Abstract
Aquatic invertebrates can be exposed to copper from various sources, including agricultural applications. For example, concentrations up to 1000 µg L−1 are found within rice fields, where copper-containing formulations are used as fungicides and algaecides. We conducted toxicity tests to study lethal and sublethal effects of copper sulfate pentahydrate on all immature stages across two generations of Culex pipiens mosquitoes as our model organism. Mortality was dose-dependent at concentrations of 500 µg L−1 and above in the first generation, and 125 µg L−1 and above in the second generation. The median lethal concentrations (LC50) of copper sulfate pentahydrate for larval Cx. pipiens were 476 ± 30.60 µg L−1 and 348.67 ± 23.20 µg L−1 for the first and second generations, respectively. Generation one pupation decreased from 96% in controls to 48% at 500 µg L−1, while the second-generation pupation decreased from 96% in controls to 17.5% at 500 µg L−1. Mortality during the pupal stage varied from 2 to 10% at 500 µg L−1 of first and second generations, respectively. Higher levels also delayed development to adulthood in both generations. The duration of the immature period was 14.8 days in controls in both generations, but when exposed at 500 µg L−1 it increased to 18.8 days in the first generation and to 20.5 days in the second generation. The chronic, multi-generation exposures in this study showed greater toxicity than reported for shorter exposures of Cx. pipiens, and confamilial taxa like Culex hortensis and Anopheles hispaniola.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
All over the world, natural ecosystems have been adversely affected by anthropogenic activities (Dirilgen, 2001; Rajaganapathy et al., 2011; Benson et al., 2016). Industrialization, modern farming and increased vehicular use have led to elevated concentrations of heavy metals such as copper, cadmium, chromium, lead, nickel, and zinc throughout the biosphere (Farombi et al., 2007; Atafar et al., 2010; Rajaganapathy et al., 2011). These potentially toxic heavy metals become part of the biogeochemical cycle when they contaminate soil, air and water regularly (Lee et al., 2006; Byrne et al., 2012). Numerous researchers have examined effects of heavy metals on aquatic ecosystems (Dirilgen, 2001; El-Sheikh et al., 2010; Malaj et al., 2012), species diversity (Yan et al., 1996; Dutta et al., 2010), community productivity (Hill et al., 1997; Farombi et al., 2007) and animal behavior (Bonnard et al., 2009; Mogren & Trumble, 2010; Thomas et al., 2016). Despite many studies concerning the effect of metal pollutants and their physiological costs, there are gaps in knowledge about the sublethal and lethal effects of metal pollutants. For example, long-term and cross-generational studies are rare (Vedamanikam & Shazilli, 2008; Brix et al., 2011; Marinković et al., 2012; Ellis et al., 2020).
Insects are popularly used as bioindicators because the Class Insecta is the most diverse group of animals found in fresh water (Hare, 1992; El-Shenawy et al., 2010). Insects can accumulate metals depending on chemical speciation and free metal ion activity in which concentrations of metal in insects is often correlated with that of the environment (De Jonge et al., 2014). Heavy metals have great acute and chronic effects on various insects, such as morphological changes, growth inhibition, developmental abnormalities, decreased hatchability and reduced reproduction (e.g., Sildanchandra & Crane, 2000; El-Sheikh et al., 2010; Perez & Noriega, 2014; Goswami et al., 2019; Mebane et al., 2020). We focused on copper because it has played a significant role in reducing the abundance and altering the community composition of aquatic insects (Brix et al., 2011, Clements et al., 2013).
We chose the mosquito Culex pipiens (Culicidae: Diptera) as our test organism for two reasons. First, mosquitoes are one of few aquatic insects that can be cultured in the laboratory and so they are useful as indicator species. It is essential to determine the susceptibility to metal pollutants of insects relative to other taxa (Brix et al., 2011). Cx. pipiens, is distributed worldwide except for Australia and Antarctica (Farajollahi et al., 2011). It is an essential vector for periodic lymphatic filariasis (Farid et al., 2001), West Nile virus (Turell et al., 2001), St. Louis encephalitis (Mackenzie et al., 2002), Western equine encephalitis, Japanese encephalitis, and Rift Valley fever (Kramer & Ebel, 2003; Farajollahi et al., 2011). It frequently inhabits very small containers as well as larger habitats (Vinogradova, 2000; Vezzani & Albicocco, 2009).
Copper is one of many metals that Cx. pipiens are exposed to in their environment. Copper is one of the trace metals that are important for functions of many enzymes like hemocyanin, cytochrome oxidase and monoamine oxidase but it could cause environmental impacts when it used in excessive amounts (Scheiber et al., 2014). Copper may enter water when used as a biocide in antifouling paint formulations or from agricultural and urban application sites. It is widely used as a fungicide, as an algaecide and as a molluscicide to control leeches and tadpole shrimps in rice farming, and for root treatment of various crops (Bishop et al., 2014; Wagner et al., 2017; Lusk & Chapman, 2020). Copper sulfate is often used as algaecide with the rate of 1000–1500 µg L−1 in freshwater systems (Houmann, 2015). Copper is used in both conventional and organic agriculture, and poses a risk to ecosystems (Goswami et al., 2019). Copper affects the nervous systems of aquatic organisms (Viarengo & Nicotera, 1991; Tilton et al., 2011; Thomas et al., 2016), insect metabolism (Bagatto & Shorthouse, 1996; Joachim et al., 2017; Ilahi et al., 2020) and it impacts non-target organisms, resulting in environmental concerns (Bishop et al., 2014; Rothmeier et al., 2020). Additionally, copper can be used to clarify main ecotoxicological concepts like biodynamics, lethality and sublethal effects (Luoma & Rainbow, 2005; Clements et al., 2013; Goswami et al., 2019).
This study aims to investigate long-term effects of copper exposure on instar development and adult emergence of Cx. pipiens across two successive generations. Due to copper’s known toxicity, we hypothesized that long-term elevated copper exposure would decrease larval survival, growth rate, and development to pupal and adult stages. In addition, we hypothesized a lower sensitivity of Cx. pipiens in subsequent generations, due to the loss of susceptible individuals and genetic adaptation.
Materials and methods
Insect collection
We obtained Cx. pipiens larvae from the Sacramento-Yolo Mosquito and Vector Control District (SYMVCD; www.fightthebitenet), and reared them through adulthood to produce first instar larvae for exposures. This laboratory population was initially obtained from Merced, CA., sometime in the 1950s, and has been maintained as a “susceptible” strain, unexposed to toxicants. While inbreeding is likely, the insects appear robust and normal. Such strains are used routinely in environmental toxicology as a precautionary approach. A domesticated strain was also the best choice for a two generations study because field populations may not breed in the lab. Field caught populations have additional issues associated with them such as epigenetic modifications and potential localized pesticide resistance. Moreover, standard mosquito strains are used in many laboratories in the world because they are more experimentally reproducible than field strains (Bonizzoni et al., 2012; Costa-Da-Silva et al., 2017). The strain we used is maintained by several California Mosquito and Vector Control Districts, which rear the insects in large colonies of hundreds to thousands. Districts exchange specimens every few years to maintain colony vigor. Due to the ubiquity of pesticide-resistance genes in wild mosquito populations outcrossing with wild mosquitoes has not been possible in many years (Personal communication, Deborah Dritz, Sacramento-Yolo Mosquito and Vector Abatement District, Elk Grove, CA). SYMVCD reared larvae and adults in an environmental chamber at 25 ± 0.5 °C and ~80% humidity, with 18:6 h day/night cycle. We followed SYMVCD’s general rearing technique. Larvae were reared in enamel trays (40 × 25 cm) filled with ~1 L reconstituted deionized water (RDiW). The reconstituted deionized water consisted of 0.096 g NaHCO3, 0.06 g MgSO4, 0.004 g KCl, and 0.06 g CaSO4.2H2O dissolved in 1 L deionized water mixed according to (Weber, 1991). Larval diet stock proportions consisted of 5 g ground fish food (Tetramin®), 5 g ground and sifted alfalfa, ½ g of nutritional yeast and ½ g of ground beef liver. About 80% of rearing water was renewed once a week. We transferred pupae into cups filled with RDiW water and kept them in cages with dimensions of (30 × 30 × 30 cm) until adult emergence. Each cage was provided with a piece of sponge soaked in 10% sugar solution. After 4 or 5 days, heparinized sheep blood meal (Hemostat Laboratories, Dixon, CA) was made available to females to feed from via a small beaker covered with thin parafilm, inverted and placed on the top of the cage. We provided oviposition cups filled with RDiW water in each cage to receive F1 egg rafts.
Copper solution
Copper sulfate pentahydrate (CuSO4.5H2O) was obtained from Sigma Aldrich, CA; purity: 99.9%. We prepared a stock solution of 10 g L−1 by weighing 0.1 g of CuSO4.5H2O, putting it in a volumetric flask, and adding RDiW water to a volume of 10 mL and mixing well. From this stock solution, we prepared four test concentrations: 125, 250, 500 and 1000 µg L−1 in RDiW in addition to controls. We chose these concentrations to be under the human safe limit of copper (1300 µg L−1) in fresh water (Fitzgerald, 1998; Potera, 2004). On the first day of the experiment, 30 mL of newly prepared control water and each concentration were saved in plastic Falcon© tubes at 4 °C for chemical analysis. Nominal concentrations of CuSO4.5H2O were designed to provide equivalent concentrations of Cu+2 of 0, 31, 63, 127, 254 µg L−1 which were measured and confirmed using an ICP-MS instrument (Interdisciplinary Center for Plasma Mass Spectrometry, UC Davis) to be 0 ± 0.001, 28 ± 0.002, 69 ± 0.008, 129 ± 0.013 and 335 ± 0.031 µg L−1 respectively under the following liquid sample digestion procedure. A volume of 120 µL concentration trace metals grade HNO3 was added to 1.5 mL of each sample (suffix-D), then samples were introduced to a hot block at 95 °C for ½ h. A volume of 75 µL 30 % H2O2 was added incrementally to hot samples (25 and 50 µL was added over 5 min) and heating was continued for an additional 1 h, before removal from the hot block. Samples were allowed to cool, capped, centrifuged and brought up to a final volume (FV) = 1.5 mL with MQW. Final matrix = 8% HNO3.
Effects of copper exposure on Cx. pipiens development and emergence
We conducted toxicity tests according to (El-Sheikh et al., 2010) under laboratory controlled conditions of 25 ± 2 °C and a 18:6 light: dark photoperiod, as described for rearing conditions. The study consisted of five replicates for controls and each concentration of the first generation, while the second generation consisted of five replicates only for controls and 125 µg L−1, and only four replicates for higher concentrations, due to fewer animals available. Each replicate was a polyethylene plastic cup containing 100 mL of copper solution and ten first instar larvae (<24 h); plastic is recommended for such tests because most metals do not bind to it (Nollet et al., 2000). We initially added 0.002 g of larval food to each container, increasing the amount to 0.004 g as larvae grew. We renewed 90% of the solution every 48 h. Each day we recorded development (instar/stage), at which time mortality was also recorded and any dead organisms removed from the vessels (Hasenbein et al., 2015). Emerged adults from the first generation were kept in cages with dimensions of (30 × 30 × 30 cm; described above) to continue with the second-generation toxicity experiment. We combined the adults from the replicates within treatments, for one cage per treatment. Sponges soaked in 10% sugar solution were provided in each cage. After 4 or 5 days, females were allowed to take heparinized sheep blood meal and we provided oviposition cups. Egg rafts resulting from control and each concentration except 1000 µg L−1 were collected separately and transferred to rearing trays without tracking of their productivity. Each tray contained not more than three egg rafts. Hatchling larvae were then chosen randomly from each tray and used in the second-generation experiment, conducted as described.
Statistics
Response variables were larval mortality, accumulated mortality through adult emergence, and complete immature period (days). Histograms and QQ-plots were used to check the normality of the data. Almost none of the response variables met the normality assumption. We could not find any transformation that normalized the data after trying several transformations. We conducted Kruskal–Wallis one-way analysis of variance (ANOVA) tests on larval mortality, accumulated mortality through adult emergence and development time. For post-hoc tests we used Dunn’s Test as a non-parametric multiple comparison method using rank sums, which controls error rate for multiple comparisons within response variables. While this does not adjust the experiment-wise alpha level, it strikes a balance between Type I and II error rates and is further justified because all tests were based on the a priori hypotheses that increasing copper sulfate exposures would have negative effects on mosquito growth and development. We used probit analysis using Minitab program to determine median lethal concentrations (LC50) of the total larval period for each Cx. pipiens generation with 95% confidence intervals. Lifetime survival was estimated using the non-parametric Kaplan-Meier survival curve. All analyses were carried out using Minitab program.
Results
Expected and measured Cu2+ concentrations were usually very similar (Table 1). Therefore, for simplicity we will present results in terms of the nominal concentrations of CuSO4.5H2O.
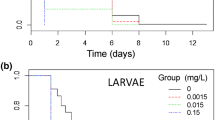
The first generation showed a sharp increase in larval mortality (H(χ2) = 19.90, p = 0.001*, df = 4) from an average of 4% in controls to 100% at the highest concentration of 1000 µg L−1 (Fig. 1). Mortality at 1000 µg L−1 was significantly greater than all treatments except 500 µg L−1 (Table 2). We calculated the LC50 of the first generation’s larvae to be 476 ± 30.60 µg L−1 (421.99–546.06 µg L−1). The accumulated mortality through adult emergence increased significantly very similar to larval mortality (H(χ2) = 19.90, p = 0.001*, df = 4) from 94% in controls to zero at 1000 µg L−1 and 54% at 500 µg L−1 (Fig. 1). There was a non-significant pupal mortality of 2% at 125 and 500 µg L−1 of this generation. The effect of concentration of 500 µg L−1 was significantly different from all other treatments except for 250 µg L−1 (Table 3). Copper also delayed development (H(χ2) = 11.68, p = 0.0128*, df = 4): surviving controls all emerged by day 16 whereas at least some larvae exposed to 1000 µg L−1 were still in the larval stage by days 20–22, although none of these pupated (Fig. 1). The effect of concentrations 250 and 500 µg L−1 also showed non-significant trends toward developmental delay (both p ~0.10; Fig. 1 and Table 4).
The second generation showed notably larger changes in larval mortality (H(χ2) = 13.61, p = 0.003*, df = 3) with copper exposure. Control and 125 µg L−1 mortalities were again about 4% but rose to 82.5% at 500 µg L−1 (Table 2 and Fig. 2). The LC50 level was substantially lower than in the first generation at 348.67 ± 23.20 µg L−1 (306.52–400.85 µg L−1). Both controls started to pupate at day 8. The second-generation cumulative mortality through adult emergence decreased significantly (H(χ2) = 13.96, p = 0.003*, df = 3) from 96% in controls to 7.5% at 500 µg L−1. There was a non-significant pupal mortality of 10% at 500 µg L−1 of this generation. The development delays of the second generation were higher than that of the first (Fig. 1 and 2). There were significant increases (H(χ2) = 13.31, p = 0.0029*, df = 3) in the immature period at all concentrations tested, of up to ~4–5 days compared to controls (Table 4 and Fig. 2). We caution that two false positives might be expected among the post-hoc contrasts presented above. However, 16 contrasts were significant, and all were in expected directions.
Discussion
Multi-generation toxicology studies are valuable in revealing whether tolerance increases or decreases across generations (Vedamanikam & Shazilli, 2008; Marinković et al., 2012; Jegede et al., 2019; Ellis et al., 2020) depending on the nature of maternal effects or selection. However, our study could not distinguish between selection and maternal effects, and selection on a laboratory strain might not reflect what could happen with field strains (Marinković et al., 2012). In contrast to our hypothesis that effects would decrease across generations due to genetic adaptation and loss of susceptible individuals, we found increased sensitivity effects across generations. In the first generation, 48% of larvae survived at 500 µg L−1. In the second-generation bred from parents that were exposed to copper during their immature stages, only 17.5% of larvae survived a 500 µg L−1 exposure. Reza & Ilmiawati (2020) also demonstrated the susceptibility of Cx. pipiens to copper in a one generation study. This might be due to copper bioaccumulation within insect tissue. They argued these findings were due to the impact of copper on the commensal bacteria in the larval midgut. In our study, the increase in sensitivity of the second generation may be due to longer-term exposure of the immature stages at the second generation than those of the first one. Phenotypic plasticity is another mechanism that could change sensitivity across generations (Marinković et al., 2012). Reza & Ilmiawati (2020) postulated the susceptibility was due to intestinal dysfunction (Matsuoka et al., 2015) that reduced the larva’s ability to produce energy to complete its development. To our knowledge, this is the first study quantifying the effects of copper on the survival and development of the widespread mosquito Cx. pipiens across two successive generations.
Chronic exposure to copper at 1000 µg L−1 resulted in 100% mortality of developing, first generation, Cx. pipiens mosquitoes. This is an approved application rate for controlling algae in rice fields (Epstein & Bassein, 2003; Stevens et al., 2014). This suggests that mosquito control may not be necessary immediately after algaecide treatment. However, further field tests are advisable because sensitivities of wild mosquitoes may differ. Reported sensitivity to pollutants, including copper exposure, varies across mosquito species. Our study species, Cx. pipiens, is considered to be somewhat tolerant of poor water conditions (Vinogradova, 2000). Members of its species complex can occur in waters contaminated with copper from industrial, domestic, and agricultural sources, and this has the potential to affect breeding success (Ilahi et al., 2020). Cx. pipiens are relatively resistant to insecticides such as deltamethrin, lambda-cyhalothrin, beta-cyfluthrin and bifenthrin (Al-Sarar, 2010; Ghorbani et al., 2018), so negative effects on this taxon are worrying for other taxa as well.
The LC50 levels determined in this study were 476 ± 30.60 µg L−1 (421.99–546.06 µg L−1) and 348.67 ± 23.20 µg L−1 (306.52–400.85 µg L–1) for the first and second generations respectively in which larvae have been exposed from the first instar. Another unigenerational study estimated the LC50 of Cx. pipiens toward copper to be 300 µg L−1 (Reza & Ilmiawati, 2020), which might be lower because they observed larval mortality until day 22 only. This is lower than previously reported in this and other mosquito species, however, most other exposure tests were shorter (24–96 h) (e.g., Bouallam & Nejmeddine, 2001; Mireji et al., 2010; Perez & Noriega, 2014). In our study most mortality occurred after 96 h. Although 24–96 h tests are standard, total mortality throughout the immature stages is also of interest.
The contrast in sensitivities to copper exposure across semiaquatic invertebrates is broad (Bouallam & Nejmeddine, 2001). Although there are few exposure tests lasting most of larval development, some tests conducted longer than 24 h also show lower effect concentrations. Since our larvae have been exposed from the first instar, they are expected to be more sensitive than those exposed at later stages. In a study determining LC50 levels of CuSO4 for chironomid fly larvae Chironomus ramosus, third instars were exposed to CuSO4 for 24, 48, 72 and 96 h which resulted in LC50 of 3280,1073, 780 and 1830 µg L−1 respectively (Majumdar & Gupta, 2012). A study on Cx. pipiens collected from the field and reared for several generations in the lab indicated that the LC50 of larval stage (starting from the second instar) for CuSO4 was 5090 µg L−1 (El-Sheikh et al., 2010). Bouallam & Nejmeddine (2001) studied the sensitivity of Cx. pipiens, Cx. hortensis and Anopheles hispaniola to CuSO4 in an experimental wastewater lagoon using 24 h exposure of the fourth larval stage, finding LC50 of 5910, 5020 and 3780 µg L−1 respectively. Another mosquito, Aedes aegypti, had an intermediate LC50 of 2060 μg L−1 in 24 h trials in the third instar (Rockefeller strain; Perez & Noriega, 2014). However, that species’ response is variable; Rayms-Keller et al. (1998) found an LC50 for 24 h of the third instar of the Puerto Rico strain of Aedes aegypti of 33000 μg L−1. Differences between studies could also be due to stage, source localities or to the number of generations that the organisms were maintained in the laboratory
To put these dipteran studies in a broader context, a review showed that among aquatic insects, the insect orders most tolerant to copper may be Trichoptera and the suborder Zygoptera, while the most sensitive orders may be Ephemeroptera, Diptera and the suborder Heteroptera (Malaj et al., 2012). However, considerable variation may be found at the species level. Similarly, Brix et al. (2011) found that Trichoptera and Plecoptera were less sensitive than Ephemeroptera and Diptera (mainly chironomids). Studies concerning the effect of Cu on non-dipteran aquatic insects, including field studies (Schmidt et al., 2010) and microcosm experiments (Clark & Clements, 2006; Clements, 2004) showed greater sensitivities of mayflies. Brinkman & Johnston (2008) found that the mayfly Rhithrogena hageni had an LC50 of waterborne Cu at 96 h of 137 µg L−1. Although a review by Iwasaki & Ormerod (2012) suggested that 6.6 µg L–1 was generally safe, it is useful to know sensitivities of different groups to inform how food webs might change with greater exposures.
In addition to causing larval mortality, copper delayed mosquito development and reduced adult emergence, both of which are relevant to mosquito abatement. Developmental delay exposes immature mosquitoes to other sources of mortality such as predation (if predators are not similarly impacted by copper), and pathogen transmission could be reduced if there are fewer and smaller generations of adults. In the first generation almost all control mosquitoes had pupated by day 14, but at concentration of 500 µg L−1 and above at least some larvae remained as larvae past day 20 (Fig. 1d). Generation two larvae were more sensitive to copper exposure, with higher developmental delays and much greater losses in emerged adults (Fig. 2). Less than 10% emerged as adults at the highest concentration 500 µg L−1 of the second generation (Fig. 2d). Other studies have also found developmental delays in dipterans. In a study of the effect of copper sulfate pentahydrates on Aedes aegypti, larval development period was 17.38 days versus 5.95 days for controls (Perez & Noriega, 2014). Another study on Culex pipiens, Aedes aegyptae and Anopheles stephensi demonstrated that copper prolonged pupation time and adult emergence (Reza & Ilmiawati, 2020). Also at 1.8 µg L−1 CuSO4 concentration, pupation of the Chironomus ramosus fly was delayed (Majumdar & Gupta, 2012). In contrast, there were few and variable sublethal effect of copper on nine generations of Chironomus riparius in which copper concentrations were 10–30 mg Cu/kg dw of sediment (Marinković et al., 2012).
We conducted this study using copper sulfate pentahydrate due to its broad use. Copper is one of the highly impactful heavy metals to aquatic organisms and ecosystems. Sensitive insects will be affected at exposures less than some current application rates, which could affect the equilibrium and the composition of the aquatic community. Impacts on mosquito numbers and development rates may be beneficial to mosquito abatement. However, when viewed as a model organism, the high toxicity of copper to a relatively pollution-tolerant mosquito is worrying. This study filled an information gap concerning long-term exposure and cumulative effects across generations. Our results revealed that current rates of application for some purposes may not be protective of aquatic insects. In addition, we found higher sensitivity of the second generation of Cx. pipiens relative to the first one, and also revealed that copper caused a developmental delay.
References
Al-Sarar AS (2010) Insecticide resistance of Culex pipiens (L.) populations (Diptera: Culicidae) from Riyadh city, Saudi Arabia: status and overcome. Saudi J Biol Sci 17:95–100. https://doi.org/10.1016/j.sjbs.2010.02.001
Atafar Z, Mesdaghinia A, Nouri J et al. (2010) Effect of fertilizer application on soil heavy metal concentration. Environ Monit Assess 160:83–89. https://doi.org/10.1007/s10661-008-0659-x
Bagatto G, Shorthouse JD (1996) Accumulation of Cu and Ni in successive stages of Lymantria dispar L. (lymantriidae, lepidoptera) near ore smelters at Sudbury, Ontario, Canada. Environ Pollut 92:7–12. https://doi.org/10.1016/0269-7491(95)00092-5
Benson NU, Asuquo FE, Williams AB et al. (2016) Source evaluation and trace metal contamination in benthic sediments from equatorial ecosystems using multivariate statistical techniques. PLoS One 11:1–19. https://doi.org/10.1371/journal.pone.0156485
Bishop WM, Johnson BM, Rodgers JH (2014) Comparative responses of target and nontarget species to exposures of a copper-based algaecide. J Aquat Plant Manag 52:65–70
Bonizzoni M, Dunn WA, Campbell CL et al. (2012) Complex modulation of the Aedes aegypti transcriptome in response to dengue virus infection. PLoS One 7:1–14. https://doi.org/10.1371/journal.pone.0050512
Bonnard M, Roméo M, Amiard-Triquet C (2009) Effects of copper on the burrowing behavior of estuarine and coastal invertebrates, the polychaete Nereis diversicolor and the bivalve Scrobicularia plana. Hum Ecol Risk Assess 15:11–26. https://doi.org/10.1080/10807030802614934
Bouallam S, Nejmeddine A (2001) Effects of heavy metals- Cu, Hg, Cd- on three species of mosquitoes larvae (Diptera: Culicidae). Annales de Limnologie 37:49–57
Brinkman SF, Johnston WD (2008) Acute toxicity of aqueous copper, cadmium, and zinc to the mayfly Rhithrogena hageni. Arch Environ Contam Toxicol 54:466–472. https://doi.org/10.1007/s00244-007-9043-z
Brix KV, DeForest DK, Adams WJ (2011) The sensitivity of aquatic insects to divalent metals: a comparative analysis of laboratory and field data. Sci Total Environ 409:4187–4197. https://doi.org/10.1016/j.scitotenv.2011.06.061
Byrne P, Wood PJ, Reid I (2012) The impairment of river systems by metal mine contamination: a review including remediation options. Crit Rev Environ Sci Technol 42:2017–2077. https://doi.org/10.1080/10643389.2011.574103
Clark JL, Clements WH (2006) The use of in situ and stream microcosm experiments to assess population- and community-level responses to metals. Environ Toxicol Chem 25:2306–2312. https://doi.org/10.1897/05-552.1
Clements WH (2004) Small-scale experiments support causal relationships between metal contamination and macroinvertebrate community responses. Ecol Appl 14:954–967. https://doi.org/10.1890/03-5009
Clements WH, Cadmus P, Brinkman SF (2013) Responses of aquatic insects to Cu and Zn in stream microcosms: understanding differences between single species tests and field responses. Environ Sci Technol 47:7506–7513
Costa-Da-Silva AL, Ioshino RS, De Araújo HRC et al. (2017) Laboratory strains of Aedes aegypti are competent to Brazilian Zika virus. PLoS One 12:1–13. https://doi.org/10.1371/journal.pone.0171951
De Jonge M, Lofts S, Bervoets L, Blust R (2014) Relating metal exposure and chemical speciation to trace metal accumulation in aquatic insects under natural field conditions. Sci Total Environ 496:11–21. https://doi.org/10.1016/j.scitotenv.2014.07.023
Dirilgen N (2001) Accumulation of heavy metals in freshwater organisms: assessment of toxic interactions. Turkish J Chem 25:173–179
Dutta A, Kumari S, Smita A, Dutta S (2010) Bioaccumulation of copper and lead in Chironomus Sp. (Diptera: Chironomidae) at different temperature under laboratory. Bioscan 2:313–322
El-Sheikh E-S, Fouda M, Hassan M et al. (2010) Toxicological effects of some heavy metal ions on Culex pipiens L. (Diptera: Culicidae). Egypt Acad J Biol Sci F Toxicol Pest Control 2:63–76. https://doi.org/10.21608/eajbsf.2010.17465
El-Shenawy NS, Ahmed RSS, Ismail FM, Abo-Ghalia A (2010) Evaluation of the efficiency of wastewater treatment and use of Chironomus calipterus (Diptera: Chironomidae) as a bioindicator in El-Tall El-Keber, Egypt. J Fish Aquat Sci 5:94–105
Ellis LJA, Kissane S, Hoffman E et al. (2020) Multigenerational exposures of Daphnia Magna to pristine and aged silver nanoparticles: epigenetic changes and phenotypical ageing related effects. Small 16:1–15. https://doi.org/10.1002/smll.202000301
Epstein L, Bassein S (2003) Patterns of pesticide use in California and the implications for strategies for reduction of pesticides. Annu Rev Phytopathol 41:351–375. https://doi.org/10.1146/annurev.phyto.41.052002.095612
Farajollahi A, Fonseca DM, Kramer LD, Marm Kilpatrick A (2011) “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect Genet Evol 11:1577–1585. https://doi.org/10.1016/j.meegid.2011.08.013
Farid HA, Hammad RE, Hassan MM et al. (2001) Detection of Wuchereria bancrofti in mosquitoes by the polymerase chain reaction: a potentially useful tool for large-scale control programmes. Trans R Soc Trop Med Hyg 95:29–32. https://doi.org/10.1016/S0035-9203(01)90322-0
Farombi EO, Adelowo OA, Ajimoko YR (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health 4:158–165. https://doi.org/10.3390/ijerph2007040011
Fitzgerald DJ (1998) Safety guidelines for copper in water. Am J Clin Nutr 67:1098–1102. https://doi.org/10.1093/ajcn/67.5.1098S
Ghorbani F, Vatandoost H, Hanafi-Bojd AA et al. (2018) High resistance of vector of West Nile Virus, Culex pipiens Linnaeus (Diptera: Culicidae) to different insecticides recommended by WHO in northern. Iran J Arthropod Borne Dis 12:24–30
Goswami S, Ahad RIA, Syiem MB (2019) Expression of copper toxicity in the rice-field cyanobacterium anabaena oryzae ind4. EurAsian J Biosci 13:57–67
Hare L (1992) Aquatic insects and trace metals: bioavailability, bioaccumulation, and toxicity. Crit Rev Toxicol 22:327–369. https://doi.org/10.3109/10408449209146312
Hasenbein S, Connon RE, Lawler SP, Geist J (2015) A comparison of the sublethal and lethal toxicity of four pesticides in Hyalella azteca and Chironomus dilutus. Environ Sci Pollut Res 22:11327–11339. https://doi.org/10.1007/s11356-015-4374-1
Hill BH, Lazorchak JM, McCormick FH, Willingham WT (1997) The effects of elevated metals on benthic community metabolism in a rocky mountain stream. Environ Pollut 95:183–190. https://doi.org/10.1016/S0269-7491(96)00123-6
Houmann C (2015) Functional characterization of green sorption media and scaling of pilot studies for copper removal in stormwater runoff. PhD Dissertation, University of Central Florida, FL.
Ilahi I, Yousafzai AM, Ali H (2020) Effect of Pb, Cd and Cu on survival and development of Culex quinquefasciatus (Diptera: Culicidae). Chem Ecol 36:205–219
Iwasaki Y, Ormerod SJ (2012) Estimating safe concentrations of trace metals from inter-continental field data on river macroinvertebrates. Environ Pollut 166:182–186. https://doi.org/10.1016/j.envpol.2012.03.028
Jegede OO, Hale BA, Siciliano SD (2019) Multigenerational exposure of populations of Oppia nitens to zinc under pulse and continuous exposure scenarios. Environ Toxicol Chem 38:896–904. https://doi.org/10.1002/etc.4369
Joachim S, Roussel H, Bonzom JM et al. (2017) A long-term copper exposure in a freshwater ecosystem using lotic mesocosms: Invertebrate community responses. Environ Toxicol Chem 36:2698–2714. https://doi.org/10.1002/etc.3822
Kramer LD, Ebel GD (2003) Dynamics of flavivirus infection in mosquitoes. Adv Virus Res 60:187–232
Lee CS, Li X, Shi W et al. (2006) Metal contamination in urban, suburban, and country park soils of Hong Kong: a study based on GIS and multivariate statistics. Sci Total Environ 356:45–61. https://doi.org/10.1016/j.scitotenv.2005.03.024
Luoma SN, Rainbow PS (2005) Why is metal bioaccumulation so variable? Biodynamics as a unifying concept. Environ Sci Technol 39:1921–1931. https://doi.org/10.1021/es048947e
Lusk MG, Chapman K (2020) Copper concentration data for water, sediments, and vegetation of urban stormwater ponds treated with copper sulfate algaecide. Data Br 31:1–7. https://doi.org/10.1016/j.dib.2020.105982
Mackenzie JS, Barrett ADT, Deubel V (2002) The Japanese encephalitis serological group of flaviviruses: a brief introduction to the group. Springer, Berlin, Heidelberg, p 1–10
Majumdar TN, Gupta A (2012) Acute and chronic toxicity of copper on aquatic insect Chironomus ramosus from Assam, India. J Environ Biol 33:139–142
Malaj E, Grote M, Schäfer RB et al. (2012) Physiological sensitivity of freshwater macroinvertebrates to heavy metals. Environ Toxicol Chem 31:1754–1764. https://doi.org/10.1002/etc.1868
Marinković M, De Bruijn K, Asselman M et al. (2012) Response of the nonbiting midge Chironomus riparius to multigeneration toxicant exposure. Environ Sci Technol 46:12105–12111. https://doi.org/10.1021/es300421r
Matsuoka H, Tomita H, Hattori R et al. (2015) Visualization of malaria parasites in the skin using the luciferase transgenic parasite, Plasmodium berghei. Trop Med Health 43:53–61. https://doi.org/10.2149/tmh.2014-18
Mebane CA, Schmidt TS, Miller JL, Balistrieri LS (2020) Bioaccumulation and toxicity of cadmium, copper, nickel, and zinc and their mixtures to aquatic insect communities. Environ Toxicol Chem 39:812–833. https://doi.org/10.1002/etc.4663
Mireji PO, Keating J, Hassanali A et al. (2010) Biological cost of tolerance to heavy metals in the mosquito Anopheles gambiae. Med Vet Entomol 24:101–107. https://doi.org/10.1111/j.1365-2915.2010.00863.x
Mogren CL, Trumble JT (2010) The impacts of metals and metalloids on insect behavior. Entomol Exp Appl 135:1–17. https://doi.org/10.1111/j.1570-7458.2010.00967.x
Nollet LML (2000) The handbook of water analysis. New york
Perez MH, Noriega FG (2014) Sublethal metal stress response of larvae of Aedes aegypti. Physiol Entomol 39:111–119. https://doi.org/10.1111/phen.12054
Potera C (2004) Fullerenes and fish brains copper in drinking water lead in Mexican children pottery use slows reductions in blood. Environ Health Perspect 112:568–569
Rajaganapathy V, Xavier F, Sreekumar D, Mandal PK (2011) Heavy metal contamination in soil, water and fodder and their presence in livestock and products: a review. J Environ Sci Technol 4:234–249
Rayms-Keller A, Olson KE, McGaw M et al. (1998) Effect of heavy metals on Aedes aegypti (Diptera: Culicidae) larvae. Ecotoxicol Environ Saf 39:41–47
Reza M, Ilmiawati C (2020) Laboratory testing of low concentration (<1 ppm) of copper to prolong mosquito pupation and adult emergence time: an alternative method to delay mosquito life cycle. PLoS One 15:1–9. https://doi.org/10.1371/journal.pone.0226859
Rothmeier LM, Martens A, Watermann B et al. (2020) Effects of copper ions on non-target species: a case study using the Grazer Theodoxus fluviatilis (Gastropoda: Neritidae). Bull Environ Contam Toxicol 105:62–66. https://doi.org/10.1007/s00128-020-02913-x
Scheiber IF, Mercer JFB, Dringen R (2014) Metabolism and functions of copper in brain. Prog Neurobiol 116:33–57. https://doi.org/10.1016/j.pneurobio.2014.01.002
Schmidt TS, Clements WH, Mitchell KA et al. (2010) Development of a new toxic-unit model for the bioassessment of metals in streams. Environ Toxicol Chem 29:2432–2442. https://doi.org/10.1002/etc.302
Sildanchandra W, Crane M (2000) Influence of sexual dimorphism in Chironomus riparius Meigen on toxic effects of cadmium. Environ Toxicol Chem An Int J 19:2309–2313
Stevens MM, Doran G, Mo J (2014) Efficacy and environmental fate of copper sulphate applied to Australian rice fields for control of the aquatic snail Isidorella newcombi. Crop Prot 63:48–56. https://doi.org/10.1016/j.cropro.2014.05.004
Thomas ORB, Barbee NC, Hassell KL, Swearer SE (2016) Smell no evil: copper disrupts the alarm chemical response in a diadromous fish, Galaxias maculatus. Environ Toxicol Chem 35:2209–2214. https://doi.org/10.1002/etc.3371
Tilton FA, Bammler TK, Gallagher EP (2011) Swimming impairment and acetylcholinesterase inhibition in zebrafish exposed to copper or chlorpyrifos separately, or as mixtures. Comp Biochem Physiol C Toxicol Pharmacol 153:9–16. https://doi.org/10.1016/j.cbpc.2010.07.008
Turell MJ, O’Guinn ML, Dohm DJ, Jones JW (2001) Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol 38:130–134. https://doi.org/10.1603/0022-2585-38.2.130
Vedamanikam VJ, Shazilli NAM (2008) The effect of multi-generational exposure to metals and resultant change in median lethal toxicity tests values over subsequent generations. Bull Environ Contam Toxicol 80:63–67. https://doi.org/10.1007/s00128-007-9317-1
Vezzani D, AlbicÓcco AP (2009) The effect of shade on the container index and pupal productivity of the mosquitoes Aedes aegypti and Culex pipiens breeding in artificial containers. Med Vet Entomol 23:78–84. https://doi.org/10.1111/j.1365-2915.2008.00783.x
Viarengo A, Nicotera P (1991) Possible role of ca2+ in heavy metal cytotoxicity. Comp Biochem Physiol Part C Comp Pharmacol 100:81–84. https://doi.org/10.1016/0742-8413(91)90127-F
Vinogradova EB (2000) Culex pipiens pipiens mosquitoes: taxonomy, distribution, ecology, physiology, genetics, applied importance and control. Pensoft Publishers, Bulgaria.
Wagner JL, Townsend AK, Velzis AE, Paul EA (2017) Temperature and toxicity of the copper herbicide (NautiqueTM) to freshwater fish in field and laboratory trials. Cogent. Environ Sci 3:1–12. https://doi.org/10.1080/23311843.2017.1339386
Weber CI (1991) Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. Environmental Protection Agency, Cincinnati, OH.
Yan ND, Keller W, Somers KM et al. (1996) Recovery of crustacean zooplankton communities from acid and metal contamination: comparing manipulated and reference lakes. Can J Fish Aquat Sci 53:1301–1327. https://doi.org/10.1139/f96-065
Acknowledgements
We thank the biostatistical consultant team Xingmei Lou, Zihan Xiao, and Jiahui Li for their help in conducting an initial statistical analysis under the supervision of Wang Jane-Ling and Cody Carroll. We are also very grateful to Deborah Dritz, Sacramento-Yolo Mosquito, and Vector Abatement District for providing us with mosquito larvae. We would like to express our special thanks to Austin Cole, UC Davis Interdisciplinary Center for Plasma Mass Spectrometry (UCD/ICPMS) who conducted the chemical analysis and to trained N. Raffat in his lab.
Funding
This research was supported by the Egyptian Cultural and Educational Bureau, Washington DC.
Author contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by NRA. NRA, SPL, and REC contributed to data analysis. The first draft of the manuscript was written by NRA. All authors reviewed at least one draft of the manuscript and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Amer, N.R., Lawler, S.P., Zohdy, N.M. et al. Effect of long-term exposure to copper on survival and development of two successive generations of Culex pipiens (Diptera, Culicidae). Ecotoxicology 30, 351–360 (2021). https://doi.org/10.1007/s10646-021-02358-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-021-02358-w