Abstract
An increasing number of studies have found tolerance variation in populations consistently exposed to contaminants, but few studies have examined whether these laboratory-derived estimates of tolerance have survival implications in field conditions. We examined four populations of the mayfly Stenacron interpunctatum for variation in tolerance to the common agricultural insecticide clothianidin. Using laboratory bioassays, we found a 2.3× range in 96 h EC50 tolerance values to clothianidin between our four populations. We then conducted a common-garden experiment with nymphs from each population placed into the collection stream most heavily impacted by upstream agricultural activities to assess whether our laboratory tolerance estimates predict survival under field conditions. We monitored survival and growth in situ for three weeks during the spring planting season, when clothianidin is applied to croplands upstream of our study site. While growth was similar across all groups, the most tolerant population, which was native to the impacted stream, had higher survival than the more sensitive populations. This suggests that population-level variation in contaminant tolerance as measured in laboratory bioassays could have real-world survival implications for sensitive aquatic macroinvertebrates in contaminated streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The deleterious effects of human-produced contaminants on the abundance and diversity of aquatic biota is well-known. Globally, 34% of accessible fresh water is diverted for use in agriculture, industry, or municipalities resulting in the addition of 300 million metric tons of synthetic organic compounds to downstream waters (Schwarzenbach et al., 2006, 2010; Stone et al., 2014). These contaminants can be directly toxic to sensitive organisms and also have sublethal impacts on growth, behavior, and development (Relyea et al., 2005; Beketov and Liess, 2008; Köhler and Triebskorn, 2013; Rumschlag et al., 2019). For instance, fertilizers can create eutrophic conditions and shift community structure in aquatic ecosystems (Woodward et al., 2012). Likewise, herbicides can impact aquatic plant growth and can interfere with amphibian growth and reproduction (Larson et al., 1998; Cedergreen and Streibig, 2005; Hayes et al., 2010). At the community level, the loss of sensitive taxa can result in trophic cascades within stream ecosystems (Englert et al., 2012; Finnegan et al., 2018). However, there is also evidence that in some cases sensitive taxa may persist despite contaminant effects by increasing their tolerance to contaminant exposure, often called evolved tolerance (Brady and Richardson, 2017; Brady et al., 2017).
Evolved tolerance occurs when the lethal or sublethal effects of contaminant exposure create a selection pressure on an organism that has heritable variation in its sensitivity to the contaminant (Hua et al., 2015; Nacci et al., 2016). Those individuals with higher tolerance to the contaminant survive at higher rates and pass their tolerance on to future generations, resulting in a more tolerant population (Montagna et al. 2012). While there are sometimes energetic consequences to evolving greater tolerance (Heim et al., 2018), if the benefits of increased tolerance outweigh the costs, then evolved tolerance can persist (Jayasundara et al., 2017; DiGiacopo and Hua 2020), although this can lead to reduced genetic diversity (Sever et al. 2020; Švara et al., 2022). Evolved tolerance is usually described in the context of pest species that display increased tolerance to routine use of target pesticides, which results in less effective pest control and thus reduced yields for agriculture (Gut et al., 2007; Jansen et al., 2011; Hawkins et al., 2019).
Beyond pest species, evolved tolerance has also been observed in wild non-target populations that are inadvertently exposed to contaminants (Brausch and Smith, 2009; Montagna et al., 2012; Dunlop et al., 2018). Most observed cases are in model species easily caught and raised for bioassays like the crustaceans Daphnia magna or Gammarus pulex (Brady et al., 2017; Shahid et al., 2018). In the crustacean Hyalella azteca, resistance to pyrethroids has been shown to persist in a population even after 22 months in a pesticide-free culture, indicating the resistance was heritable (Weston et al., 2013; Heim et al., 2018; Sever et al., 2020). Evolved tolerance is also seen in amphibians, with populations from ponds that are closer to agricultural fields having higher tolerance to pesticides, implying a link with the frequency of exposure (Cothran et al., 2013). With abundant evidence that evolved tolerance can occur in non-target organisms, there is a need to take the next step to understand the real-world implications for stream organisms and ecosystem health (Becker and Liess, 2017; Amiard-Triquet, 2019).
Many ecotoxicological studies of evolved tolerance, including those listed above, involve laboratory bioassays on wild-caught specimens from a single population exposed to a single contaminant of interest in a controlled setting. While these studies are beneficial for their ability to examine specific toxicants at specific concentrations, they often use contaminant concentrations higher than are observed in the environment and, appropriately, expose organisms in a highly controlled way which may only loosely reflect exposure in a natural system (Skelly and Kiesecker, 2001). These experiments also exclude the full variability of environmental conditions, often looking at only a few factors at a time and thus missing potential interactions between stressors (Berghahn et al., 2012; van Dijk et al., 2013). To understand how evolved tolerance develops in wild systems, we need to link our laboratory studies with field experiments to see if the population-level differences in pesticide tolerance observed in lab studies have implications under real world contaminant exposure patterns (Becker and Liess, 2017).
We previously used laboratory bioassays to examine ten populations of Stenacron interpunctatum mayflies for their sensitivity to the common neonicotinoid insecticide clothianidin, finding a 6.5× range in 96 h EC50s between the least and most sensitive population (Rackliffe and Hoverman, 2020). While this range in tolerance suggests that some populations may be adapting to pesticides more than others, it remains unknown whether these population-level differences in tolerance have survival benefits in real-world conditions or if they represent a heritable evolutionary response or an acclimatization response. Our goal was to assess whether population-level differences in pesticide sensitivity determined from a laboratory bioassay to a single contaminant could predict population responses when under real-world conditions. We used the same mayfly species, S. interpunctatum (Say 1839), as our previous study as it is a relatively sensitive species that remains widespread in heavily impacted streams of the midwestern United States (Lewis 1974; Chang et al., 2014; Rackliffe and Hoverman 2020). We conducted additional bioassays using clothianidin on four populations that showed significant variation in tolerance in our earlier study. We then collected specimens from the four populations and placed them in cages at one of the collection sites, which was a stream embedded in an agricultural landscape. This site, Little Pine Creek, receives high loads of agricultural contaminants during the spring planting season (Rackliffe and Hoverman, 2020), similar to other streams in the region (Douglas and Tooker, 2015; Hladik et al., 2018). We hypothesized that populations showing lower tolerance to clothianidin would experience impaired growth or greater mortality when exposed to the impacted stream compared to populations that showed higher tolerance to clothianidin.
Methods
Collection sites
Our S. interpunctatum mayfly populations came from four streams that drain agricultural landscapes in Indiana, USA. Three streams; Little Pine Creek, Wildcat Creek, and Burnett’s Creek; are in northern Indiana within a 20 km area in Tippecanoe County, where 73–80% of the land area in their watersheds is planted as corn or soybean crops based on 2016 Indiana land use data from the state GIS portal (IndianaMap). Each collection site has a distinct watershed, with none of them upstream from another site or adjacent to the watershed of another site. The closest two watersheds are 5 km apart with two watersheds between them. Downstream, the three watersheds each connect in the Wabash River. The fourth collection site; the Muscatatuck River; was 200 km south of the other sites and has 38% of its watershed under corn or soybean cultivation (Table 1). We collected mayfly nymphs from each site by manually picking them off stream rocks with soft forceps within five days of starting the experiments. They were held in buckets of their water of origin with an aerator in a temperature-controlled environmental chamber until each of the four population samples were collected. One of the collection streams, Little Pine Creek, served as the location of the common garden experiment. This stream is small and wadable with a silty bottom and slow current approximately 0.66 m deep at the deepest part during base flow. It has forested banks and was immediately upstream from a highway bridge resulting in partial shade. Of our study sites, it has the largest percentage of the watershed land area under agricultural use and the most tolerant mayfly population based on our bioassays (Table 1).
Bioassays
Two weeks prior to beginning the common garden experiment, we collected samples of each population and performed median effective toxicity bioassays (EC50) over 96 h with the neonicotinoid clothianidin. These bioassays were used to assess the relative sensitivity of our populations. We used clothianidin because it is highly toxic to insects, commonly used on the major crops in the area, and we have detected it at higher concentrations in our study creek than any other insecticide (Hladik et al., 2014; Rackliffe and Hoverman, 2020). We used aquatic larvae for each bioassay, with 10 individuals separately exposed in 100 mL of water per treatment of five concentrations of technical grade clothianidin (Chem Service Inc West Chester, PA): 6, 13, 36, 67, and 124 µg/L and a control. Thus, each exposure was replicated 10 times and 60 total animals were used. The effect of the pesticide was determined by either death or paralysis within 96 h. This procedure followed our previous study with this species (Rackliffe and Hoverman, 2020).
To calculate 96 h EC50s for each population, we fit our data to a probit model using package lme4 in R (Bates et al., 2015) in RStudio v1.3.1093 for Windows (RStudio Team, 2020). For each population’s EC50, we used a GLM with affected individuals as the response variable and concentration as the explanatory variable with a binomial family and a probit link function to calculate EC50 values. We then used 10,000 bootstrapping iterations to calculate the 95% confidence intervals for each EC50 value. We also compared between sites by combining the response data for all sites in a single GLM model and including site as a covariate. We then conducted a Chi-squared test on that model to test for among-populations differences.
Common-garden experiment
We used 10 mayflies for each experimental unit with 10 replicates for a total of 100 mayflies from each population and placed them in the study stream on 7 May 2020 following a common-garden style experiment. Prior to placement, the aerated buckets holding the mayflies were given a minimum of 1 h to acclimate to the stream temperature by placing them in the stream. We counted and verified identification of 10 mayflies and placed them in a white tray with a ruler to be photographed before adding them to each experimental unit. Experimental units were plastic cylinders with 1 mm mesh covering the top and bottom of the container so that stream water could easily flow through them. The cages were randomly assigned inside concrete cinderblocks, restrained with mesh, and completely submerged in the stream parallel to the bank in about 30 cm of water. Every seven days we removed the mayflies from the cages, placed them in the white tray to be photographed, counted the survivors, then returned them to the cages and the stream. We did not feed the mayflies as they had access to the stream water. On 4 June 2020, we noticed some of the mayflies emerging as adults. Being unable to distinguish between mortality due to emergence vs mortality due to stream conditions, we used only the data up to May 28 for our analysis, prior to the mayflies reaching their final instars and subsequent increase in emergence rate.
We used our photographs of the nymphs in the scaled white trays to measure the length of each mayfly nymph using the software ImageJ (Schneider et al., 2012; Rasband, 2015). Length was defined from the tip of the head to the base of the abdomen. Mayfly length was compared using the mean length per cage in a one-way ANOVA on population. Sizes were compared between populations for each week independently. When normality was violated for a week (weeks 0–2), we transformed the data for that week using a Tukey Ladder of Power from package rcompanion (Mangiafico, 2021). We analyzed the survival of the mayflies between populations and over time using a two-way, between-within (mixed) ANOVA using the R package rstatix (Kassambara, 2021). We found several violations (normality, homogeneity of variance, homogeneity of covariance, and sphericity) of ANOVA assumptions that we attempted to correct using a transformation of x2.225 from the Tukey Ladder of Powers. This corrected some, but not all, of our violations. So, we additionally ran a Wilcox robust mixed ANOVA without trimming means on the transformed data using the command “bwtrim” in the package WRS2 (Mair and Wilcox, 2020). We used one-way ANOVAs and pairwise T-tests on the mixed ANOVA and a robust bootstrap group comparison test on medians with 2000 iterations for post hoc comparisons. We ran an additional robust bootstrap comparison test that included our starting sample size on May 7 to better analyze population changes over the full experiment.
Temperature, planting, and pesticide monitoring
We placed a HOBO temperature logger (Onset, Bourne, MA) on a cinder block in the center of our cages to record water temperature during the experiment. Planting progress for the upstream watershed came from the USDA National Agricultural Statistic Service, which reports weekly statewide progress in the planting of major crops. As our study site is in the northern part of the state, the planting progress lags slightly behind the statewide average reported by the USDA. We assessed the impact of rain events on stream flow, and thus pulses of pesticide exposure, by monitoring the USGS stream gage (#033356848) on Big Pine Creek, which is 16 km due west of our study site. While the flow in Big Pine Creek exceeds Little Pine Creek due to the larger watershed, we consider the temporal patterns of high-flow events to be reflective of the local conditions.
We monitored clothianidin loads at the study site during the experiment by taking grab water samples from the surface of the moving creek where the water was well-mixed. While we have detected other pesticides at this site previously (e.g., carbaryl, thiamethoxam, metolachlor, atrazine), we focused on clothianidin because it was the most abundant insecticide we detected and reasoned it would have the strongest effect on mayflies based on our previous work (Rackliffe and Hoverman, 2020). Furthermore, as in-stream concentrations of clothianidin are driven by rain events, concentrations of other contaminants are likely to spike at the same time. We sampled weekly and focused additional sampling during rain events. In all, we collected 9 water samples during our three-week study period in 50 mL falcon tubes, which were frozen and stored in the dark until they could be analyzed by the Purdue University Bindley Bioscience Center. For chemical testing, 15 mL of water was spiked with an internal standard and then extracted through 3 cc Oasis SPE cartridges (Waters INC, Milford, MA, USA). We then used QQQ LC/MS to measure the concentrations. This same method was used to verify the clothianidin concentrations used in our EC50 bioassays.
Results
96h EC50s
Our clothianidin 96 h EC50 estimates for our four populations showed a gradient from the most sensitive at Wildcat Creek (96 h EC50 = 13.57 µg/L, 95% CI = 7.97–19.47) to least sensitive at Little Pine Creek (96 h EC50 = 31.17 µg/L, 95% CI = 18.65–44.23; Table 1). Overall, we found significant differences among the four populations (Χ2 = 8.08, df = 3, p = 0.044) with the GLM indicating that Wildcat Creek (p = 0.026) and Muscatatuck River (p = 0.03) populations were more sensitive than Little Pine Creek.
Environmental Conditions
We found that clothianidin concentrations in Little Pine Creek averaged 0.20 µg/L (SE = 0.04, n = 9). There was one major rain event during the three-week period on May 19, which is readily visible in the stream gage data. The highest clothianidin concentration detected was 0.41 µg/L following that rain event. Smaller rain events occurred May 10, May 14, and May 25 and are visible as a slight decline in temperature (likely due to cloud cover) but did not increase stream flow. Stream temperature gradually increased during the experiment (Fig. 1). Planting of corn and soybeans also increased during the experiment from the statewide estimate of 28% of fields planted on May 4 to 82% on May 31 (Fig. 1).
A Clothianidin concentrations in Little Pine Creek measured during the experiment and stream discharged measured at midnight daily in the nearby Big Pine Creek at USGS stream gage #033356848. As Big Pine Creek is larger, the magnitude of the flow differs from Little Pine Creek but the temporal patterns are driven by the same weather events. B Temperature was measured hourly by a HOBO sonde in the creek and the daily average is presented here. Planting progress is the statewide average for Indiana as reported by the USDA Agricultural Statistics service and is the combined corn and soybean crop, which are the most abundant crops in the area
Field experiment
After transforming the non-normal data, we found no differences in mayfly length between populations (Week 0: F3,36 = 2.56, p = 0.07, week 1: F3,27 = 0.12, p = 0.95, week 2: F3,34 = 0.24, p = 0.87, week 3: F3,34 = 2.61, p = 0.07). However, all populations increased in size over the course of the experiment suggesting that they were able to obtain food from access to stream water (Fig. 2). Furthermore, noticeable emergence after 4 June 2020 in all populations indicated experimental conditions allowed for growth and development.
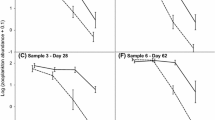
Using a traditional mixed ANOVA on our transformed survival data with a Huynh-Feldt correction (HF ε = 0.83), we found there was no population effect (F 3,36 = 1.6, p = 0.21, η2 = 0.11), but there was an effect of time (F1.7,59.6 = 65.4, p < 0.001, η2 = 0.15) and the interaction (F5.0,59.6 = 2.4, p = 0.049, η2 = 0.02). To explore the interaction, we divided our dataset by week and examined the effects of populations on survival. On May 14, we found survival was higher in Little Pine Creek compared to Wildcat Creek (p = 0.037). On May 21, survival was higher in Little Pine Creek compared to Burnett Creek (p = 0.035). On May 28, there were no differences between the populations (Fig. 3).
Wilcox’s robust mixed ANOVA on the transformed data confirmed the traditional analysis by finding no effect of population (Q 3,19.6 = 2.0, p = 0.15) but still an effect of time (Q 2,28.0 = 42.6, p < 0.001) and the interaction (Q 6, 20.8 = 4.5, p = 0.004). The robust bootstrap post-hoc comparisons indicated significant comparisons overall (p = 0.026) but do not identify specific comparisons, rather it indicates relative effect size for each comparison. This test indicated declining survival in the Muscatatuck River population from May 14–28. The test from May 7–28 was also significant (p = 0.013) and indicated higher survival in Little Pine Creek compared to other creeks.
Discussion
Adapting to anthropogenic contaminants is an opportunity for sensitive populations to persist and perhaps recover in environments that have been heavily impacted (Brady et al., 2017). Understanding how this adaptation develops in real-world settings, whether through acclimatization to previous exposures or heritable traits passed on from previous generations, will help us allocate conservation resources and adapt biomonitoring programs to account for current sensitivities (Becker and Liess, 2017). Our experiment assessed the consequences of population-level differences in pesticide tolerance on survival within a heavily impacted system. We found that the population that was native to our common garden stream and possessed the highest level of clothianidin tolerance under laboratory conditions had the highest rates of survival in our common-garden experiment. This provides support for our hypothesis that populations more tolerant to clothianidin would survive better in a heavily contaminated stream. Our stream experienced a strong pesticide pulse (max 0.41 µg/L clothianidin) during our experiment, which was consistent in magnitude to pulses we have measured in previous years (Rackliffe and Hoverman, 2020), suggesting that similar pulse/contaminant dynamics are likely to occur in future years and in similar streams embedded in agricultural landscapes.
We found an interaction between time and population suggesting that populations varied in their survival over the course of the experiment, although the difference between populations was low. This is not surprising as the difference in sensitivity between the least tolerant and most tolerant populations as indicated by the EC50s was only 2.3×. Additionally, there is uncertainty inherent to field studies due to interactions with the environment, which can dampen effects observed in laboratory studies (Mertz and McCauley, 1980; Skelly and Kiesecker, 2001). A study incorporating populations with a wider range of tolerance values may find stronger effects. Interestingly, the interaction effect seems largely driven by higher survival rates in the native Little Pine Creek population, while the other three populations survived at similar rates to each other. This could be explained by the greater tolerance that Little Pine Creek mayflies had to clothianidin as we hypothesized, but there are other factors to consider. For instance, we have also detected traces of other neonicotinoids, carbaryl, metolachlor, atrazine, deet, and other pesticides in Little Pine Creek (Rackliffe and Hoverman, 2020). We did not assess sensitivity to these other contaminants or their possible mixtures. It may be that each population varies independently in sensitivity to each contaminant and so our observed effect is the combination of the impacts of each contaminant. The contaminant mixtures likely interact with the physical and chemical conditions of the creek, which can also induce an adaptive response by native populations (Eliason et al., 2011; Camp and Buchwalter, 2016; Macaulay et al., 2021). Thus, the increased survival of the native population may be due to adaptation to the combined suite of local conditions rather than just clothianidin or just pesticides. A review of 500 evolutionary studies found that populations often adapt to local conditions and that this local adaptation often softens broader ecological patterns (Urban et al., 2020). Either way, local evolution is a potential explanation for the differences we found between mayfly populations.
However, we must also acknowledge alternative explanations for our results. While the population with the highest EC50 did survive at the highest rates, it was also the native population to the experimental conditions. It is possible that the local population had adapted to other environmental factors within the stream that deleteriously effected the other populations. As one would expect with a real-world experiment, there were many uncontrolled factors, natural and artificial, such as water temperature, conductivity, pH, other contaminants, photoperiod, or available food types that could benefit the local population over the non-local populations. Because of the design of our experiment, we must use caution in attributing the differences in survival to evolved tolerance or heritable resistance to clothianidin. As it was beyond our resources to conduct genetic analysis or investigate multiple generations of organisms, our patterns could be produced by acclimatation to the contaminants through previous exposures during earlier life stages of the mayflies.
While we found no differences in growth rate between our populations, there may be other sublethal effects of pesticide exposure that we did not measure. Our experiment ended when emergence began to affect our specimens. Future studies could incorporate a malaise trap into the cage design to allow the capture and quantification of emerging insects. Delayed emergence (18–25 d) due to exposure to similar concentrations of neonicotinoids (0.5 µg/L) has also been documented in other aquatic insects like Chironomidae and Zygoptera (Cavallaro et al., 2018). Previous studies have also shown that chronic exposure to insecticides like clothianidin can alter emergent body size in mayflies, even at concentrations as low as 0.1 µg/L, which is the lowest concentration of clothianidin we detected in the stream during our study and may represent the baseline concentration for this creek (Alexander et al., 2008). Such chronic sublethal effects could contribute to the evolution of tolerance in contaminated streams.
Evolved tolerance has been observed in fish (Nacci et al., 2010), amphibians (Hua et al., 2015; Brady et al., 2017), crustaceans (Anguiano et al. 2017), dipterans (Montagna et al., 2012), and many other insects (Becker and Liess, 2017). However, few studies have explored whether the observations from laboratory studies translate to patterns that would occur under field conditions. While we cannot definitively attribute our population-level variation to evolved tolerance, our study does suggest that even small differences in tolerance measured in a laboratory can have real-world survival implications for organisms especially when their sensitivities are close to environmental concentrations of contaminants. Evolved tolerance will not likely occur for every species or every contaminant scenario, yet adaption can help sensitive species persist despite contamination. However, it can be misinterpreted by those making habitat or contaminant assessments. For instance, in biomonitoring programs, the relative sensitivity of taxa to contaminants is assumed to be constant across populations. However, populations of a sensitive species that evolve tolerance to common contaminants would be expected to persist even as water quality declines below the species’ presumed tolerance range. This can result in a biomonitoring conclusion that overlooks the decline in water quality (Brady et al., 2017). Regulators may allow higher environmental contaminant loads in streams due to a few populations of key species with evolved tolerance while more sensitive species that have not evolved tolerance face increased risk (Becker and Liess, 2017). We need to watch for adaptive responses and account for them as we monitor the environmental impacts of contaminants.
References
Alexander AC, Heard KS, Culp JM (2008) Emergent body size of mayfly survivors. Freshw Biol 53:171–180. https://doi.org/10.1111/j.1365-2427.2007.01880.x
Amiard-Triquet C (2019) Pollution tolerance in aquatic animals: From fundamental biological mechanisms to ecological consequences. In: Ecotoxicology: New Challenges and New Approaches. Elsevier, 33–91
Anguiano OL, Vacca M, Rodriguez Araujo ME et al. (2017) Acute toxicity and esterase response to carbaryl exposure in two different populations of amphipods Hyalella curvispina. Aquatic Toxicol 188:72–79. https://doi.org/10.1016/j.aquatox.2017.04.013
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Becker JM, Liess M (2017) Species diversity hinders adaptation to toxicants. Environ Sci Technol 51:10195–10202. https://doi.org/10.1021/acs.est.7b02440
Beketov MA, Liess M (2008) Potential of 11 pesticides to initiate downstream drift of stream macroinvertebrates. Arch Environ Contam Toxicol 55:247–253. https://doi.org/10.1007/s00244-007-9104-3
Berghahn R, Mohr S, Hübner V et al. (2012) Effects of repeated insecticide pulses on macroinvertebrate drift in indoor stream mesocosms. Aquat Toxicol 122–123:56–66. https://doi.org/10.1016/j.aquatox.2012.05.012
Brady SP, Richardson JL (2017) Road ecology: shifting gears toward evolutionary perspectives. Front Ecol Environ 15. https://doi.org/10.1002/fee.1458
Brady SP, Richardson JL, Kunz BK (2017) Incorporating evolutionary insights to improve ecotoxicology for freshwater species. Evol Appl 10:829–838. https://doi.org/10.1111/eva.12507
Brausch JM, Smith PN(2009) Pesticide resistance from historical agricultural chemical exposure in Thamnocephalus platyurus (Crustacea: Anostraca) Environ Pollut 157:481–487. https://doi.org/10.1016/j.envpol.2008.09.010
Camp AA, Buchwalter DB (2016) Can’t take the heat: Temperature-enhanced toxicity in the mayfly Isonychia bicolor exposed to the neonicotinoid insecticide imidacloprid. Aquat Toxicol 178:49–57. https://doi.org/10.1016/j.aquatox.2016.07.011
Cavallaro MC, Liber K, Headley JV et al. (2018) Community-level and phenological responses of emerging aquatic insects exposed to 3 neonicotinoid insecticides: An in situ wetland limnocorral approach. Environ Toxicol Chem 37:2401–2412. https://doi.org/10.1002/ETC.4187
Cedergreen N, Streibig JC (2005) The toxicity of herbicides to non-target aquatic plants and algae: assessment of predictive factors and hazard. Pest Manag Sci 61:1152–1160. https://doi.org/10.1002/ps.1117
Chang FH, Lawrence JE, Rios-Touma B, Resh VH (2014) Tolerance values of benthic macroinvertebrates for stream biomonitoring: Assessment of assumptions underlying scoring systems worldwide. Environ Monit Assess 186:2135–2149. https://doi.org/10.1007/s10661-013-3523-6
Cothran RD, Brown JM, Relyea RA (2013) Proximity to agriculture is correlated with pesticide tolerance: Evidence for the evolution of amphibian resistance to modern pesticides. Evol Appl 6:832–841. https://doi.org/10.1111/eva.12069
DiGiacopo DG, Hua J (2020) Evaluating the fitness consequences of plasticity in tolerance to pesticides. Ecol Evol 10:4448–4456. https://doi.org/10.1002/ece3.6211
Douglas MR, Tooker JF (2015) Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. Field crops. Environ Sci Technol 49:5088–5097. https://doi.org/10.1021/es506141g
Dunlop ES, McLaughlin R, Adams JV et al. (2018) Rapid evolution meets invasive species control: The potential for pesticide resistance in sea lamprey. Can J Fish Aquat Sci 75:152–168. https://doi.org/10.1139/cjfas-2017-0015
Eliason EJ, Clark TD, Hague MJ et al. (2011) Differences in thermal tolerance among sockeye salmon populations. Science (1979) 332:109–112. https://doi.org/10.1126/science.1199158
Englert D, Bundschuh M, Schulz R(2012) Thiacloprid affects trophic interaction between gammarids and mayflies Environ Pollut 167:41–6. https://doi.org/10.1016/j.envpol.2012.03.024
Finnegan MC, Emburey S, Hommen U et al. (2018) A freshwater mesocosm study into the effects of the neonicotinoid insecticide thiamethoxam at multiple trophic levels. Environ Pollut 242:1444–1457. https://doi.org/10.1016/j.envpol.2018.07.096
Gut L, Schilder A, Isaacs R, McManus P (2007) Managing the Community of Pests and Beneficials.
Hawkins NJ, Bass C, Dixon A, Neve P (2019) The evolutionary origins of pesticide resistance. Biol Rev 94:135–155. https://doi.org/10.1111/brv.12440
Hayes TB, Khoury V, Narayan A et al (2010) Atrazine induces complete feminization and chemical castration in male African clawed frogs (Xenopus laevis) Proc Natl Acad Sci USA 107:4612–4617. https://doi.org/10.1073/pnas.0909519107
Heim JR, Weston DP, Major K, et al (2018) Are there fitness costs of adaptive pyrethroid resistance in the amphipod, Hyalella azteca? Environ Pollut 235. https://doi.org/10.1016/j.envpol.2017.12.043
Hladik ML, Corsi SR, Kolpin DW et al. (2018) Year-round presence of neonicotinoid insecticides in tributaries to the Great Lakes, USA. Environ Pollut 235:1022–1029. https://doi.org/10.1016/j.envpol.2018.01.013
Hladik ML, Kolpin DW, Kuivila KM (2014) Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ Pollut 193:189–196. https://doi.org/10.1016/j.envpol.2014.06.033
Hua J, Jones DK, Mattes BM et al. (2015) Evolved pesticide tolerance in amphibians: Predicting mechanisms based on pesticide novelty and mode of action. Environ Pollut 206:56–63. https://doi.org/10.1016/j.envpol.2015.06.030
Jansen M, Coors A, Stoks R, de Meester L (2011) Evolutionary ecotoxicology of pesticide resistance: A case study in Daphnia. Ecotoxicology 20:543–551
Jayasundara N, Fernando PW, Osterberg JS et al. (2017) Cost of tolerance: physiological consequences of evolved resistance to inhabit a polluted environment in Teleost Fish Fundulus heteroclitus. Environ Sci Technol 51:8763–8772. https://doi.org/10.1021/acs.est.7b01913
Kassambara A (2021) rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R package version 0.5.0
Köhler HR, Triebskorn R (2013) Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science (1979) 341:759–765
Larson DL, McDonald S, Fivizzani AJ et al. (1998) Effects of the herbicide atrazine on Ambystoma tigrinum metamorphosis: Duration, larval growth, and hormonal response. Physiol Zool 71:671–679. https://doi.org/10.1086/515999
Lewis PA (1974) Taxonomy and ecology of Stenonema mayflies (Heptageniidae:Ephemeroptera). U.S. EPA Environmental Monitoring Series EPA-670/4-:1–89
Macaulay SJ, Hageman KJ, Piggott JJ, Matthaei CD (2021) Time-cumulative effects of neonicotinoid exposure, heatwaves and food limitation on stream mayfly nymphs: A multiple-stressor experiment. Sci Total Environ 754:141941. https://doi.org/10.1016/j.scitotenv.2020.141941
Mair P, Wilcox R (2020) Robust statistical methods in R using the WRS2 package. Behav Res Methods 52:464–488. https://doi.org/10.3758/s13428-019-01246-w
Mangiafico S (2021) “rcompanion” Functions to Support Extension Education Program Evaluation. R package version 2.3.27
Mertz DB, McCauley DE (1980) The domain of laboratory ecology. Synthese 43:95–110. https://doi.org/10.1007/BF00413858
Montagna CM, Gauna LE, de D’Angelo AP, Anguiano OL (2012) Evolution of insecticide resistance in non-target black flies (Diptera: Simuliidae) from Argentina. Mem Inst Oswaldo Cruz 107:458–465. https://doi.org/10.1590/S0074-02762012000400003
Nacci D, Proestou D, Champlin D et al. (2016) Genetic basis for rapidly evolved tolerance in the wild: adaptation to toxic pollutants by an estuarine fish species. Mol Ecol 25:5467–5482. https://doi.org/10.1111/mec.13848
Nacci DE, Champlin D, Jayaraman S (2010) Adaptation of the estuarine fish fundulus heteroclitus (Atlantic Killifish) to polychlorinated biphenyls (PCBs). Estuar Coasts 33:853–864. https://doi.org/10.1007/s12237-009-9257-6
Rackliffe DR, Hoverman JT (2020) Population-level variation in neonicotinoid tolerance in nymphs of the Heptageniidae. Environ Pollut 265:114803. https://doi.org/10.1016/j.envpol.2020.114803
Rasband W (2015) ImageJ [Software]. U S National Institutes of Health, Bethesda, Maryland, USA
Relyea RA, Schoeppner NM, Hoverman JT (2005) Pesticides and amphibians: The importance of community context. Ecol Appl 15:1125–1134. https://doi.org/10.1890/04-0559
RStudio Team (2020) RStudio: Integrated Development Environment for R. RStudio
Rumschlag SL, Halstead NT, Hoverman JT et al. (2019) Effects of pesticides on exposure and susceptibility to parasites can be generalised to pesticide class and type in aquatic communities. Ecol Lett 22:962–972. https://doi.org/10.1111/ele.13253
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Schwarzenbach RP, Egli T, Hofstetter TB, et al (2010) Global Water Pollution and Human Health. Annu Rev Environ Resour. https://doi.org/10.1146/annurev-environ-100809-125342
Schwarzenbach RP, Escher BI, Fenner K et al. (2006) The challenge of micropollutants in aquatic systems. Science (1979) 313:1072–1077. https://doi.org/10.1126/science.1127291
Sever HC, Heim JR, Lydy VR, et al (2020) Recessivity of pyrethroid resistance and limited interspecies hybridization across Hyalella clades supports rapid and independent origins of resistance. Environ Pollut 266. https://doi.org/10.1016/j.envpol.2020.115074
Shahid N, Becker JM, Krauss M et al. (2018) Adaptation of Gammarus pulex to agricultural insecticide contamination in streams. Sci Total Environ 621:479–485. https://doi.org/10.1016/j.scitotenv.2017.11.220
Skelly DK, Kiesecker JM (2001) Venue and outcome in ecological experiments: manipulations of larval anurans. Oikos 94:198–208. https://doi.org/10.1034/j.1600-0706.2001.t01-1-11105.x
Stone WW, Gilliom RJ, Ryberg KR (2014) Pesticides in U.S. streams and rivers: Occurrence and trends during 1992-2011. Environ Sci Technol 48:11025–11030. https://doi.org/10.1021/es5025367
Švara V, Michalski SG, Krauss, Martin, et al. (2022) Reduced genetic diversity of freshwater amphipods in rivers with increased levels of anthropogenic organic micropollutants. https://doi.org/10.1111/eva.13387
Urban MC, Strauss SY, Pelletier F, et al (2020) Evolutionary origins for ecological patterns in space. Proceedings of the National Academy of the Sciences 117:17482–17490. https://doi.org/10.1073/pnas.1918960117/-/DCSupplemental
van Dijk TC, van Staalduinen MA, van der Sluijs JP (2013) Macro-invertebrate decline in surface water polluted with Imidacloprid. PLoS One 8: https://doi.org/10.1371/journal.pone.0062374
Weston DP, Ramil HL, Lydy MJ (2013) Pyrethroid insecticides in municipal wastewater. Environ Toxicol Chem 32: https://doi.org/10.1002/etc.2338
Woodward G, Gessner MO, Giller PS et al. (2012) Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science (1979) 336:1438–1440. https://doi.org/10.1126/science.1219534
Acknowledgements
DR Rackliffe is grateful for financial support from the Department of Forestry and Natural Resources at Purdue University in the pursuit of this project. He is also grateful for the help of members of the Jason Hoverman lab for assistance in the field aspects of this project and for the intellectual contributions of his PhD committee (Mark Christie, Rueben Goforth, Christian Krupke, and Jason Hoverman) in redesigning the experiment after the first failure and reviewing the final manuscript.
Author contributions
RR conceived, conducted, analyzed, and prepared the manuscript for this experiment under the guidance of JH.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This work required no ethical approval under the guidelines of the Purdue Animal Care and Use Committee as only invertebrate organisms were used.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rackliffe, D.R., Hoverman, J.T. Population-level variation in pesticide tolerance predicts survival under field conditions in mayflies. Ecotoxicology 31, 1477–1484 (2022). https://doi.org/10.1007/s10646-022-02603-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-022-02603-w