Abstract
Bioinsecticides based on Bacillus thuringiensis (Bt) Berliner, 1915 are widely used to control lepidopteran in several crops. However, surviving insects exposed to the sub-lethal concentration of Bt-based bioinsecticides can suffer a multitude of effects on the biological conditioning known as hormesis. Here, we aimed to provide a clearer understanding of the biological conditioning of Anticarsia gemmatalis (Hübner, 1818), exposed to different concentrations of a Bt-based bioinsecticide, by assessing life table parameters over three generations. We defined five sub-lethal concentrations (LC5, LC10, LC15, LC20, and LC25) from the response curve estimate of A. gemmatalis. Deionized water was used as a control. We assessed the parameters of eggs-viability and the duration of the stages, incubation, larval, pre-pupal, pupal, adult, pre-oviposition and total biological cycle. Data were used to construct the fertility life table using the two-sex program. The survival curves showed greater variation in the proportion of individuals at each development stage using the LC25. The sub-lethal concentrations did not influence the incubation-eggs period, pre-pupal and pupal. However, the larval and adult stages using LC25 and LC10 were the most affected. Changes in sex ratio were observed using LC20 and LC5. The toxic effect of Bt-based bioinsecticide interfered mainly in the parameters of fertility, sex ratio, net reproduction rate (R0), and gross reproduction rate (GRR).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Velvet bean caterpillar Anticarsia gemmatalis Hübner, 1818, is one of the main defoliating caterpillars in the soybean crop (Haase et al. 2015). However, the losses provided by the species in the field vary from 3 to 75% in conventional soybean cultivars (Silva et al. 2003; Moscardi et al. 2012).
In the 1990s, the control of A. gemmatalis in Brazil was carried out, starting with chemical insecticides such as organochlorines and organophosphates. However, problems provided to man and the environment have led to restrictions on the use of these pesticides (Moscardi et al. 2012). Currently, this pest control is carried out with insecticides selective to the environment and cultivars of transgenic soybean that expresses only the toxin Cry1Ac from the bacterium Bacillus thuringiensis Berliner, 1915 (Bernardi et al. 2012).
The pressure exerted by the consumer market to reduce dependence on chemical insecticides in agriculture, combined with growing reports of insect resistance to transgenic plants, renewed the worldwide interest in Bt-based bioinsecticides (Lacey 2017; Konecka et al. 2018; Amaral et al. 2019; Horikoshi et al. 2019).
Agriculture over the years has made significant leaps in technology; however, it still faces challenges in microbial control to reach the biologically active product in the target and in the correct concentration (Frye et al. 1973; Sedaratian et al. 2013). Surviving insects, exposed to sub-lethal concentration of Bt-based bioinsecticides due to biotic factors (incompatibility with other products, inadequate spray calibration, and drift) and abiotic (temperature, ultraviolet radiation, precipitation, and others) can suffer a multitude of effects in biological conditioning (Van-Rie and Fereé 2000).
The phenomenon is known as hormesis and occurs in surviving insects and their descendants. Studies show that the phenomenon can provide positive consequences, called hormoligosis (Guedes et al. 2009; Guedes and Culter 2013). The theory of hormoligosis is poorly studied; however, part of the assumption that sub-lethal concentration, instead of harming the insect, ends up having the opposite effect, stimulating biological development through, for example, an increase in the fertility parameter (physiological hormoligosis) or oviposition behavior (behavioral hormoligosis) of the species, leading to a significant increase in its abundance (Abivardi 2004; Dutcher 2007).
Studies are evaluating the effects of insecticides on the biological parameters of pests based on the theory of physiological and behavioral hormoligosis, however, there are no studies performed with Bt-based bioinsecticides at this moment. The lack of understanding about the theory prevents us from understanding the flaws in the pest control programs, outbreaks and resurgences of insects, among other factors (Abivardi 2004; Cohen 2006; Dutcher 2007). Thus, this study aimed to evaluate the biological conditioning of A. gemmatalis exposed to sub-lethal concentration of Bt-based bioinsecticides Dipel® through the parameters of the life table over three generations.
Material and methods
Insect rearing
The population of A. gemmatalis was obtained at the Laboratory of Insect Biology of the College of Agriculture “Luiz de Queiroz” (ESALQ-Piracicaba) and the insect rearing was established at the laboratory of microbial control of arthropod pests at Paulista State University “Júlio de Mesquita Filho” (UNESP - Jaboticabal), maintained on an artificial diet (Greene et al. 1976).
For the colony establishment, 2000 eggs of A. gemmatalis were placed in Petri dishes (9 cm Ø) with filter paper moistened in distilled water. The insect colony was kept under 25 ± 1 °C, RH of 70 ± 10%, and 12:12 (L:D).
Commercial formulation lethal concentrations
The commercial formulation toxicity was evaluated using the spore-crystal suspensions of the Bt-based bioinsecticide (Dipel®). The suspensions were defined by plating on nutrient agar to determine the CFU, which was evaluated after seven days (Sedaratian et al. 2013). The curve response was estimated using the Six Error Problems analysis (Sas University 2013). 200 μL on the surface of the artificial diet (4.8 cm3) were previously distributed in polyethylene cups (3.5 cm Ø). A hundred insects were used to estimate a curve response for each treatment, distributed in 10 repetitions. Deionized water was applied in equal volume as a control (Santos et al. 2019). The bioassay evaluations were carried out after seven days.
Sub-lethal concentrations
Based on mortality data from bioassays, artificial diet preparations containing sub-lethal concentrations of Dipel® or controls (untreated diet) were prepared and used to study sublethal effects of Bt on A. gemmatalis. The sub-lethal concentrations LC5, LC10, LC15, LC20, and LC25 (0.20509, 0.38126, 0.57929, 0.80776, and 1.07438 µg Bt.mL diet−1) were chosen by the estimate response curve (Sedaratian et al. 2013).
In these assays, a 200 μL aliquot of each sub-lethal concentration was applied to the surface of the artificial diet (4.8 cm3), previously distributed in polyethylene cups (3.5 cm Ø) (Sas University 2013). The surviving caterpillars were fed an artificial diet containing their respective sub-lethal concentrations for three generations (F1, F2, and F3). For each generation, 100 insects were used for each treatment, considering each caterpillar as a sampling unit. Deionized water was applied in an equivalent volume in the control. The evaluations were carried out daily.
Sub-lethal effects bioassay
All generations were evaluated daily until the pupae phase, which was sexed for up to 24 h (Butt and Cantu 1962). The newly emerged adults were separated into couples and placed inside a PVC cage (10 cm × 20 cm), lined with white A4 sulfite paper (used as an oviposition substrate). At the bottom, a Petri dish with filter paper was used and an upper part sealed with voile fabric.
The adults were fed with a 10% honey solution moistened with cotton wool, placed in a polyethylene petri dish (49 × 12 mm) at the bottom of the cage. The papers used as a laying substrate were removed daily and the eggs counted with the aid of a stereoscopic microscope (Leica-S8 APO).
Data analysis
Mortality data were submitted to Probit regression analysis and sub-lethal concentrations values LC5, LC10, LC15, LC20, and LC25 were obtained using the SAS software (P > 95%) (Sas University 2013). The experimental design was a completely randomized design (CRD) for the variables duration of the biological cycle and egg viability, caterpillar, pre-pupa, pupa, adult, and pre-oviposition phases.
The following parameters were recorded in each treatment (LC5, LC10, LC15, LC20, LC25, and control) and generation (F1, F2, and F3): pre-oviposition period (APOP: period from the adult emergence to the first oviposition), total pre-oviposition (TPOP: period from the egg eclosion to the first oviposition), period of oviposition and daily fertility (Colinet et al. 2015).
The Two-Sex fertility life table program (Chi and Liu 1985) was used to analyze the biological parameters of egg, larva, pupa, adult pre-oviposition period, total pre-oviposition period, and fertility (Chi 1988, 2019). In the age stage, the Two-Sex fertility life table values, lx, mx, and R0 are calculated as:
where k is the number of stages, sxj is the survival rate of the velvet bean caterpillar, x = age in days, and j = stage. fxj is the stage-specific fertility, lx is the stage-specific survival rate, mx is the stage-specific fertility stage, exj life expectancy, vxj reproductive value in the stage, R0 is the net reproductive rate, k finite rate of increase, and T is the mean generation.
In this study, the iterative bisection method of the Euler–Lotka formula was used to estimate r (r is the intrinsic rate of increase) using the age indexed to 0 (Goodman 1982), as shown in Eq. (2).
The exj is defined as the period of duration that an individual or insect of x and j is predictable to live (Chi and Su 2006) as:
where S’iy is defined as the probability that individuals and individuals will survive to age and stage and it’s found assuming Siy = 1. The vxj was estimated following the methodology of Abbas et al. (2014) and was calculated as:
Standard errors and means were estimated technically using bootstrap (Efron and Tibshirani 1994).
Results
Susceptibility of A. gemmatalis larvae to Dipel®
The results of bioassays with Dipel® incorporated in the artificial diet for neonates of A. gemmatalis are presented in Table 1. The estimated value of LC50 and LC99 after seven days was 3.396 and 180.009 (µg Bt.mL diet−1), while no mortality was recorded in controls. Based on data from these bioassays, we calculated the sub-lethal Dipel® concentrations, from LC5 to LC25, to use in our study (Table 1).
Sub-lethal effects on the biological parameters of A. gemmatalis
Anticarsia gemmatalis submitted to sub-lethal concentrations LC5, LC10, LC15, and LC20 and the control completed the biological development in the three generations evaluated with different responses in the biological conditioning of the species. The LC25 treatment was the only one that did not reach the third generation.
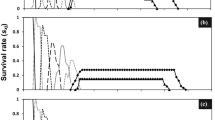
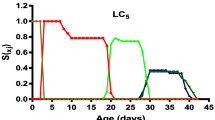
The survival curves show the proportion of A. gemmatalis at each development stage related to the first eggs. The overlaps observed are due to differences in the speed of development among individuals. The proportions reached maximum values, with the subsequent reduction due to changes in the next phase or mortality or because they died in adulthood (Fig. 1).
The most significant variation was observed in the proportion of individuals in the LC25 in the first generation. In the next generation for the LC5 and LC15, adult male individuals had a longer survival time than females. Furthermore, in the last generation, the results show an increase in the survival in treatments LC15 and LC20 (Fig. 1).
The sub-lethal concentrations did not influence the eggs incubation period of A. gemmatalis (Table 2). The duration of the pre-pupa and pupa phases was not affected by the treatments. In the adult phase, treatments LC25 in the first generation and LC10 in the second and third generations reached the lowest values.
The pre-oviposition period (APOP) was significant in the 2nd generation with the LC10 and in the 3rd generation using the LC5, LC10 treatments. In the total pre-oviposition period (TPOP) was observed difference in the third generation with the LC10 (Table 3). The greater longevity of females and males in the first generation was observed in the second and third generations. The oviposition period and fertility parameters were affected by the LC20 and LC25 over the evaluated generations (Table 3). The sex ratio was affected only in the treatments LC20 in the first generation and LC5 in the second generation, since the mean in the generations was 0.47 between the first generation and the second generation and 0.54 in the third generation, respectively (Table 3).
The fertility life table was generated using descendants treated with the respective sub-lethal concentration. The witness had a higher mean in all parameters evaluated over the three generations. In the generation time parameter (T), the CL15 treatment reached ~27.93 days, representing the lowest mean among all treatments in the three evaluated generations (Table 4).
The parameters of the life table showed an association with the applied concentration of the bioinsecticide; with the increase in concentration, there is a reduction in the net reproduction rate (R0) (80.64%), gross reproductive rate (GRR) (75.87%), intrinsic population growth rate (r) (35.85%) and the finite population growth rate (λ) (5.87%) (Table 4).
Discussion
Susceptibility of A. gemmatalis larvae to Dipel®
The lethal concentration required to kill 99% of the population of A. gemmatalis (LC99) proves differences of 60 (LC50), 180 (LC25), 225 (LC20), 360 (LC15), 600 (LC10), and 900 (LC5) times about the proportion of the bioinsecticide Dipel®. The results demonstrate, a larger interval between the lethal concentrations of the LC5 and LC10 treatments about the LC20 and LC25 treatments. Studies determining the susceptibility of A. gemmatalis populations revealed that the LC50 for the susceptible population was 0.25 µg Bt.mL diet−1, lower than that found in the present study (Gholmie et al. 2018).
These differences are attributed to the genetic variability between the populations collected in the state of Parana and that of the present study in Sao Paulo, Brazil. Dipel® is composed of B. thuringiensis var. kurstaki, line HD-1, and presents the toxins Cry1Aa, 1Ab, and 1Ac, having in more significant quantities Cry1Ab and Cry1Ac. The toxins can also act individually or together, which can potentiate the individual toxicity of each toxin (Xue et al. 2005; Wei et al. 2015) or the combination of the toxins can reduce the insecticidal effect (Ameen et al. 1998; Garbutt et al. 2011), probably due to competition for the same receptor in the intestine of the caterpillar (Gómez et al. 2007).
Differences in susceptibility may be the main cause of the presence or absence of specific receptors for Cry toxins in the apical microvilli of columnar cells in the caterpillar’s midgut (Gómez et al., 2007). Any interference associated with the mode of action of Bt helps in the survival of the insect and, therefore, in the development of resistance (Tabashnik, 1994).
Sub-lethal effects on the biological parameters of A. gemmatalis
The effect of sub-lethal concentration on A. gemmatalis varied according to treatments. This observation suggests that sub-lethal must be considered and can be used as a tool in integrated pest management (Bauce et al. 2006). The ingestion of different sub-lethal concentrations of B. thuringiensis by caterpillars of the second instar of A. gemmatalis had several consequences on biological conditioning and resulted in the prolongation of the larval period in some treatments. These observations occurred in other species, such as Lymantria dispar L., 1758 (Erb et al. 2001), Sesamia nonagrioides Lefèbvre, 1827 (Eizaguirre et al. 2005) and Helicoverpa armigera Hübner, 1808 (Sedaratian et al. 2013).
As a survival strategy, organisms can start to inhibit food, prolong development time, or even increase the incidence of polymorphism with the aim of biological compensation (Moreau and Bauce 2003). Fast and Regniere (1984) found that fourth-instar caterpillars of L. dispar can recover their development when exposed to B. thuringiensis, with an increased number of instars without changing the pupae weight (Ramachandran et al. 1993). Also, in field conditions, the behavior may be different due to the continuous inlet of the sub-lethal concentration over the generations, which may make it difficult to acquire a lethal dose due to organoleptic properties that cause food inhibition (Van-Frankenhuyzen et al. 2000).
Intoxication caused by the bacterium B. thuringiensis can result in physiological changes resulting from complex interactions between δ endotoxin and the intestinal epithelium of the insect pest (Fathipour et al. 2019). A process that can occur after the death of enterococci caused by the action of δ endotoxin is the activation of the healing mechanism in the damaged regions of the intestine. This process is regulated by proteins that control larval development, justifying the prolongation of this phase (Retnakaran et al. 1983; Sedaratian et al. 2013). Digestibility reduction due to changes in the number of proteases is another justification for slow development. Larvae exposed to δ endotoxin may have a prolonged stage to compensate for the costs associated with recovery from sub-lethal exposure, with increased food consumption (Martinez-Ramirez et al. 1999; Gujar et al. 2001; Dmitriew 2011).
Another fact that can occur is the change in the number of instars in response to the nutritional quality of the food (Sehnal 1985; Kidd and Orr 2001; Mayntz et al. 2003; Verdinelli and Sanna-Passino 2003). Studies carried out with S. nonagrioides showed that larvae fed with sub-lethal concentrations of the Cry1Ac toxin increased seedlings and had a longer development period. This larval delay can interfere with the emergence of resistant populations in the agroecosystem, providing susceptible insects to mate with resistant insects (Liu et al. 1999; Eizaguirre et al. 2005). When the larval stage is prolonged in field conditions, the likelihood of predation or parasitism may increase. This is observed in transgenic plants, the positive effect in generalist predators, as in Podisus nigrispinus Dallas, 1851 due to the indiscriminate reduction in the application of pesticides that directly contributes to the permanence, constancy and increase in the population of these natural enemies in field conditions. (Malaquias et al. 2014; Malaquias et al. 2014).
In addition, development parameters, such as survival and fertility, can be affected due to reduced digestibility of the food eaten (Erb et al. 2001; Janmaat et al. 2014). In the present study, our results using LC25 corroborate these findings.
Studies carried out with Choristoneura fumiferana Clemens, 1865 exposed to sub-lethal concentration of B. thuringiensis demonstrated that the pupal phase did not affect longevity since the larvae recovered from the exposure (Fast and Regniere 1984; Ramachandran et al. 1993). These studies corroborate our results; however, even with recovery, there was a reduction in adult longevity. The emerged moths showed a reduction in longevity with variation in egg production according to the treatment. Changes in fertility after treatment with the bacterium B. thuringiensis have also been reported for other species such as C. fumiferana and Spodoptera littoralis Boisduval, 1833 (Salama and Zaki 1986; Pedersen et al. 1997).
The reduction in fertility impacted the pest species’ population, affecting the population dynamics of the pest in the field. The reduction in egg production caused by the bacteria has been reported in other species such as S. littoralis and C. fumiferana (Salama and Zaki 1986; Bauce et al. 2006). These observations prove that the treatments have a direct effect on the reproductive system of A. gemmatalis. Another factor observed was deformations in the pre-pupa, pupa and adult phases, mainly in the anterior and/or posterior wings, increasing according to the increase in sub-lethal concentration. Insects have basic nutritional requirements such as lipids, carbohydrates and proteins (Dadd 1983). These nutria nts are required for the production of structural proteins and specific enzymes. However, some fatty acids are not synthesized by insects, such as linoleic and linolenic acid and must be ingested from the diet (Parra et al. 2012; Cohen 2015). These fatty acids are referred to as essential for the orders Orthoptera (Dadd 1960), Coleoptera (Vanderzant and Richardson 1964) and, mainly, Lepidoptera (Meneguim et al. 1997).
The insecticidal capacity of B. thuringiensis possibly caused some physiological imbalance in the insect pest, contributing to the lack of these fatty acids, which resulted in pre-pupae, pupae and deformed adults (Levinson and Navon 1969; Sivapalan and Gnanapragasam 1979; Bracken 1982; Dadd 1983). Physiologically, a greater energy allocation is expected for processes, such as growth with less allocation for metabolism and/or reproduction. The change in the allocation pattern can have positive or negative consequences depending on each individual (Dadd 1983). Under stress conditions, insects exposed to concentrations of bioinsecticides based on B. thuringiensis, spend more energy responding to infection. These additional energy expenditures result in less energy available for reproduction (Parsons 2000).
The adult pre-oviposition period (APOP) and the total pre-oviposition period (TPOP) were affected by the sub-lethal concentrations and changes in the proportion of males and females were observed. It is believed that these changes may be associated with differential susceptibility between the sexes and physiological effects on egg fertilization (Alix et al. 2001; Desneux et al. 2007).
Other studies showed sexual distortion in the face of insecticides (Morais et al. 2016; Delpuech and Meyet 2003). However, this is the first report on Bt-based bioinsecticides to act in the ratio between males and females. It should be noted that this study was carried out under laboratory conditions, where insects are subjected to maximum exposure to the bioinsecticide. Thus, it is possible that under field conditions, the effects on the sex ratio are lower than those observed in the present study. However, field studies must be carried out to verify the sub-lethal effects in the A. gemmatalis population and subsequent generations.
The Two-Sex fertility life table parameters of A. gemmatalis that characterize possible adequacy costs were significantly affected over the three generations assessed. These parameters can also be used to estimate the product’s effectiveness related to the target insect (Özgökçe et al. 2018; Rostami et al. 2018). The longer the period of development, the lower the survival rate, which results in less population increase. Therefore, population projection using the two-sex life table helps estimate variations in the different stages of the population development of the species under study and observe the separate behavior between both sexes (Chi 1990; Koner et al. 2019).
The gross reproductive rate (GRR) indicates the rapid increase in the population that depends on the number of eggs, hatched eggs and emergence of adults affected by the nutritional quality of the food eaten observed in the present study (Khaliq et al. 2007). Green leafhoppers Nephotettix virescens Distant, 1908 and Nephotettix cincticeps Uhler, 1896 exposed to sub-lethal concentrations of imidacloprid had a lower reproductive rate than those exposed to untreated and hormoligosis did not occur (Widiarta et al. 2001). Studies of behavioral hormoligosis, evaluating the oviposition preference of Bemisia tabaci Gennadius, 1889 revealed changes in the biochemical components of cotton leaves treated with insecticides. The results revealed that the plants treated with Carbaril and Endosulfan caused significant changes in the total phenols, in the pH value of the plants, reduction in total sugars and increase in the total free amino acids (Abdullah et al. 2006).
However, when using Fenvalerate, whiteflies preferred them for oviposition because these biochemical changes do not occur, which may be one of the causes of their resurgence in plants repeatedly treated with these insecticides (Abdullah et al. 2006). As the present study was conducted under laboratory conditions, there is a need to evaluate the effects of sub-lethal concentrations of Bt-based bioinsecticides in field conditions. Because the different sub-lethal concentrations can alter the biochemical components of the leaves of the plant and cause positive or negative effects on the pest. Hormoligosis can occur whenever the pest is exposed to a sub-lethal concentration. And pesticides, applied in lethal concentrations, tend to be reduced to sub-lethal concentrations over time and exposure to climatic conditions in the field (Dutcher, 2007).
The intrinsic population growth rate (r) is a useful parameter to describe the dynamic population of the pest species, which encompasses survival, development and reproduction, and the finite population growth rate (λ) revealed the total population decrease over a while under exposure to the treatments used (Farhadi et al. 2011; Rostami et al. 2018; Das et al. 2019). The life table parameters provided evidence of the sub-lethal effects of the Bt-based bioinsecticides in A. gemmatalis.
Hormoligosis is a phenomenon that occurs in the measurement of the dose-response to a series of concentrations of a treatment. A low dose (sub-lethal concentration) causes a stimulating response, and a high dose (recommended concentration) causes an inhibitory response (Calabrese and Baldwin, 2003). In these cases, the LC25 treatment showed an inhibitory response. For the other treatments, even with the alterations observed in the parameters of biological development, according to the theory of hormoligosis, the surviving insects are looking for new ways or improve the ones that have survived to deal with the exposure to sub-lethal concentrations, contributing to the emergence of populations with average levels of tolerance (Abivardi, 2004).
For integrated pest management, it means the permanence of insects in the field that will cause damage to the crop or the subsequent ones with the possibility of acquiring resistance due to hormoligosis. However, the lack of studies on the sub-lethal effects leaves this possible method unanswered and the adoption of the recommended concentration, together with selective pesticides, conservation of natural enemies and resistance of the host plant, becomes effective A. gemmatalis control with a reduction in the possibility of the emergence of resistant insect populations.
Conclusions
The variation of the sub-lethal concentration interfered in the biological parameters of A. gemmatalis, with emphasis on the LC25 as it did not provide subsequent descendants. The toxic effect of Bt-based bioinsecticide interfered mainly in the parameters of fertility, sex ratio, net reproduction rate (R0), and gross reproduction rate (GRR). It is unlikely that the theory of hormoligosis will not have a substantial impact on the performance of A. gemmatalis observed in its population growth rate; however, it should not be neglected because the induction of pest outbreaks in the agroecosystems is challenging to assess since other complex environmental factors are likely to be involved.
Data availability
The authors are available for availability of data and materials.
References
Abbas N, Khan HAA, Shad SA (2014) Resistance of the house fly Musca domestica (Diptera: Muscidae) to lambda-cyhalothrin: Mode of inheritance, realized heritability, and cross-resistance to other insecticides. Ecotoxicology 23:791–801. https://doi.org/10.1007/s10646-014-1217-7
Abdullah NMM, Singh J, Sohal BS (2006) Behavioral hormoligosis in oviposition preference of Bemisia tabaci on cotton. Pestic Biochem Physiol 84:10–16. https://doi.org/10.1016/j.pestbp.2005.03.011
Abivardi C (2004) Pesticide Hormoligosis. In: Encyclopedia of Entomology. Dordrecht: Springer. https://doi.org/10.1007/0-306-48380-7_3178
Alix A, Corteesero AM, Nénon JP, Anger JP (2001) Selectivity assessment of chlorfenvinphos reevaluated by including physiological and behavioral effects on an important beneficial insect. Environ Toxicol Chem 20:2530–2536. https://doi.org/10.1897/1551-5028(2001)020<2530:saocrb>2.0.co;2
Amaral FSA, Guidolin AS, Salmeron E, Kanno RH, Padovez FEO, Fatoretto JF, Omoto C (2019) Geographical distribution of Vip3Aa20 resistance allele frequencies in Spodoptera frugiperda (Lepidoptera: Noctuidae) populations in Brazil. Pest Manag Sci 76:169–178. https://doi.org/10.1002/ps.5490
Ameen AO, Fuxa JR, Richter AR (1998) Antagonism between formulations of different Bacillus thuringiensis subspecies in Heliothis virescens and Helicoverpa zea (Lepidoptera: Noctuidae). J Entomol Sci 33:129–134. https://doi.org/10.18474/0749-8004-33.2.129
Bauce E, Carisey N, Dupont A (2006) Carry over effects of the entomopathogen Bacillus thuringiensis subsp. Kurstaki on Choristoneura fumiferana (Lepidoptera: Tortricidae) progeny under various stressful environmental conditions. Agr Forest Entomol 8:63–76. https://doi.org/10.1111/j.1461-9555.2006.00283.x
Bernardi O, Malvestiti GS, Dourado PM, Oliveira WS, Martinelli S, Berger U, Head GP, Omoto C (2012) Assessment of the high-dose concept and level of control provided by MON 87701 × MON 89788 soybean against Anticarsia gemmatalis and Pseudoplusia includens (Lepidoptera: Noctuidae) in Brazil. Pest Manag Sci 68:1083–1091. https://doi.org/10.1002/ps.3271
Bracken GK (1982) The bertha armyworm, Mamestra configurata (Lepidoptera: Noctuidae) effects of dietary linoleic acid on pupal syndrome, wing syndrome, survival, and pupal fat composition. Can Entomol 114:567–573. https://doi.org/10.4039/Ent114567-7
Brand RJ, Pinnock DE, Jackson KJ, Milstead JE (1976) Viable spore count as an index of effective dose of Bacillus thuringiensis. J Invertebr Pathol 27:141–148. https://doi.org/10.1016/0022-2011(76)90139-7
Butt BA, Cantu E (1962) Sex determination of lepidopterous pupae. Washington: USDA, 7p
Calabrese EJ, Baldwin LA (2003) Hormesis: the dose-response revolution. Annu Rev Pharmacol Toxicol 43:175–197. https://doi.org/10.1146/annurev.pharmtox.43.100901.140223
Chi H (1988) Life-table analysis incorporating both sexes and variable development rates among individuals. Environ Entomol 17:26–34. https://doi.org/10.1093/ee/17.1.26
Chi H (1990) Timing of control based on the stage structure of pest populations: a simulation approach. J Econ Entomol 83:1143–1150. https://doi.org/10.1093/jee/83.4.1143
Chi H (2019) TwoSex-MSChart: a computer program for the age-stage, two-sex life table analysis. Disponível em: http://140.120.197.173/ecolo gy/Downl oad/Twose x-MSCha rt.rar>. Acesso em 20 de janeiro de 2020
Chi H, Liu H (1985) Two new methods for the study of insect population ecology. Bull Inst Zool 24:225–240
Chi H, Su HY (2006) Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ Entomol 35:10–21. https://doi.org/10.1603/0046-225X-35.1.10
Cohen AC (2015) Insect diets: Science and technology. Boca Raton: CRC Press 164p
Cohen E (2006) Pesticide-mediated homeostatic modulation in arthropods. Pestic Biochem Phys 85:21–27. https://doi.org/10.1016/j.pestbp.2005.09.002
Colinet H, Sinclair BJ, Vernon P, Renault D (2015) Insects in fluctuating thermal environments. Annu Rev Entomol 60:123–140. https://doi.org/10.1146/annurev-ento-010814-021017
Dadd RH (1960) The nutritional requirements of locusts. Development of synthetic diets and lipid requirements. J Insect Physiol 4:319–347. https://doi.org/10.1016/0022-1910(60)90057-3
Dadd RH (1983) Long-chain polyenoics and the essential dietary fatty acid requirement of the waxmoth, Galleria mellonella. J Insect Physiol 29:779–786. https://doi.org/10.1016/0022-1910(83)90007-0
Das S, Koner A, Barik A (2019) Biology and life history of Lema praeusta (Fab.) (Coleoptera: Chrysomelidae), a biocontrol agent of two Commelinaceae weeds, Commelina benghalensis and Murdannia nudiflora. Entom Res 109:463–471. https://doi.org/10.1017/S0007485318000731
Delpuech JM, Meyet J (2003) Reduction in the sex ratio of the progeny of a parasitoid wasp (Trichogramma brassicae) surviving the insecticide chlorpyrifos. Arch Environ Con Tox 45:203–208. https://doi.org/10.1007/s00244-002-0146-2
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106. https://doi.org/10.1146/annurev.ento.52.110405.091440
Dmitriew CM (2011) The evolution of growth trajectories: what limits growth rate? Biol Rev 86:97–116. https://doi.org/10.1111/j.1469-185X.2010.00136.x
Dutcher JD (2007) A Review of resurgence and replacement causing pest outbreaks in IPM. In: Ciancio A, Mukerji KG (Eds.) General Concepts in Integrated Pest and Disease Management. Integrated Management of Plants Pests and Diseases. Dordrecht: Springer. https://doi.org/10.1007/978-1-4020-6061-8_2
Efron B, Tibshirani RJ (1994) An introduction to the bootstrap. Boca Raton: CRC Press 456p
Eizaguirre M, Tort S, Lopez C, Albajes R (2005) Effects of sublethal concentrations of Bacillus thuringiensis on larval development of Sesamia nonagrioides. J Econ Entomol 98:464–470. https://doi.org/10.1093/jee/98.2.464
Erb SL, Bourchier RS, Van- Frankenhuyzen K, Smith SM (2001) Sublethal effects of Bacillus thuringiensis Berliner subsp. kurstaki on Lymantria dispar (Lepidoptera: Lymantriidae) and the Tachinid parasitoid Compsilura concinnata (Diptera: Tachinidae). Environ Entomol 30:1174–1181. https://doi.org/10.1603/0046-225X-30.6.1174
Farhadi R, Allahyari H, Chi H (2011) Life table and predation capacity of Hippodamia variegata (Coleoptera: Coccinellidae) feeding on Aphis fabae (Hemiptera: Aphididae). Biol Control 59:83–89. https://doi.org/10.1016/j.biocontrol.2011.07.013
Fast PG, Regniere J (1984) Effect of exposure time to Bacillus thuringiensis on mortality and recovery of the spruce budworm (Lepidoptera: Tortricidae). Can Entomol 116:123–130. https://doi.org/10.4039/Ent116123-2
Fathipour Y, Sedaratian A, Bagheri A, Talaei-Hassanlouei (2019) Increased food utilization indices and decreased proteolytic activity in Helicoverpa armigera larvae fed sublethal Bacillus thuringiensis-treated diet. Physiol Entomol 1–9. https://doi.org/10.1111/phen.12288
Frye RD, Scholl CG, Scholz EW, Funke BR (1973) Effect of weather on a microbial insecticide. J Invertebr Pathol 22:50–54. https://doi.org/10.1016/0022-2011(73)90009-8
Garbutt J, Michael BB, Wright DJ, Raymond B (2011) Antagonistic competition moderates virulence in Bacillus thuringiensis. Ecol Lett 14:765–772. https://doi.org/10.1111/j.1461-0248.2011.01638.x
Gholmie MAR, Lopes ION, Sosa-Gomez DR (2018) Suscetibilidade de Anticarsia gemmatalis (resistente e suscetível a toxina Cry1Ac) a inseticidas biológicos à base de Bacillus thuringiensis. In: Oliveira-Junior A, Leite RMVBC; Cattelan AJ. Goiânia: VIII Congresso Brasileiro de Soja, 8:201–203
Gómez I, Pardo-López L, Munoz-Garay C, Fernandez LE, Pérez C, Sánchez J, Soberón M, Bravo A (2007) Role of receptor interaction in the mode of action of insecticidal Cry and Cyt toxins produced by Bacillus thuringiensis. Peptides 28:169–173. https://doi.org/10.1016/j.peptides.2006.06.013
Goodman D (1982) Optimal life histories, optimal notation, and the value of reproductive value. Am Nat 119:803–823
Greene GL, Leppla NC, Dickerson WA (1976) Velvetbean caterpillar: A rearing procedure and artificial medium. J Econ Entomol 69:487–497. https://doi.org/10.1093/jee/69.4.487
Guedes RNC, Cutler GC (2013) Insecticide-induced hormesis and arthropod pest management. Pest Manag Sci 70:690–697. https://doi.org/10.1002/ps.3669
Guedes RNC, Magalhães LC, Cosme LV (2009) Stimulatory sublethal response of a generalist predator to permethrin: Hormesis, hormoligosis, or homeostatic regulation? J Econ Entomol 102:170–176. https://doi.org/10.1603/029.102.0124
Gujar GT, Kalia V, Kumari A (2001) Effect of sublethal concentration of Bacillus thuringiensis var. kurstaki on food and developmental needs of the American bollworm, Helicoverpa armigera (Hübner). Indian J Exp Biol 39:1130–1135
Haase S, Sciocco-Cap A, Romanowski V (2015) Baculovirus insecticides in Latin America: Historical overview, current status and future perspectives. Viruses 7:2230–2267. 3390/v7052230
Habib MEM, Andrade CFS (1998) Bactérias entomopatogênicas. In: Alves SB (Eds) Controle microbiano de insetos. Piracicaba: Fealq 2: 383–432
Horikoshi RJ, Bernardi O, Amaral FSA, Miraldo LL, Durigan MR, Bernardi D, Silva SS, Omoto C (2019) Lack of relevant cross-resistance to Bt insecticide XenTari in strains of Spodoptera frugiperda (J. E. Smith) resistant to Bt maize. J Invertebr Pathol 161:1–6. https://doi.org/10.1016/j.jip.2018.12.008
Janmaat AF, Bergmann L, Ericsson J (2014) Effect of low levels of Bacillus thuringiensis exposure on the growth, food consumption and digestion efficiencies of Trichoplusia ni resistant and susceptible to Bt. J Invertebr Pathol 119:32–39. https://doi.org/10.1016/j.jip.2014.04.001
Khaliq A, Attique M, Sayyed A (2007) Evidence for resistance to pyrethroids and organophosphates in Plutella xylostella (Lepidoptera: Plutellidae) from Pakistan. Entom Res 97:191–200. https://doi.org/10.1017/S0007485307004877
Kidd KA, Orr DB (2001) Comparative feeding and development of Pseudoplusia includens (Lepidoptera: Noctuidae) on kudzu and soybean foliage. Ann Entomol Soc Am 94:219–225. https://doi.org/10.1603/0013-8746(2001)094[0219:CFADOP]2.0.CO;2
Konecka E, Czarniewska E, Adam-Kaznowski A, Grochowska J (2018) Insecticidal activity of Bacillus thuringiensis crystals and thymol mixtures. Ind Crop Prod 117:272–277. https://doi.org/10.1016/j.indcrop.2018.03.010
Koner A, Debnath R, Barik A (2019) Age-stage, two-sex life table and food utilization efficiencies of Galerucella placida Baly (Coleoptera: Chrysomelidae) on two Polygonaceae weeds. J Asia-Pac Entomol 22:1136–1144. https://doi.org/10.1002/ps.5490
Lacey LA (2017) Insect and Mite Pests. From Theory to Practice. Amsterdam: Academic Press 484p
Levinson HZ, Navon A (1969) Ascorbic acid and unsaturated fatty acids in the nutrition of the Egyptian cotton leafworm, Prodenia litura. J Insect Physiol 15:491–495. https://doi.org/10.1016/0022-1910(69)90257-1
Liu YB, Tabashnik BE, Denehy TJ, Patin AL, Bartlett AC (1999) Development time and resistance to Bt crops. Nature 400:519. https://doi.org/10.1038/22919
Malaquias JB, Omoto C, Ramalho FS, Wesley WAC, Silveira RF (2015) Bt cotton and the predator Podisus nigrispinus (Dallas) (Heteroptera: Pentatomidae) in the management of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) resistance to lambda-cyhalothrin. J Pest Sci 88:57–63. https://doi.org/10.1007/s10340-014-0585-3
Malaquias JB, Ramalho FS, Omoto C, Godoy WAC, Silveira RF (2014) Imidacloprid affects the functional response of predator Podisus nigrispinus (Dallas) (Heteroptera: Pentatomidae) to strains of Spodoptera frugiperda (J.E. Smith) on Bt cotton. Ecotoxicology 23:192–200. https://doi.org/10.1007/s10646-013-1162-x
Martinez-Ramirez AC, Gould F, Ferre J (1999) Histopathological effects and growth reduction in a susceptible and a resistant strain of Heliothis virescens (Lepidoptera: Noctuidae) caused by sublethal doses of pure Cry1A crystal proteins from Bacillus thuringiensis. Biocontrol Sci Technol 9:239–246. https://doi.org/10.1080/09583159929811
Mayntz D, Toft S, Vollrath F (2003) Effects of prey quality and availability on the life history of a trap-building predator. Oikos 101:631–638. https://doi.org/10.1034/j.1600-0706.2003.12408.x
Meneguim AM, Parra JRP, Haddad ML (1997) Comparação de dietas artificiais, contendo diferentes fontes de ácidos graxos, para criação de Elasmopalpus lignosellus (Zeller) (Lepidoptera: Pyralidae). An Soc Entomol Bras 26:35–43. https://doi.org/10.1590/S0301-80591997000100005
Morais MR, Zanardi OZ, Rugno GR, Yamamoto PT (2016) Impact of five insecticides used to control citrus pests on the parasitoid Ageniaspis citricola Longvinovskaya (Hymenoptera: Encyrtidae). Ecotoxicology 25:1011–1020. https://doi.org/10.1007/s10646-016-1658-2
Moreau G, Bauce E (2003) Lethal and sublethal effects of single and double applications of Bacillus thuringiensis variety kurstaki on spruce budworm (Lepidoptera: Tortricidae) larvae. J Econ Entomol 96:280–286. https://doi.org/10.1603/0022-0493-96.2.280
Moscardi F, Bueno AF, Sosa-Gómez DR, Roggia S, Hoffmann-Campo CB, Pomari AF, Corso IC, Yano SAC (2012) Artrópodes que atacam as folhas da soja. In: Hoffmann-Campo CB, Corrêa-Ferreira BS, Moscardi F (Eds.) Manejo Integrado e Insetos e outros Artrópodes Praga. Brasília: Embrapa, p 213–334
Özgökçe MS, Chi H, Atlıhan R, Kara H (2018) Demography and population projection of Myzus persicae (Sulz.) (Hemiptera: Aphididae) on five pepper (Capsicum annuum L.) cultivars. Phytoparasitica 46:153–167. https://doi.org/10.1007/s12600-018-0658-6
Parra JRP, Panizzi AR, Haddad ML (2012) Nutritional indices for measuring food intake and utilization. In: Panizzi AR, Parra JRP (Eds.) Insect bioecology and nutrition for integrated pest management. Boca Ratón: CRC Press p 13–49
Parsons PA (2000) Hormesis: an adaptive fitness response and an evolutionary expectation in stressed free-living populations, with particular reference to ionizing radiation. J Appl Toxicol 20:103–112. https://doi.org/10.1002/(SICI)1099-1263(200003/04)20:23.0.CO;2-O
Pedersen A, Dedes J, Gauthier D, Van-Frankenhuyzen K (1997) Sublethal effects of Bacillus thuringiensis on the spruce budworm, Choristoneura fumiferana. Entomol Exp Appl 83:253–262. https://doi.org/10.1023/A:1002965901086
Pinnock DE, Brand RJ, Milstead JE (1971) The Field Persistence of Bacillus thuringiensis Spores. J Invertebr Pathol 18:405–411. https://doi.org/10.1016/0022-2011(71)90046-2
Pinto CPG, Azevedo EB, Santos ALZ, Cardoso CP, Fernandes FO, Rossi GD, Polanczyk RA (2019) Immune response and susceptibility to Cotesia flavipes parasitizing Diatraea saccharalis larvae exposed to and surviving an LC25 dosage of Bacillus thuringiensis. J Invertebr Pathol 166:107209. https://doi.org/10.1016/j.jip.2019.107209
Ramachandran R, Raffa KF, Miller MJ, Ellis DD, Mccown BH (1993) Behavioral responses and sublethal effects of spruce budworm (Lepidoptera: Tortricidae) and fall webworm (Lepidoptera: Arctiidae) larvae to Bacillus thuringiensis Cry1A(a) toxin in diet. Environ Entomol 22:197–211. https://doi.org/10.1093/ee/22.1.197
Retnakaran A, Lauzon H, Fast PG (1983) Bacillus thuringiensis induced anorexia in the spruce budworm, Choristoneura fumiferana. Entomol Exp Appl 34:233–239. https://doi.org/10.1111/j.1570-7458.1983.tb03327.x
Rostami N, Maroufpoor M, Sadeghi A, Ghazi MM, Atlıhan R (2018) Demographic characteristics and population projection of Phytonemus pallidus fragariae reared on different strawberry cultivars. Exp App. Acarol 23:2224–2236. https://doi.org/10.1007/s10493-018-0326-z
Salama HS, Zaki FN (1986) Effect of Bacillus thuringiensis Berliner on prepupal and pupal stages of Spodoptera littoralis (Boisd.) (Lepidoptera: Noctuidae). Int J Trop Insect Sci 7:747–749. https://doi.org/10.1017/S1742758400011838
Santos MS, Dias NP, Costa LL, Bortoli CP, Souza EH, Santos ACF, Bortoli SA, Polanczyk RA (2019) Interactions of Bacillus thuringiensis strains for Plutella xylostella (L.) (Lepidoptera: Plutellidae) susceptibility. J Invertebr Pathol 168:107255. https://doi.org/10.1016/j.jip.2019.107255
Sas University (2013) SAS® 9.4 Statements. Third Edition SAS Instit. Inc., USA
Sedaratian A, Fathipour Y, Talaei-Hassanloui R, Jurat-Fuentes JL (2013) Fitness costs of sublethal exposure to Bacillus thuringiensis in Helicoverpa armigera: a carryover study on offspring. J Appl Entomol 137:540–549. https://doi.org/10.1111/jen.12030
Sehnal F (1985) Growth and life cycles. In: Kerkut GA, Gilbert LI (Eds.) Comprehensive insect physiology, biochemistry, and pharmacology. Oxford: United Kingdom, p 121–123
Silva MTB, Costa EC, Boss A (2003) Control of Anticarsia gemmatalis Hübner (Lepidoptera: Noctuidae) larvae with insect growth regulators. Ciênc Rural 33:601–605. https://doi.org/10.1590/S0103-84782003000400002.
Sivapalan P, Gnanapragasam NC (1979) The influence of linoleic acid and linolenic acid on adult moth emergence of Homona caffearia from meridic diets in vitro. J Insect Physiol 25:393–398. https://doi.org/10.1590/S0301-80591997000100005
Tabashnik BE (1994) Evolution of resistance to Bacillus thuringiensis. Annu Rev Entomol 39:47–79. https://doi.org/10.1146/annurev.en.39.010194.000403
Vanderzant ES, Richardson CD (1964) Nutrition of the adult boll weevil: lipid requirements. J Insect Physiol 10:267–272. https://doi.org/10.1016/0022-1910(64)90010-1
Van-Frankenhuyzen K, Nystrom C, Dedes J, Seligy V (2000) Mortality, feeding inhibition, and recovery of spruce budworm (Lepidoptera: Tortricidae) larvae following aerial application of a high-potency formulation of Bacillus thuringiensis subsp. kurstaki. Can Entomol 132:505–518. https://doi.org/10.4039/Ent132505-4
Van-Rie J, Ferré J (2000) Insect resistance to Bacillus thuringiensis crystal proteins. In: Charles JF, Delecluse A, Leroux N (Eds.) Entomopathogenic bacteria. Netherlands: Kluwer Academic Publications, p 219–237
Verdinelli M, Sanna-Passino G (2003) Development and feeding efficiency of Malacosoma neustrium larvae reared with Quercus spp. leaves. Ann Appl Biol 143:161–167. https://doi.org/10.1111/j.1744-7348.2003.tb00282.x
Wei J, Guo Y, Liang G, Wu K, Zgang J, Tabashnik BE, Li X (2015) Cross resistance and interactions between Bt toxins Cry1Ac and Cry2Ab against the cotton bollworm. Sci Rep 5:7714. https://doi.org/10.1038/srep07714
Widiarta IN, Matsumura M, Suzuki Y, Nakasuji F (2001) Effects of sublethal doses of imidacloprid on the fecundity of green leafhoppers, Nephotettix spp. (Hemiptera: Cicadellidae) and their natural enemies. Appl Entomol Zool 36:501–507. https://doi.org/10.1303/aez.2001.501
Xue JL, Cai QX, Zheng DS, Yuan ZM (2005) The synergistic activity between Cry1Aa e Cry1Ac from Bacillus thuringiensis against Spodoptera exigua and Helicoverpa armigera. Lett Appl Microbiol 40:460–465. https://doi.org/10.1111/j.1472-765X.2005.01712.x
Author contributions
FOF, JAD, and RAP conceived and designed the research. FOF, TDS, and ACS conducted experiments. FOF and TDS analyzed the data. FOF wrote the first draft. NPD and RAP revised and edited the manuscript. All authors read and approved the manuscript.
Funding
We thank the Coordination for the Improvement of Higher Education Personnel (CAPES) and the Paulista State University “Júlio de Mesquita Filho” for the scholarship and infrastructure grants. This study was financed in part by the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) - Financial Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Informed consent was obtained from all individual participants included in the study.This study was conducted with a pest species reared under laboratory conditions, in accordance with the standards required by the Department of Agricultural Entomology of the State University “Júlio de Mesquita Filho”, Unesp / Jaboticabal, São Paulo - Brazil.
Consent to participate
All authors were informed individually and approved the publication of the study result.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Research involving human participants and/or animals’ note
This study does not involve endangered or protected species
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernandes, F.O., de Souza, T.D., Sanches, A.C. et al. Sub-lethal effects of a Bt-based bioinsecticide on the biological conditioning of Anticarsia gemmatalis. Ecotoxicology 30, 2071–2082 (2021). https://doi.org/10.1007/s10646-021-02476-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02476-5