Abstract
Since it has been demonstrated that urban effluents can have adverse effects on aquatic organisms, a multibiomarker study was used to evaluate the effects of wastewater treatment plant (WWTP) effluents discharged into the marine and freshwater environments on clams in Cádiz, Spain. One bioassay was performed in the Bay of Cádiz, exposing Ruditapes philippinarum (marine) to a reference site as well as two sites close to WWTP discharges for 14 days. A second bioassay was performed in the Guadalete River, exposing Corbicula fluminea (fresh water) to three sites for 21 days. The biomarkers analysed included defence mechanisms and various toxic effects. Results indicated that WWTP effluents activated defence mechanisms and induced toxic effects in clams exposed to both environments, thus indicating bioavailability of contaminants present in water. Elevated enzymatic activity was found in clams deployed in La Puntilla and El Trocadero compared to control clams and those exposed to the reference site, and 96% of clams deployed at G2 in the Guadalete River died before day 7. Clams exposed to G1 and G3 indicated significant differences in all biomarkers analysed with respect to control clams (p < 0.05). Both species were sensitive to contaminants present in studied sites. This is the first time that these species were used in cages to assess the environmental risk of wastewater effluent discharges in freshwater and marine column environments. The multibiomarker approach provided important ecotoxicological information and is useful for the assessment of the bioavailability and effect of contaminants from WWTP effluents on marine and fresh water invertebrates.
Highlights
-
A multibiomarker study evaluated WWTP effluent effects on clams in water columns.
-
WWTP effluents were toxic to clams in adjacent marine and fresh water environments.
-
Defence mechanisms increased in marine clams nearest to WWTP discharges.
-
96% of clams exposed directly to WWTP effluent in Guadalete River died before day 7.
-
We showed that the multibiomarker approach provides important ecotoxicological data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Urban effluents are a major source of pollutants that release a complex mixture of chemicals into receiving waters, including polycyclic aromatic hydrocarbons (PAHs), pesticides, surfactants, steroids, metals, pharmaceuticals and personal care products (Abessa et al. 2005; Gagné et al. 2004a, 2008; Lara-Martín et al. 2014; Metcalfe et al. 2003, Zaborska et al. 2019). Aquatic organisms can be exposed to the contaminants released in urban wastewater discharges, often leading to toxic effects (Gagné et al. 2002). Bioassays involving the use of biomarkers are recognized as a powerful tool for studying pollutants in situ (Depledge and Fossi 1994). By considering the biological effects of a contaminant, the biomarker approach can offer more complete and relevant information regarding the potential impact of a pollutant on the health of an animal (Van der Oost et al. 1996). This is one advantage that the biomarker approach has over conventional biomonitoring programmes, which directly measure the concentration of a toxin in an organism, not accounting for the biological effects (Depledge 1993). The application of a biomarker approach has been recommended by environmental agencies such as UNEP, OECD, IOC and the international OSPAR and ICES conventions (Cajaraville et al. 2000; Solé et al. 2009). Exposure of organisms to bioavailable environmental contaminants can trigger the activation of defence mechanisms and the performance that determines the toxic effect on the organisms (Barreto et al. 2018; Livingstone 2001; Parenti et al. 2019). With the biomarker approach, it is either the activated defence mechanisms or the toxic effect that is measured in an organism, or both (Quinn et al. 2005). It has been established that the assessment of effects of contaminants should include the evaluation of general stress as a toxicity screening tool (Viarengo et al. 2007), as well as the use of biochemical biomarkers (i.e. defence mechanisms and biomarkers of effect) (Broeg et al. 2005; Van der Oost et al. 2003; Viarengo et al. 2007). An example of said biomarkers includes the activity of biotransformation enzymes (etoxyresorufin O-deethylase [EROD], dibenzylfluorescein dealkylase [DBF], gluthathione-S-transferase [GST]), antioxidant enzymes (gluthathione reductase [GR] and gluthathione peroxidase [GPX]) and biomarkers of effect (Lipid peroxidation [LPO] and DNA damage). These biomarkers have been applied as parameters that indicate the presence, availability and even the toxicity of compounds (Aguirre-Martínez et al. 2013a, 2014; Gagné et al. 2007; Martín-Díaz et al. 2004; Morales-Caselles et al. 2008; Ramos-Gómez et al. 2011; Viarengo et al. 2007).
WWTP effluents are indistinctively discharged worldwide into marine and freshwater environments. Environmental conditions that are different in these ecosystems could affect the availability of the contaminants present in the effluents discharged into aquatic environments (Seyfried et al. 2014), and this availability may have an effect on the toxic response of each organism. It has been stated that bivalves such as clams are suitable bioindicators for evaluating the impacts of municipal effluents (Díaz-Garduño et al. 2018; Maranho et al. 2015; Solé et al. 2009). Their filter feeding behaviour puts them in direct contact with contaminants present in water.
For this reason, a biomarker study was performed in the water column of marine and fresh water environments using two sentinel species of clams: Ruditapes philippinarum (marine) and Corbicula fluminea (fresh water). These species are abundant and easy to collect and maintain under laboratory conditions. Moreover, they are sensitive to aquatic contaminants, and have been shown to be useful in the laboratory (Aguirre-Martínez et al. 2014; Cooper and Bidwell 2006) and in situ assessments in various ecotoxicological studies (Bonnail et al. 2019; Morales-Caselles et al. 2008). Moreover, biomarker responses have been measured by different authors in R. philippinarum (Díaz-Garduño et al. 2018; Maranho et al. 2015; Matozzo et al. 2016; Martín-Díaz et al. 2008a, 2008b) and in C. fluminea (Aguirre-Martínez et al. 2018; Bonnail et al. 2019; Cataldo et al. 2001; Legeay et al. 2005).
Because WWTP effluents are discharged directly into aquatic environments, the purpose of this study was to assess the effects of whole WWTP discharges in the water column from six sites in Cádiz (SW, Spain) using caged clams. In order to do so, a multibiomarker approach including activated defence mechanisms and toxicity was evaluated in R. philippinarum after 14 days of exposure to areas near WWTP effluent discharges in the Bay of Cádiz, and in C. fluminea exposed for 21 days to areas near WWTP effluent discharges in the Guadalete River.
Materials and methods
General approach
Bay of Cádiz (marine environment)
The Bay of Cádiz, located in the Province of Cádiz, Spain (Fig. 1), has been considered as a natural park since 1996 (Solé et al. 2009); however, the bay receives constant input from WWTPs from Puerto Real and Puerto Santa Maria. The plant processes consist of a primary grit removal, followed by an activated sludge process and a secondary treatment consisting in an anaerobic process. After the treatment, the water is pumped into underwater effluents located in El Trocadero and La Puntilla (respectively), and the effluents discharge the treated or untreated water directly into the Bay of Cádiz. The WWTP from Puerto Real has an average flow of 29,000 m3 per day from agricultural, urban and industrial origins; this plant receives wastewater directly from the hospital in Puerto Real without previous treatment. The sediment from this area is characterized by the presence of metal and organic compounds such as polycyclic aromatic hydrocarbons (PAHs) and pharmaceutical products (Maranho et al. 2015). The WWTP from Puerto Santa Maria has an average flow of 27,000 m3 per day (sometimes reaching 50,000 m3). This plant receives wastewater from households, industries and directly from the hospital without previous treatment. La Puntilla also receives effluents coming from the upper part of Guadalete River. Sediment from this area contains metals, PAHs, pharmaceuticals (Maranho et al. 2015) and surfactants (Lara-Martín et al. 2006).
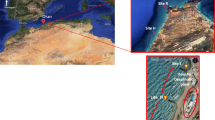
Map of the coastal area of the Bay of Cádiz showing the locations of the sampling sites (La Puntilla, Trocadero) and the reference site (Chorrillos); map of the Guadalete River showing the locations of the sampling sites G1 Upstream, reference site G2 outside the waste water treatment plant (WWTP) and G3 Downstream
Two sites in the Bay of Cádiz were selected because they were adjacent to the underwater effluents from El Trocadero (Puerto Real) and La Puntilla (Puerto Santa Maria). One site in Playa Chorrillos (Rota) located far from known wastewater discharges and with high water circulation was chosen as a reference site for the marine environment.
Guadalete River (fresh water environment)
The Guadalete River (Fig. 1) is 157 km long and its basin covers an area of 3677 km2. It flows across the province of Cádiz, Spain from the Sierra de Grazalema Natural Park and enters the sea in the northern part of the Bay of Cádiz (Corada-Fernández et al. 2011). This river is a sewage-receiving environment from the municipal WWTP located in Jerez de la Frontera. The plant processes consist of a primary grit removal, followed by an activated sludge process and a secondary treatment consisting in an anaerobic process. This WWTP receives untreated domestic wastewater from a total population of ~691,200 inhabitants, including Jerez city, small villages around Guadalcacín, Estella, Graciagos, Los Albarizones, La Corta, and El Portal, and directly from hospitals located in these towns without previous treatment. The WWTP from Jerez de la Frontera has an average flow of 35,000 m3 per day. Studies have demonstrated the presence of surfactants, pharmaceuticals and personal care products in the Guadalete River close to the WWTP (Corada-Fernández et al. 2017; Lara-Martín et al. 2008). Three sites were selected in the Guadalete River. Site G1 was located upstream from the treated sewage outfall, site G2 was a treated sewage receiving area (WWTP effluent discharge), and the third site, G3, was located downstream, ~1 km from the treated sewage outfall (Fig. 1). For this experiment, exposed clams were compared to those from day 0.
Selection of organisms
R. philippinarum were purchased from an aquaculture farm located in the bay of Cádiz (SW Spain). All organisms used in this research were of similar size and length (45 ± 0.9 mm). Once in the laboratory, the clams were acclimated for one week in a 300 l tank and supplied with constant aeration; sea water temperature (15 ± 1 °C), salinity (33.8 ± 0.3), pH (7.8–8.2) and dissolved oxygen (7.5 ± 1.1 mg l−1, 90% saturation) were strictly maintained under a 12 h light-dark regime. On the other hand, C. fluminea were collected at 22 km from the mouth of the Miño River in the locality of Pontevedresa de Amorín in Galicia (NW Spain). This river presents a naturalized invasive population of Asian clams. All individuals used in this research were of similar size and length (40 ± 0.5 mm). Once in the laboratory, these clams were acclimated for one week in a 300 l tank containing tap water that was previously dechlorinated for at least 96 h and supplied with constant aeration; water temperature (16 ± 0.2 °C), pH (8 ± 0.3) and dissolved oxygen (7.6 ± 1.3 mg l−1, 94.5% saturation) were constantly monitored in a 12 h light-dark regime.
Bioassay design
During the previous day of the field bioassay, 10 clams were introduced to mesh bags, and plastic bridles separated each clam (Fig. 2a). After the load, the mesh bags were left overnight in acclimation tanks. Physical-chemical parameters of water in the tanks were similar to those applied during the acclimation period. On the day of the bioassay, the mesh bags were transported carefully in plastic containers with ice and humid cloths. At the site, eight loaded mesh bags were attached to a PVC frame by both extremes and covered by a plastic grid to protect the clams from predators (Fig. 2b). Two cages were deployed at each site at least 1 m below the water’s surface. Field experiments were performed at different time periods during 2013. The bioassay exposing R. philippinarum in the Bay of Cádiz was performed for 14 days in March, while the bioassay exposing C. fluminea to the Guadalete River was performed for 21 days in October. Both in situ experiments were intended to last 21 days (in both experiments we intended to sample at 0, 7, 14 and 21 days), however over the course of the experiment we lost some cages in the marine environment after 14 days of experiment, so we could not record the 21 day exposure data for this area (this loss could have been due to robbery in the areas close to WWTP and in the reference site (which was located far from human activity), the loss of the cages could have been due to the underwater current in the area. Therefore, In the marine environment, we collected data at 0, 7 and 14 days only. With regard to the fresh water environment during the day 14 of the experiment due to flood conditions we did not have access to the site to collect the cages thus we could not sample site G1. Then on day 21 of the experiment we could access the two sites located on the Guadalete River to recover all the cages. Therefore, In the fresh water environment, we collected data at 0, 7 and 21 days only.
The sampling of clams was performed every seven days by randomly extracting two mesh bags with 10 clams each from each cage. Physical and chemical parameters were measured at each site during the sampling process (see Table 1). Organisms were transported carefully from the field to the laboratory in containers with ice and wet cloths.
We decided to present the results of the longer exposure period of each species compared to controls in order to analyse the biomarker response during the longer exposure time so the complete data corresponded to the 14 days of exposure in the marine environment and 21 days in the Guadalete River.
Lysosomal membrane stability
Once in the laboratory, haemolymph was extracted in vivo from all clams sampled in the field, and lysosomal membrane stability (LMS) was evaluated using the neutral red retention time assay (NRRA) following the methodology reported in detail by Aguirre-Martínez et al. (2013b), adapted from Martínez-Gómez et al. (2008). Briefly, 100 mM stock solution of neutral red was prepared by dissolving 28.8 mg of dye powder in 1 ml of DMSO, and 3 μl of neutral red stock was then dissolved in 197 μl of physiological saline. Clam physiological saline (436 M NaCl, 10 mM KCl, 10 mM CaCl2, 53 mM MgSO4 and 20 mM Hepes sodium salt adjusted to pH 7.3 with 1 N NaOH) was prepared following the protocol of Lowe et al. (1995) and Marchi et al. (2004). Two replicates of 40 µl samples were transferred onto microscope slides at room temperature and placed in a lightproof humidity chamber where the haemocytes were left to settle and attach to the slide surface for 30 min Later, 40 µl of 0.2 mM neutral red solution was added. Retention time was examined under a microscope after 15, 30, 60, 90, 105 and 120 min Results were expressed as destabilization time, representing the time at which more than 50% of the lysosomes released the dye into the cytosol. LMS was evaluated in clam haemocytes at the beginning of the assay (day 0) and at the end of assay (day 14).
Tissue preparation
Clams collected at the beginning (day 0) and at the end of the bioassay were dissected. Digestive gland tissues were extracted from 40 R. phillipinarum and 64 C. fluminea per site, combined into 8 pools from 5 and 8 organisms, respectively. Samples were stored at −80 °C. All analyses were performed on pooled samples (n = 8) in triplicate.
Pooled samples were homogenized following the procedure described by Lafontaine et al. (2000), centrifuged at 15,000 × g for 20 min at 4 °C to obtain the supernatant fraction S15, and centrifuged at 3000 × g for 20 min at 4 °C to obtain the supernatant fraction S3. The total protein concentration (TP) was determined in the homogenate, S15 and S3 following an adaptation of the methodology of Bradford (1976). Protein content was expressed as mg TP.
Ethoxyresorufin O-deethylase (EROD) activity
Mixed-function oxidase activity was measured using an EROD assay, which was initially adapted for fingerling rainbow trout (Gagné and Blaise 1993) and for the species in this study. In dark microplates (96 flat bottom wells), 50 µl of S15 was added to 160 µl 7-ethoxyresorufin, and 10 µl reduced NADPH, to initiate the reaction (final volume of well 220 µl). 7-Hydroxyresorufin was detected fluorometrically every 10 min for 60 min at 30 °C (kinetic microplate reader, Infinite® M200, 516 nm excitation and 600 nm emission wavelengths). Calibration was achieved through a standard curve of 7-hydroxyresorufin. The results were standardized to total protein (TP) content. EROD activity was expressed as pmol/min/mg TP.
Dibenzylfluorescein dealkylase (DBF) activity
DBF activity was measured following the methodology described by Quinn et al. (2004). In dark microplates (96 flat bottom wells), 50 µl of supernatant (S15) was briefly added to 50 µM dibenzylfluorescein, and 100 µM reduced NADPH in 125 mM NaCl, buffered with 10 mM Hepes–NaOH, pH 7.4. Samples were incubated at 30 °C, and the release of fluorescein was measured at 0, 15, 30 and 60 min Fluorescein was determined by fluorometry in a microplate reader, Infinite®M200 using 485 nm (excitation) and 532 nm (emission) filters. Fluorescein in samples was measured using a standard calibration curve developed with concentrations of a standard solution of 5 µM fluorescein. The results were expressed as nmol/min/mg TP.
Gluthatione S-transferase (GST) activity
The procedure used to determine GST activity was adopted from Boryslawskyj et al. (1988). In a transparent microplate (96 flat bottom wells), a sample of 50 µl of supernatant (S15) was added to 200 µl of 1 mM GSH and 1 mM 1-chloro-2.4-dinitrobenzene in a buffer of 10 mM Hepes–NaOH, pH 6.5, containing 125 mM NaCl. GST activity was measured spectrophotometrically. Absorbance based on the appearance of the glutathione conjugate was measured at 340 nm at 0, 5, 10, 15, 20, 25 and 30 min Results were expressed as OD/min/mg TP.
Glutathione peroxidase (GPX) activity
The procedure applied for GPX activity was adapted from Mcfarland et al. (1999). In a transparent microplate (96 flat bottom wells), 20 µl of the homogenate sample (10 µl homogenate + 10 µl MilliQ Water) was measured spectrophotometrically in a microplate reader (Infinite®M200) at 340 nm, at 3 s intervals for 3 min using as substrate 1 mM cumene hydroperoxide. The decrease in NADPH absorbance measured at 340 nm during the oxidation of NADPH to NADP was indicative of GPX activity. Results were expressed as pmol/min/mg TP.
Glutathione reductase (GR) activity
GR activity was determined utilizing an adaptation of the McFarland et al. (1999) procedure, previously conducted by Martín-Díaz et al. (2007). In a transparent microplate (96 flat bottom wells), a 20 µl of the S15 sample (10 µl S15 + 10 µl MilliQ Water) was measured spectrophotometrically in a microplate reader (Infinite®M200) at 340 nm, every 2 min for 10 min at 30 °C. GR, together with the co-factor NADPH, catalysed the reduction of oxidized glutathione (GSSG) to GSH. The consumption of NADPH produced a decrease in absorbance at 340 nm, which was directly proportional to the glutathione reductase activity in the sample. The samples were added to the reaction mixture (substrates 10 mM oxidized glutathione and 1 mM NADPH in 200 mM phosphate buffer with a pH of 7.6, kept at 30 °C). The results were expressed as nmol/min/mg TP.
Lipid peroxidation (LPO)
An adaptation of the thiobarbituric acid reactive substances (TBARS) method by Wills (1987) was used to determine LPO. Oxidative stress leads to malondialdehyde (MDA) production from the degradation of initial products of free radical attacks on fatty acids (Janero 1990). MDA reacts with 2-thiobarbituric acid, producing tetramethoxypropane (TMP). This was measured spectrophotometrically, allowing the indirect determination of MDA. Standard solutions and homogenate samples were prepared separately in 1.5 ml Eppendorfs. Standard solutions were used of TMP 0.0001% (0, 0.6, 1.5, 3, 4, 6, 10 and 15 µM), 300 µl of trichloroacetic acid (TCA) 10%, 1 mM FeSO4, 150 µl of thiobarbituric acid (TBA) 0.67%, then 150 µl of diluted homogenate (75 µl of the sample + 75 µl milliQ water), 300 µl of trichloroacetic acid (TCA) 10%, 1 mM FeSO4 and 150 µl of thiobarbituric acid (TBA) 0.67%. Standards and samples were incubated in a Unitronic 320 OR P Selecta Heater® at 70 °C for 10 min Later, 200 µl of the standard solution was added to transparent microplates (96 flat bottom wells), and passed through a microplate reader (Infinite®M200) to measure absorbance at 540 nm, in order to set the standard curve of TMP. Finally, 200 µl of the samples in duplicate were measured in the same way. LPO was expressed as μgTBARS/mgTP.
DNA damage
DNA damage was measured using a ‘DNA precipitation’ assay described by Olive (1988). This methodology is based on the K-SDS precipitation of DNA-protein crosslink, which uses fluorescence to quantify the DNA strand breaks. Denatured single-stranded DNA was released from a physical matrix (cellular proteins). The physical separation of single stranded DNA from double stranded DNA during the denaturation process allowed quantifying the amount of the two DNA species at the end of the assay (Shugart 2000). In dark microplates (96 flat bottom wells), the homogenate (25 µl) was mixed with 200 µl of 2% SDS containing 10 mM EDTA, 10 mM Tris-base and 40 mM NaOH. After mixing for 1 min, 200 µl of 0.12 M KCl was added and the solution was heated at 60 °C for 10 min, mixed by inversion, and cooled at 4 °C for 30 min to precipitate the genomic DNA linked to SDS-associated nucleoproteins. This mixture was then centrifuged at 8000 × g for 5 min (4 °C). In a 96-well plate, 50 µl of the supernatant was added to 150 µl of Hoescht dyein a concentration of 0.1 µg mL−1 (diluted with a buffer containing 0.4 M NaCl, 4 mM sodium cholate and 0.1 M Tris-acetate, pH 8.5). Fluorescence was measured in a microplate reader (Infinite®M200) using 360 nm (excitation) and 450 nm (emission) filters against blanks, containing identical constituents without the homogenate. Salmon sperm genomic DNA standards (Sigma) were added for DNA calibration, and the results were expressed as µg/mgTP.
Acetylcholinesterase activity (AChE)
Post-mitochondrial AChE activity was determined in the S3 fraction, using acetylthiocholine as the substrate and Ellman’s reagent for detection (Bonacci et al. 2004). A standard solution of freshly prepared reduced glutathione was used for calibration. Data were expressed as the formation of thiols in μmol DNTB/min/mgTP (Guilhermino et al. 1996).
Statistical analysis
EC50 values from the NRRA test were estimated by the statistical programme ICPin®. Data was analysed using the SPSS/PC+ statistical package®. Normality of the data and homogeneity of variance were analysed prior to the use of the parametric test. Significant differences between control clams (organisms from day 0) and clams exposed to WWTP effluent discharges were determined using a one-way ANOVA, followed by the Dunnett’s multiple comparisons test. The significance level was set at p < 0.05. Biomarker responses were analysed using a SPSS/PC+ statistical package®. Significant differences between controls (organisms exposed to the reference site in the case of R. philippinarum, and organisms from day 0 in the case of C. fluminea) with respect to organisms exposed to the studied areas were determined using a one-way ANOVA; the data was not transformed followed by a Dunnett’s multiple comparisons test. The significance level was set at p < 0.05.
An integrated biomarker response index (IBR index) was calculated by standardizing the data to a common scale; to calculate the index, the response of each biomarker (LMS, EROD, DBF, GST, GPX, GR LPO, AChE and DNA damage) was divided by the control average. The one-way ANOVA was applied, followed by Dunnett’s test. The IBR index for R. philippinarum was calculated by comparing the biomarker responses in clams exposed to Trocadero and La Puntilla to the responses from clams exposed to the reference site (Chorrillos). The IBR index for C. fluminea was calculated comparing clams exposed to G1 (upstream) and G3 (downstream) to responses from clams analysed on day 0. The IBR index was represented in star plots.
Results
Bay of Cádiz
No significant mortality compared to the control site (Chorrillos) was observed in R. philippinarum exposed for 14 days to El Trocadero and La Puntilla in the Bay of Cádiz. Results of LMS measured in marine clams are indicated in Fig. 3.
Lysosomal membrane stability (LMS) evaluated by the neutral red retention time (NRRT) assay in haemocytes of R. philippinarum exposed for 14 days to El Trocadero, La Puntilla and Chorrillos (reference site) in the Bay of Cádiz, and in C. fluminea exposed for 21 days to points G1 (upstream), G2 (WWTP) and G3 (downstream) in Guadalete River. Asterisks indicate significant differences compared to Day 0 (one way ANOVA, p < 0.05). Data are means ± SE, n = 10. Black lines indicate the health status threshold for R. philippinarum and C fluminea proposed by Aguirre-Martínez et al. (2013b, 2015), respectively (adapted from Martínez-Gómez et al. 2008)
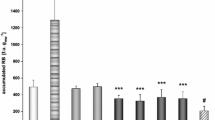
It was observed that the NRRT on day 0 of the bioassay evaluated in haemocytes from R. philippinarum was 134 ± 13 min (n = 10). At the end of the bioassay (day 14), the mean time of the red dye retention exposed to the reference site was 128 ± 9 min (n = 10), which was similar to the response from day 0. Clams exposed to the studied areas in the marine environment presented significant differences with respect to clams exposed to the reference site (p < 0.05). The threshold values for general stress are indicated in Fig. 3. R. philippinarum measured on day 0 and those that were exposed to Chorrillos were considered to be healthy (NRRT ≥80 min). The NRRT in clams exposed to El Trocadero was 64 ± 8 min, indicating a general stress syndrome (NRRT <80 but ≥45 min). The NRRT of clams exposed to La Puntilla was 40 ± 13 min, and these clams presented what was considered to be a diminished health status (NRRT <45 min). Biochemical responses studied in the digestive glands of R. philippinarum after 14 days of the experiment in the field are shown in Fig. 4. Significant induction in EROD activity was found in R. philippinarum exposed to El Trocadero and La Puntilla, compared to control organisms (1.9 and 1.3 fold p < 0.05). DBF activity was induced significantly in clams exposed to El Tocadero compared to control clams (1.5 and 1 fold p < 0.05). Clams exposed to El Trocadero and La Puntilla showed significant induction of GST activity (both 3 fold p < 0.05) compared to the control. The same trend was found for antioxidant responses: GPX and GR activity were observed to be significantly increased in Trocadero (2.2 and 3.3 fold control p < 0.05) and La Puntilla (1.7 and 5.9 fold control p < 0.05), respectively. Levels of AChE and LPO were significantly lower in clams exposed to El Trocadero and La Puntilla compared to control clams (p < 0.05), and DNA damage was not observed.
Biochemical biomarkers including ethoxyresorufin O-deethylase (EROD), dibenzylfluorescein dealkylase (DBF), glutathione S-transferase (GST), glutathione peroxidase (GPX), glutathione reductase (GR), Acetyl cholinesterase (AChE) and lipid peroxidation (LPO) and DNA damage measured in digestive gland tissues of R. philippinarum exposed for 14 days to El Trocadero, La Puntilla and Chorrillos in the Bay of Cádiz. Asterisks indicate significant difference
Guadalete River
Results obtained from C. fluminea exposed to points G1, G2 and G3 were compared to the results measured in clams from day 0 of the bioassay (control). During the experiment performed in the Guadalete River, significant mortality was observed in C. fluminea exposed to G2 compared to G1 (p < 0.05). It is important to mention that 96% of the mortalities registered in this site occurred before day 7 of the bioassay. Results of LMS in fresh water clams and the threshold values for general stress for fresh water clams are indicated in Fig. 3. The NRRT, measured in C. fluminea on day 0, was 120 ± 4 min (n = 10). These clams were considered to be healthy (NRRT ≥110 min). After 21 days of exposure, studied areas G1 and G3 presented significant differences compared to clams from day 0 (p < 0.05). The NRRT of clams exposed to G1 was 74 ± 17 min, and these clams were considered to be stressed but compensating (NRRT <110 but ≥50 min). There are no results for the NRRT in clams exposed to G2 on day 21, as all organisms died. It is important to mention that the NRRT of the survivors (n = 7) on day 7 was 6 ± 2 min, indicating diminished health status (NRRT <50 min). The NRRT of clams exposed to G3 was 77 ± 19 min, and these clams were considered to be stressed but compensating (NRRT <110 but ≥50 min).
Biochemical responses studied in the digestive glands of C. fluminea after 21 days of exposure are shown in Fig. 5. Significant induction in EROD, DBF and GST activity was found in fresh water clams exposed for 21 days to G3 compared to individuals from day 0 (1.9, 2.7 and 2.7 fold day 0, respectively, p < 0.05). With regard to antioxidant responses, a significant increase was noted compared to clams from day 0 for GPX activity measured in clams exposed to G1 (5.8 fold day 0, p < 0.05) and G3 (4.3 fold day 0, p < 0.05). Similarly, GR activity significantly increased in clams exposed to sites G1 and G3 compared to the activity measured in clams from day 0 (4.1 and 3.7 fold day 0, p < 0.05). Levels of AChE were significantly reduced in the digestive gland tissues of clams exposed to sites G1 and G3 when compared to the AChE levels in clams from day 0 (p < 0.05). LPO levels significantly increased in clams exposed to G1 and G3 compared to clams from day 0 (3 and 2 fold day 0, p < 0.05). DNA damage significantly increased in caged clams exposed to G1 and G3 of the Guadalete River compared to clams from day 0 (2.8 and 2.5 fold day 0, p < 0.05).
Biochemical biomarkers including ethoxyresorufin O-deethylase (EROD), dibenzylfluorescein dealkylase (DBF), glutathione S-transferase (GST), glutathione peroxidase (GPX), glutathione reductase (GR), Acetyl cholinesterase (AChE) and lipid peroxidation (LPO) and DNA damage measured in digestive gland tissues of C. fluminea exposed for 21 days to points G1 (reference site) and G3 in the Guadalete River. Asterisks indicate significant differences from the control (one way ANOVA, p < 0.05). Data are means ± SE, n = 5 pools from n = 20 clams
The IBR values calculated for each site in the Bay of Cádiz are demonstrated through star plots in Fig. 6. Maximum IBR values in El Trocadero were observed for the antioxidant response GR (4.2), for the enzyme activity from Phase I EROD (3.4), followed by the antioxidant GPX (2.6) and AChE (2.6). Maximum IBR values in La Puntilla were established for antioxidant response GR (7.4), followed by AChE (3.4), LMS (3.2), EROD (2.3), GST (2.1) and GPX (2.1). The IBR index calculated for the Guadalete River is demonstrated through star plots in Fig. 7. The highest IBR value in site G1 was recorded for the antioxidant responses GPX (5.8), followed by GR (4.1), LPO (3.0), DNA damage (2.8), and AChE (2.5). The maximum IBR score on site G3 was observed for the antioxidant GPX (4.3), followed by GR (3.7), the enzyme activity from Phase I DBF (2.7), GST (2.7), and DNA damage (2.5).
The Integrated biomarker response (IBR) analysed in the digestive glands of R. philippinarum exposed for 14 days to Trocadero and La Puntilla in the bay of Cádiz compared to the responses obtained from clams exposed to the reference site, Chorrillos. Biomarker responses considered for this index were: LMS, EROD, DBF, GST, GPX, GR, LPO, DNA damage and AChE
Discussion
The Bay of Cádiz and Guadalete River are characterized by a mixture of contaminants. The Bay of Cádiz has been studied in some depth and is characterized by an absence of significant contamination compared to other enclosed areas such as ports (DelValls and Chapman 1998; Martín-Díaz et al. 2008a, 2008b; Riba et al. 2004, 2005). Nevertheless, the chemical characterization of sediment samples in the Bay of Cádiz indicated a mixture of contaminants: metals, surfactants (Lara-Martín et al. 2005, 2006), polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs) (Martin-Díaz et al. 2008a) and pharmaceutical active compounds (Maranho et al. 2015). The WWTP effluents in El Trocadero from Puerto Real, and La Puntilla from Puerto de Santa Maria, receive urban wastewater directly from hospitals without previous treatment. The Guadalete River receives direct discharges from farms and individuals’ households, and the WWTP in Jerez de la Frontera receives untreated domestic wastewater from Jerez city and small villages, as well as from hospitals located in these towns (Corada-Fernández et al. 2011). This plant is sometimes forced to discharge some of the wastewater without prior purification through a secondary effluent located upstream throughout the winter seasons and during periods of heavy rainfall (Corada-Fernández et al. 2011, 2017; Lara-Martín et al. 2008). The use of biomarkers in assessing the toxicity of contaminants present in WWTP effluents is continuously developing, but improvements are still crucial. Therefore, it is necessary to validate biomarker responses considering the determination of biomarker responses in situ and the sentinel species for testing.
NRRT measures the lysosomal content efflux into the cytosol, which reflects a physiological process in stressed organisms after membrane damage and comparatively measures the capacity of cellular processes to adapt to stress conditions (Lowe and Pipe 1994). In this study, the biomarker of general stress, determined by LMS, indicated that averages values of NRRT (128 ± 9 min) from R. philippinarum exposed to the reference site Chorrillos were above the values measured previously in this species exposed under field conditions for 14 days to a reference site in Rota during the winter (average NRRT 100 min; Maranho et al. 2015), and for 28 days to a reference site in the Bay of Cádiz (NRRT = 70 ± 10 min; Buratti et al. 2010). In a previous study from our group under laboratory conditions, the NRRT measured in R. philippinarum exposed to the control treatment after 35 days was 94 ± 7 min (Aguirre-Martínez et al. 2013b), and reached over 100 min when exposed for 7 days (Díaz-Garduño et al. 2018). Just as R. philippinarum had reached an NRRT 134 ± 13 min on day 0, the NRRT determined in C. fluminea on day 0 was 120 ± 4 min These values are also higher than the NRRT recorded in other fresh water bivalves under laboratory and field conditions; for example, the NRRT observed in the zebra mussel species Dreissena polymorpha exposed in the laboratory to a control treatment was about 90 min (Binelli et al. 2009).
LMS is considered to be a sensitive early-warning biomarker of animal health status (Martínez-Gómez et al. 2008; Viarengo et al. 2007), and supports the development of a stress response to contaminants. Thus, it was applied as screening biomarker to identify changes in health status associated with contaminated environments (Buratti et al. 2012). Considering the health status thresholds proposed for R. philippinarum and C. fluminea by Aguirre-Martínez et al. 2013b, 2015, respectively, results from the present experiment indicated that R. philippinarum exposed to Chorrillos (reference site during 14 days) were healthy. Clams exposed to El Trocadero were stressed but compensating, and clams exposed to La Puntilla showed diminished health status. Similarly, the controls for the fresh water species C. fluminea evaluated on day 0 of the assay were considered healthy, and clams exposed G1 (upstream) and to G3 (downstream) showed signs of stress. Decreased values of NRRT in the studied areas from Cádiz and the Guadalete River compared to the controls could be attributed to increased pollution levels prevailing in the respective areas. These results showing decreased NRRT are consistent with those reported for bivalves, where NRRT decreased following exposure to heavy metals (Matozzo et al. 2001; Viarengo et al. 2000), as well as organic pollutants (Lowe et al. 1995) and whole effluents (Díaz-Garduño et al. 2018; Maranho et al. 2015). It has been reported that in field studies, NRRT assays are either minimally or not at all affected by natural factors, such as temperature and salinity, but are mainly influenced by pollutants (Ringwood et al. 1998). LMS evaluated in this study was therefore a sensitive tool for the evaluation of general stress in both species of clams under field conditions, and was shown to be a suitable screening biomarker for contamination in aquatic environments due to its ability to demonstrate a detrimental contaminant effect in the marine and fresh water environment.
EROD activity is involved in Phase I of metabolism, unmasking or adding reactive functional groups (oxidation, reduction or hydrolysis) (Goeptar et al. 1995). The increase of EROD activity observed in clams exposed to the Bay of Cádiz and to the Guadalete River indicates a metabolization of the organic compounds that are present and bioavailable in the water column. Increases in EROD activities have been reported in many species of invertebrates, including clam and crab species, following exposure to organic pollutants including polycyclic aromatic hydrocarbon (PAH’s), Polychlorinated biphenyl (PCBs), chlorophenols (CPs), hexachlorobenzene (HCB), Tributyltin (TBT), pharmaceutical active compounds and antibacterial agents (Binelli et al. 2005, 2006; Lafontaine et al. 2000; Aguirre-Martínez et al. 2013b, 2014, 2016). PAH and pharmaceutical products have been reported as being responsible for the activation of EROD activity in R. philippinarum and freshwater clams in the field (Maranho et al. 2015) and under laboratory conditions (Aguirre-Martínez et al. 2015, 2016). Results from this study are also consistent with the increase of EROD activity demonstrated in the digestive gland tissues of E. complanata exposed for 7 weeks to different concentrations of a primary treatment effluent (Gagné et al. 2007). This is consistent with a study that indicated an increase of EROD activity in digestive gland tissues of E. complanata after an injection of effluent extract (Martín-Díaz et al. 2009). In addition to and supporting our results, it has been shown that EROD activity increases in the digestive glands of C. fluminea exposed for 15 days to domestic land field leachate, thus demonstrating its toxicity (Oliveira et al. 2014). Similarly, EROD activity was induced in Oncorhynchus mykiss hepatocytes after exposure to municipal effluents after 48 h (Gagné and Blaise 1999), and in O. mykiss liver microsomes exposed in vitro (Gagné et al. 2006).
The activity of DBF from Phase I of detoxification metabolism is linked to cytochrome P4503A4, which is involved in the metabolism of many pharmaceutical products, and detoxification against xenobiotics (Smith 2009; Taxak and Bharatam 2010) was significantly induced in R. philippinarum exposed to El Tocadero and in C. fluminea exposed to G3 (downstream). Results from this study do not coincide with a previous study indicating that DBF activity was reduced in the digestive gland of E. complanata exposed to different concentrations of a municipal effluent for seven weeks (Gagné et al. 2007). The increase of DBF activity in El Trocadero and in G3 might suggest the presence and bioavailability of pharmaceutical active compounds during the experiment and their metabolization by R. philippinarum and C. fluminea after 14 and 21 days of exposure, respectively. In this sense, it has been reported that municipal effluents contain large amounts of relatively polar compounds such as pharmaceutical products and steroids (Boyd et al. 2003).
Glutathione S-transferase activity was found to be significantly induced in R. philippinarum and C. fluminea after exposure to areas near WWTP effluents. Glutathione transferases are a family of enzymes that use glutathione (GSH) as a substrate in reactions, which permit the biotransformation and disposal of a wide range of exogenous compounds (Contreras-Vergara et al. 2004). These compounds may be xenobiotics, drugs or products of oxidative stress, but are mainly polar organic compounds. These enzymes are Phase II type enzymes and catalyse the synthetic conjugation reactions of the xenobiotic parent compounds and their metabolites in order to facilitate the excretion of chemicals. Results from this study indicating increases in GST activity in digestive glands from both species of clams exposed to WWTP effluents are consistent with a previous study reporting significant increases of GST activity in fresh water mussels, Elliptio complanata, located downstream from a primary treated municipal effluent for one year, suggesting the presence of organic contaminants (Gagné et al. 2004b). This finding coincides with the observation that municipal effluents are a major source of polyaromatic hydrocarbons (PAHs), as represented by pyrene concentrations and miscellaneous organic chemicals including pharmaceutical drugs and personal-care products (Chambers et al. 1997; Kümmerer 2001). In addition, an increase in hepatic GST activity has been reported in several studies following exposure to PAHs and PCBs (Van der Oost et al. 2003). Moreover, exposure of trout hepatocytes to municipal effluents was shown to induce GST activity after 48 h in a study conducted by Gagné and Blaise (1999).
Defence systems that tend to inhibit oxyradical formation include antioxidant enzymes such as glutathione peroxidase (GPX) and glutathione reductase (GR). GPX and GR activity protects the organism from oxidative damage, and GPX is involved in the inhibition of oxyradical formation in the presence of redox-active compounds such as PCBs and PAHs. It has been demonstrated that GPX activity increases in fish exposed to PCBs and PAHs (Van der Oost et al. 2003), but more research is required on invertebrate species. GR is responsible for oxidized glutathione reduction (GSS G) via a NADPH-dependent process. Therefore, it is essential for the regeneration of reduced GSH, which is necessary for the operation of GPXs and many other cell enzymes (Manduzio et al. 2003). Many pollutants may induce toxicity reactions related to oxidative stress. Oxygen toxicity may act as a potent oxidant, capable of reacting with critical cellular macromolecules, possibly leading to DNA damage and cell death. Lipid peroxidation is an important effect of oxidative stress, and GPX and GR enzymatic activity are efficient in protecting against damage caused by LPO (Winston and Di Giulio 1991). LPO levels decreased in R. philippinarum exposed to Trocadero and La Puntilla in the Bay of Cádiz; on the contrary, LPO levels increased in C. fluminea exposed to G1 and G3 in the Guadalete River. In this regard, a previous study by Gagné et al. (2007) demonstrated that LPO levels increased in gill tissues of E. complanata exposed for 7 weeks to different concentrations of municipal effluents. Conversely, LPO levels decreased in a study on E. complanata exposed for one year to a primary-treated effluent plume (Gagné et al. 2004b). These authors have also shown that municipal effluents can induce LPO in O. mykiss liver microsomes exposed in vitro, indicating that products present in municipal effluents have the potential to produce a toxic response in aquatic organisms (Gagné et al. 2006).
DNA damage could be a consequence of oxidative stress (Winston and Di Giulio 1991). The number of DNA strand breaks in tissues is a function of DNA repair/synthesis activity and indicates the degree of DNA damage. It was noted in this study that R. philippinarum exposed to WWTP effluents in the marine environment did not show DNA damage. Similarly, another study performed in the Gulf of Gdańsk indicated no genotoxic effects of sewage effluents on sampled blue mussels, Mytilus edulis trossulus, that were close to point sources (Larsson et al. 2018). A possible explanation for the lack of DNA damage in digestive gland tissues of R. philippinarum exposed to WWTP effluents in the Bay of Cádiz could be that defence mechanisms, such as enzyme activity from Phase I, biotransformation enzymes form Phase II, and antioxidant responses were efficient in preventing severe damage, and were effective in providing cellular protection against oxidative damage. Bivalves have the capacity to biotransform foreign organic chemicals such as Phase I and II biotransformation enzymes. On the other hand, fresh water clams exposed to G1 (upstream) and G3 (downstream) in the Guadalete River did show DNA damage. Our results regarding clams exposed to The Guadalete River differ from previous studies, where DNA damage did not vary significantly in the digestive glands of E. Complanata exposed to an upstream site of a municipal effluent plume for a year (Gagné et al. 2004b), or in the digestive glands of E. complanatta exposed for 62 days to the same municipal plume, but at a distance of 4 km (Gagné et al. 2002). Results from our study are consistent with a study that reported that DNA damage increased in the digestive glands of E. complanata exposed for seven weeks to different concentrations of a primary-treated municipal effluent (Gagné et al. 2007). Other studies have recognized the genotoxic effect of effluents. Enzymatic activity from Phase I and Phase II studied and induced in this research provides evidence for the activation of the defence mechanisms in R. philippinarum and C. fluminea, and shows the bioavailability of contaminants present in the water column from each site.
AChE is a group of enzymes crucial to the transmission of nervous impulses, and is found in all animal phyla, including mollusks (Walker and Thompson 1991). AChE converts acetylcholine into choline and acetate, and neurotransmission is stopped by the AChE effect (Pohanka 2011, 2012, 2013). Inhibition of AChE activity was analysed in order to determine whether the WWTP effluent outfalls selected were able to cause a neurotoxic effect in R. philippinarum and C. fluminea. Results from this study indicated an effect demonstrated by the inhibition of AChE activity observed in R. philippinarum exposed to WWTP in El Trocadero and La Puntilla, and in C. fluminea exposed to G1 (upstream) and G3 (downstream). In general, many field studies have demonstrated the value of measuring AChE activity in invertebrates as exposure biomarkers in coastal waters and rivers (Dailianis et al. 2003; Gagné et al. 2010; Rank et al. 2007; Stien et al. 1998). AChE activity is inhibited in the presence of organic and inorganic compounds such as pesticides, hydrocarbons, antibacterial agents, detergents and metals (Brown et al. 2004; Damásio et al. 2011; Guilhermino et al. 1998; Lionetto et al. 2003; Matozzo et al. 2012; Payne et al. 1996). AChE is a well-recognized biomarker for detecting general physiological stress in aquatic organisms caused by exposure to contaminants (Rank et al. 2007). Coinciding with the results from this study, other studies have demonstrated a decrease of AChE activity in gills from the clam Venerupis philippinarum exposed to fluoxetine concentrations (Munari et al. 2014), and in the digestive glands of Carcinus aestuarii when exposed to lower levels of organic contaminants (Ricciardi et al. 2010). A decrease of AChE was also reported in the sea urchin species Paracentrotus lividus when exposed to pesticides (Pesando et al. 2003). In contrast, it has been demonstrated that mussels injected with an effluent extract have significantly increased in AChE (Gagné et al. 2010). The U.S. EPA (1998) suggests that a decrease of 20% or more in AChE activity can be considered as a clear toxicological effect of xenobiotic exposure. In this regard, the present study noted a decrease of 61% in AChE activity measured in R. philippinarum exposed to El Trocadero, and a 70% decrease found in clams exposed to La Puntilla. When C. fluminea were exposed to G1, a decrease of 61% in AChE was noted, and clams exposed to G3 presented a decrease of 57%. These results indicate a clear toxicological effect produced by these WWTP effluents.
The IBR index was applied to integrate the biomarker responses in organisms after exposure to effluents (Díaz-Garduño et al. 2018; Kim and Jung 2016; Maranho et al. 2015; Serafim et al. 2013) in order to identify the effects of stress on both clam species. Based on this index, similar responses between clams exposed to El Trocadero and La Puntilla were noted, with maximum IBR values of GR indicating the activation of antioxidant responses in both sites. Similarly, LPO and DNA damage were not observed in these sites. Both sites induced EROD and DBF activity, nevertheless, El Trocadero presented higher IBR values of these responses than La Puntilla, demonstrating the presence of bioavailable organic compounds, as previously discussed. A higher IBR score was calculated for AChE in La Puntilla, revealing neurotoxic compounds in this area. The IBR index revealed distinctions between the affected sites in the Bay of Cadiz, indicating that clams were more stressed in La Puntilla than El Trocadero. The IBR index calculated for the Guadalete River demonstrated that all biomarkers were activated indistinctively. The maximum IBR values for LMS, GPX, GR, LPO, DNA damage and AChE were observed in G1 (upstream), indicating that organisms were more stressed in this site, and the IBR index for DBF was higher in G3 (downstream), suggesting that there are likely pharmaceuticals products in this area.
Caged organisms have widely been used to determine various biological responses in order evaluate the environmental quality of areas impacted by wastewater effluents (Díaz-Garduño et al. 2018; Maranho et al. 2015; Nobles and Zhang 2015). The caged R. philippinarum and C. fluminea used in this study exhibit changes in the biomarkers when indistinctively exposed to studied sites, indicating that chemicals from wastewater had a measurable impact on the health status of both species. Upon comparing both species, the greatest biomarker activation was visible in C. fluminea, which may be due to the longer exposure time (21 days) compared to the experiment with R. phillipinarum (14 days). This was also likely related to the bioavailability of contaminants present in the fresh water environment that induced oxidative stress and DNA damage. Nevertheless, both species were found to be sensitive and susceptible to environmental contaminants present in the water column, thus suggesting their potential value in environmental risk assessments.
Conclusion
This study evaluated the effects of WWTP discharges in marine and fresh water environments on caged clams via the application of biomarkers of general stress, exposure and effect and the biomaker of neurotoxicity. It was concluded that lysosomal damage is a sensitive tool for the evaluation of the general stress of caged clams under field conditions, and is a suitable screening biomarker for contamination in aquatic environments due to its ability to demonstrate a detrimental contaminant effect in both species. Results from this study indicated that Phase I, Phase II, antioxidant enzymes, neurotoxic effect, lipid peroxidation and DNA damage selected as sublethal responses, constituted suitable measurements to be incorporated in a battery of biomarkers to assess toxicity in marine and fresh water environments. The digestive gland tissues analysed were found to be reliable for biomarker analysis. Contaminants present in the studied areas from the Bay of Cádiz (El Tocadero and La Puntilla) and the Guadalete River (G1 and G3) may affect the health status of R. philippinarum and C. fluminea, as well as other aquatic organisms. The effluent discharge from WWTP in Jerez de la Frontera led to the death of 96% of the clams within a week of the bioassay, indicating a high level of toxicity. Nevertheless, more research should be performed in coastal and fresh water environments affected by constant WWTP discharges. Finally, the data reported in this study, including toxic effects and defence mechanisms, represent important ecotoxicological information and will provide a useful reference for the assessment of areas adjacent to WWTPs and the effect their effluents might have on marine and fresh water organisms, using the clam R. philippinarum and C. fluminea as bioindicator species. This study also demonstrates the importance of in situ bioassays in evaluating the adverse effects of WWTP effluents, as they indicate realistic conditions of organisms exposed to mixtures of contaminants.
References
Abessa DM, Carr RS, Rachid BR, Sousa EC, Hortelani MA, Sarkis JE (2005) Influence of a Brazilian sewage outfall on the toxicity and contamination of adjacent sediment. Mar Pollut Bull 50:875–855. https://doi.org/10.1016/j.marpolbul.2005.02.034
Aguirre-Martínez GV, André C, Gagné F, Martín-Díaz LM (2018) The effects of human drugs in Corbicula fluminea. Assessment of neurotoxicity, inflammation, gametogenic activity, and energy status. Ecotoxicol Environ Saf 148:652–663. https://doi.org/10.1016/j.ecoenv.2017.09.042
Aguirre-Martínez GV, Buratti S, Fabbri E, DelValls AT, Martín-Díaz ML (2013b) Using lysosomal membrane stability of haemocytes in Ruditapes philippinarum as a biomarker of cellular stress to assess contamination by caffeine, ibuprofen, carbamazepine and novobiocin. J Environ Sci 25:1408–1418. https://doi.org/10.1016/S1001-0742(12)60207-1
Aguirre-Martínez GV, Del Valls TA, Martín-Díaz ML (2013a) Identification of biomarkers responsive to chronic exposure to pharmaceuticals in target tissues of Carcinus maenas. Mar Environ Res 87:1–11. https://doi.org/10.1016/j.marenvres.2013.02.011
Aguirre-Martínez GV, DelValls TA, Martín-Díaz ML (2015) Yes, caffeine, ibuprofen, carbamazepine, novobiocin and tamoxifen have an effect on Corbicula fluminea (Müller, 1774). Ecotoxicol Environ Safe 120:142–154. https://doi.org/10.1016/j.ecoenv.2015.05.036
Aguirre-Martínez GV, DelValls TA, Martín-Díaz ML (2016) General stress, detoxification pathways, neurotoxicity and genotoxicity evaluated in Ruditapes philippinarum exposed to human pharmaceuticals. Ecotoxicol Environ Safe 124:18–31. https://doi.org/10.1016/j.ecoenv.2015.09.031
Aguirre-Martínez GV, Morales-Caselles C, DelValls TA, Martín-Díaz ML (2014) Applicative implications of Carcinus maenas and Ruditapes philippinarum in biomonitoring studies after oil spills. Chem Ecol 31:77–91. https://doi.org/10.1080/02757540.2014.932780.
Barreto A, Luis LG, Paíga P, Santos LH, Delerue-Matos C, Soares AM, Hylland K, Loureiro S, Oliveira M (2018) A multibiomarker approach highlights effects induced by the human pharmaceutical gemfibrozil to gilthead seabream Sparus aurata. Aquat Toxicol 200:266–274. https://doi.org/10.1016/j.aquatox.2018.05.012
Bonnail E, Riba I, de Seabra AA, DelValls TÁ (2019) Sediment quality assessment in the Guadalquivir River (SW, Spain) using caged Asian clams: a biomarker field approach. Sci Total Environ 650:1996–2003. https://doi.org/10.1016/j.scitotenv.2018.09.346
Binelli A, Ricciardi F, Riva C, Provini A (2005) Screening of POP pollution by AChE and EROD activities in Zebra mussels from the Italian Great Lakes. Chemosphere 61(8):1074–1082. https://doi.org/10.1016/10.1016/j.chemosphere.2005.03.047
Binelli A, Cogni D, Parolini M, Riva C, Provini A (2009) In vivo experiments for the evaluation of genotoxic and cytotoxic effects of triclosan in zebra mussel hemocytes. Aquat Toxicol 91(3):238–244. https://doi.org/10.1016/j.aquatox.2008.11.008
Binelli A, Ricciardi F, Riva C, Provini A (2006) New evidences for old biomarkers: effects of several xenobiotics on EROD and AChE activities in Zebra mussel (Dreissena polymorpha). Chemosphere 62(4):510–519. https://doi.org/10.1016/j.chemosphere.2005.06.033
Bonacci S, Browne MA, Dissanayake A, Hagger JA, Corsi I, Focardi S, Galloway TS (2004) Esterase activities in the bivalve mollusc Adamussium colbecki as a biomarker for pollution monitoring in the Antarctic marine environment. Mar Pollut Bull 49(5–6):445–455. https://doi.org/10.1016/j.marpolbul.2004.02.033
Boryslawskyj M, Garrood AC, Pearson JT, Woodhead D (1988) Elevation of glutathione-S-transferase activity as a stress response to organochlorine compounds, in the freshwater mussel, Sphaerium corneum. Mar Environ Res 24(1–4):101–104. https://doi.org/10.1016/0141-1136(88)90263-2
Boyd GR, Reemtsma H, Grimm DA, Mitra S (2003) Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci Total Environ 311(1–3):135–149. https://doi.org/10.1016/S0048-9697(03)00138-4
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Broeg K, Westernhagen HV, Zander S, Körting W, Koehler A (2005) The bioeffect assessment index (BAI) A concept for the quantification of effects ofmarine pollution by an integrated biomarker approach. Mar Pollut Bull 50:495–503. https://doi.org/10.1016/j.marpolbul.2005.02.042Get
Brown RJ, Galloway TS, Lowe D, Browne MA, Dissanayake A, Jones MB, Depledge MH (2004) Differential sensitivity of three marine invertebrates to copper assessed using multiple biomarkers. Aquat Toxicol 66(3):267–278. https://doi.org/10.1016/j.aquatox.2003.10.001
Buratti S, Fabbri E, Pereira CD, Ramos-Gómez J, Del Valls TA, Martín-Díaz ML (2010) Application of neutral red retention assay in the clam Ruditapes philippinarum and the crab Carcinus maenas as a screening tool for sediment quality assessment in marine environment. Abstracts/Comp Biochem Phys A 157:S27. https://doi.org/10.1016/j.cbpa.2010.06.077
Buratti S, Ramos-Gómez J, Fabbri E, DelValls TA, Martín-Díaz ML (2012) Application of neutral red retention assay to caged clams (Ruditapes decussatus) and crabs (Carcinus maenas) in the assessment of dredged material. Ecotoxicology 21(1):75–86. https://doi.org/10.1007/s10646-011-0767-1
Cajaraville MP, Bebianno MJ, Blasco J, Porte C, Sarasquete C, Viarengo A (2000) The use of biomarkers to assess the impact of pollution in coastalenvironments of the Iberian Peninsula: a practical approach. Sci Total Environ 247:295–311. https://doig.org/10.1016/s0048-9697(99)00499-4
Cataldo D, Colombo JC, Boltovskoy D, Bilos C, Landoni P (2001) Environmental toxicity assessment in the Paraná river delta (Argentina): simultaneous evaluation of selected pollutants and mortality rates of Corbicula fluminea (Bivalvia) early juveniles. Environ Pollut 112(3):379–389. https://doi.org/10.1016/S0269-7491(00)00145-7
Chambers PA, Allard M, Walker SL, Marsalek J, Lawrence J, Servos M, Busnarda J, Munger KS, Adare K, Jefferson C, Kent RA (1997) Impacts of municipal wastewater effluents on Canadian waters: a review. Water Qual Res J 32(4):659–714. https://doi.org/10.2166/wqrj.1997.038
Contreras-Vergara CA, Harris-Valle C, Sotelo-Mundo RR, Yepiz-Plascencia G (2004) A mu-class glutathione S-transferase from the marine shrimp Litopenaeus vannamei: molecular cloning and active-site structural modeling. J Biochem Mol Toxicol 18(5):245–252. https://doi.org/10.1002/jbt.20033
Cooper NL, Bidwell JR (2006) Cholinesterase inhibition and impacts on behavior of the Asian clam, Corbicula fluminea, after exposure to an organophosphate insecticide. Aquat Toxicol 76:258–267. https://doi.org/10.1016/j.aquatox.2005.09.012
Corada-Fernández C, Candela L, Torres-Fuentes N, Pintado-Herrera MG, Paniw M, González-Mazo E (2017) Effects of extreme rainfall events on the distribution of selected emerging contaminants in surface and groundwater: the Guadalete River basin (SW, Spain). Sci Total Environ 605:770–783. https://doi.org/10.1016/j.scitotenv.2017.06.049
Corada-Fernández C, Lara-Martín PA, Candela L, González-Mazo E (2011) Tracking sewage derived contamination in riverine settings by analysis of synthetic surfactants. J Environ Monit 13(7):2010–2017. https://doi.org/10.1039/C1EM10150A
Dailianis S, Domouhtsidou GP, Raftopoulou E, Kaloyianni M, Dimitriadis VK (2003) Evaluation of neutral red retention assay, micronucleus test, acetylcholinesterase activity and a signal transduction molecule (cAMP) in tissues of Mytilus galloprovincialis (L.), in pollution monitoring. Mar Environ Res 56(4):443–470. https://doi.org/10.1016/S0141-1136(03)00005-9
Damásio J, Barceló D, Brix R, Postigo C, Gros M, Petrovic M, Sabater S, Guasch H, de Alda ML, Barata C (2011) Are pharmaceuticals more harmful than other pollutants to aquatic invertebrate species: a hypothesis tested using multi-biomarker and multi-species responses in field collected and transplanted organisms. Chemosphere 85(10):1548–1554. https://doi.org/10.1016/j.chemosphere.2011.07.058
DelValls TA, Chapman PM (1998) Site-specific sediment quality values for the Gulf of Cádiz (Spain) and San Francisco Bay (USA), using the sediment quality triad and multivariate analysis. Ciencias Marinas 24(3):313–336
Depledge MH (1993) The Rational Basis for the Use of Biomarkers as Ecotoxicological tools. In: Fossi MC, Leonzio C (eds) NondestructiveBiomarkers in Vertebrates, Lewis Publisher, Boca Raton, 271–295
Depledge MH, Fossi MC (1994) The role of biomarkers in environmental assessment (2). Invertebrates. Ecotoxicology 3(3):161–172
Díaz-Garduño B, Perales JA, Garrido-Pérez C, Martín-Díaz ML (2018) Health status alterations in Ruditapes philippinarum after continuous secondary effluent exposure before and after additional tertiary treatment application. Environ Pollut 235:720–729. https://doi.org/10.1016/j.envpol.2018.01.017
Gagné F, André C, Cejka P, Gagnon C, Blaise C (2007) Toxicological effects of primary-treated urban wastewaters, before and after ozone treatment, on freshwater mussels (Elliptio complanata). Comp Biochem Phys C 145(4):542–552. https://doi.org/10.1016/j.cbpc.2007.01.019
Gagné F, André C, Cejka P, Hausler R, Fournier M, Blaise C (2008) Immunotoxic effects on freshwater mussels of a primary-treated wastewater before and after ozonation: a pilot plant study. Ecotoxicol Environ Safe 69(3):366–373. https://doi.org/10.1016/j.ecoenv.2007.10.027
Gagné F, André C, Gélinas M (2010) Neurochemical effects of benzodiazepine and morphine on freshwater mussels. Comp Biochem Phys C 152(2):207–214. https://doi.org/10.1016/j.cbpc.2010.04.007
Gagné F, Blaise C (1993) Hepatic metallothionein level and mixed function oxidase activity in fingerling rainbow trout (Oncorhynchus mykiss) after acute exposure to pulp and paper mill effluents. Water Res 27(11):1669–1682. https://doi.org/10.1016/0043-1354(93)90131-Z
Gagné F, Blaise C (1999) Toxicological effects of municipal wastewaters to rainbow trout hepatocytes. Bull Environ Contam Toxicol 63(4):503–510
Gagné F, Blaise C, André C (2006) Occurrence of pharmaceutical products in a municipal effluent and toxicity to rainbow trout (Oncorhynchus mykiss) hepatocytes. Ecotox Environ Safe 64:329–336. https://doi.org/10.1016/j.ecoenv.2005.04.004
Gagné F, Blaise C, Aoyama I, Luo R, Gagnon C, Couillard Y, Campbell P, Salazar M (2002) Biomarker study of a municipal effluent dispersion plume in two species of freshwater mussels. Environ Toxicol 17(3):149–159. https://doi.org/10.1002/tox.10046
Gagné F, Blaise C, Hellou J (2004b) Endocrine disruption and health effects of caged mussels, Elliptio complanata, placed downstream from a primary-treated municipal effluent plume for 1 year. Comp Biochem Phys C 138(1):33–44. https://doi.org/10.1016/j.cca.2004.04.006
Gagné F, Fournier M, Blaise C (2004a) Serotonergic effects of municipal effluents: induced spawning activity in freshwater mussels. Fres Environ Bull 13(11):1099–1103. https://doi.org/10.1016/j.ecoenv.2005.04.004
Goeptar AR, Scheerens H, Vermeulen NP (1995) Oxygen and xenobiotic reductase activities of cytochromeP450. Crit Rev Toxicol 25(1):25–65. https://doi.org/10.3109/10408449509089886
Guilhermino L, Barros B, Silva MC, Soares AMVM (1998) SHORT COMMUNICATION, Should the use of inhibition of cholinesterases as a specific biomarker for organophosphate and carbamate pesticides be questioned. Biomarkers 3(2):157–163. https://doi.org/10.1080/135475098231318
Guilhermino L, Lopes MC, Carvalho AP, Soared AM (1996) Inhibition of acetylcholinesterase activity as effect criterion in acute tests with juvenile Daphnia magna. Chemosphere 32(4):727–738. https://doi.org/10.1016/0045-6535(95)00360-6
Janero DR (1990) Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med 9(6):515–540
Kim W-K, Jung J (2016) In situ impact assessment of wastewater effluents by integrating multilevel biomarker responses in the pale chub (Zacco platypus). Ecotox Environ Safe 128:246–251. https://doi.org/10.1016/j.ecoenv.2016.02.028
Kümmerer K (2001) Drugs in the environment: emission of drugs, diagnostic aids and disinfectants into wastewater by hospitals in relation to other sources—a review. Chemosphere 45(6–7):957–969. https://doi.org/10.1016/S0045-6535(01)00144-8
de Lafontaine Y, Gagné F, Blaise C, Costan G, Gagnon P, Chan HM (2000) Biomarkers in Zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St. Lawrence River (Canada). Aquat Toxicol 50:51–70. https://doi.org/10.1016/S0166-445X(99)00094-6
Lara-Martín PA, Gómez-Parra A, González-Mazo E (2005) Determination and distribution of alkyl ethoxysulfates and linear alkylbenzene sulfonates in coastal marine sediments from the Bay of Cadiz (southwest of Spain). Environ Toxicol Chem 24(9):2196–2202. https://doi.org/10.1897/04-446R.1
Lara-Martín PA, Gómez-Parra A, González-Mazo E (2008) Sources, transport and reactivity of anionic and non-ionic surfactants in several aquatic ecosystems in SW Spain: a comparative study. Environ Pollut 156(1):36–45. https://doi.org/10.1016/j.envpol.2008.01.005
Lara-Martín PA, González-Mazo E, Petrovic M, Barceló D, Brownawell BJ (2014) Occurrence, distribution and partitioning of nonionic surfactants and pharmaceuticals in the urbanized Long Island Sound Estuary (NY). Mar Pollut Bull 85(2):710–719. https://doi.org/10.1016/j.marpolbul.2014.01.022
Lara-Martín PA, Petrovic M, Gómez-Parra A, Barceló D, González-Mazo E (2006) Presence of surfactants and their degradation intermediates in sediment cores and grabs from the Cadiz Bay area. Environ Pollut 144(2):483–491. https://doi.org/10.1016/j.envpol.2006.01.033
Larsson J, Smolarz K, Świeżak J, Turower M, Czerniawska N, Grahn M (2018) Multi biomarker analysis of pollution effect on resident populations of blue mussels from the Baltic Sea. Aquat Toxicol 198:240–256. https://doi.org/10.1016/j.aquatox.2018.02.024
Legeay A, Achard-Joris M, Baudrimont M, Massabuau JC, Bourdineaud JP (2005) Impact of cadmium contamination and oxygenation levels on biochemical responses in the Asiatic clam Corbicula fluminea. Aquat Toxicol 74(3):242–253. https://doi.org/10.1016/j.aquatox.2005.05.015
Lionetto MG, Caricato R, Giordano ME, Pascariello MF, Marinosci L, Schettino T (2003) Integrated use of biomarkers (acetylcholinesterase and antioxidant enzymes activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Pollut Bull 46(3):324–330. https://doi.org/10.1016/S0025-326X(02)00403-4
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666
Lowe DM, Fossato VU, Depledge MH (1995) Contaminant-induced lysosomal membrane damage in blood cells of mussels Mytilus galloprovincialis from the Venice Lagoon: an in vitro study. Mar Ecol Prog Ser 129:189–196
Lowe DM, Pipe RK (1994) Contaminant induced lysosomal membrane damage in marine mussel digestive cells: an in vitro study. Aquat Toxicol 30:357–365. https://doi.org/10.1016/0166-445X(94)00045-X
Manduzio H, Monsinjon T, Rocher B, Leboulenger F, Galap C (2003) Characterization of an inducible isoform of the Cu/Zn superoxide dismutase in the blue mussel Mytilus edulis. Aquat Toxicol 64:73–83. https://doi.org/10.1016/S0166-445X(03)00026-2
Maranho LA, DelValls TA, Martín-Díaz ML (2015) Assessing potential risks of wastewater discharges to benthic biota: an integrated approach to biomarker responses in clams (Ruditapes philippinarum) exposed under controlled conditions. Mar Pollut Bull 92(1–2):11–24. https://doi.org/10.1016/j.marpolbul.2015.01.009
Marchi B, Burlando B, Moore MN, Viarengo A (2004) Mercury- and copper induced lysosomal membrane destabilisation depends on [Ca2+]I dependent phospholipase A2 activation. Aquat Toxicol 66:197–204. https://doi.org/10.1016/j.aquatox.2003.09.003
Martín-Díaz ML, Blasco J, Sales D, DelValls A (2004) Biomarkers as tools to assess sediment quality. Laboratory and fields surveys. Trends Anal Chem 23(10–11):807–818. https://doi.org/10.1016/j.trac.2004.07.012
Martín-Díaz ML, Blasco J, Sales D, DelValls TA (2007) Biomarkers study for sediment quality assessment in Spanish ports using the crab Carcinus maenas and the clam Ruditapes philippinarum. Arch Environ Contam Toxic 53(1):66–76. https://doi.org/10.1007/s00244-006-0121-4
Martín-Díaz ML, Blasco J, Sales D, DelValls TA (2008a) Field validation of a battery of biomarkers to assess sediment quality in Spanish ports. Environ Pollut 151(3):631–640. https://doi.org/10.1016/j.envpol.2007.03.019
Martín-Díaz ML, Gagné F, Blaise C (2009) The use of biochemical responses to assess ecotoxicological effects of pharmaceutical and personal care products (PPCPs) after injection in the mussel Elliptio complanata. Environ Toxicol Pharm 28(2):237–242. https://doi.org/10.1016/j.etap.2009.04.009
Martín-Díaz ML, Jiménez-Tenorio N, Sales D, DelValls TA (2008b) Accumulation and histopathological damage in the clam Ruditapes philippinarum and the crab Carcinus maenas to assess sediment toxicity in Spanish ports. Chemosphere 71(10):1916–1927. https://doi.org/10.1016/j.chemosphere.2008.01.022
Martínez-Gómez C, Benedicto J, Campillo JA, Moore M (2008) Application and evaluation of the neutral red retention (NRR) assay for lysosomal stability in mussel populations along the Iberian Mediterranean coast. J Environ Monit 10(4):490–499. https://doi.org/10.1039/B800441M
Matozzo V, Ballarin L, Pampanin DM, Marin MG (2001) Effects of copper and cadmium exposure on functional responses of hemocytes in the clam, Tapes philippinarum. Arch Environ Contam Toxic 41(2):163–170. https://doi.org/10.1007/s002440010234
Matozzo V, Bertin V, Battistara M, Guidolin A, Masiero L, Marisa I, Orsetti A (2016) Does the antibiotic amoxicillin affect haemocyte parameters in non-target aquatic invertebrates? The clam Ruditapes philippinarum and the mussel Mytilus galloprovincialis as model organisms. Mar Environ Res 119:51–58. https://doi.org/10.1016/j.marenvres.2016.05.017
Matozzo V, Formenti A, Donadello G, Marin MG (2012) A multi-biomarker approach to assess effects of Triclosan in the clam Ruditapes philippinarum. Mar Environ Res 74:40–46. https://doi.org/10.1016/j.marenvres.2011.12.002
McFarland VA, Inouye LS, Lutz CH, Jarvis AS, Clarke JU, McCant DD (1999) Biomarkers of oxidative stress and genotoxicity in livers of field-collected brown bullhead, Ameiurus nebulosus. Arch Environ Contam Toxic 37(2):236–241. https://doi.org/10.1007/s002449900510
Metcalfe CD, Miao XS, Koenig BG, Struger J (2003) Distribution of acidic and neutral drugs in surface waters near sewage treatment plants in the lower Great Lakes, Canada. Environ Toxicol Chem 22(12):2881–2889. https://doi.org/10.1897/02-627
Morales-Caselles C, Martín-Díaz ML, Riba I, Sarasquete C, DelValls TÁ (2008) Sublethal responses in caged organisms exposed to sediments affected by oil spills. Chemosphere 72(5):819–825. https://doi.org/10.1016/j.chemosphere.2008.02.060
Munari M, Marin MG, Matozzo V (2014) Effects of the antidepressant fluoxetine on the immune parameters and acetylcholinesterase activity of the clam Venerupis philippinarum. Mar Environ Res 94:32–37. https://doi.org/10.1016/j.marenvres.2013.11.007
Nobles T, Zhang Y (2015) Survival, growth and condition of freshwater mussels: effects of municipal wastewater effluent. PloS ONE 10(6):e0128488. https://doi.org/10.1371/journal.pone.0128488
Olive PL (1988) DNA precipitation assay: a rapid and simple method for detecting DNA damage in mammalian cells. Environ Mol Mutagen 11(4):487–495. https://doi.org/10.1002/em.2850110409
Oliveira LF, Silva SM, Martinez CB (2014) Assessment of domestic landfill leachate toxicity to the Asian clam Corbicula fluminea via biomarkers. Ecotoxicol Environ Safe 103:17–23. https://doi.org/10.1016/j.ecoenv.2014.01.034
Payne JF, Mathieu A, Melvin W, Fancey LL (1996) Acetylcholinesterase, an old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundland. Mar Pollut Bull 32(2):225–231. https://doi.org/10.1016/0025-326X(95)00112-Z
Pesando D, Huitorel P, Dolcini V, Angelini C, Guidetti P, Falugi C (2003) Biological targets of neurotoxic pesticides analysed by alteration of developmental events in the Mediterranean sea urchin, Paracentrotus lividus. Mar Environ Res 55(1):39–57. https://doi.org/10.1016/S0141-1136(02)00215-5
Pohanka M (2011) Cholinesterases, a target of pharmacology and toxicology. Biomed Pap 155(3). https://doi.org/10.5507/bp.2011.036
Pohanka M (2012) Acetylcholinesterase inhibitors: a patent review (2008–present). Expert Opin Ther Pat 22(8):871–886. https://doi.org/10.1517/13543776.2012.701620
Pohanka M (2013) Cholinesterases in biorecognition and biosensors construction: a review. Anal Lett 46(12):1849–1868. https://doi.org/10.1080/00032719.2013.780240
Quinn B, Gagné F, Blaise C (2004) Oxidative metabolism activity in Hydra attenuata exposed to carbamazepine. Fresen Environ Bull 13(8):783–788
Quinn B, Gagné F, Weber JP, Blaise C (2005) Ecotoxicological effects of a semi-submerged municipal dump (Castle harbour, Bermuda) on the Calico scallop Argopecten gibbus. Mar Pollut Bull 51(5–7):534–544. https://doi.org/10.1016/j.marpolbul.2005.07.019
Rank J, Lehtonen KK, Strand J, Laursen M (2007) DNA damage, acetylcholinesterase activity and lysosomal stability in native and transplanted mussels (Mytilus edulis) in areas close to coastal chemical dumping sites in Denmark. Aquat Toxicol 84:50–61. https://doi.org/10.1016/j.aquatox.2007.05.013
Ramos-Gómes J, Coz A, Viguri JR, Luque A, Martiń -Diáz ML, Del Valls TA (2011) Biomarker responsiveness in different tissues of caged Ruditapes philippinarum and its use within an integrated sediment quality assessment. Environ Pollut 159(7):1914–1922. https://doi.org/10.1016/j.envpol.2011.03.030
Riba I, Blasco J, Jiménez-Tenorio N, DelValls TÁ (2005) Heavy metal bioavailability and effects: II. Histopathology–bioaccumulation relationships caused by mining activities in the Gulf of Cádiz (SW, Spain). Chemosphere 58(5):671–682. https://doi.org/10.1016/j.chemosphere.2004.02.016
Riba I, Forja JM, Gómez-Parra A, DelValls TÁ (2004) Sediment quality in littoral regions of the Gulf of Cádiz: a triad approach to address the influence of mining activities. Environ Pollut 132(2):341–353. https://doi.org/10.1016/j.envpol.2004.03.021
Ricciardi F, Matozzo V, Binelli A, Marin MG (2010) Biomarker responses and contamination levels in crabs (Carcinus aestuarii) from the Lagoon of Venice: an integrated approach in biomonitoring estuarine environments. Water Res 44(6):1725–1736. https://doi.org/10.1016/j.watres.2009.11.042
Ringwood AH, Conners DE, Hoguet J (1998) Effects of natural and anthropogenic stressors on lysosomal destabilization in oysters Crassostrea virginica. Mar Ecol Prog Ser 166:163–171. https://doi.org/10.3354/meps166163
Parenti CC, Ghilardi A, Della Torre C, Mandelli M, Magni S, Del Giacco L, Binelli A (2019) Environmental concentrations of triclosan activate cellular defence mechanism and generate cytotoxicity on zebrafish (Danio rerio) embryos. Sci Total Environ 650:1752–1758. https://doi.org/10.1016/j.scitotenv.2018.09.283
Seyfried M, Boschung A, Miffon F, Ohleyer E, Chaintreau A (2014) Elucidation of the upper pathway of alicyclic musk Romandolide®degradation in OECD screening tests with activated sludge. Environ Sci Pollut Res 21:9487–9494. https://doi.org/10.1007/s11356-013-2347-9
Serafim A, Lopes CB, Fonseca VF, Franca S, Vasconcelos RP, Bebianno MJ, Cabral HN (2013) Application of an integrated biomarker response index (IBR) to assess temporal variation of environmental quality in two Portuguese aquatic system. Ecol Indic 19:215–225. https://doi.org/10.1016/j.ecolind.2011.08.009
Shugart LR (2000) DNA damage as a biomarker of exposure. Ecotoxicology 9(5):329–340. https://doi.org/10.1023/A:1026513009527
Smith HS (2009) Opioid metabolism. Mayo Clinic Proceedings 84:613–624
Solé M, Kopecka-Pilarczyk J, Blasco J (2009) Pollution biomarkers in two estuarine invertebrates, Nereis diversicolor and Scrobicularia plana, from a Marsh ecosystem in SW Spain. Environ Int 35(3):523–531. https://doi.org/10.1016/j.envint.2008.09.013
Stien X, Percic P, Gnassia-Barelli M, Roméo M, Lafaurie M (1998) Evaluation of biomarkers in caged fishes and mussels to assess the quality of waters in a bay of the NW Mediterranean Sea. Environ Pollut 99(3):339–345. https://doi.org/10.1016/S0269-7491(98)00013-X
Taxak N, Bharatam PV (2010) An insight into the concept and details of mechanism-based inhibition of CYP450. Curr Res Inform Pharm Sci 11:62–67
US EPA (1998) US Environmental Protection Agency. SCE policy issues related to the food quality protection act. Office of pesticide program_s science policy on the use of cholinesterase inhibition for risk assessment of organophosphate and carbamate pesticides. OOP Docket # 00560. Federal register 63 (214). Washington, USA
Van der Oost R, Goksøyr A, Celander M, Heida H, Vermeulen NP (1996) Biomonitoring aquatic pollution with feral eel (Anguilla anguilla): II. Biomarkers: pollution-induced Biochemical responses. Aquat Toxicol 36:189–222. https://doi.org/10.1016/S0166-445X(96)00802-8
Van der Oost R, Beyer J, Vermeulen NP (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharm 13(2):57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A (2007) The use of biomarkers in biomonitoring: a 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Phys C 146(3):281–300. https://doi.org/10.1016/j.cbpc.2007.04.011
Viarengo A, Marro A, Marchi B, Burlando B (2000) Single and combined effects of heavy metals and hormones on lysosomes of haemolymph cells fromthe mussel Mytilus galloprovincialis. Mar Biol 137:907–912. https://doi.org/10.1007/s002270000391
Walker CH, Thompson HM (1991) Phylogenetic distribution of cholinesterases and related esterases, In:Mineau P (ed.) Cholinesterase-inhibiting insecticides.Elsevier, Amsterdam, p 1–17
Wills ED (1987) Evaluation of lipid peroxidation in lipids and biological membranes. In: Snell K, Mullack B (eds) Biochemical toxicology: a practical approach. IRL Press, Oxford, p 127–152
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161. https://doi.org/10.1016/0166-445X(91)90033-6
Zaborska A, Siedlewicz G, Szymczycha B, Dzierzbicka-Głowacka L, Pazdro K (2019) Legacy and emerging pollutants in the Gulf of Gdańsk (southern Baltic Sea)—loads and distribution revisited. Mar Poll Bull 139:238–255. https://doi.org/10.1016/j.marpolbul.2018.11.060
Acknowledgements
This work was conducted under the framework of the project P09-RNM-5136 (Andalusian Government, Spain). GVA-M would like to thank the financial support from Fondos Europeos de Desarrollo Regional (FEDER), Consejería de Economía, Innovación y Ciencia (Regional Government of Andalusia) and Becas Chile (Chilean Government). The authors would like to thank Rocio Antón, Luciane Alves and Victor Almagro for their valuable help during the field work, as well as the scuba divers Damian in Bay of Cádiz, and Manuel in Guadalete River, for their valuable help during the deployment of cages and sampling processes in the field. Thanks also to the crew members of the different vessels used in this research and the various car drivers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aguirre-Martínez, G.V., Martín-Díaz, M.L. A multibiomarker approach to assess toxic effects of wastewater treatment plant effluents and activated defence mechanisms in marine (Ruditapes philippinarum) and fresh water (Corbicula fluminea) bivalve species. Ecotoxicology 29, 941–958 (2020). https://doi.org/10.1007/s10646-020-02216-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02216-1