Abstract
Bifenthrin (BF) and acetochlor (AT) are widely used as an insecticide and herbicide, respectively, which are introduced to the aquatic environment as a natural result. Although the thyroid active substances may coexist in the environment, their joint effects on fish have not been identified. We examined the joint toxicity of BF and AT in zebrafish (Danio rerio) in this study. An acute lethal toxicity test indicated that the median lethal concentration (LC50) values of BF and AT under 96 h treatment were 0.40 and 4.56 µmol L−1, respectively. The binary mixture of BF + AT displayed an antagonistic effect on the acute lethal toxicity. After 14 days post fertilization (dpf) with exposure to individual pesticides at sub-lethal concentrations of, no effects were observed on the catalase (CAT) and peroxidase (POD) activities, while the binary mixtures (except for the 7.2 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT exposure group) significantly induced the CAT activity. The superoxide dismutase (SOD) activity and triiodothyronine (T3) level were significantly increased in all exposure groups. The thyroxine (T4) level remained unchanged after exposure to individual pesticides, but significantly increased in the 7.2 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT group. The expressions of the genes Dio2, TRa, TSHβ and CRH in the thyroid hormone (TH) axis were significantly up-regulated in the 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT group. Our data indicated that the binary mixture of BF + AT significantly altered the antioxidant enzyme activities and gene expressions in the hypothalamic–pituitary–thyroid (HPT) axis and changed the TH levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pesticides are not designed for aqueous systems, but may enter water bodies through run-off in spray drift applications, resulting in deteriorated water quality. Many pesticides may interfere with endocrine disrupting chemicals (EDCs) or have been proven to be such chemicals, which might affect the reproductive and neuroendocrine systems of humans and wildlife, especially those of aquatic organisms directly exposed to environmental pollutants, causing aging and even carcinogenesis (Eva et al. 2015; Patrick 2009; Rhind 2005). Pesticide residues in natural waters do not exist separately, but exist in mixtures in most cases (Hua et al. 2016; Runnalls et al. 2015). The mixture of these substances may have higher toxicity than a single chemical, and the joint effects of pesticides need to be addressed in risk assessment (Zhao et al. 2015; Zucchi et al. 2013).
Bifenthrin (BF) is a pyrethroid pesticide, widely used in the world (Spurlock and Lee 2008; Brander et al. 2016a), and its environmentally relevant concentrations in the San Francisco Bay estuary of water can reach up to 43.0 ng L−1 (Brander et al. 2013). According to the United States Geological Survey (USGS), 33.3 ng L−1 of BF was detected at various sample sites in the Central Valley of California in 2015 and 2016. Pyrethroids are considered less harmful to birds and mammals than other pesticides (Spurlock and Lee 2008). However, most of them have been proven to be EDCs in fish (Brander et al. 2016a, b; Forsgren et al. 2013).
Acetochlor (AT), a chloroacetamide herbicide, is among the most widely used herbicides worldwide (Lengyel and Foldenyi 2003; Lerro et al. 2015; Mokry and Hoagland 1990). Because of its wide application, AT residues have become ubiquitous in aqueous systems (Yuan et al. 2018). Flows of up to 2.5 μg L−1 of AT have been found in water in the Midwestern United States (Foley et al. 2008). Many studies have also shown that AT is a thyroid chemical disorder-causing (Crump et al. 2002; Jiang et al. 2015; Li et al. 2009; Yang et al. 2016). Therefore, AT may lead to tissue modified expression of thyroid hormone-related genes in fish (Li et al. 2009). AT can affect amphibian development by accelerating T3-dependent metamorphosis (Crump et al. 2002). AT and BF have been widely used to control crop pests and diseases, and their residues often coexist in water environments (Wang et al. 2014).
Fish is arguably the most directly exposed to pollutants in water and is more susceptible to their influence. Zebrafish (Danio rerio) is an ordinary vertebrate model for using genomic technologies to assess the toxicity activities of environmental chemicals because of its short lifecycle and mature genes technologies (Chen et al. 2012; Hill et al. 2005; Tu et al. 2013). TH disrupting chemicals are considered important endocrine disruptors in the environment. The hypothalamic–pituitary–thyroid (HPT) axis of fish is used to regulate the synthesis, transport, and metabolism of TH and plays vital roles in the process of fish growth and development, especially during the early life stages (Carr and Patiño 2011; Kawakami et al. 2008). At present, genes related to the HPT axis have become specific biomarkers for the study of TH disrupting chemicals (Huang et al. 2016). Reactive oxygen species can be cleared by cells’ antioxidant defense to maintain the oxidant-antioxidant balance (Lushchak 2011). Superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) are crucial antioxidant enzymes that play a key role in becoming the first line of defense against excessive free radicals in the body (Lin et al. 2009; Jin et al. 2010; Slaninova et al. 2009).
Many previous researches have shown that thyroid hormone receptor (TR) and deiodinase gene expressions are sensitive molecular biomarkers for detecting thyroid dysfunctions in fish exposed to environmental pollutants. Most ecotoxicological studies focus only on the effects of AT or BF, and therefore it is of great significance to study the combined toxic effects of AT and BF on water systems. In this study, zebrafish embryos were treated by BF, AT and their mixtures at different concentrations, and the toxicities of BF, AT and their mixtures to zebrafish embryos in terms of TH were evaluated. Our findings elucidated the potential effects of BF, AT and their binary mixture on the thyroid endocrine system in zebrafish.
Materials and methods
Chemicals
BF (technical grade AI: 97%) and AT (technical grade AI: 95%) were purchased from Shandong Qiaochang Chemical Co., Ltd (Binzhou, Shandong, China). Dimethyl sulfoxide (DMSO; >99.9% purity; Amresco, Solon, OH, USA) was used as the solvent and they were dissolved and kept at 4 °C for 1 month. The regenerated water containing 0.5 mmol L−1 Mg2+, 2 mmol L−1 Ca2+, 0.074 mmol L−1 K+ and 0.75 mmol L−1 Na2+, was further diluted to prepare working solutions. Kits for TRIzol reagent, reverse transcriptase, SYBR Green system, and oxidative stress enzyme biomarker assays were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, Jiangsu, China). ELISA kits for the detection of enzyme activities and THs were purchased from Cusabio (Wuhan, China) and USCN Life (Wuhan, China), respectively.
Experimental fish
Adult AB strain zebrafish was obtained from China Zebrafish Resource Center (Wuhan, China), cultured in a fish facility maintained at 26 ± 1 °C (Esen Corp.), and circulated in a filtered carbon containing water system in a 14 h:10 h light/dark.
The fish was fed twice daily with freshly hatched brine shrimp. Male and female adult fish (ratio of 2/1) were kept in a spawning aquarium with grids near the bottom. Spawning was induced in the following morning by light. Fertilized eggs were siphoned, washed several times by water from the fish system, and then measured under microscopy (Leica Microsystems, Wetzlar, Germany). Normal embryos at the 2–4 cell development stage were stored in beakers for exposure experiments. All zebrafish breeding and egg harvesting conditions were based on the protocols (DeMicco et al. 2010).
Acute toxicity of single pesticides
Fish embryo toxicity (FETs) tests were performed based on the OECD TG 236. Embryos were randomly transferred into 24-well plates. The wenty wells of each plate were used, and each well consisted of an embryo and 2 mL test solution. Reconstituted water containing 0.001% (v/v) DMSO served as the blank control. The tests for both the treatment and control groups were repeated three times. To establish a relationship between the concentration and mortality, embryos were treated with five different concentrations in a geometrical ratio (Table 1). The 24-well plates were incubated at 26 ± 1 °C and covered by transparent self-adhesive sealing film (NuncTM) at a 14/10 h (light/dark) cycle. Exposure solutions were refreshed every 24 h in order to maintain the compound concentrations and water quality. After treatment for 72 and 96 h, the mortality of test organisms was determined, which was a basic for determination of the lethal concentration (LC50).
Acute toxicity of binary mixtures
The BF + AT mixture experiment was conducted on fish embryos for 96 h. Each treatment used the same toxic mixture containing each pesticide, and the test results were obtained from the LC50 value of each pesticide. Dilutions of the BF + AT combination were tested at a fixed dilution facto. In acute toxicity tests, mortality of the test animals was determined per 24 h. Toxic interactions were characterized by Marking’s additive index (AI) (Marking 1977).
Subchronic toxicity on fish embryos
The embryos were randomly placed into glass beaker containing 0.50 L of test solution at various concentrations and repeated in triplicate from 1 h post fertilization (hpf) (4-cell stage) to 14 days. A total of 200 embryos were distributed into each beaker. The design of the pesticide concentration was based on acute toxicity experimental data, and the high concentrations of each pesticide did not exceed 1/20 of 96-h LC50 value. The embryos were exposed to BF at the concentrations of 2.4 × 10−3 and 7.2 × 10−3 µmol L−1, to AT at concentrations of 0.4 × 10−2 and 1.2 × 10−2 µmol L−1 or their binary mixtures at the concentrations of 2.4 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT, 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT, 2.4 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT, and 7.2 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT. Embryos in the control groups were exposed to water only with solvent containing the 0.001% (v/v) DMSO. The beakers were incubated at 26 ± 1 °C for a 14/10 h (light/dark) cycle. Every 24 h, the culture solution was updated to maintain the proper concentrations and water quality. Zebrafish larvae were incubated for six days and then fed egg yolk twice a day. After exposure for 14 days, 190 larvae were randomly selected for each treatment, and immediately frozen by liquid nitrogen and stored at −80 °C for TH, mRNA expression and antioxidant activity analyses.
Quantitative real-time PCR (q-RT PCR)
Six key genes related to the HPT axis of zebrafish were detected (Table S1). The primer sequences of zebrafish were designed with Primer 6.0 (Premier Biosoft International). Thirty larvae were homogenized in each repetition, and TRIzol reagent (Takara, Dalian, China) was used for total RNA extracting. Reverse transcription (Takara, Dalian, China) was performed using reverse transcription transcriptase kit (Takara, Dalian, China) according to the instruction manual, and q-RT PCR (Biorad, CA, USA) was performed using SYBR Green reagents (Takara, Dalian, China). In short, after denaturation for 1 min at 95 °C, the samples were amplified were performed 40 cycles at 95 °C for 10 s, extensioned for 30 s at 72 °C, and annealed at 60 °C for 15 s. The geNorm algorithm (http://medgen.ugent.be) was adopted to identify the most stable reference genes from five commonly used ones (18s rRNA, β-actin, RPL-7, GAPDH, and rpl8), and the gene (18s rRNA) with the lowest M value was set as the housekeeping gene which was used as an internal standard. The gene expression levels were quantified according to the cycle threshold (Ct) by normalizing the housekeeping gene (Livak and Schmittgen 2001) and using the of 2−ΔΔCt method. A fold change in comparison to the control was showed for the target gene expressions.
Thyroid hormones and enzyme activity assay
The TH levels were determined by ELISA kits (Scnlife, Wuhan, China) following the operation manual. The whole-body TH of zebrafish larvae was extracted according to the method as previously described (Tu et al. 2013). In short, 80 larvae were homogenized for per treatment by 0.2 mL of ELISA buffer. The buffer samples were then crushed on ice by means of intermittent sonic oscillation for 5 min. At 5000 × g the samples were centrifuged for 10 min at 4 °C to collect the supernatant and were then stored at −80 °C for TH assays. The detection limits of 3,3′,5-triiodo-l-thyronine (T3) and l-thyroxine (T4) were 0.6 ng mL−1 and 0.3 ng mL−1, respectively.
The 80 zebrafish larvae were homogenized in precooled phosphate buffer (pH 7.2, 0.1 M) (1:9, w/v) on ice. The homogenates samples were centrifuged by 3500 rpm at 4 °C for 15 min before the supernatant solutions were collected. The activities of SOD, CAT, and POD were determined by ELISA kits (Nanjing Jiancheng Bioengineering Institute, China).
Chemical analysis
The actual concentrations of BF, AT and their mixture were detected at the beginning (0 h) of the exposure period and before water renewal (24 h). The collected 30 mL samples were filtered using a Whatman filter for analysis of BF (diam., 25 mm; pore size, 0.45 µmol−1). BF was extracted twice using cyclohexane/ethyl acetate with the volume ratio of 1:1. The extracts were then evaporated under 40 °C, redissolved by 0.5 mL of methanol and stored at 4 °C for analysis. The samples collected of AT analysis were filtered through a 0.45 µmol L−1 filter and extracted with acetone/hexane at the volume ratio of 1:1, and the supernatants were eluted by anhydrous Na2SO4 columns and then evaporated at 40 °C, redissolved by 0.5 mL of methanol and stored at 4 °C for analysis. The concentrations of BF and AT were analyzed using a gas chromatograph/mass spectrometer (97890A-5975C, Agilent, USA) with a HP-5 MS fused quartz capillary column (30 × 0.25 × 0.25 mm), and helium (99.999%) as the carrier gas at a flow rate of 1.0 mL min−1.The oven temperature was maintained as follows: 45 °C (1 min); raised to 170 °C by 25 °C min−1; then raised to 290 °C by 10 °C min−1, and held at 290 °C for 1 min. The injector temperature was maintained at 250 °C. The ionization energy of the electron ionization (EI) was 70 eV, and the ion source temperature was 230 °C. The characteristic ions (m/z) of each herbicide were measured in selective ion monitoring (SIM) mode: AT: 269, 162 and 146; BF: 188, 166 and 153. During the whole experiment, the actual concentrations of BF, AT and their mixtures changed no more than 20% compared with the nominal content, indicating that the actual and nominal concentrations of the chemicals were maintained well.
Assessment methods for combined toxicity
All values obtained were shown as the mean ± standard error (SEM). SPSS 16.0 was used for all statistical analysis. The Kolmogorov Smirnov function was applied to test the normality of all values. Spss 16.0 (SPSS, Chicago, USA) software was used for probit regression analysis, and the nominal LC50 value was calculated (Finney 1971).
Marking’s additive index (AI) (Marking 1977) for aquatic toxicology synergy was used to evaluate the combined effects as follows:
where S means the sum of the toxicity effect of the pesticide mixture A and B; Am is the LC50 of A in the mixture; Ai is the LC 50 of single A; Bm is the LC50 of B in the mixture; Bi is the LC50 of single B.
The AI value was calculated from S based on the following formula:
Interactions of the pesticides were classified into three types: antagonistic (AI ≤ −0.2), additive (−0.2 < I ≤ 0.25), and synergistic effect (AI > 0.25). The greater the AI value when >0.25, the greater the pesticide synergy.
At the mRNA level, the differences in Dio1, Dio2, TRa, TRβ, TSHβ and CRH expressions during treatments were assessed by ANOVA. When ANOVA showed significant treatment effects Tukey’s post-hoc test was used. The results p < 0.05 were considered statistically significant different.
Results
Acute toxicity effects of the single pesticides and binary mixture
Exposure to BF and AT in a dose-dependent manner (shown in Table 1) resulted in zebrafish mortality during its developing stage. After zebrafish embryos were treated by BF and AT for 72 h, the LC50 values were 0.60 and 17.7 µmol L−1, respectively, LC50 values under 96 h treatment were 0.40 and 4.56 µmol L−1, respectively (Table 1). The toxicity of BF was 11-fold higher than that of AT after exposure for 96 h (Table 1).
According to the individual LC50 values of pesticides under 96 h treatment, fish embryos were treated with a set of pesticide dilutions and mixture with fixed equitoxic constant. The AI values were −1.22 and −1.85, after treatment for 72 and 96 h, respectively, implying potential antagonistic effect (shown in Table 2).
Subchronic toxicity on fish embryos
Antioxidant enzyme activities
In the present study, antioxidant activities of CAT, POD and SOD on zebrafish embryos were determined to reflect the stresses of BF, AT, and their mixture. The CAT activity was not significantly changed significantly for single pesticide. However, significant increases were obtained in their combinations of 2.4 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1AT, 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT, and 7.2 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT compared with the control (shown in Fig. 1a). In addition, the SOD activity was increased significantly in response to exposures to BF, AT and binary mixtures (Fig. 1b). The POD activity was not significantly altered in the BF, AT and binary mixture treatment groups compared with the control (Fig. 1c).
Effects of AT, BF, and their binary mixture on the activities of CAT (a), SOD (b), and POD (c) in zebrafish embryos after exposure from 1 h post fertilization to 14 days. Values are presented as means ± SEM (n = 3 replicates of 100 embryos each). Different letters indicate significant differences among treatments (p < 0.05)
Contents of the whole body T3 and T4
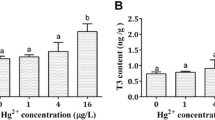
In the control group, the T3 content was 0.32 ± 0.01 ng mg−1, which was significantly increased in all individual and binary mixture treatment groups compared with the control (Fig. 2a). The T4 content in control group was 0.16 ± 0.03 ng mg−1, which was not significantly changed in all individual exposure groups compared with the control (Fig. 2b). In contrast, the T4 content in the 3 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT exposure group was 0.76 ± 0.09 ng mg−1, which was significantly increased compared with the control group (Fig. 2b).
Whole-body T4 (a) and T3 content (b) in zebrafish larvae exposed to different concentrations of AT, BF, and their binary mixture from 1 h post fertilization to 14 days. Values are presented as means ± SEM (n = 3 replicates of 100 zebrafish each). Different letters indicate significant differences among treatments (p < 0.05)
Profile of gene expression
The mRNA levels of the genes related to the HPT axis were determined at 14 dpf. The Dio1 in zebrafish larvae showed no significantly different expression in any concentrations of BF, AT and their mixture treatment (Fig. 3a). The expression of Dio2 in the 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT exposure groups were significantly up-regulated by 1.5-fold compared with the control and also up-regulated compared with individual pesticide treatment groups (Fig. 3b).
The gene expression of Dio1, Dio2, TRa, TRβ, Tshb, and CRH in zebrafish embryos after exposure to AT, BF, and their binary mixture for 14 days. mRNA levels of genes were normalized to 18s rRNA and values are presented as means ± SEM (n = 3 replicates of 30 embryos each). Different letters indicate significance among treatments (p < 0.05)
TRa and TRβ (two isoforms of TRs) were measured in assay. The expression of TRa in zebrafish larvae was not significantly changed any individual pesticide’ treatment. However, in the 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT exposure group, the expression of TRa was up-regulated significantly compared with the individual pesticide exposure groups (Fig. 3c). No significant change in expression of TRβ among any exposure groups were obtained (Fig. 3d).
The expression of TSHβ tended to be normal for individual exposures to BF and AT at 14 dpf. However, in the presence of 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT the expression of TSHβ significantly up-regulated compared with the control (Fig. 3e). Upon exposure to the binary mixtures of 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT and 7.2 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT for 14 days, the CRH expression was significantly up-regulated compared with the control and that treated by single chemicals (Fig. 3f).
Discussion
In this research, we assessed the potential toxicities of single and compound pesticides using fish embryos. The results of the acute toxicity tests demonstrated that the toxic effects of BF, AT and their binary mixtures were time- and concentration-dependent. BF can be highly toxic, while AT exhibits a moderate toxicity. Pyrethroids were considered less harmful to birds and mammals (Spurlock and Lee 2008), but they were reported to be highly toxic to aquatic life (Brander et al. 2016a, b). The study of DeMicco et al. (2010) revealed that BF had an LC50 value of 0.19 mg L−1 under 144 h exposure, which was similar to the result of a high toxicity of BF to aquatic organisms. Traditional risk assessment usually focuses on exposure to single and additive behaviors of pesticide exposure (Barata et al. 2006). The toxicity risk assessment of the mixed pollutants can better reflect the reality of aquatic ecosystems exposure. Although BF has been found to highly toxic to aquatic organisms, the co-occurrence of BF and AT exerted an antagonistic effect on the acute toxicity. Therefore, when BF and AT exist simultaneously, the acute toxicity to aquatic organisms in water may be reduced.
In natural water, pesticide mixtures mostly exist in low-dose state, and long-term exposure of water organisms to low-dose pesticide environments may produce certain toxicological effects. Therefore, in addition to studying the toxic effect of the lethal dose, his paper also studied the single and combined toxic effect of low-dose and long-term exposure on zebrafish. Increasing evidence has demonstrated that many pesticides cause oxidative stress and thyroid disruption in zebrafish. However, there are few studies on the toxicity effects of environmentally relevant concentrations of combined pesticides on oxidative stress and thyroid disruption. In present study, we explored the potential thyroid-disrupting effects and oxidative stress of BF, AT, and their binary mixture at sublethal concentrations, Pyrethroids (cypermethrin, fenvalerate and BF) may induce severe oxidative stress in mice and rats (Dar et al. 2013; Jin et al. 2014; Kale et al. 1999). In this study, the CAT activity was increased by 118.3, 88.1, and 97.8% following exposure to mixtures for 14 days at sub-lethal (lowest, intermediate, highest) concentrations, respectively, which might indicate increasing contributions to elimination of free radicals. The mixture of BF and AT did not show greater effects on antioxidant activities than they did individually. In terms of toxic equivalency, if the oxidative toxicity of each chemical is dose dependant, there should be a difference between single chemical exposures and mixture exposure, simply due to the quantitative load of the chemicals in the medium.
EDCs interfere with thyroid through a feedback mechanism triggered by the change of circulating TH, leading to a TSH expression change, and affect the bioavailability of TH by targeting relative binding of proteins (e.g. transthyroxine and deiodinase) (Opitz et al. 2006; Power et al. 2001). In this study, after 14 days of pesticides poisoning on zebrafish embryos, BF, AT and their binary mixture significantly increased the T3 level in zebrafish larvae. In addition to the significantly increase in T4 level in the binary mixture of 7.2 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT compared with that for the control. BF, AT and their binary mixture in this study could produce an endocrine disrupting effect by changing TH level. Due to the important physiological role of TH, the change in the TH level would impede normal development of the thyroid function in juvenile zebrafish. Many previous researches reported that different synthetic pyrethroids (SP) treatments resulted in significant varied TH levels (Maiti and Kar 1998; Kaul et al. 1996). Shi et al. (2009) reported that perfluorooctane sulfonic acid treatment (100–400 µmol L−1) significantly increased the T3 level, while the T4 level remained unchanged. The previous study has demonstrated that co-exposure to butachlor and triadimefon causes a greater up-regulation of the T3 and T4 levels (Cao et al. 2016).
The secretion of CRH and TSH in the adjustment of the fish HPT axis will change because of the TH level in the circulation system, so we can detect CRH and TSHβ gene transcription level change to assess the environmental compounds will cause thyroid function isorder, and to clarify the mechanism of action of compound disrupt thyroid function (DeGroot and Brander 2014; Yu et al. 2011). In the present study, the binary mixture of 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT significantly up-regulated TSHβ expression compared with the control treatment. Moreover, binary mixtures of 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT and 7.2 × 10−3 µmol L−1 BF + 1.2 × 10−2 µmol L−1 AT significantly up-regulated CRH expression compared with individual pesticides. TSHβ coded TSH, and through the negative feedback mechanism, T4 could modulates the transcription of CRH and TSH in fish (Zhang et al. 2010) and the increased T4 levels and upregulation of related gene expression should be positively regulated.
In vertebrates, deiodinase (Dio1 and Dio2) plays a crucial role in the regulation of the TH content (Orozco and Valverde-R 2005). Deiodinase is more sensitive to environmental chemicals, which makes its transcription level a sensitive biomarker in fish for thyroid disruption identification (Li et al. 2009; Shi et al. 2009). In our assay, Dio2 was up-regulated significantly under the treatment of 1.2 × 10−2 µmol L−1 AT, but not altered in BF exposure groups. Moreover, Dio1 was significantly up-regulated in 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT compared with individual pesticides. As previously studied, hypothyroidism in fish can lead to increased Diol and Dio2 enzyme activities or gene transcription levels. Previous reports have shown that deiodinases (Dio1 and Dio2) are not significantly induced when zebrafish embryos are exposed to a certain concentration of AT for 96 h (Jiang et al. 2015). Tu et al. (2013) reported that Dio1 remained unaltered in zebrafish embryos exposed to various concentrations (1 × 10−3 µmol L−1, 3 × 10−3 µmol L−1, and 1 × 10−2 µmol L−1) of BF until 72 hpf, while Dio2 was significantly up-regulated in all BF exposure groups. The bioactivity of TH was mediated by two isoforms of TRs named TRα and TRβ (Power et al. 2001). According to previous study, some environmental pollutants could affect the transcription levels of TRs (Chen et al. 2012). In our study, TRa expression was significantly increased after exposure to 7.2 × 10−3 µmol L−1 BF + 0.4 × 10−2 µmol L−1 AT compared with individual pesticides. T3 was reported to enhance TR expression, while exposure to a variety of chemicals results in an increased TR expression, which was associated with enhanced T3 levels through a feedback mechanism (Crump et al. 2008). Accordingly, we speculated that the up-regulation of TRα in our study was attributed to the increase of the T3 level and had an impact on the circulation of TH. This study showed that the applications of BF and AT at the concentrations affecting the environment had adverse effects on embryonic development of zebrafish and led to thyroid endocrine disruption, while co-exposure to BF and AT could coordinate these effects.
Conclusions
In this study, a binary mixture of BF + AT exerted antagonistic effects on acute toxicity against zebrafish embryos. Moreover, the binary mixture of BF + AT could significantly alter the activities of antioxidants and certain gene expressions of the HPT axis compared with those caused by individual pesticides, leading to changes in the TH levels. Although gene expression analyses have the improved understanding of the mechanisms of this combination, our data may not be sufficient to elucidate complex chemical interactions and toxicity mechanisms. Therefore, in depth studies are advocated to illustrate the underlying mechanisms of interactions between these binary mixtures of pesticides.
References
Barata C, Baird DJ, Nogueira AJA, Soares A, Riva MC (2006) Toxicity of binary mixtures of metals and pyrethroid insecticides to Daphnia magna Straus. Implications for multi-substance risks assessment. Aquat Toxicol 78:1e14
Brander SM, Connon RE, He G, Hobbs JA, Smalling KL, Teh SJ, White JW, Werner I, Denison MS, Cherr GN (2013) From Omics to Otoliths: responses of an estuarine fish to endocrine disrupting compounds across biological scales. PLoS ONE 8(9):74251–74266
Brander SM, Gabler MK, Fowler NL, Connon RE, Schlenk D (2016a) Pyrethroid pesticides as endocrine disruptors: molecular mechanisms in vertebrates with a focus on fishes. Environ Sci Technol 50:8977–89923
Brander SM, Jeffries KM, Cole BJ, DeCourten BM, Wilson White J, Hasenbein S, Fangue NA, Connon RE (2016b) Transcriptomic changes underlie altered egg protein production and reduced fecundity in an estuarine model fish exposed to bifenthrin. Aquat Toxicol 174:247–260
Cao CY, Wang QW, Jiao F, Zhu GN (2016) Impact of co-exposure with butachlor and triadimefon on thyroid endocrine system in larval zebrafish. Exp Toxicol Pathol 68:463–469
Carr JA, Patiño R (2011) The hypothalamus-pituitary-thyroid axis in teleosts and amphibians: endocrine disruption and its consequences to natural populations. Gen Comp Endocrinol 170(2):299–312
Chen Q, Yu LQ, Yang LH, Zhou BS (2012) Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol 110–111:141–148
Crump D, Jagla MM, Chiu S, Kennedy SW (2008) Detection of PBDE effects on mRNA expression in chicken (Gallus domesticus) neuronal cells using real-time RT-PCR and a new differential display method. Toxicol in Vitro 22:1337–1343
Crump D, Werry K, Veldhoen N, Van Aggelen G, Helbing CC (2002) Exposure to the herbicide acetochlor alters thyroid hormone-dependent gene expression and metamorphosis in Xenopus Laevis. Environ Health Perspect 110:1199–1205
Dar MA, Khan AM, Raina R, Verma PK, Sultana M (2013) Effect of repeated oral administration of bifenthrin on lipid peroxidation and anti-oxidant parameters in Wistar rats. Bull Environ Contam Toxicol 91:125–128
DeGroot BC, Brander SM (2014) The role of P450 metabolism in the estrogenic activity of bifenthrin in fish. Aquat Toxicol 156:17–20
DeMicco A, Cooper KR, Richardson JR, White LA (2010) Developmental neurotoxicity of pyrethroid insecticides in zebrafish embryos. Toxicol Sci 113:177–186
Eva RK, Monica SR, Imon R (2015) A review on endocrine disruptors and their possible impacts on human health. Environ Toxicol Pharmacol 40:241–258
Finney DJ (1971) Probit analysis, 2001. In: Graymore M, Stagnitti F, Allison G (Eds.) Impacts of atrazine on equatic ecosystems, vol. 26. Cambridge University Press, Cambridge, p 483–495
Foley ME, Sigler V, Gruden CL (2008) A multiphasic characterization of the impactof the herbicide acetochlor on freshwater bacterial communities. ISME J 2:56–66
Forsgren KL, Riar N, Schlenk D (2013) The effects of the pyrethroid insecticide, bifenthrin, on steroid hormone levels and gonadal development of steelhead (Oncorhynchus mykiss) under hypersaline conditions. Gen Comp Endocrinol 186:101–107
Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 86:6–19
Hua JH, Hanc J, Wang XF, Guo YY, Zhou BS (2016) The binary mixtures of megestrol acetate and 17α-ethynylestradiol adversely affect zebrafish reproduction. Environ Pollut 213:776–784
Huang GM, Tian XF, Fu JJ (2016) Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere 147:188–194
Jiang JH, Wu SG, Liu XJ, Wang YH, An XH, Cai LM, Zhao XP (2015) Effect of acetochlor on transcription of genes associated with oxidative stress, apoptosis, immunotoxicity and endocrine disruptionin the early life stage of zebrafish. Environ Toxicol Pharmacol 40:516–523
Jin Y, Pan X, Fu Z (2014) Exposure to bifenthrin causes immunotoxicity and oxidative stress in male mice. Environ Toxicol 29:991–999
Kale M, Rathore N, John S, Bhathagar D (1999) Lipid peroxidative damage on pyrethroid exposure and alteration in antioxidant status in rat erythrocytes. A possible involvement of reactive oxygen species. Toxicol Lett 105:197–205
Kaul P, Rastogi A, Hans R, Seth T, Seth P, Srimal R (1996) Fenvalerate-induced alterations in circulatory thyroid hormones and calcium stores in rat brain. Toxicol Lett 89:29–33
Kawakami Y, Yokoi K, Kumai HH (2008) The role of thyroid hormones during the development of eye pigmentation in the Pacific bluefin tuna (Thunnus orientalis). Comp Biochem Physiol B Biochem Mol Biol 150(1):112–6
Lengyel Z, Foldenyi R (2003) Acetochlor as a soil pollutant. Environ Sci Pollut Res Int 10:13–18
Lerro CC, Koutros S, Andreotti G, Hines CJ, Blair A, Lubin J, Ma XM, Zhang YW, Beane Freeman LE (2015) Use of acetochlor and cancer incidence in the Agricultural Health Study. Int J Cancer 137:1167–1175
Lin CT, Tseng WC, Hsiao NW, Chang HH, Ken CF (2009) Characterization, molecular modelling and developmental expression of zebrafish manganese superoxide dismutase. Fish Shellfish Immunol 27:318–324
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCP and the 2 (-Delta Delta C (T)) method. Methods 25:402–408
Li W, Zha JM, Spear PA, Li ZL, Yang LH, Wang ZJ (2009) Changes of thyroid hormone levels and related gene expression in Chinese rare minnow (Gobiocypris rarus) during 3-amino-1,2,4-triazole exposure and recovery. Aquat Toxicol 92:50–57
Yu LQ, Lam JCW, Guo YY, Wu RSS, Lam PKS, Zhou BS (2011) Parental transfer of polybrominated diphenyl ethers (PBDEs) and thyroid endocrine disruption in zebrafish. Environ Sci Technol 45(24):10652–10659
Lushchak VI (2011) Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101:13–30
Maiti PK, Kar A (1998) Is triiodothyronine capable of ameliorating pyrethroid-induced thyroid dysfunction and lipid peroxidation? J Appl Toxicol 18:125–128
Marking LL (1977) Method for assessing additive toxicity of chemical mixtures, aquatic toxicology and hazard evaluation. In: Mayer FL, Hamelink JL (Eds.) Aquatic toxicology and hazard evaluation. American Society for Testing and Materials, Philadelphia, PA, p 99–108
Mokry LE, Hoagland KD (1990) Acute toxicities of five synthetic pyrethroid insecticides to Daphnia magna and Ceriodaphnia dubia. Environ Toxicol Chem 9:1045–1051
Opitz R, Lutz I, Nguyen NH, Scanlan TS, Kloas W (2006) Analysis of thyroid hormone receptor betaa mrna expression in xenopus laevis tadpoles as a means to detect agonism and antagonism of thyroid hormone action. Toxicol Appl Pharmacol 212(2):1–13
Orozco A, Valverde-R C (2005) Thyroid hormone deiodination in fish. Thyroid 15:799–813
Patrick L (2009) Thyroid disruption: mechanism and clinical implications in human health. Altern Med Rev 14:326–346
Power DM, Llewellyn L, Faustino M, Nowell MA, Bjornsson BT, Einarsdottir IE, Canario AV, Sweeney GE (2001) Thyroid hormones in growth and development of fish. Comp Biochem Physiol 130C:447–459
Runnalls TJ, Beresford N, Kugathas S, Margiotta-Casaluci L, Scholze M, Scott AP, Sumpter JP (2015) From single chemicals to mixtures—reproductive effects of levonorgestrel and ethinylestradiol on the fathead minnow. Aquat Toxicol 169:152–167
Rhind SM (2005) Are endocrine disrupting compounds a threat to farm animal health, welfare and productivity? Reprod Domest Anim 40:282–290
Shi XJ, Liu CS, Wu GQ, Zhou BS (2009) Waterborne exposure to PFOS causes disruption of the hypothalamus–pituitary–thyroid axis in zebrafish larvae. Chemosphere 77:1010–1018
Slaninova A, Smutna M, Modra H, Svobodova Z (2009) A review: oxidative stress in fish induced by pesticides. NeuroEndocrinol Lett 30(Suppl 1):2–12
Spurlock F, Lee M (2008) Synthetic pyrethroid use patterns, properties, and environmental effects. In: Gan J, Spurlock F, Hendley P, Weston DP (eds) Synthetic pyrethroids: occurrence and behavior in aquatic environments, ACS symposium series, vol. 991. American Chemical Society, Washington, DC
Tu WQ, Niu LL, Liu WP, Xu C (2013) Embryonic exposure to butachlor in zebrafish (Danio rerio): endocrine disruption, developmental toxicity and immunotoxicity. Ecotoxicol Environ Saf 89:189–199
Wang S, Tang X, Wang L, Zhang Y, Wu Q, Xie W (2014) Effects of sublethal concentrations of bifenthrin on the two-spotted spider mite Tetranychus urticae (Acari: tetranychidae). Syst Appl Acarol 19:481–490
Yang M, Hu JJ, Li SY, Ma YN, Gui WJ, Zhu GN (2016) Thyroid endocrine disruption of acetochlor on zebrafish (Danio rerio) larvae. J Appl Toxicol 36(6):844–852
Jin YX, Zhang XX, Shu LJ, Chen LF, Sun LW, Qian HF, Liu WP, Fu ZW (2010) Oxidative stress response and gene expression with atrazine exposure in adult female zebrafish (Danio rerio). Chemosphere 78:846–852
Yuan C, Chin YP, Weavers LK (2018) Photochemicalacetochlor degradation induced by hydroxyl radical in Fe-amended wetland waters: impact of pH and dissolved organic matter. Water Res 132:52–60
Zhang ZY, Yu XY, Wang DL, Yan HJ, Liu XJ (2010) Acute toxicity to zebrafish of two organophosphates and four pyrethroids and their binary mixtures. Pest Manag Sci 66:84–89
Zhao YB, Castiglioni S, Fent K (2015) Synthetic progestins medroxyprogesterone acetate and dydrogesterone and their binary mixtures adversely affect reproduction and lead to histological and transcriptional alterations in zebrafish (Danio rerio). Environ Sci Technol 49:4636–4645
Zucchi S, Castiglioni S, Fent K (2013) Progesterone alters global transcription profiles at environmental concentrations in brain and ovary of female zebrafish (Danio rerio). Environ Sci Technol 47:12548–12556
Acknowledgements
The research was supported by the National Key research and Development Program (2018YFC1603004), and National Nature Fund Project (31701519).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
All authors have approved this version of the work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Rights and permissions
About this article
Cite this article
Guo, D., Liu, W., Qiu, J. et al. Changes in thyroid hormone levels and related gene expressions in embryo–larval zebrafish exposed to binary combinations of bifenthrin and acetochlor. Ecotoxicology 29, 584–593 (2020). https://doi.org/10.1007/s10646-020-02206-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02206-3