Abstract
Environmental geochemistry classifies elements into essential, non-essential and toxic elements in relationship to human health. To assess the environmental impact of mining at Datoko-Shega area, the distributions and concentrations of trace elements in stream sediments and soil samples were carried out. X-ray fluorescence analytical technique was used to measure the major and trace element concentrations in sediments and modified fire assay absorption spectrometry in soils. The results showed general depletion of major elements except titanium oxide (TiO2) compared to the average crustal concentrations. The retention of TiO2 at the near surface environment probably was due to the intense tropical weathering accompanied by the removal of fine sediments and soil fractions during the harmattan season by the dry north-east trade winds and sheet wash deposits formed after flash floods. The results also showed extreme contamination of selenium (Se), cadmium (Cd) and mercury (Hg), plus strong contaminations of arsenic (As) and chromium (Cr) in addition to moderate contamination of lead (Pb) in the trace element samples relative to crustal averages in the upper continental crust. However Hg, Pb and Cd concentrations tend to be high around the artisanal workings. It was recognised from the analysis of the results that the artisanal mining activity harnessed and introduces some potentially toxic elements such as Hg, Cd and Pb mostly in the artisan mine sites. But the interpretation of the trace element data thus invalidates the elevation of As concentrations to be from the mine operations. It consequently noticed As values in the mine-impacted areas to be similar or sometimes lower than As values in areas outside the mine sites from the stream sediment results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Composition of minerals that occur in local rocks influences the elemental diversity in the environment as the landscape evolves. The concentrations of major and trace elements in the environment are caused by geogenic and anthropogenic contaminations. Secondly, among the elements in the environment, some are essential and others are toxic whose concentrations, distributions and redistributions may be through natural and/or human activities. Knowing the elemental concentrations and mode of their distributions in the surface environments may enrich our understanding of the numerous primary health issues particularly in the mining regions. There are several reports that show the effects of trace elements on human health (Selinus et al. 2004). Their impact on health depends on trace elements excesses and deficiencies relative to accepted baseline values. The chemical contents of the trace elements can be so tiny, but the associated human health risks depending on the degree of exposure, role of speciation, route of exposure and length of exposure can be detrimental (Selinus et al. (2004). Sulphide-rich rocks have an association with chalcophile minerals (pyrite (FeS), arsenopyrite (AsFeS), chalcopyrite (CuFeS2), galena (PbS) and sphalerite [(ZnFe)S] which after weathering releases both essential and potentially toxic elements (PTE) to the environment. The study area falls into the Birimian of Ghana and has similar geology to the world class AngloGold Ashanti mine at Obuasi in Ghana where high As and Hg contaminations have been reported to occur in stream sediments, soils and water bodies (Smedley 1996; Smedley et al. 1996; Foli et al. 2012). The favourability of the underlying geology for the study area to host potential gold (Au) mineralisation has resulted in several artisanal mine workings in the area. Therefore, to identify chemical variations of trace elements that may have implications on human and animal health this paper:
-
1.
Assessed the levels of trace element contaminations due to artisan mining with respect to natural background value prior to mining
-
2.
Established spatial geochemical maps that highlight environments that need epidemiological studies for possible health issues due to toxicities and deficiencies of trace elements.
Location, geology, physiography and regolith of the study area
Location and geology
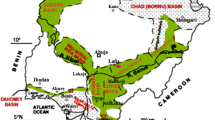
Datoko-Shega is a village located in the Bole–Navrongo–Nangodi Birimian gold belt, one of the seven Greenstone belts in Ghana (Kesse 1985; Griffis et al. 2002; Fig. 1). It is 600 km north of Kumasi and 850 km from Accra the national capital. Datoko-Shega is about 45 km southeast of Bolgatanga the capital of the Upper East Region and forms part of a newly created Talensi district, formerly part of Talensi-Nabdam district. Mafic Birimian volcanic rocks comprising basalt, andesite, dacite, etc., and Birimian volcano-sedimentary rocks such as amphibole schist, biotite–hornblende schist, sericite schist and tuff underlie the area (Milési et al. 1989; Fig. 1).
Physiography
The climatic regime of the study area is dry savannah with annual rainfall of about 600–1200 mm (Webber 1996). The area has a single rainy season. The monthly rainfall totals increase slowly from March and peak in August after which there is a sharp decrease after October (Dickson 1972). The average rainfall is about 60–100 mm per month, and temperatures can vary between 22 °C at night and >40 °C during the day. The mean annual temperature is 35 °C with the maximum temperature in excess of 40 °C usually recorded around March to April. Lowest temperatures of ≤22 °C occur between November and January due to the influence of the north-easterly winds known as Harmattan. Relative humidity is between 70 and 90 % during the month of May and October but can be as low as 20 % during the long dry season (Webber 1996).
The general stream pattern is dendritic with the major streams in draining north–south whilst the 2nd order streams drains westerly towards the main north–south stream. The stream systems carry most of the weathered materials as well as elements released to the environment by weathering processes.
Regolith of the study area
The area is characterised by deeply weathered profiles consisting of residual and transported cover materials. The two regolith types are spatially discontinuous and have subunits that are classified genetically based on the mode of formations. Those formed residually from the weathered rocks are classified as relict, and those formed from redistributed sediments that deposit over bedrocks different from bedrocks of source exotic sediments are considered as depositional regolith. Areas where the pre-existing preserved top layers are partly eroded or fully eroded that shows the mottled zone, plasmic zone, saprolite and bedrock, etc., at surface are referred to as erosional regolith. Patches of ferruginous materials of unknown origin are not uncommon. These regolith units are formed as a result of disintegration and decomposition of underlying rocks, secondary re-cementation of both residual weathered materials and exotic sediments overlying the coherent bedrock by Fe-oxides and clay minerals (Arhin and Nude 2009; Anand 2001). Depending on the source of materials undergoing re-cementation, the regolith may exhibit residual or transported regolith characteristics. Any of the regolith domains may have influence on the element transports in the environment, and hence, the trace element concentrations will depend on geochemical and physical processes acting at the place.
Testing small-scale mine area and the surrounding environ for geochemical variability
Underlying rocks are made up of different minerals that weather and liberate varying minerals and elements at different weathering fronts. The mobilisation of the minerals and elements will depend on the chemical and physical environments. Some of the minerals may be stable in the reducing environment, while others may transform to secondary minerals and major, minor and trace elements in the oxidised zones. From Kesse (1985), the metavolcanic and the Tarkwaian bedrocks host varieties of gold deposits that have an association with sulphide minerals (pyrite (FeS2), chalcopyrite (CuFeS2), arsenopyrite (AsFeS), sphalerite [ZnFe)S] and galena (PbS) that are widely distributed in the Birimian rocks. The sulphide minerals stable in the reducing environments will be unstable in the oxidised environments and will release the contained elements such as iron (Fe), copper (Cu), arsenic (As), zinc (Zn) and lead (Pb) in the surface environments. Even not considering the bulk geochemistry of the rocks in the Birimian system, the sulphide minerals may contain these main elements Ag, As, Cu, Zn, Fe, Pb, Cd, S, Hg and Te. Some of these elements Pb, As, Hg and Cd are established PTE. Copper (Cu), Zn and Fe are known essential elements for human and animal growth, but their usefulness still depend on dose. The mining operations enhance weathering and also introduce foreign elements/chemicals into the environment during metal extraction. The concern now is all trace elements in general are toxic to plants and animals if present in the soil in concentrations appreciably in excess of the normal or average. In some cases (e.g. boron, copper, fluorine, molybdenum and selenium), their toxic effects appear even with relatively low concentrations. These factors, together with the large number of trace elements and their complicated functions in biological processes, and the difficulties in identifying deficiency or toxicity symptoms, if not severe, make the correction of trace element problems often laborious and time-consuming and in cases of toxicity even risky. For instance, normal soils inherit their trace elements primarily from the rocks through geochemical and pedochemical weathering processes to which the soil forming materials have been subjected. In addition to the natural trace elements sources emanating from the underlying rocks, there are also secondary sources obtained from products of plants and animals decays, as well as trace elements from materials from the atmosphere, fertilisers, insecticides and fungicides.
The variable nature of underlying rocks and minerals lends their trace element composition to the surface regolith during weathering and soil formation processes. In areas where there has not been heterogeneous mixing of weathered products, trace element contents in the surface soils reflect the composition of the materials from which they have been derived, as has been shown by several authors (Mitchel 1963; Wells 1960). But the element concentrations between trace element in soil and in its parent material may vary in terms of element values for satisfactory quantitative estimation of the former from the latter (Oertel 1961). This may be common in complex regolith environment like Datoko-Shega area where flash floods leading to sheet wash deposits enhancing mixing of heterogeneous parent materials. The complexity of the regolith due to the climatic and other factors predominating during the process of soil formation coupled with the type and intensity of weathering and the artisanal mine operations may have local influence on the trace elements concentrations and distributions. For instance, sediment transport carries along secondary minerals and elements that may either dilute or enhance trace elements concentrations relative to the average crustal abundance. The depletion and enrichments of the trace elements may impact on the local ecology and human health, but knowledge of their relative distributions and concentrations has their merits and demerits.
Additionally, landscape evolution processes produces surface environments where the surface materials or regolith tends to have geochemistry different from the underlying rocks. This explains why soils and sediments serve as both sources and sinks of PTE and essential elements. The known occurrence of chalcophile minerals in the Birimian of Ghana (Kesse 1985) suggests the opportunity for release of Ag, As, Cd, Cu, Hg, Pb, S, Te and Zn. The liberation of these elements in the near surface and surface environments may have different geochemical levels depending on the underlying geology, type and intensity of weathering, climatic conditions, pedochemical processes and other factors predominating at the environment during the process of soil formation. Elements introduced to the soil and sediment environment may be immobilised, for example, by sorption and complexation with iron and manganese oxides as well as organic/humic substances, but some may still go into solution and can be mobilised to distant places. The elements mobility in the oxidised environments is, however, dependent on a number of geochemical conditions such as pH and the prevailing redox conditions. Datoko-Shega area is characterised by savannah climate and exhibits deep weathering characteristics. But with a single and short rainy season and a long dry season as indicated by Dickson (1972), physical weathering will predominate. This implies that underlying rocks and mineralisation may generally create an opportunity for the release of the trace elements. The released trace elements and weathered materials will be transported physically by flash flood and wind in addition to chemical mobilisation and leaching of trace elements based on the environmental conditions.
Apparently, most known attention paid to the artisanal mining environments only relate to the land degradation and the associated pollutions of water. There is no reported evidence that reveal work done in the area to show empirically the concentrations of trace elements particularly for PTE as has been identified to occur in similar geological terrain in Obuasi-AngloGold Ashanti mine in southern Ghana (Boateng et al. 2012; Foli et al. 2012). The concentrations and mode of occurrences of the PTE’s and the deficiencies of essential elements are the most important issues as the excess of the PTE’s and the deficiencies of the essential elements will have implications on human health.
Sampling and methodology
During the fieldwork, ten stream sediment samples and 54 soil samples were collected. The stream sediment survey devised for the regional concentrations of multi-elements across the landscape of the Datoko-Shega environments was taken from sediments of streams draining the artisanal mine sites and areas farther away from the mine operations. The samples were collected specifically at 10–20 m off confluences, at bends, behind rock bars and meandering sites of streams. To obtain representative samples, 5–8 scoops of sediment across the stream channel were collected after removing the organic and plant debris forming the top layer. Dead tree twigs and oversize quartz and lithic fragments of about >4 cm were removed from the collected samples. Regional stream sediment anomalies were delineated from the stream sediment survey, which form the basis of the soil survey. The enriched and deficient areas of some trace elements (e.g. Pb, Cd, As, Cu Zn) detrimental to human health on the account of dose–response ratios were subjected to geochemical soil survey. A circular hole of 30-cm nominal diameter dimension was dug up to 20 cm depth. A sample was collected from top to bottom from each of the 54 holes dug. Thousand grams of sample was collected from the hole with no considerations to the regolith types. The 54 samples were sieved to <125 µm size fractions after which FAAS analytical technique was performed on the sieved samples to analyse for only 5.0 elements. The 5.0 elements were selected for further studies after the appraisal of the stream sediment survey which showed elevated values compared with the crustal averages. Normally, environmental trace element samples against threats to human health generally are obtained from <2 mm sieve size samples, but this work used <125 µm where metal elements tend to concentrate most. This implies that elemental concentrations in the current work will have higher metal contents as a result of the collection method relative to the standard sample preparation protocol of sieving up to <2 mm particle size. Though there are no guideline values for stream sediments, the study included stream sediments survey because of the ephemeral nature of streams in northern Ghana where sediments are exposed to the atmosphere for most part of the year. This field soil survey covered three small-scale mining sites labelled mine sites A, B and C (Fig. 1), Au extraction sites 1 and 2, and some agricultural and non-agricultural areas. Sampling information such as soil type, lithology of sampling environment and possible weathering and geomorphic histories was recorded. The data collected during the soil survey allowed documentation and comparison of measured elements in areas where mining activities occurred in the past with average continental crustal values.
The collected soil samples were sun-dried and later reduced by sieving to <125 µm fine fraction. Information such as the percentages of fragment lithology and general description of the sample whether clayey, silt or sandy was recorded. The sieved and logged samples were then sent to a commercial laboratory for FAAS analysis.
Laboratory analysis of sediments and soils
The trace elements and major oxides concentrations in sediments and soils were measured by X-ray fluorescence (XRF) and fire assay with atomic absorption (FAAS) techniques, respectively. In determining the total trace element concentration in the sediments, seven (7) grams of the milled powder was placed into a small plastic beaker and then weighed using a beam balance. Pressed pellets were formed from the powdered mixture. The pellets were put in XRF machine which takes 20 samples at a time. This machine was connected to a computer with the Spectro X-Lab software that records the elements analysed. The soil samples, however, were analysed using modified FAAS whose detailed method description is documented by Apea (2012) and Loon (1980).
Results
The results of the major and trace elements geochemistry from the stream sediments and soils collected are presented in Tables 1 and 2. The tables show the crustal averages and soil guideline values for some PTE by Inter-Departmental Committee for the Redevelopment of Contaminated lands (ICRCL) and United States Environmental Protection Agency (USEPA) and published concentrations of elements in stream sediments in a similar environment in West Africa.
Element enrichment factors (EFs) were calculated in order to assess the extent of enrichment and /or depletion of trace elements in sediments and soils relative to their crustal concentrations. The average upper continental crust concentrations (Bn) of the elements were used as baseline or background values, and the enrichment factor (EF) was calculated by dividing the mean element concentrations (Cn) by the average continental crustal values. The EF for some of the elements is presented in Tables 1 and 2 for stream sediments and soils, respectively.
Indices of geoaccumulations (Igeo) were also computed following Muller (1969) method to estimate the enrichment of the elemental concentrations above and below the background values. His classification as to the severity of pollution using seven enrichment classes based on an increase in the numerical value of the scale is presented in Table 3. The last columns of Tables 1 and 2 labelled Igeo show the index of geoaccumulations in stream sediments and soil samples. The geoaccumulation index is calculated as:
where Cn is the concentration of the element measured in a sample and Bn is the average crustal value, while 1.5 is a constant which is introduced to minimise the effect of the variation of background values.
Pearson correlation coefficient test was performed also on the stream sediment and soil samples to obtain relationship between the elements in the geosphere. Results obtained are presented in Figs. 2 and 3, respectively. Spatial maps showing locations and geochemical levels of some of the PTE as well as some essential elements are shown in Figs. 4, 5 and 6 for stream sediments and Figs. 7, 8, 9 and 10 for soils. The overlap map of four contaminated toxic elements As, Hg, Cd and Pb obtained from stream sediment samples showing pollution intensities is shown in Fig. 10. The extremely polluted areas for all the toxic elements is shown red and grades to deep green for environments of one or none of the established toxic elements.
Discussions
Major element geochemistry
From Table 1, SiO2, Al2O3 and Fe2O3 show depletion in element concentrations except TiO2 that showed no change with the average crustal abundances. Similarly, mean concentrations of magnesium oxide (MgO) calcium oxide, (CaO), potassium oxide (K2O) and sodium oxide (Na2O), except manganese oxide (MnO), were all lower than their average crustal values. The depletion of most resistant oxides such as the silicon oxide (SiO2), aluminium oxide (Al2O3) and iron oxide (Fe2O3) in the tropical savannah region of northern Ghana may be attributed to the deep weathering conditions enhanced by climate and environmental conditions. The depletion of these oxides, especially Fe-oxide, may affect human health because Fe-oxide can decrease the bioavailability and toxicity of water-borne metals, and Fe is essential for most animal species. It is the central unit in haemoglobin molecules and plays a key role in many physiological reactions, including oxygen exchange. Lack of it in humans lead to anaemia; hence, the depleted amount as shown in Table 1 implies limited quantity of Fe will be available to the people via the food chain which are produced locally. Likewise, depletion of aluminium and alkalis in sediments and soil samples analysed was lower relative to the upper continental crustal abundances and this may be attributed to the maturity of the chemical weathering process, which involves the progressive loss of alkalis and other major oxides [e.g. (SiO2, Al2O3 and Fe2O3)] as shown in Tables 1 and 2. Conversely, TiO2 and MnO maintained the global average values of the continental crust, while CaO, K2O, Na2O, P2O5, MgO and SO3 were transformed and moved into solution during weathering, erosion and deposition controlled generally by groundwater and surface waters.
Essential and potentially toxic elements geochemistry
The trace elements geochemistry obtained in the study were classified into essential and PTE. The results as presented in Table 1 showed essential elements V, Cr, Co and Se to have mean concentrations to be above the average crustal concentrations, whereas Ni, Cu, Zn and Mo recorded mean concentrations lower than the average crustal abundances. The PTE’s in the sediment samples had As, Cd and Hg mean concentrations exceeding the average crustal values (Figs. 4, 5), whereas the mean Pb concentration was lower than its crustal abundance in sediment samples (Fig. 5). Conversely, Pb in soils has concentrations in excess of the average crustal value (Fig. 7). The five (5) elements analysed in the soil samples consisted of three essential elements (Zn, Cu and Mn) and two established PTE’s Cd and Pb. The revelation from Table 3 showed the two (2) PTE’s Cd and Pb recorded mean concentrations far above the crustal abundances. This is shown spatially also in Figs. 7 and 8. The three (3) essential elements have low mean concentrations compared with the crustal averages (Figs. 9, 10). The testament is the essential elements that support life appear to be deficient, whereas the hazardous elements to human and animal life seem to be above the crustal concentrations in sediments and soils where their livelihood will depend.
From the enrichment ratios (EF) presented in Tables 1 and 2, the essential and toxic elements that showed an enrichment ranging from >1 to 14 are Co, V, I, W, Cd, Cr, As, Hg and Se. Among these elements, Cr, As, Se and Hg in stream sediments have greater than fivefold enrichments compared with the crustal averages and those in soils with elemental excesses are Cd and Pb. Meanwhile, there is a hidden flaw that has been noticed by Reimann and de Caritat (2000) in the use of EF in identifying and quantifying human interference with global element cycles. The study consent to their notion because the natural fractionation of elements during the elements transfer from the crust to the near surface environment through processes such as weathering, erosion, deposition and local geochemical and anthropogenic processes are not incorporated in the background value estimation. The EF computed in the current study only shows theoretically the elements excesses and deficiencies in the environment with respect to the average crustal concentrations. From Tables 1 and 2, the enrichment factor, in particular, for Pb varies for the two media analysed for the essential and toxic elements, while over seven enrichment ratios were obtained for Pb in soils and only 0.33 EF was obtained for the sediment samples. The enrichment ratios in secondary environment samples do not provide hints about the source of the pollutants but just indicate the variation of a particular element to the background crustal averages at a place. Establishing background values at the study area was very challenging because of the general land usage; the scattered artisan mine operations and agricultural activities. The 0.33 EF for Pb in sediments could mean that there are trace amount of Pb in the underlying rocks or Pb has desorb from the chalcophile mineral and has been weathered and transformed to a secondary mineral. The sevenfold Pb enrichment perhaps can be an attribute of the anthropogenic contamination from the artisan mines and fungicides and pesticides use by the mine workers since sediments in streams draining the study area recorded Pb values below the average crustal value. The high concentrated Pb occurs at mine sites A and B and also at the gold extraction sites 1 and 2 with Pb values ranging from 146 to 303 ppm. Trace element samples collected upstream and downstream of mine sites A and B returned insignificant Pb contents below the average crustal abundance (Fig. 7) with the elevated Pb values occurring either at the mine sites or at the gold extraction sites. The NE trade winds that bring Harmattan dust from the north-eastern side of the study area did not influence Pb concentrations outside the mine and the gold extraction sites. This suggests that Pb contents in soils in the artisanal mining sites were introduced by the mine operators. The calculated geoaccumulation classes predicting the pollution intensities using Table 3 designed by Muller (1969) show different pollution intensities for Pb in sediments and in soils. Concentration of Pb in soils showed moderate pollutions (Table 4), but the same element was recognised to be depleted in stream sediments which may probably be due to erosion mechanisms as well as the chemical and hydraulic processes in the streams. The depletion of Pb in sediments but enrichment in soils suggests that Pb could be an attribute of the mining operations or may be from fungicide use. Another element of similar concern like Pb is Cd. It also has varying geochemical levels in sediments and in soils of which mean levels in both media exceeds the average crustal abundances. The EF of about 8.0 and 33.0 was obtained for sediments and soils and, respectively, showed strong and extreme pollutions (Table 4). The geochemical pattern for Cd is similar to Pb. The two elements have elevated values at the mine sites A and B as well as the gold extraction sites 1 and 2. The exception between the two elements is that there are some isolated Cd high areas outside the artisan mine workings (Fig. 8).This is shown in both sediments and soil samples. Pb on the contrary has high Pb levels in soils compared with average crustal values at the mine working areas in soils but depleted in stream sediments (Figs. 4, 5 and Figs. 7, 8). Also from Fig. 8, Cd is polluted mostly at the mine areas at the centre, south-eastern and north-western sections of the mine sites with concentrations of 5–15 ppm. However, the average upper continental crustal concentration of Cd is 0.11 ppm (Taylor and McLennan 1985). The minimum and maximum concentrations from the soil results showed Cd values of 0.06 and 14.1 ppm (Table 3). As shown in Fig. 4, it is only one point out of the ten samples collected in the stream sediment samples that had Cd content equalled or close to the background value calculated by Taylor and McLennan (1985). This, thus, confirms the extreme contamination of Cd computed for elements geoaccumulation in soils and presented in Table 4. Also from Fig. 4 using the Cd crustal concentration value as the geochemical baseline, the entire area can be marked out as polluted. This again confirms the strong contamination of Cd in stream sediments shown in Table 4. There is a positive and strong correlation of 0.9 between Cd and Pb in soil samples (Fig. 3) but weak correlation of 0.3 between the same elements in stream sediments (Fig. 2). Figures 7 and 8 show the coincidence of extreme pollutions of Cd and Pb at the artisanal working areas. Lead (Pb) concentrations in sediments fall far below the Pb crustal concentration of 70 ppm. The median for Pb in the stream sediment samples is 4.7 and suggests probable absence of Pb in the underlying rocks. It is also possible to attribute the Cd contamination in the area to both natural and anthropogenic sources, but this requires further studies.
Moreover, as noticed from Table 1, As, Hg and Se have EF of approximately 14, 11 and 14, respectively. Hg and As are known PTE’s to humans, and depending on the dose–response concentration of Se, it may become toxic to human and animal health too. Trace element samples collected at mine site A and assayed for Hg showed elevated Hg levels of 1.0 ppm, while a sample taken upstream of mine site B equally registered Hg content of 0.8 ppm (Fig. 5). Elsewhere, in the area outside the artisan workings, Hg levels of 1.4 and 0.1 ppm were obtained from the stream sediment samples (Fig. 5). Upstream of mine sites A and B, Hg values of 1.0 ppm representing 93 % in excess of the continental crustal abundances were recorded (Fig. 5). North of mine site C and gold extraction site 3, Hg levels of 0.8–1.4 ppm were chemically measured from the stream sediment samples. The location of gold extraction site 3 seems to be higher on elevation than the surrounding areas. This implies that any chemical used in metal processing will mechanically be transported to the streams in the low-lying areas. It is probable that Hg used to amalgamate gold at extraction site 3 was washed down into the nearby streams. Similarly, natural weathering and mechanical movement of materials from mine site C will transport terrestrial sediments and elements that may include Hg into the nearby streams. The combined Hg from the mine site C and the extraction site 3 will redistribute and re-concentrate at a point in the stream probably explaining the 1.4 ppm Hg upstream of mine site C and extraction site 3. Contrastingly, the controlled sample collected at the northern part of the study has Hg content of 0.1 ppm. The nearest artisanal operation site is 7 km south-west from this point measured from mine site A. The Hg content at the sample control site varies from the average crustal concentration in excess of 3 %. However, the minimum Hg concentration around the artisanal mines is 0.8 ppm representing 73 % in excess of the average crustal levels. From Fig. 5, Hg concentrations in sediments appear to be above the crustal concentrations at all sample points. The Hg crustal average is 0.067 ppm, but the minimum and maximum obtained in this work are 0.1–1.4 ppm (Table 1). This gives an enrichment factor of about 14 and geoaccumulation index of six interpreted as extremely polluted (Table 4, Muller 1969). The sharp Hg variations spatially between the artisan mine sites and the control point suggest that Hg contamination is of anthropogenic source and should be attributed to the artisanal mining operations.
Furthermore, from the spatial maps developed for the different elements (Figs. 4, 5, 6) in streams and (Figs. 7, 8, 9, 10) in soils, it appears the elements behave differently in the near surface environment. The high As levels in streams do not necessarily appear excessively higher at and near the artisan mine areas but showed higher value at the non-mining areas too (Fig. 4). This uneven distribution of As in the stream sediments may also be an attribute of the hydraulic properties of the streams and re-weathering of the exotic sediments in the stream sediments materials sampled. As seen in the study, the average As concentrations in the sediment samples were not high enough to detect Au mineralisation, but they were above the trigger concentrations in sediments and soils to result in health problems. Comparing the results of As values from areas located outside the artisanal workings to the mining areas (Fig. 4), the results show no significant elevation of As levels in areas severely affected by artisanal gold mining and processing. Although As content in stream sediment samples was found to be consistently higher and above the average crustal values, it does not show any significant variation between As concentration in sediments in the vicinity of the mine, at the artisan workings and the controlled site (Fig. 4). Arsenic content at a sample taken from stream sediment at the control site equalled the average crustal abundance of 1.5 ppm in soils (Taylor and McLennan 1995). It is possible that the As anomaly around the artisan workings and other areas outside the artisan working is derived from natural weathering and mechanical movement of materials. Though the range of As values recorded at the mine area is above the average crustal value, it is considerably lower than some locations outside the mine area. It is probable that the source of As in the area is from the weathered underlying rocks and the associated chalcophile minerals particularly from arsenopyrite and concentration levels have been enhanced from the activities of the artisanal operations. This, thus, suggests geogenic contamination and since exposure of this element can be detrimental to human health, it is better if these areas are demarcated for epidemiological studies.
Copper and Zn are known essential elements, but the research realised general depletion of these elements in the environment (Figs. 9, 10). Copper showed isolated high concentrations at places but showed overall depletion with an EF of 0.59. The mean Cu level in the environment is 14.7 ppm as against crustal average of 31.6 ppm. Zinc, however, showed depletion with EF of 0.45 but had some occasional high values ranging from 127 to 255 ppm. The elevated values occur upstream of the mining areas with background Zn levels occurring at the mine sites A and B as well as the gold extractions areas 1 and 2, respectively. It therefore appears that the artisan mines do not have any influence on the migrations and concentrations of Cu and Zn at the near surface environments. As observed from Fig. 9 and 10, the essential elements Cu and Zn from the soil sample results appear to be enriched at some localities but generally deficient for the entire area as presented in Table 2. The geoaccumulation indices of 0.3 were calculated for Cu, and 0.2 was computed for Zn. These geoaccumulated values from Muller (1969) suggest depletion with respect to the upper continental crustal averages. The only enriched essential elements in the area are Se, V and Cr (Table 1). Selenium (Se) is extremely contaminated which is good because it can be taken up to humans and animals via the food chain, but the only quandary is excess exposure to humans can be poisonous. There is, therefore, the need to monitor dose–response concentrations after spatially defining areas of high and low concentrations of the essential elements for possible epidemiological studies.
Conclusion
The results from the stream sediment geochemical samples show depletion of resistant oxides (SiO2, Al2O3 and Fe2O3) and base cations but show no change in TiO2. Background values estimation for the PTE’s was very challenging because of the dual contamination sources especially for some trace elements in and outside the artisan mine sites. Mercury for example has a background value of 0.1 ppm compared to 0.067 ppm of the average crustal abundance. This background value was obtained from the controlled sample away from the artisan workings, whereas background for As is <1.5 ppm since the minimum obtained at the controlled sample area was 0.3 ppm. The minimum concentration of As at the artisan workings is 3.0 ppm which is 50 % of the established background value in the upper continental crust. It was very difficult to establish the background value for Cd because its mobilisation, distributions and concentrations seem to come from natural and anthropogenic sources. More work is therefore required to establish background value particularly for Cd. However, at the control site, the minimum level recorded was 0.6 ppm. This value is being adopted as the background value for Cd for the study area. Meanwhile, the lowest Cd value at and near the artisan workings was 3.7 ppm. This high Cd value at the artisan workings is attributed to geogenic and anthropogenic contaminations. Background value for Pb outside the mine area in soils is 75 ppm, whereas the values close to the artisan workings have minimum Pb levels at 108 ppm. The study therefore noticed the environments that have not been influenced anthropogenically by the artisan mine had background values not more than 7 % for Pb, 3 % for Hg and zero per cent for As. It concludes that the extreme and strong pollutions are from the unguided artisan mining activities and the authors fear for possible human health problem due to the bioavailability and bioaccumulation of the elevated high concentrations of PTE’s and deficiencies of some essential elements in stream sediments and soil samples at Datoko-Shega area.
On the whole, an urgent attention is definitely needed to curtail and abate, especially Hg distribution, dispersion and transportation to currently uncontaminated sites so as to avoid a major environmental disaster which could arise with continued release of Hg into the ecosystem. The time has, therefore, come for scientists in Ghana to launch intensive studies into the various environmental media so as to establish the levels of trace elements in the environment and assess their possible social, economic and human health impacts. It is obvious that scientific information would be needed to aid in designing appropriate sanitary and remedial measures for PTE-impacted areas.
References
Anand, R. R. (2001). Evolution, classification and use of ferruginous regolith materials in gold exploration Yilgarn Craton, Western Australia. Geochemistry: Exploration, Environment, Analysis, 1, 221–236.
Apea, O. B. (2012). Modelling the role of humid substances in the distribution of trace metals in selected ecosystems in Ghana. Unpublished PhD dissertation, Kwame Nkrumah University of Science and Technology Kumasi, Ghana.
Arhin, E., & Nude, P. M. (2009). Significance of regolith mapping and its implication for gold exploration in northern Ghana: A case study at Tinga and Kunche. Geochemistry: Exploration, Environment, Analysis, 9, 63–69.
Boateng, E., Dowuona, G. N. N., M. Nude, P. M., Foli, G., Gyekye P., and Hashim, M. (2012). Geochemical assessment of the impact of mine tailings reclamation on the quality of soils at anglogold concession, Obuasi, Ghana. Research Journal of Environmental and Earth Sciences 4(4): pp. 466–474, 2012 ISSN: 2041–0492.
Dickson, K. B. (1972). A historical geography of Ghana. Journal of Asian and African Studies, Centre for Global Metallogeny, 7, 253–255.
Foli, G., Nude, P. M., and Ohene O. Boansi (2012). Geochemical characteristics of soils from selected districts in the upper east region, Ghana: Implications for trace element pollution and enrichment. Research Journal of Environmental and Earth Sciences, 4(2): pp. 186–195, ISSN: 2041–0492. Maxwell Scientific Organization.
Griffis, J., Barning, K., Agezo, F. L., & Akosa, F. (2002). Gold deposits of Ghana, prepared on behalf of Ghana mineral commission. Ghana: Accra. p. 432.
Kesse, G. O. (1985). The rock and mineral resources of Ghana. Rotterdam, Netherlands: A. A. Balkema. p. 610.
Loon, Jon C. Van., (1980). Analytical Atomic Absorption Spectroscopy Selected Methods. ISBN: 0127140506/0-12-714050-6, Academic Press, New York.
Milési, J. P., Feybesse, J. L., Ledru, P., Dommanget, A., Ouédraogo, M. F., Marcoux, E., Prost, A., Vinchon, C., Sylvain, J. P., Johan, V., Tegyey, M., Calvez, J. Y., & Lagny, P., (1989), West African gold deposits. Chronique de la recherché minière, BRGM Report No. 497, 3–98.
Mitchell, R. L. (1963). Soil aspects of trace element problems in plants and animals. J. Royal Agriculture Society England, 124, 75–86.
Muller, G. (1969). Index of geoaccumulation in sediments of the Rhine River. GeoJournal, 2, 108–118.
Oertel, A. C. (1961). Relation between trace-element concentrations in soil and parent 1161 material. Journal of Soil Science, 12, 119–128.
Reimann, C., & De Caritat, P. (2000). Intrinsic flaws of element enrichment factors (EFs) in environmental geochemistry. Environ. Sci. Technol, 34, (24), pp. 5084–5091. Publishers American Chemical Society. 22: pp. 459–470. Academic press Inc. San Diego.
Selinus, O., Alloway B., Centeno J. A., Finkelman, R. B., Fuge, R., Lindh, U & Smedley P. (2004) Essentials of Medical Geology: impacts of the natural environment on Public Health, Elsevier academic press, 812 pp. ISBN: 0-12-636341-2.
Smedley, P. L. (1996). Arsenic in rural groundwater in Ghana. Journal of African Earth Sciences, 22(4), 459–470.
Smedley, P. L., Edmunds, W. M., & PeligBa, K. B. (1996). Mobility of arsenic in groundwater in the Obuasi area of Ghana. In J. D. Appleton, R. Fuge, & G. J. H. McCall (Eds.), Environmental geochemistry and health (pp. 163–181). London: Geological Society Specific Publication 113 Geological Society.
Taylor, S. R., & McLennan, S. M. (1985). The continental crust: Its composition and evolution. Carlton: Blackwell Scientific Publication. p. 312
Taylor, S. R., & McLennan, S. M. (1995). The geochemical evolution of continental crust. Review Geophysics, 33(2), 241–265.
Webber, P. (1996). News from the village: Agrarian change in Ghana. Geography Review, 9(3), 25–30.
Wells, N. (1960). Total elements in top soils from igneous rocks: An extension of geochemistry. Journal Soil Science, 11, 409–424.
Acknowledgments
This paper is one of the several medical geology researches aimed at preventing many of the primary health diseases emanating from geological processes by the Ghana Chapter of Medical Geology Association. The authors wish to thank the International Medical Geology Association (IMGA) for their moral support but wish that financial support will be available for the next phase of the research. We are grateful also to Veritas Ebiyatakyi and Samson Boadi for helping in sample collection and GIS work. Those whose names are not mentioned but contributed one way or the other we say big thank you.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arhin, E., Boansi, A.O. & Zango, M.S. Trace elements distributions at Datoko-Shega artisanal mining site, northern Ghana. Environ Geochem Health 38, 203–218 (2016). https://doi.org/10.1007/s10653-015-9705-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-015-9705-0