceedings of the Royal Society of London A: Mathematical, Physical and Engineering Sciences. Vol. 406A, pp. 325–344.
Altmaier, M., Gaona, X., & Fanghaenel, T. (2013). Recent advances in aqueous actinide chemistry and thermodynamics. Chemical Reviews, 113, 901–943.
Banerjee, K., Ligon, A., & Spiteller, M. (2006). Environmental fate of trifloxystrobin in soils of different geographical origins and photolytic degradation in water. Journal of Agriculture and Food Chemistry, 54, 9479–9487.
Batrakov, Y., & Puchkova, E. (2003). Effect of the LIII absorption edge of uranium on chemical shifts of the Lβ1 line of uranium in UO2+x. Radiochemistry, 45, 25327.
Beauchamp, J. (1976). Properties and reactions of uranium hexafluoride by ion cyclotron resonance spectroscopy. The Journal of Chemical Physics, 64, 718–723.
Beebe, K., Pell, R., & Seasholtz, M. (1998). Chemometrics: A practical guide (pp. 1–348). New York: Wiley.
Berthinier, C., Rado, C., Chatillon, C., & Hodaj, F. (2013). Thermodynamic assessment of oxygen diffusion in non-stoichiometric UO2±x from experimental data and Frenkel pair modeling. Journal of the Nuclear Materials, 433, 265–286.
Best, S., Clark, R., & Cooney, R. (1988). Infrared spectroscopic studies of aqueous solutions of dioxouranium(VI) and its hydrolysed products and of in situ electra-generated dioxouranium(V). Inorganica Chimica Acta, 145, 141–147.
Chandrasekaran, K., Karunasagar, D., & Arunachalam, J. (2011). Dispersive liquid–liquid micro extraction of uranium(VI) from groundwater and seawater samples and determination by inductively coupled plasma–optical emission spectrometry and flow injection–inductively coupled plasma mass spectrometry. Analytical Methods, 3, 2140–2147.
Chappaz, A., Gobeil, C., & Tessier, A. (2010). Controls on uranium distribution in lake sediments. Geochimica et Cosmochimica Acta, 2010(74), 203–214.
Chaumont, A., Klimchuk, O., Gaillard, C., Billard, I., Ouadi, A., Hennig, C., & Wipff, G. (2012). Perrhenate complexation by uranyl in traditional solvents and in ionic liquids: A joint molecular dynamics/spectroscopic study. The Journal of Physical Chemistry B, 116, 3205–3219.
Choi, J., Lamshoeft, M., Zuehlke, S., Park, K., Shim, J., & Spiteller, M. (2012). Determination of sedatives and adrenergic blockers in blood meal using accelerated solvent extraction and Orbitrap mass spectrometry. Journal of Chromatography A, 1260, 111–119.
Clark, D., Hobart, I., & Neda, M. (1995). Actinide carbonate complexes and their importance in actinide environmental chemistry. Chemical Reviews, 95, 25–48.
Compton, R. (1977). On the formation of positive and negative ions in gaseous UF6. The Journal of Chemical Physics, 66, 4478–4485.
Conradson, S., Manara, D., Wastin, F., Clark, D., Lander, G., Morales, L., et al. (2004). Local structure and charge distribution in the UO2–U4O9 system. Inorganic Chemistry, 43, 6922–6935.
Crawford, C., Fugate, G., Cable-Dunlap, P., Wall, N., Siems, W., & Hill, H. (2012). The novel analysis of uranyl compounds by electrospray-ion mobility-mass spectrometry,. doi:10.1016/j.ijms.2012.08.004.
Denning, R. (1992). Electronic structure and bonding in actinyl ions. In: Complexes, clusters and crystal chemistry. Structure and bonding (pp. 215–276). Heidelberg: Springer.
Denning, R. (2007). Electronic structure and bonding in actinyl ions and their analogs. The Journal of Physical Chemistry A, 111, 4125–4143.
Downs, A., & Gardner, C. (1984). Formation and characterization of uranium (VI) chloride fluorides, UFnCln (n = 1–5). Journal of the Chemical Society, Dalton Transactions, 1984, 2127–2132.
Dreissig, I., Weiss, S., Hennig, C., Bernhard, G., & Zaenker, H. (2011). Formation of uranium(IV)–silica colloids at near-neutral pH. Geochimica et Cosmochimica Acta, 75, 352–367.
Duff, M., Coughlin, J., & Hunter, D. (2002). Uranium co-precipitation with iron oxide minerals. Geochimica et Cosmochimica Acta, 66, 3533–3547.
Environmental Contamination from Uranium Production Facilities and their Remediation Proceedings of an International Workshop Lisbon, 11–13.02.2004, International Atomic Energy Agency, Wienn, 2005, pp. 1–262.
Euroatom Supply Agency—Annual report 2011, European Commission, Luxembourg, Publications Office of the European Union, 2012, pp. 1–43.
Frimmel, F., Abbt-Braun, G., Heumann, K., Hock, B., Luedeman, H., & Spiteller, M. (2002). Refractory organic substances in the environment, environmental chemistry. Weinheim: Wiley-VCH.
Gorshkov, N., Izosimov, I., Kazimov, A., Kolychev, S., Kudryashev, N., & Mashirov, L. (2001). The role of hydroxide ions in reduction of uranyl ion stimulated by nitrogen laser radiation (337.1 nm). Radiochemistry, 43, 3354–3359.
Gresham, G., Dinescu, A., Benson, M., Van Stipdonk, M., & Groenewold, G. (2011). Investigation of uranyl nitrate ion pairs complexed with amide ligands using electrospray ionization ion trap mass spectrometry and density functional theory. Journal of Physical Chemistry A, 115, 3497–3508.
Gu, B., Brooks, S., Roh, Y., & Jardine, P. (2003). Geochemical reactions and dynamics during titration of a contaminated groundwater with high uranium, aluminum, and calcium. Geochimica et Cosmochimica Acta, 67, 2749–2761.
Guerra, D., Silva, E., & Airoldi, C. (2010). Application of modified attapulgites as adsorbents for uranyl uptake from aqueous solution-Thermodynamic approach. Process Safety and Environmental Protection, 88, 53–61.
Guimbretiere, G., Desgranges, L., Canizares, A., Carlot, G., Caraballo, R., Jegou, C., Duval, F., Raimboux, N., Ammar, M., & Simon P. (2012). Determination of in-depth damaged profile by Raman line scan in a pre-cut He2+ irradiated UO2. Applied Physics Letters, 100, 251914.
Hall, D., Rea, A., & Waters, T. (1965). The crystal structure of bisnitratodiaquodioxo-uranium (VI) tetrahydrate (uranyl nitrate hexahydrate). Acta Crystallographica, 19, 389–395.
He, H., Qin, Z., & Shoesmith, D. (2010). Characterizing the relationship between hyperstoichiometry, defect structure and local corrosion kinetics of uranium dioxide. Electrochimica Acta, 56, 53–60.
He, H., Wang, P., Allred, D., Majewski, J., Wilkerson, M., & Rector, K. (2002). Characterization of chemical speciation in ultrathin uranium oxide layered films. Analytical Chemistry, 84, 10380–10387.
Hocking, H., Burggraf, L., Duan, X., Gardella, J, Jr, Yatzor, B., & Schuler, W. (2013). Composition of uranium oxide particles related to TOF-SIMS ion distributions. Surface and Interface Analysis, 45, 545–548.
Hubbard, H., & Griffiths, T. (1987). An investigation of defect structures in single-crystal UO 2+x by optical absorption spectroscopy. Journal of the Chemical Society, Faraday Transactions 2, 83, 1215–1227.
Ilton, E., Wang, Z., Boily, J., Qafoku, Q., Rosso, K., & Smith, S. (2012). The effect of pH and time on the extractability and speciation of uranium(VI) sorbed to SiO2. Environmental Science and Technology, 46, 6604–6611.
Ivanova, B., & Kolev, T. (2012). Linearly polarized IR spectroscopy: Theory and applications for structural analysis (pp. 1–240). Taylor and Francis Group LLC: CRC Press, Boca Raton, FL. ISBN 978-1-4398-2559-4.
Ivanova, B., & Spiteller, M. (2012a). Quantitative analysis of substituted N, N-dimethyl-tryptamines in the presence of natural type XII alkaloids. Natural Products Communications, 7, 1273–1276.
Ivanova, B., & Spiteller, M. (2012b). Matrix-assisted laser desorption/ionization mass spectrometric analysis of herbicides in dication-containing organic crystals. Analytical Methods, 4, 4360–4367.
Ivanova, B., & Spiteller, M. (2012c). A quantitative solid-state Raman spectroscopic method for control of fungicides. Analyst, 137, 3355–3364.
Ivanova, B., & Spiteller, M. (2014). Uranyl–water containing complexes—Solid-state UV-MALDI mass spectrometric and IR spectroscopic approach for selective quantitation. Environmental Science and Pollution Research, 21, 1163–1177.
Jennings, K., Kemp, T., & Read, P. (1989). Cluster formation in the fast atom bombardment (FAB) mass spectra of dioxouranium(VI) dinitrate and diacetate. Inorganica Chimica Acta, 157, 157–159.
Jung, H., Boyanov, M., Konishi, H., Sun, Y., Mishra, B., Kemner, K., et al. (2012). Redox behavior of uranium at the nanoporous aluminum oxide-water interface: implications for uranium remediation. Environmental Science and Technology, 46, 7301–7309.
Kalinin, B., Atanov, V., & Aleksandrov, O. (2002). Metastable ions in the mass spectrum of uranium hexafluoride. Technical Physics, 47, 648–650.
Kalkowski G., Kaindl G., Brewer W., & Krone W. (1987). Near-edge x-ray-absorption fine structure in uranium compounds. Physical Review B, 35, 2667–2677.
Kelley, C. (1999). Iterative methods for optimization. Society for Industrial and Applied Mathematics, 18, 1–188.
Klinkhammer, G., & Palmers, L. (1991). Uranium in the oceans: Where it goes and why. Geochimica et Cosmochimica Acta, 55, 1799–1806.
Knope, K., & Soderholm, L. (2013). Solution and solid-state structural chemistry of actinide hydrates and their hydrolysis and condensation products. Chemical Reviews, 113, 944–994.
Koleva, B., Kolev, T., Simeonov, V., Spassov, T., & Spiteller, M. (2008). Linearly polarized IR-spectroscopy of partially oriented solids as a colloid suspension in nematic host: A tool for spectroscopic and structural elucidation of the embedded chemicals. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 61, 319–333.
Kulyukhin, S., & Kamenskaya, A. (2010). Decomposition of uranyl nitrate in the silica gel matrix under the action of microwave radiation. Radiochemistry, 52, 237–244.
Kusari, S., Deivasigamani, P., Lamshoeft, M., & Spiteller, M. (2009). In vitro residual anti-bacterial activity of difloxacin, sarafloxacin and their photoproducts after photolysis in water. Environmental Pollution, 157, 2722–2730.
Lamshoeft, M., Grobe, N., & Spiteller, M. (2011). Picomolar concentrations of morphine in human urine determined by dansyl derivatization and liquid chromatography-mass spectrometry. Journal of Chromatography B, 879, 933–937.
Lee, C., Wang, S., & Lii, K. (2009). Cs2K(UO)2Si4O12: A mixed-valence uranium(IV, V) silicate. Journal of the American Chemical Society, 131, 15116–15117.
Lesher, E., Honeyman, B., & Ranville, J. (2013). Detection and characterization of uranium-humic complexes during 1D transport studies. Geochimica et Cosmochimica Acta, 109, 127–142.
Liang, B., Hunt, R., Kushto, G., Andrews, L., Li, J., Wiley, W., & Bursten, B. (2005). Reactions of laser-ablated uranium atoms with H2O in excess argon: A matrix infrared and relativistic DFT investigation of uranium oxyhydrides. Inorganic Chemistry, 55, 2159–2168.
Lotnik, S., Khamidullina, L., & Kazakov, V. (2001). Formation of excited uranyl in oxidation of U(IV) with oxygen in HClO4 aqueous solutions: I. Effect of pH on kinetic and chemiluminescence parameters of the reaction. Radiochemistry, 43, 51355.
Madsen, K., Nielsen, H., & Tingleff, O. (2004). Informatics and mathematical modelling (2nd ed.). Copenhagen: DTU Press.
Malaviya, P., & Singh, A. (2012). Phytoremediation strategies for remediation of uranium-contaminated environments: A review. Critical Reviews in Environmental Science and Technology, 42, 2575–2647.
Malliakas, C., Yao, J., Wells, D., Jin, G., Skanthakumar, S., Choi, E., et al. (2012). Oxidation state of uranium in A6Cu12U2S15 (A = K, Rb, Cs) compounds. Inorganic Chemistry, 51, 6153–6163.
Massart, D., Vandeginste, B., Deming, S., Michotte, Y., & Kaufman, L. (1988). Chemometrics, 2 (pp. 1–488). Amsterdam: Elsevier.
Mayer, K., Wallenius, M., & Varga, Z. (2013). Nuclear forensic science: Correlating measurable material parameters to the history of nuclear material. Chemical Reviews, 113, 884–900.
Meinrath, G., Lis, S., Strylad, Z., & Noubactep, C. (2000). Lifetime and fluorescence quantum yield of uranium(VI) species in hydrolyzed solutions. Journal of Alloys and Compounds, 300–301, 107–112.
Miller, J. (2000). Statistics and chemometrics for analytical chemistry (4th ed., pp. 1–271). Essex: Prentice Hill.
Moore, E., Gueneau, C., & Crocombette, J. (2013). Diffusion model of the non-stoichiometric uranium dioxide. Journal of Solid State Chemistry, 203, 145–153.
Mueller, K., Foerstendorf, H., Tsushima, S., Brendler, V., & Bernhard, G. (2009). Direct spectroscopic characterization of aqueous actinyl(VI) species: A comparative study of Np and U. The Journal of Physical Chemistry A, 113, 6626–6632.
Nagaishi, R., Kimura, T., Inagawa, J., & Kato, Y. (1998). Isotope and temperature effects on photochemical reactions of uranyl ion in H O–DO mixtures. Journal of Alloys and Compounds, 271–273, 794–798.
Pajo, L., Tamborini, G., Mayer, K., & Koch, L. (2001). Use of thermal ionization mass spectrometry for measuring the oxygen isotope ratio in uranium oxides1, 2. Radiochemistry, 43, 453–454.
Park, Y., Sakai, Y., Abe, R., Ishii, T., Harada, M., Kojima, T., & Tomiyasu, H. (1990). Deactivation mechanism of excited uranium(V1) complexes in aqueous solutions. Journal of the Chemical Society, Faraday Transactions, 86, 55–60.
Perez-Bendito, D., & Rubio, S. (1999). Environmental analytical chemistry. In S. Weber (Ed.), Wilson and Wilson’s comprehensive analytical chemistry (Vol. XXXII, pp. 1–842). Amsterdam: Elsevier Science B.V.
Perry, D. (2015). The tris(carbonato)dioxouranium(VI) ion: A structural model for uranium 4f7/2, 5/2 X-ray photoelectron spectra satellite structures for oxide and oxygen coordination cores. Vacuum, 114, 162–165.
Petiau, J., Calas, G., Petitmaire, D., Bianconi, A., Benfatto, M., & Marcelli, M. (1986). Delocalized versus localized unoccupied 5f states and the uranium site structure in uranium oxides and glasses probed by X-ray-absorption near-edge structure. Physical Review B, 34, 7350–7361.
Qiu, J., & Burns, P. (2013). Clusters of actinides with oxide, peroxide, or hydroxide bridges. Chemical Reviews, 113, 1097–1120.
Ray, A., Bargar, J., Sivaswamy, V., Dohnalkova, A., Fujita, Y., Peyton, B., & Magnuson, T. (2011). Evidence for multiple modes of uranium immobilization by an anaerobic bacterium. Geochimica et Cosmochimica Acta, 75, 2684–2695.
Rios, D., Michelini, M., Lucena, A., Marcalo, J., Bray, T., & Gibson, J. (2012). Gas-phase uranyl, neptunyl, and plutonyl: hydration and oxidation studied by experiment and theory. Inorganic Chemistry, 51, 6603–6614.
Rutkowski, P., Rios, D., Gibson, J., & Van Stipdonk, M. (2011). Gas-phase coordination complexes of UVIO2 2+, NpVIO2 2+, and PuVIO2 2+ with dimethylformamide. Journal of the American Society for Mass Spectrometry, 22, 2042–2048.
Schoenes, J. (1987). Recent Spectroscopic Studies of UO2. Journal of the Chemical Society, Faraday Transactions 2, 83, 1205–1213.
Scott, T., Tort, O., & Allen, G. (2007). Aqueous uptake of uranium onto pyrite surfaces; reactivity of fresh versus weathered material. Geochimica et Cosmochimica Acta, 71, 5044–5053.
Smejkalova, S., Piccolo, A., & Spiteller, M. (2006). Oligomerization of humic phenolic monomers by oxidative coupling under biomimetic catalysis. Environmental Science and Technology, 40, 6955–6962.
Spiteller, M. (1985). Extraction of soil organic matter by supercritical fluids. Organic Geochemistry, 8, 111–113.
Spiteller, M. (1987). Isolation and characterisation of dissolved organic carbon from natural and lysimeter waters by ultrafiltration. Science of the Total Environment, 62, 47–54.
Spiteller, M. (2012). Highlights of morphine research with prof. M. Zenk. Pharmaceutical Biology, 50, 616.
Spiteller, M., & Saiz-Jimenez, C. (1990). A two step degradative procedure for structural studies of aquatic humic acids Org. Geochemistry, 15, 449–455.
Steppert, M., Walther, C., Fuss, M., & Buchner, S. (2012). On the polymerization of hexavalent uranium. An electrospray mass spectrometry study. Rapid Communications in Mass Spectrometry, 26, 583–591.
Stoliker, D., Kent, D., & Zachara, J. (2011). Quantifying differences in the impact of variable chemistry on equilibrium uranium(VI) adsorption properties of aquifer sediments. Environmental Science and Technology, 45, 8733–8740.
Sukul, P., Zuehlke, S., Lamshoeft, M., Rosales-Conrado, N., & Spiteller, M. (2010). Dissipation and metabolism of 14C-spiroxamine in soil under laboratory condition. Environmental Pollution, 158, 1542–1550.
Sun, Y., Li, J., & Wang, X. (2014). The retention of uranium and europium onto sepiolite investigated by macroscopic, spectroscopic and modeling techniques. Geochimica et Cosmochimica Acta, 140, 621–643.
Sundararajan, M., Rajaraman, G., & Ghosh, S. (2011). Speciation of uranyl ions in fulvic acid and humic acid: a DFT exploration. Physical Chemistry Chemical Physics: PCCP, 13, 18038–18046.
Um, W., Icenhower, J., Brown, C., Serne, R., Wang, Z., Dodge, C., & Francis, A. (2010). Characterization of uranium-contaminated sediments from beneath a nuclear waste storage tank from Hanford, Washington: Implications for contaminant transport and fate. Geochimica et Cosmochimica Acta, 74, 1363–1380.
Van Horn, J., & Huang, H. (2006). Uranium(VI) bio-coordination chemistry from biochemical, solution and protein structural data. Coordination Chemistry Reviews, 250, 765–775.
Walton, S., & Mitchell, D. (2013). A novel rapid detection approach for the analysis of radionuclides in environmental samples using graphite MALDI mass spectrometry. Journal of Radioanalytical and Nuclear Chemistry,. doi:10.1007/s10967-012-2176-1.
Wick, F., & McDowell, L. (1918). A preliminary study of the luminescence of the uranyl salts under cathode ray excitation. Physical Review, 11, 421–429.
Winkler, J., & Gray, H. (2012). Electronic structures of oxo-metal ions. Structure and Bonding, 142, 17–28.
Yakub, E. (2014). Molecular dynamics simulation of fast processes in non-stoichiometric ionic solids. High Temperatures High Pressures, 43, 87–99.
Yusov, A., & Shilov, V. (2007). Interaction of U(VI), Np(VI), and Pu(VI) ions with unsaturated heteropolytungstates of the 17th and 11th series in aqueous solutions. Radiochemistry, 49, 144–151.
Acknowledgments
The authors thank Deutscher Akademischer Austausch Dienst, the Deutsche Forschungsgemeinschaft, the central instrumental laboratories for structural analysis at Dortmund University (Germany) and the analytical and computational laboratory cluster at Institute of Environmental Research at the same University. Conflict of interests: Michael Spiteller has received research grants (Deutsche Forschungsgemeinschaft, 255/22-1, 1315); Bojidarka Ivanova has received research grant (Deutsche Forschungsgemeinschaft, 255/22-1).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10653_2015_9752_MOESM1_ESM.doc
Supplementary material 1 Conventional and polarized IR-LD analysis (Fig. S1–S5,S7,S8, Table S2); Thermodynamics (Table S1); Experimental MS data (Fig. S6); Quantum chemical Z-matrix and thermodynamics; Computational details with relating references (DOC 3972 kb)
Rights and permissions
About this article
Cite this article
Ivanova, B., Spiteller, M. Environmental modeling of uranium interstitial compositions of non-stoichiometric oxides: experimental and theoretical analysis. Environ Geochem Health 38, 1051–1066 (2016). https://doi.org/10.1007/s10653-015-9752-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9752-6