Abstract
Soils can be contaminated with substances arising from anthropogenic sources, but also with natural bioactive compounds produced by plants, such as terpenes and flavonoids. While terpenes and flavonoids have received much less attention from research studies than metals, the effects that phytocompounds can have on soil organisms such as beneficial microorganisms should not be neglected. Herein we report the sole and combined exposure of Rhizobium to cadmium, to the monoterpene alpha-pinene and to the flavanol quercetin. A range of environmentally relevant concentrations of the phytocompounds was tested. Physiological (growth, protein content and intracellular Cd concentration), oxidative damage (lipid peroxidation, protein carbonylation) and antioxidant mechanisms (superoxide dismutase, catalase, glutathione, glutathione-S-transferases, protein electrophoretic profiles) were assessed. Results suggest that exposure to both phytocompounds do not influence Rhizobium growth, but for combined exposure to phytocompounds and Cd, different responses are observed. At low concentrations, phytocompounds seem to relieve the stress imposed by Cd by increasing antioxidant responses, but at high concentrations this advantage is lost and membrane damage may even be exacerbated. Thus, the presence of bioactive phytocompounds in soil may influence the tolerance of microorganisms to persistent toxicants, and may change their impact on the environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pressure to produce food for an ever-expanding world population has boosted agricultural practices such as the application of chemical fertilizers and biocides with the aim of increasing productivity. Soils receive many of these products, and organisms living in the soil are especially vulnerable to changes induced by fertilizers (Geisseler and Scow 2014) and biocides (Kalia and Gosal 2011). Soil microorganisms provide important services in soil fertility maintenance and ecosystem productivity (Gattinger et al. 2008), both in agricultural and natural areas. Rhizobia in particular were reported to be involved in mineralization of organic compounds, solubilization of nutrients, synthesis of plant growth promoting substances, production of antimicrobials and nitrogen fixation (Marschner 1995; Gopalakrishnan et al. 2015). Changes in soil microorganism communities may impact services provided to ecosystems with consequences to fertility and productivity. Thus, it is important to identify pressures imposed on soil bacteria in order to understand how bacteria are affected and what are the repercussions to the environment.
Cadmium (Cd) is classified as the seventh most toxic substance in the 2017 Priority List of Hazardous Substances by the Agency for Toxic Substances and Disease Registry (Agency for Toxic Substances and Disease Registry 2017). Cd interacts with calcium and zinc metabolism, reacts with thiol groups, displaces essential biological metals and induces oxidative stress, thus inhibiting protein function and electron transport chains (Nies 1999; Pacheco et al. 2008; Prévéral et al. 2009; Cuypers et al. 2010), inducing membrane and protein damage and formation of DNA adducts (Nies 1999; Lemire et al. 2013; Cardoso et al. 2017). These multitude of effects entail reduced growth, long log phases, lower cell densities, and ultimately bacterial death (Les and Walker 1984; Sinha and Mukherjee 2009). Therefore, even at low concentrations the potential to affect soil microbial communities should be high (Shentu et al. 2008). More than half of the Cd present in some agricultural soils comes from the application of fertilizers made from phosphate rock, which contain approximately 32 milligrams of cadmium per kilogram (mg Cd/kg) of phosphorus fertilizer, but the level can be as high as 200 mg Cd/kg (Gilbert 2018). Additionally, industry, sludge amendment and mining activities are also routes of Cd entry into soils (Volesky and Holan 1995; Garbisu and Alkorta 2001). Cd concentrations in agricultural soils are generally above reference levels. In soil solution, Cd concentrations are usually found between 0.3 μg and 6 mg L−1 (0.0027–53 μM) (Helmke 1999; Kabata-Pendias 2011), but this element can reach concentrations higher than 300 mg L−1 (2669 μM) at highly contaminated sites (Itoh et al. 1979). Thus, plans to cut Cd levels in phosphate fertilizers as a way to decrease exposure of European citizens to Cd are currently on the European Union agenda (Gilbert 2018).
Other constraints to soil microorganisms have a natural origin and result from the activity of other communities occupying the same area. Plants synthesize compounds with proven insecticidal (Ibanez et al. 2012), bactericidal (Cowan 1999) and fungicidal activity (Kuc 1992), enabling them to endure environmental conditions and to persist in those systems, but with potential side effects to organisms inhabiting the same site which are not the direct targets of this biological war, like many soil microorganisms. Two of the compounds most commonly synthesized by plants are alpha-pinene (α-pinene), a monoterpene, and quercetin, a flavonol (Noma and Asakawa 2010; Ay et al. 2016). In soils, volatile isoprenoids have important ecological roles (Loreto et al. 2014) that influence the rhizosphere community, including nutrient cycling (Asensio et al. 2008) and as growth substrates (Kleinheinz et al. 1999; Demyttenaere et al. 2000; Vokou et al. 2002; Yoo and Day 2002; Owen et al. 2007). Monoterpenes, in particular, were reported to inhibit the net mineralization of nitrogen and net nitrification in soil (White 1991, 1994).
α-pinene is one of the major phytocompounds released by a wide range of species throughout the world, namely conifers such as those from the genus Pinus (Yang et al. 2016), for example Pinus silvestris (Judzentiene and Kupcinskiene 2008) and Pinus halepensis (Llusia and Penuelas 2000), but also by other plants such as several Eucalyptus species (Faria et al. 2011), Juniperus communis (Sybilska et al. 1994), Cistus ladanifer (Verdeguer et al. 2012), and Bupleurum fruticosum (Llusia and Penuelas 2000), among others. At ecologically relevant concentrations, α-pinene disrupts energy metabolism by acting as an uncoupler of oxidative phosphorylation, and by inhibiting the electron transport chain (Abrahim et al. 2003), generating reactive oxygen species (ROS). The oxidative stress originated by ROS induces lipid peroxidation, damaging the membrane, and activates antioxidant enzyme systems (Singh et al. 2006). Plants emitting high levels of α-pinene include Eucalyptus sp., Pinus sp. and Quercus sp. (Singh et al. 2006). Conifers produce high amounts of α-pinene which accumulate preferentially in the needles, being needle litter the main source of α-pinene (Asensio et al. 2008). On pinewood soils, α-pinene was detected at concentrations reaching 3000 μM (Asensio et al. 2008), whereas in soils occupied by other species concentrations are much lower. On beech rich-areas, α-pinene was detected in concentrations between 0.15 and 2 μM (Maurer et al. 2008).
Quercetin is produced by different plants species ranging different taxonomic groups, for instance Camellia sinensis (Jeganathan et al. 2016), Helianthus annuus (Karamać et al. 2012) and Allium sativum (Pan et al. 2018). In soils covered by white clover quercetin concentrations can surpass 827 nmol/Kg soil (Carlsen et al. 2012). Flavonoids can have suppressive effects on germination and growth of other plant species (Grant and Sallans 1964; Newman and Rovira 1975; Macfarlane et al. 1982), diseases (Carlsen and Fomsgaard 2008) and soil rhizobacteria (Hale and Mathers 1977; Hale et al. 1979). Quercetin can be used as a carbon source by soil microorganisms (Inderjit 2005) and to overcome oxidative stress since quercetin is a powerful antioxidant (Zhang et al. 2011). Additionally, the ability of quercetin to make complexes with metals, including with Cd, was reported (Ravichandran et al. 2014). Thus, in addition to the antioxidant ability quercetin may also help microorganisms to tolerate stress induced by Cd by complexing the metal, and decreasing its toxicity.
In stable ecosystems, communities are well adapted to the prevailing conditions. However, the consequences for microorganisms arising from phytocompounds accumulation in soils are still poorly understood and in communities additionally challenged by anthropogenic (cadmium) constraints little is known about the effects of the interaction of these two types of influences on soil microorganisms. Thus, the present study aims to evaluate the effect that a toxic element (Cd) and naturally occurring bioactive compounds (α-pinene and quercetin) have on soil microorganisms, especially those playing important roles in soil fertility such as rhizobia. For this, a Rhizobium strain was grown in the presence of Cd and two concentrations of α-pinene and quercetin. The concentrations of the compounds chosen represent concentrations found in areas occupied by plants that synthesize low and high concentrations of the two compounds. The effects of individual and combined exposure to the metal and each compound were evaluated by the response signatures of Rhizobium to oxidative stress, cellular damage and antioxidant endpoints.
Materials and methods
Experimental conditions
Rhizobium sp. strain E20-8 (partial 16S rRNA sequence Genbank accession number KY491644), isolated from Pisum sativum L. root nodules grown in a non-contaminated field in Southern Portugal, and previously described as tolerant to Cd (Corticeiro et al. 2013), was grown in tubes containing 5 mL of yeast mannitol broth (YMB) medium (Somasegaran and Hoben 1994) supplemented with cadmium (0, 7.5, 10 and 20 μM), α-pinene (0, 0.3, 30, 300 and 3000 μM) and quercetin (0, 0.01, 0.025, 0.05, 0.10, 0.50 and 1.00 μM), in order to determine the tolerance to Cd, α-pinene and quercetin and to choose the conditions for further work. Quercetin (Cayman Chemical Company) and α-pinene ((1-S)-(-)alpha-Pinene, 98%, Acros Organics) stock solutions were prepared with ethanol (100%) and sterilized deionized water (1:1). Ethanol concentration used was confirmed to have no effect on rhizobia growth. Inoculated tubes were incubated at 26 °C in an orbital shaker (150 rpm) until late exponential phase (14 h). Growth was determined by measuring optical density at 620 nm. The relationship between optical density and cell concentration was obtained by direct cell counting in a Neubauer chamber. Cell concentration was expressed in million cells per milliliter (M cells mL−1).
Considering Rhizobium tolerance to Cd (10 μM Cd inhibited growth significantly compared to control) and to both organic compounds (none of the quercetin and α-pinene concentrations inhibited growth), two Cd (0 and 10 μM), three α-pinene (0, 0.3 and 3000 μM) and three quercetin (0, 0.01 and 1 μM) concentrations were chosen for the toxicity tests. Cells were grown in triplicate in YMB medium in a total of six different conditions for the Cd and α-pinene experiment: 0 μM Cd + 0 μM α-pinene (control); 0 μM Cd + 0.3 μM α-pinene; 0 μM Cd + 3000 μM α-pinene; 10 μM Cd + 0 μM α-pinene; 10 μM Cd + 0.3 μM α-pinene; and 10 μM Cd + 3000 μM α-pinene; cells were also grown in six different conditions for the Cd and quercetin experiment: 0 μM Cd + 0 μM quercetin (control); 0 μM Cd + 0.01 μM quercetin; 0 μM Cd + 1 μM quercetin; 10 μM Cd + 0 μM quercetin; 10 μM Cd + 0.01 μM quercetin; and 10 μM Cd + 1 μM quercetin. Both α-pinene and quercetin experiments were repeated three times, so in total 3 × 3 samples were obtained for each condition. The cadmium concentration used (10 µM) is environmentally relevant (Kabata-Pendias 2011). The concentrations of quercetin and α-pinene chosen represent concentrations found in areas occupied by plants that synthesize low and high concentrations of the two compounds (Asensio et al. 2008; Maurer et al. 2008; Carlsen et al. 2012). Although compounds are accumulated in soils (solid phase) and their concentrations reported by soil mass (e.g., µmole/Kg) and not by soil volume, the uptake route for toxic compounds (and nutrients) for organisms is the aqueous phase, since a compound is only absorbed if it is in solution, regardless of their concentration in the soil. Thus, the trial was performed in liquid medium.
Intracellular cadmium concentration
Cells were collected by centrifugation at 10,000 × g for 10 min, washed twice in deionized water and centrifuged. In order to remove Cd ions associated to cell walls, intact cell pellets were suspended in 2 mL of 0.1 M HNO3, sonicated in a water bath for 5 min and centrifuged at 10,000 × g for 10 min (Lima et al. 2006). This procedure is crucial since it limits contamination of intracellular Cd by extracellular Cd ions. Supernatant was discarded and cell pellets were suspended in 1 mL of deionized water. The resulting suspension was transferred to polytetrafluoroethylene (Teflon) bombs and 1.8 mL of H2O2 (30%) and 0.2 mL of concentrated HNO3 (65%) were added. Cells were digested overnight at 115 °C. The cooled digest was made up to 5 mL with deionized water, and the concentration of intracellular cadmium was determined by inductively coupled plasma mass spectrometry (ICP-MS) analysis. Cadmium concentration was expressed as ng Cd per million cells (ng Cd M cells−1).
Biochemical parameters
Cells were collected after centrifugation at 10,000 × g for 10 min at 4 °C, washed twice with deionized water, and frozen at −80 °C for further use. Frozen cells were suspended in specific extraction buffers and lysed in an ultrasonic probe for 20 s, keeping tubes in an ice bath, and extracts centrifuged at 12,000 × g for 10 min at 4 °C. For lipid peroxidation (LPO), samples were extracted using 20% (v/v) trichloroacetic acid (TCA). For catalase (CAT), superoxide dismutase (SOD), glutathione-S-transferases (GSTs), reduced glutathione (GSH), oxidized glutathione (GSSG), protein carbonylation (PC) and protein content, sodium phosphate buffer (50 mM sodium dihydrogen phosphate monohydrate; 50 mM disodium hydrogen phosphate dihydrate; 1 mM ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA); 1% (v/v) Triton X-100; 1% (v/v) polyvinylpyrrolidone (PVP); 1 mM dithiothreitol (DTT), pH 7.0) was used.
Protein content
Protein content was determined by the Biuret method (Robinson and Hogden 1940). The amount of protein was determined spectrophotometrically at 540 nm, using bovine serum albumin (BSA, Sigma) as standard (1.25–10 mg mL−1). Results were expressed in µg protein per million cells (µg M cells−1).
Oxidative damage
Lipid peroxidation (LPO) was measured by quantification of thiobarbituric acid reactive substances (TBARS), according to the protocol described by Buege and Aust (Buege and Aust 1978), based on the reaction of lipid peroxidation products such as malondialdehyde (MDA) with 2-thiobarbituric acid (TBA), forming TBARS. The amount of TBARS was quantified spectrophotometrically at 532 nm and calculated using the molar extinction coefficient of MDA (1.56 × 105 M−1 cm−1). Results were expressed in nmol of MDA equivalents per million cells (nmol MDA eq M cells−1).
Protein carbonylation (PC) was measured by the quantification of carbonyl groups (CG) based on the reaction of 2,4-dinitrophenylhydrazine (DNPH) with CG, according to the DNPH alkaline method described by Mesquita et al. (2014) with modifications described by Udenigwe et al. (2016). The amount of CG was quantified spectrophotometrically at 450 nm (22,308 M−1 cm−1 extinction coefficient) and results were expressed in μmol of CG per million cells (μmol CG.M cells−1).
Antioxidant enzymes
Superoxide dismutase (SOD) activity was determined spectrophotometrically by the reaction of nitro blue tetrazolium (NBT) with superoxide radicals to form NBT diformazan, based on the method described by Beauchamp and Fridovich (Beauchamp and Fridovich 1971). Absorbance was measured at 560 nm and SOD standards (0.01–60 U) were used. One unit of enzyme activity (U) corresponds to a 50% reduction of NBT. Results were expressed in milliunits (mU) per million cells (mU SOD.M cells−1).
Catalase (CAT) activity was determined by the reaction of CAT with methanol in the presence of H2O2 (Johansson 1988). A standard curve was built using formaldehyde standards (2.5–30 μM). One unit (U) was defined as the amount of enzyme that caused the formation of 1.0 µmol formaldehyde, per min. Results were expressed in milliunits (mU) per million cells (mU CAT.M cells−1).
Glutathione-S-transferases (GSTs) activity was determined using the method described by Habig et al. (1974) and modified by Corticeiro et al. (2013). GSTs catalyze the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with glutathione (GSH), forming a thioester product. The reaction can be followed by the absorbance increase at 340 nm and GSTs activity determined using CDNB extinction coefficient (9.6 mM cm−1). Results were expressed in milliunits (mU) per million cells (mU GSTs M cells−1).
Reduced (GSH) and oxidized (GSSG) glutathione contents were determined by the method described by Rahman et al. (2006). Absorbance was read at 412 nm for both determinations. Standard curves were built using GSH and GSSG standards (1–90 μM) and results were expressed in μmol of GSH and GSSG per million cells (μmol GSH M cells−1 and μmol GSSG M cells−1).
Protein profiles
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), carried out in bis-Tris precast gels (4–20%) 0.75 mm thick (Bio-Rad) following the system of Laemmli (Laemmli 1970). Molecular weight standards used were purchased from Bio-Rad (Prestained SDS-PAGE standard—Broad Range). Gels were stained with Coomassie Brilliant Blue R-250 (Bio-Rad). Densitometric readings were performed using a Bio-Rad densitometer, Model GS 710. The molecular weight and relative amount of proteins corresponding to each band were calculated using Quantity-One Software (Bio-Rad) as described by Figueira et al. (2005).
Statistical analysis
One-way ANOVA followed by Tukey’s multiple comparisons test was performed using GraphPad Prism version 6.00 for Windows (GraphPad Software, La Jolla California USA). Parameters were analyzed following a one-way hierarchical design, with the exposure to different Cd and α-pinene conditions or to different Cd and quercetin conditions, alone or in combination, as fixed factor. The null hypothesis tested was: no significant differences exist among tested conditions. Significant differences were considered for p < 0.05, and were identified in figures with different lowercase letters. Heatmaps were built using Metaboanalyst 3.0 (Xia et al. 2015). A matrix gathering the descriptors (growth, LPO, Prot, PC, SOD, CAT, GSTs, GSSG and GSH) per condition was used to calculate a Euclidean distance similarity matrix. This similarity matrix was simplified through the calculation of the distance among centroids matrix based on the conditions, which was then submitted to ordination analysis performed by Principal Coordinates (PCO) using Primer+Permanova (Primer-E, Plymouth). Pearson correlation vectors of biomarkers (correlation > 0.93) were provided as supplementary variables and superimposed on the PCO graph, allowing to identify the descriptors that contributed the most to differences among conditions.
Results
Toxicity tests
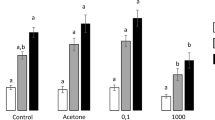
α-pinene (0.3 and 3000 μM) did not induce growth alterations in Rhizobium sp. strain E20-8 compared to control (no addition of α-pinene). Exposure to 10 μM Cd significantly inhibited cell growth. Addition of either 0.3 or 3000 μM of α-pinene to 10 μM Cd did not change the growth inhibition caused by Cd (Fig. 1a). Addition of quercetin (0.01 and 1 μM) did not affect Rhizobium growth in the absence of Cd. Quercetin attenuated the negative effect of Cd on Rhizobium growth (7% at 1 μM and 56% at 0.01 μM), but this effect was not statistically significant (p > 0.05) (Fig. 1b).
Growth of Rhizobium cells. a α-pinene experiment (conditions: 0 μM Cd and 0 μM α-pinene (CTL), 0 μM Cd and 0.3 μM α-pinene (0.3 α-P), 0 μM Cd and 3000 μM α-pinene (3000 α-P), 10 μM Cd and 0 μM α-pinene (Cd), 10 μM Cd and 0.3 μM α-pinene (Cd + 0.3 α-P), 10 μM Cd and 3000 μM α-pinene (Cd + 3000 α-P)). b quercetin experiment (conditions: 0 μM Cd and 0 μM quercetin (CTL), 0 μM Cd and 0.01 μM quercetin (0.01 Qc), 0 μM Cd and 1 μM quercetin (1 Qc), 10 μM Cd and 0 μM quercetin (Cd), 10 μM Cd and 0.01 μM quercetin (Cd + 0.01 Qc), 10 μM Cd and 1 μM quercetin (Cd + 1 Qc)). Significant differences (p < 0.05) among tested conditions for each experiment (α-pinene and quercetin) are represented with different letters. Values are means of 3 independent experiments with 3 replicates each
Intracellular cadmium concentration
In conditions without Cd (CTL, 0.3 μM α-pinene, 3000 μM α-pinene, 0.01 μM Quercetin and 1 μM Quercetin) Cd concentration was lower than the detection limit (10−4 ng Cd M cells−1). Cells grown in medium with 10 μM Cd accumulated similar concentrations in the α-pinene and in quercetin experiments (0.86 ng and 0.88 ng Cd per million cells, respectively). Addition of α-pinene increased around 40% cell Cd concentration, although not significantly (Fig. 2a). Addition of 0.01 μM quercetin did not change significantly intracellular Cd concentration compared to Cd alone. However, addition of 1 μM quercetin significantly increased (73%) Cd inside cells (Fig. 2b).
Intracellular cadmium concentration in Rhizobium cells. a α-pinene experiment (conditions: 10 μM Cd and 0 μM α-pinene (Cd), 10 μM Cd and 0.3 μM α-pinene (Cd + 0.3 α-P), 10 μM Cd and 3000 μM α-pinene (Cd + 3000 α-P)). b quercetin experiment (conditions: 10 μM Cd and 0 μM quercetin (Cd), 10 μM Cd and 0.01 μM quercetin (Cd + 0.01 Qc), 10 μM Cd and 1 μM quercetin (Cd + 1 Qc)). Significant differences (p < 0.05) among tested conditions for each experiment (α-pinene and quercetin) are represented with different letters. Values are means of 3 independent experiments with 3 replicates each
Oxidative damage
α-pinene increased LPO compared to control, not significantly (23% increase) at 0.3 μM but significantly (98% increase) at 3000 μM. Cd also increased significantly LPO. Co-exposure to Cd and α-pinene decreased LPO to levels similar to control for 0.3 μM, but resulted in the highest LPO concentrations for 3000 μM α-pinene (Fig. 3a).
Cell damage of Rhizobium cells in α-pinene experiment (conditions: the same of Fig. 1a) and in quercetin experiment (conditions: the same of Fig. 1b). a lipid peroxidation in α-pinene experiment. b lipid peroxidation in quercetin experiment. c protein carbonylation in α-pinene experiment. d protein carbonylation in quercetin experiment. Significant differences (p < 0.05) among tested conditions for each experiment (α-pinene and quercetin) are represented with different letters. Values are means of 3 independent experiments with 3 replicates each
Quercetin also increased LPO, not significantly (59% increase) at 0.01 μM and significantly (257% increase) at 1 μM. Cd induced LPO levels similar to the highest quercetin concentration (1 μM). Co-exposure to Cd and quercetin decreased LPO to levels similar to control for 0.01 μM + Cd, and did not change LPO concentrations compared to 1 μM and Cd alone for 1 μM + Cd (Fig. 3b).
α-pinene and quercetin did not change significantly PC levels compared to control. Cd increased significantly PC levels compared to control (Fig. 3c, d). Co-exposure to Cd and α-pinene resulted in PC levels similar to sole exposure to Cd, although for 0.3 μM α-pinene values were not significantly different from control (Fig. 3c). The presence of quercetin in cells exposed to Cd decreased PC for 0.01 μM, resulting in levels similar to control, but did not change PC levels compared to Cd alone (Fig. 3d).
Antioxidant and biotransformation enzymes
SOD activity was increased by α-pinene, Cd and co-exposure to both. Sole exposure to Cd and 3000 μM α-pinene and co-exposure to Cd and 0.3 μM α-pinene induced identical SOD activities (p < 0.05). Co-exposure to Cd and 3000 μM α-pinene generated a significant increase in SOD activity relatively to all other conditions (Fig. 4a).
Enzyme activity of Rhizobium cells in α-pinene experiment (conditions: the same of Fig. 1a) and in quercetin experiment (conditions: the same of Fig. 1b). a Superoxide dismutase (SOD) activity in α-pinene experiment. b SOD activity in quercetin experiment. c Catalase (CAT) activity in α-pinene experiment. d CAT activity in quercetin experiment. e Glutathione S-transferases (GSTs) activity in α-pinene experiment. f GSTs activity in quercetin experiment. Significant differences (p < 0.05) among tested conditions for each experiment (α-pinene and quercetin) are represented with different letters. Values are means of 3 independent experiments with 3 replicates each
The two quercetin concentrations originated contradictory responses in SOD activity: 0.01 μM significantly increased SOD activity and 1.0 μM decreased it 32% compared to control, although not significantly. Cd significantly increased SOD activity. Combined exposure to Cd and 1 μM quercetin did not influence SOD activity relatively to Cd alone, but co-exposure of Cd and 0.01 μM quercetin significantly increased SOD activity relatively to the remaining conditions (Fig. 4b).
CAT activity was not influenced by α-pinene or quercetin (Fig. 4c, d). Conditions with Cd (alone or in combination with both phytocompounds) significantly induced CAT activity compared to control, with the lowest concentration of both compounds inducing similar CAT activities compared to Cd alone and the highest concentration further increasing CAT activity (Fig. 4c, d).
α-pinene did not change GSTs activity but Cd increased it compared to control. Co-exposure to Cd and α-pinene increased GSTs activity not significantly in 0.3 μM (29%), but significantly in 3000 μM (Fig. 4e). Quercetin increased (58 and 42% for 0.01 and 1 μM, respectively) GSTs activity although not significantly compared to control. Cadmium significantly increased GSTs activity. Co-exposure to Cd and 0.01 μM quercetin did not induce further changes than those observed for sole exposure to Cd; but co-exposure to Cd and 1 μM significantly induced higher GSTs activity than any other condition (Fig. 4f).
Glutathione
Both α-pinene concentrations induced significant increases in GSH. Cd influence was higher (p < 0.05) than α-pinene. Co-exposure to Cd and α-pinene further increased cells GSH content (≈25%) but not significantly compared to Cd alone (Fig. 5a).
Glutathione content of Rhizobium cells in α-pinene experiment (conditions: the same of Fig. 1a) and in quercetin experiment (conditions: the same of Fig. 1b). a Reduced glutathione (GSH) in α-pinene experiment. b GSH in quercetin experiment. c Oxidized glutathione (GSSG) in α-pinene experiment. d GSSG in quercetin experiment. Significant differences (p < 0.05) among tested conditions for each experiment (α-pinene and quercetin) are represented with different letters. Values are means of 3 independent experiments with 3 replicates each
Quercetin did not influence GSH concentration. Cd significantly increased GSH (>200%). Co-exposure to Cd and 0.01 μM quercetin decreased GSH to control levels. Addition of 1 μM quercetin to Cd did not reduce significantly GSH compared to Cd alone (Fig. 5b).
Both α-pinene concentrations induced significant increases in GSSG and Cd further increased these concentrations (p < 0.05). Co-exposure to Cd and both α-pinene concentrations did not significantly change GSSG levels compared to Cd alone (Fig. 5c). Quercetin did not influence GSSG concentration and Cd significantly increased it (≈300%). Co-exposure to Cd and 0.01 μM quercetin decreased GSSG to control levels. Addition of 1 μM quercetin to Cd also reduced significantly GSSG but not to control levels (Fig. 5d).
Protein profiles
The changes in protein content and electrophoretic profiles of Rhizobium cells grown in sole or combined presence of Cd, α-pinene and quercetin, compared to control, were analyzed (Fig. 6). In α-pinene experiment, the overall amount of protein was not changed by α-pinene, but increases were noticed in Cd exposed cells. The simultaneous exposure to Cd and α-pinene resulted in a decrease in protein to control level at Cd + 0.3 µM α-pinene. At Cd + 3000 µM α-pinene the highest protein concentration was observed, although not significantly different from sole exposure to Cd (Fig. 6a).
Protein analysis of Rhizobium cells in α-pinene experiment (conditions: the same of Fig. 1a) and in quercetin experiment (conditions: the same of Fig. 1b). Protein content (prot) in α-pinene (a) and quercetin (e) experiments. Venn diagrams showing common upregulated proteins in different conditions of α-pinene (b) and quercetin (f) experiments, and common downregulated proteins in different conditions of α-pinene (c) and quercetin (g) experiments. Heatmaps of protein expression in different conditions of of α-pinene (d) and quercetin (h) experiments. For protein content (A and E) significant differences (p < 0.05) among tested conditions for each experiment (α-pinene and quercetin) are represented with different letters and values are means of 3 independent experiments with 3 replicates each
Differences in the expression of 41 proteins were observed (Fig. 6b–d). In over-expressed proteins (Fig. 6b) 0.3 µM α-pinene induced few changes (4 proteins). Exposure to 3000 µM α-pinene (alone or in combination with Cd) induced the highest number of proteins over-expressed, unique of each condition (30% and 44%, respectively) or common to other conditions. Cd alone induced the overexpression of 9 proteins, but only one was unique of this condition. In Cd + 0.3 µM α-pinene 15 proteins were over-expressed, but only one was unique of this condition (Fig. 6b). Cd + α-pinene co-exposure conditions (0.3 and 3000 µM) were those with the highest number of proteins down-expressed (15 and 17, respectively) some of which were specific for each condition (4 and 7, respectively). Fewer proteins (11 to 14) were downregulated when cells were exposed to a single factor (Cd or α-pinene) (Fig. 6c).
The heatmap evidences that the greatest differences in protein expression were observed between control and Cd + 3000 α-pinene. In control high molecular weight (>45 KDa) and low molecular (<40 KDa) proteins are less and more abundant respectively than in other conditions, especially than in Cd + 3000 α-pinene for which the opposite trend is observed (higher and lower expression in proteins with higher and lower molecular than 50 KDa, respectively). In 0.3 and 3000 α-pinene the majority of alterations were over-expression of proteins with lower molecular weight proteins and down-expression of high molecular weight proteins. At 0.3 α-pinene few proteins were over-expressed, with most changes occurring by downregulation (78%), mainly of high molecular weight proteins. In Cd condition the number of proteins over (both high and low molecular weight) and down-expressed (especially low molecular weight) were similar (Fig. 6d).
In quercetin experiment, the overall amount of protein was increased, although not significantly, by the phytocompound. Increases were noticed in Cd exposed cells. The simultaneous exposure to Cd and quercetin (both concentrations) resulted in a decrease in protein to levels not significantly different from sole exposure to quercetin, but significantly higher than control (Fig. 6e). Differences were observed in the expression of 18 proteins (Fig. 6f–h). When compared to control most of the over-expressed proteins were specific of a condition or common to two conditions. Exposure to 0.01 µM quercetin induced the highest number of proteins over-expressed relatively to control (12). Exposure to other conditions induced the expression of a lower number of proteins (3–6) (Fig. 6f). Cd and Cd + 0.01 µM quercetin decreased the expression of a high number of proteins (8 and 11, respectively) some of which were specific of a condition. Fewer proteins (1–5) were downregulated when cells were exposed just to quercetin (0.01 and 1 µM) or to Cd + 1 µM quercetin (Fig. 6g).
The heatmap (Fig. 6h) evidences the differences in protein profiles among conditions in quercetin experiment. In control high molecular weight (>60 KDa) and low molecular (<50 KDa) proteins are less and more abundant respectively than in other conditions. The influence of 0.01 μM quercetin mainly increased the expression (9 proteins were upregulated and just one was downregulated) of proteins of different molecular weights. Exposure to Cd + 0.01 μM quercetin and 1 μM quercetin resulted in an identical number of increases and decreases of protein expression (5 or 6), but with opposite effects on the smaller proteins (<60 KDa), with increases on 1 μM quercetin and decreases on Cd + 0.01 μM quercetin. The presence of Cd alone or in combination with 1 μM quercetin also altered proteins expression, mostly downregulations (Fig. 6h).
Principal components ordination
Principal Components Ordination (PCO) diagram evidenced PCO1 as the main axis explaining most of the variation (70.8%) obtained among conditions (Fig. 7). Along PCO1, two groups are clearly separated, conditions without Cd (control, 0.01 µM quercetin, 1 µM quercetin, 0.3 µM α-pinene and 3000 α-pinene) on the negative side and conditions with Cd (Cd, Cd + 0.01 µM quercetin, Cd + 1 µM quercetin, Cd + 0.3 µM α-pinene and Cd + 3000 α-pinene) on the positive side of the axis. PCO2 explained 12.8% of total variation, separating co-exposure of Cd and low concentration of both phytocompounds (Cd + 1 µM quercetin and Cd + 0.3 µM α-pinene) on the positive side from high quercetin conditions (1 µM quercetin and Cd + 1 µM quercetin) on the negative side of the axis. The remaining conditions (control, Cd, 0.01 µM quercetin, 0.3 µM α-pinene, 3000 µM α-pinene and Cd + 3000 µM α-pinene) were located near Axis 2 origin. Conditions without Cd were highly correlated (r > 0.95) with growth, thus reflecting the low impact of phytocompounds on Rhizobium. High quercetin concentration was highly correlated with LPO (r ≥ 0.93), evidencing the damage that high quercetin concentration caused in membranes.
Principal Coordinate Ordination (PCO) diagram based on descriptors (lipid peroxidation (LPO), protein carbonyl groups (CG), reduced (GSH) and oxidized (GSSG) glutathione concentrations, superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferases (GSTs) activity and growth) of Rhizobium cells in α-pinene experiment (conditions: the same of Fig. 1a) and in quercetin experiment (conditions: the same of Fig. 1b). Pearson correlation vectors of descriptors were superimposed (r > 0.93)
Co-exposure to Cd and low concentration of both phytocompounds evidenced high correlation with SOD (r > 0.93) and low correlation with LPO (r < 0.2). Conditions with Cd were highly correlated with antioxidant and biotransformation responses (CAT, GSTs, SOD, GSH, GSSG) especially Cd + 3000 µM α-pinene (r > 0.93), evidencing the higher activation of these mechanisms at this condition.
Discussion
It is well known that cadmium negatively affects soil microorganisms (Vig et al. 2003), yet litle information is available on the effects of phytocompounds towards these organisms. Moreover, different phytocompounds exhibit distinct biological activities (Sikkema et al. 1995; Cushnie and Lamb 2005). α-pinene and quercetin, two of the most common phytocompounds in nature (Noma and Asakawa 2010; Ay et al. 2016), were described to have antimicrobial activity, including at concentrations used in this study (Leite et al. 2007; Silva et al. 2012). However, most information is related to pathogenic bacteria (Leite et al. 2007; Wang et al. 2018) and the mode of action is poorly documented (Cabiscol et al. 2000). Results obtained in this study brought novel information on the effects of α-pinene and quercetin on soil bacteria, under sole exposure or in combination with other environment stressors, such as Cd.
The two concentrations of the phytocompounds used represent the range limits found in the environment and are in different orders of magnitude, two for quercetin and four for α-pinene. Despite these differences, the concentrations tested did not cause effects on the growth and protein content of Rhizobium, evidencing that the general cell metabolism was marginally affected.
α-pinene effects on bacterial growth were previously reported, with 31 and 126 µM α-pinene inhibiting the growth of Staphylococcus aureus, S. epidermidis, Streptococcus pneumoniae and S. pyogenes (Leite et al. 2007). Andrews et al. (1980) studied the toxic effects of α-pinene and other terpenes produced by Douglas fir on Bacillus strains and reported 100% inhibition at 0.13 mg/mL (0.95 mM) α-pinene concentrations. In methicillin-resistant Staphylococcus aureus the minimum inhibitory concentration was 30.4 mM (Silva et al. 2012). Thus, bacterial tolerance to α-pinene is highly variable and species dependent.
Quercetin was also reported to inhibit bacteria growth. S. aureus, Escherichia coli, Proteus vulgaris, and Pseudomonas aeruginosa growth was reduced when cells were exposed to concentrations between 66 and 1323 µM (Jaisinghani 2017). Wang et al. (2018) reported that quercetin significantly inhibited P. aeruginosa, Salmonella enterica, S. aureus, and E. coli growth, being the bacteriostatic effect of quercetin stronger on gram-positive than on gram-negative bacteria. Antimicrobial activity of quercetin was atributed to inhibition of DNA gyrase (Ohemeng et al. 1993; Plaper et al. 2003), cell wall damage (Wang et al. 2018), increased permeability of the inner bacterial membrane and the dissipation of membrane potential (Mirzoeva et al. 1997) and bacterial motility inhibition due to proton motive force disruption (Mirzoeva et al. 1997; Stepanović et al. 2003).
In our study, sole exposure to both phytocompounds had an oxidizing effect on membranes, since LPO increased significantly at high concentrations, but damages were not extensive to cytosolic proteins. Due to their lipophilic character α-pinene and quercetin can easily intercalate into the organized structures of phospholipids within membranes (van Dijk et al. 2000). This interaction may lead to membrane oxidation, influencing membrane permeability and altering the electrochemical potential and ion transport (Sikkema et al. 1995; Mirzoeva et al. 1997; van Dijk et al. 2000). Membrane lipid oxidation also destabilizes processes associated with this cellular structure, such as the electron transport chain, increasing O2− production and further oxidizing membranes (Cabiscol et al. 2000). Damage caused by O2− can efficiently be combated through SOD isoforms located near the O2− generation site, i.e., close to membranes. In fact, membrane associated Cu, Zn SOD was already reported in bacteria (Battistoni 2003), allowing a rapid and efficient O2− scavenge, and thus protecting other cellular structures from the toxic effects of membrane generated O2−. In our work, SOD activity was enhanced in the two α-pinene concentrations and in the low quercetin concentration, which may constitue a way of controlling the generated O2− burst, but at high quercetin concentration SOD activity decreased to values near control, leaving cells more vulnerable to oxidative stress and explaning the high LPO levels at this condition. Oxidized polyunsaturated fatty acids are degraded to a variety of products. Some of them, such as aldehydes, are very reactive and can damage molecules such as proteins (Humphries and Szweda 1998; Cardoso et al. 2017). Unlike reactive free radicals, aldehydes are rather long lived and can therefore diffuse from the site of their origin and reach and attack targets which are distant from the initial free-radical event (Cabiscol et al. 2000). GSTs (a family of biotransformation enzymes) use GSH to convert toxic aldehydes to alcohols (Korpi et al. 2009; Schmidt et al. 2015), which are less toxic and easily excreted (Sharma et al. 2004). GSTs associated to membranes were detected in bacteria (Allocati et al. 2009). If Rhizobium hold GSTs catalyzing aldehyde degradation associated to membranes, then aldehydes would be destroyed near generation site and interference with cytosolic proteins would be minimized. Indeed our results show that proteins were not damaged (PC did not increase) by the two concentrations of both phytocompounds, proving that damage did not reach cytosol and did not affect cell metabolism, as shown by the similar growth among control and exposure to both phytocompounds.
Though not altering growth, both concentrations of α-pinene and quercetin evidenced bioactivity in Rhizobium, increasing the content of low molecular weight antioxidants (GSH at 0.3 and 3000 μM α-pinene) and the activity of antioxidant enzymes (SOD), without significantly altering cellular redox status, protein content and growth, but causing significant damage to membranes at high concentrations. Since these concentrations are environmentally relevant, sites with phytocompounds concentrations close to the low concentration used in this study do not seem to affect soil bacteria such as Rhizobium, whereas high concentrations may induce membrane damage and alter membrane functions, such as ion transport or oxidative phosphorilation. On the other hand, cell metabolism (growth, protein content, protein carbonylation) was little affected. These results are relevant since little impact on soil microbial communities is to be expected if exposure to phytocompounds is the only constraint experienced by organisms, but in systems also impacted by other constraints the consequences of co-exposure are difficult to predict.
In the case of Cd, the ions accumulated in the cytosol interfered both with membrane and cytosolic components. Cadmium directly inactivates important proteins of cell metabolism (Figueiredo-Pereira et al. 1998), can displace redox active metals such as Fe and Cu, thus leading to reactive oxygen species (ROS) burst (Pacheco et al. 2008; Cuypers et al. 2010). Highly reactive ROS interact with lipids, proteins and DNA, causing lipid peroxidation (LPO), protein carbonylation (PC), affecting gene and protein expression, membrane fluidity and permeability and enzymes activity, disturbing cell homeostasis (Cuypers et al. 2010; Monteiro et al. 2012), and ultimately compromising growth and survival.
In the present study, Cd caused a significant growth reduction in Rhizobium (≈60%). Protein content significantly increased (>90%) and protein expression was highly impacted both by up and downregulation, evidencing the cellular effort to metabolically adapt to the changes induced by Cd, being some pathways induced and their enzymes (proteins) over-expressed and other repressed and their enzymes down-expressed. In Cupriavidus taiwanensis, protein folding-mediated GroEL and CdS biotransformation were upregulated and pathways involved in ATP utilization, such as cell motility were downregulated (Siripornadulsil et al. 2014). When exposed to Cd stress, Rhodobacter capsulatus also increased protein expression, upregulating cadmium-binding and heatshock proteins (Mohamed Fahmy Gad El-Rab et al. 2006). In Rhizobium leguminosarum protein expression was also changed by Cd, evidencing the metabolic changes necessary to maintain cell fitness under Cd stress (Nunes et al. 2018).
Cells tried to counteract Cd toxicity by inducing glutathione synthesis. GSH detoxification includes immobilization of cytosolic Cd ions in Cd-GSH complexes (Lima et al. 2006; Corticeiro et al. 2013; Cardoso et al. 2018), allowing cells to avoid Cd toxicity. When mechanisms of toxicity avoidance are not effective free Cd ions inactivate enzymes and originate ROS (Falasco et al. 2009; Cuypers et al. 2010). Thus, repairing and antioxidant mechanisms have to be activated. Siripornadulsil and collaborators (Siripornadulsil et al. 2014) reported that the chaperone GroEL was the most upregulated protein in the bacteria C. taiwanensis exposed to Cd, evidencing the importance of repairing protein alterations originated by Cd. The antioxidant response can either be achieved by low molecular weight antioxidants (GSH), which can directly scavenge several ROS (Apel and Hirt 2004) or by the action of antioxidant enzymes. In our study both GSH and antioxidant enzymes activity (SOD and CAT) increased as a response to fight ROS burst originated by Cd. When the antioxidant response is not sufficient to abrogate oxidative stress damage overcomes. The toxic effects generated by aldehydes (originated from peroxidized lipids) can be minimized by the GSTs catalysed conversion of toxic aldehydes to alcohols, decreasing protein oxidation (PC) and restraining oxidative damage in cells.
In this work, Cd induced both membrane and cytosolic damage, implying that mechanisms triggered were not able to neutralize Cd toxicity, cell fitness was affected and growth was decreased. A decresease in growth of bacteria exposed to metals was also observed in other studies. Corticeiro et al. (2006) observed that under exposure to Cd, R. leguminosarum decreased growth and induced antioxidant mechanisms. Chaudri et al. (1992) reported that Zn, Cu and Ni reduced the number of R. leguminosarum cells in the soil. Khalid and Jin (2013) observed that Zn and Pb induced oxidative stress, and enhanced the activity of antioxidant enzymes in P. putida and B. subtilis. Pacheco et al. (2008) described Cd to inhibit growth, interfere with respiratory chain and generate ROS in E. coli.
The lower damage relatively to sole exposure to Cd observed in cells exposed to low concentration of both phytocompounds can be related to SOD activity enhancement (110% for quercetin and 17% for α-pinene) and higher (24%) GSH levels for α-pinene compared to sole Cd exposure. When exposed to Cd + 0.01 μM quercetin it seems that SOD decreased oxidative stress so effectively that GSH synthesis and cell damage (LPO and PC) significantly decreased to control levels, resulting in the relieve of growth impairment imposed by Cd alone.
Co-exposure to Cd and α-pinene or quercetin at high concentration did not relieve or even aggravated (LPO at 3000 μM α-pinene + Cd) cell damage imposed by sole exposure to Cd. The further increase in the antioxidant (SOD, CAT) and biotransformation (GSTs) activity and the maintenance of glutathione synthesis were not able to restrain cell damage, since lipid and protein oxidation was mantained or even increased (LPO at 3000 µM α-pinene + Cd) compared to sole Cd exposure. Co-exposure to high phytocompounds concentration and Cd did not induce higher cytoplasm damage even when intracellular Cd concentration was higher (73%) compared to sole Cd exposure (1 µM quercetin + Cd). The increase of antioxidant mechanisms (CAT activity for both phytocompounds, and SOD for α-pinene) and toxic compounds degradation resulting from lipid peroxidation (GSTs activity), compensated the extra toxicity resulting from co-exposure to high phytocompounds concentration and Cd, resulting in growth reduction similar to sole Cd exposure. Although both α-pinene and quercetin induced similar effects in Rhizobium, other phytocompounds affected bacterial cells differently. In a previous study, farnesol decreased lipid peroxidation levels, evidencing protective and stabilizing effects on membranes, and the increase in farnesol concentration further protected cells from Cd toxicity due to a decrease in intracellular Cd concentration (Nunes et al. 2018).
Changes in protein expression show that the pathways activated are not common to all conditions. In cells exclusively exposed to 0.3 μM α-pinene few mechanisms were activated. In the remaining conditions other pathways are activated and these can be characteristic of one condition or common to more than one. The combined exposure to 3000 μM α-pinene and Cd lead to over- and down-expressed 85% of proteins, 8 and 7 of them only increasing or decreasing in this condition. Down-expressed proteins also evidence the cellular effort to adapt metabolically to changes in the intracellular environment, being some pathways induced and their enzymes (proteins) over-expressed and other repressed and their enzymes down-expressed. Thus, a higher number of down-expressed proteins also evidences higher adaptation of cells to the new intracellular conditions. The high impact on protein expression observed in combined exposure to 3000 μM α-pinene and Cd evidences the cell’s effort to adapt to the stress imposed, which biochemical parameters proved to be the condition imposing higher damage.
Conclusions
This study showed that exposure to different phytocompounds does not appear to influence Rhizobium fitness even at high concentrations but combined with other stress inducing agents, such as Cd, different and even opposite responses were observed. At low concentrations phytocompounds seemed to relieve the stress imposed by Cd by increasing antioxidant responses. At high concentrations this protective effect vanished, and stress was even exacerbated by co-exposure with Cd, although growth was not further affected. It was also shown that microorganisms growth is affected by many factors besides toxic substances, like Cd. Factors such as the presence of phytocompounds that can accumulate in soil and that present bioactivity may also influence the tolerance of microorganisms to toxicants and may change their impact on the environment. Consequently, the composition and concentration of phytocompounds present in soils are factors that should be taken into consideration when evaluating the impact of metals or other oxidizing agents in microbial communites. Phytocompounds should also be regarded as opportunities to accellerate the development of more efficient tools and technologies to remediate and restore impacted areas. Therefore, the use of plant material with phytocompounds that increase the tolerance of microorganisms to toxicants, such as Cd, should be a strategy to be considered in the future to reduce the impact of Cd contamination and possibly other metals and oxidizing agents in affected soil microbial communities.
References
Abrahim D, Francischini AC, Pergo EM et al. (2003) Effects of α-pinene on the mitochondrial respiration of maize seedlings. Plant Physiol Biochem 41:985–991. https://doi.org/10.1016/J.PLAPHY.2003.07.003
Allocati N, Federici L, Masulli M, Di Ilio C (2009) Glutathione transferases in bacteria. FEBS J 276:58–75. https://doi.org/10.1111/j.1742-4658.2008.06743.x
Andrews RE, Parks LW, Spence KD (1980) Some effects of douglas fir terpenes on certain microorganisms. Appl Environ Microbiol 40:301–4
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Asensio D, Owen SM, Llusià J, Peñuelas J (2008) The distribution of volatile isoprenoids in the soil horizons around Pinus halepensis trees. Soil Biol Biochem 40:2937–2947. https://doi.org/10.1016/J.SOILBIO.2008.08.008
Ay M, Charli A, Jin H et al. (2016) Quercetin. In: Gupta RC (ed) Nutraceuticals: Efficacy, Safety and Toxicity. Academic Press - Elsevier, Amsterdam, pp 447–452
Battistoni A (2003) Role of prokaryotic Cu, Zn superoxide dismutase in pathogenesis Biochem Soc Trans 31:1326–1329
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 287:276–287
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. https://doi.org/10.1016/S0076-6879(78)52032-6
Cabiscol E, Tamarit J, Ros J (2000) Oxidative stress in bacteria and protein damage by reactive oxygen species. Int Microbiol 3:3–8
Cardoso P, Corticeiro S, Freitas R, Figueira E (2018) Different efficiencies of the same mechanisms result in distinct Cd tolerance within Rhizobium. Ecotoxicol Environ Saf 150:260–269. https://doi.org/10.1016/j.ecoenv.2017.12.002
Cardoso P, Santos M, Freitas R et al. (2017) Response of Rhizobium to Cd exposure: a volatile perspective. Environ Pollut 231:802–811. https://doi.org/10.1016/j.envpol.2017.08.067
Carlsen SCK, Fomsgaard IS (2008) Biologically active secondary metabolites in white clover (Trifolium repens L.)—a review focusing on contents in the plant, plant–pest interactions and transformation. Chemoecology 18:129–170. https://doi.org/10.1007/s00049-008-0402-7
Carlsen SCK, Pedersen HA, Spliid NH, Fomsgaard IS (2012) Fate in soil of flavonoids released from white clover (Trifolium repens L.). Appl Environ Soil Sci 2012:1–10. https://doi.org/10.1155/2012/743413
Chaudri AM, McGrath SP, Giller KE (1992) Survival of the indigenous population of Rhizobium leguminosarum biovar trifolii in soil spiked with Cd, Zn, Cu and Ni salts. Soil Biol Biochem 24:625–632
Corticeiro S, Freitas R, Figueira E (2013) The role of GSTs in the tolerance of Rhizobium leguminosarum to cadmium Biometals 26:879–886. https://doi.org/10.1007/s10534-013-9664-9
Corticeiro SC, Lima AIG, Figueira EM de AP (2006) The importance of glutathione in oxidative status of Rhizobium leguminosarum biovar viciae under Cd exposure. Enzyme Microb Technol 40:132–137. https://doi.org/10.1016/j.enzmictec.2005.10.053
Cowan MM (1999) Plant products as antimicrobial agents Clin Microbiol Rev 12:564–582
Cushnie TPT, Lamb AJ (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26:343–356. https://doi.org/10.1016/J.IJANTIMICAG.2005.09.002
Cuypers A, Plusquin M, Remans T et al. (2010) Cadmium stress: an oxidative challenge. BioMetals 23:927–940. https://doi.org/10.1007/s10534-010-9329-x
Demyttenaere JCR, del Carmen Herrera M, De Kimpe N (2000) Biotransformation of geraniol, nerol and citral by sporulated surface cultures of Aspergillus niger and Penicillium sp. Phytochemistry 55:363–373. https://doi.org/10.1016/S0031-9422(00)00330-7
Falasco E, Bona F, Badino G et al. (2009) Diatom teratological forms and environmental alterations: a review. Hydrobiologia 623:1–35. https://doi.org/10.1007/s10750-008-9687-3
Faria JMS, Lima AS, Mendes MD et al. (2011) Eucalyptus from Mata Experimental do Escaroupim (Portugal): evaluation of the essential oil composition from sixteen species. Acta Hortic 61–66. https://doi.org/10.17660/ActaHortic.2011.925.7
Figueira E, Lima A, Pereira S (2005) Cadmium tolerance plasticity in Rhizobium leguminosarum bv. viciae: glutathione as a detoxifying agent. Can J Microbiol 51:7–14. https://doi.org/10.1139/w04-101
Figueiredo-Pereira ME, Yakushin S, Cohen G (1998) Disruption of the intracellular sulfhydryl homeostasis by cadmium-induced oxidative stress leads to protein thiolation and ubiquitination in neuronal cells. J Biol Chem 273:12703–12709. https://doi.org/10.1074/JBC.273.21.12703
Garbisu C, Alkorta I (2001) Phytoextraction: a cost-effective plant-based technology for the removal of metals from the environment. Bioresour Technol 77:229–236. https://doi.org/10.1016/S0960-8524(00)00108-5
Gattinger A, Palojärvi A, Schloter M (2008) Soil microbial communities and related functions. In: Schröder, Pfadenhauer, Munch (eds) Perspectives for Agroecosystem Management: Balancing Environmental and Socio-Economic Demands. Amsterdam, pp 279–292
Geisseler D, Scow KM (2014) Long-term effects of mineral fertilizers on soil microorganisms—a review. Soil Biol Biochem 75:54–63. https://doi.org/10.1016/j.soilbio.2014.03.023
Gilbert N (2018) European Union debates controversial plans to limit cadmium in fertilizer. Science. https://doi.org/10.1126/science.aat8448
Gopalakrishnan S, Sathya A, Vijayabharathi R et al. (2015) Plant growth promoting rhizobia: challenges and opportunities. 3 Biotech 5:355–377. https://doi.org/10.1007/s13205-014-0241-x
Grant EA, Sallans WG (1964) Influence of plant extracts on germination and growth of eight forage species. Grass Forage Sci 19:191–197. https://doi.org/10.1111/j.1365-2494.1964.tb01161.x
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139. https://doi.org/10.1017/CBO9781107415324.004
Hale CN, Lowther WL, Lloyd JM (1979) Effect of inoculant formulation on survival of Rhizobium trifolii and the establishment of oversown white clover (Trifolium repens). N Z J Exp Agric 7:311–314. https://doi.org/10.1080/03015521.1979.10429091
Hale CN, Mathers DJ (1977) Toxicity of white clover seed diffusate and its effect on the survival of Rhizobium trifolii. N Z J Agric Res 20:69–73. https://doi.org/10.1080/00288233.1977.10427305
Helmke PA (1999) Chemistry of cadmium in soil solution. In: McLaughlin MJ, Singh BR (eds) Cadmium in soils and plants. Kluwer Academic Publishers, Dordrecht, The Netherlands, p 39–64
Humphries KM, Szweda LI (1998) Selective inactivation of α-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-Hydroxy-2-nonenal. Biochemistry 37:15835–15841. https://doi.org/10.1021/bi981512h
Ibanez S, Gallet C, Després L (2012) Plant insecticidal toxins in ecological networks. Toxins 4:228–243. https://doi.org/10.3390/toxins4040228
Inderjit (2005) Soil microorganisms: an important determinant of allelopathic activity. Plant Soil 274:227–236. https://doi.org/10.1007/s11104-004-0159-x
Itoh S, Yumura Y (1979) Studies on the contamination of vegetable crops by excessive absorption of heavy metals. Bull Veg Ornam Crop Res Stn 6:123–145
Jaisinghani RN (2017) Antibacterial properties of quercetin. Microbiol Res (Pavia) 8. https://doi.org/10.4081/mr.2017.6877
Jeganathan B, Punyasiri PAN, Kottawa-Arachchi JD et al. (2016) Genetic variation of flavonols quercetin, myricetin, and kaempferol in the Sri Lankan tea (Camellia sinensis L.) and their health-promoting aspects. Int J Food Sci 2016:1–9. https://doi.org/10.1155/2016/6057434
Johansson LH, Borg LA (1988) A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem 174:331–336
Judzentiene A, Kupcinskiene E (2008) Chemical composition on essential oils from needles of Pinus sylvestris L. Grown in Northern Lithuania. J Essent Oil Res 20:26–29. https://doi.org/10.1080/10412905.2008.9699413
Kabata-Pendias A (2011) Trace elements in soils and plants, 4th edn. CRC Press—Taylor & Francis Group, United States of America
Kalia A, Gosal SK (2011) Effect of pesticide application on soil microorganisms. Arch Agron Soil Sci 57:569–596. https://doi.org/10.1080/03650341003787582
Karamać M, Kosińska A, Estrella I et al. (2012) Antioxidant activity of phenolic compounds identified in sunflower seeds. Eur Food Res Technol 235:221–230. https://doi.org/10.1007/s00217-012-1751-6
Khalid aH, Jin HJ (2013) Heavy metal resistance of bacteria and its impact on the production of antioxidant enzymes. African J Microbiol Res 7:2288–2296. https://doi.org/10.5897/AJMR12.1764
Kleinheinz B, St John WP et al. (1999) Characterization of alpha-pinene-degrading microorganisms and application to a bench-scale biofiltration system for VOC degradation. Arch Environ Contam Toxicol 37:151–157
Korpi A, Järnberg J, Pasanen A-L (2009) Microbial volatile organic compounds. Crit Rev Toxicol 39:139–193. https://doi.org/10.1080/10408440802291497
Kuc J (1992) Antifungal compounds from plants. In: Phytochemical resources for medicine and agriculture. Springer, Boston, MA, pp 159–184
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leite AM, de Oliveira Lima E, Souza EL et al. (2007) Inhibitory effect of beta-pinene, alpha-pinene and eugenol on the growth of potential infectious endocarditis causing Gram-positive bacteria Rev Bras Ciênc Farm 43:121–126. https://doi.org/10.1590/S1516-93322007000100015
Lemire JA, Harrison JJ, Turner RJ (2013) Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Microbiol 11:371–384. https://doi.org/10.1038/nrmicro3028
Les A, Walker RW (1984) Toxicity and binding of copper, zinc, and cadmium by the blue-green alga, Chroococcus paris. Water Air Soil Pollut 23:129–139. https://doi.org/10.1007/BF00206971
Lima A, Corticeiro S, Figueira E (2006) Glutathione-mediated cadmium sequestration in Rhizobium leguminosarum. Enzyme Microb Technol 39:763–769. https://doi.org/10.1016/j.enzmictec.2005.12.009
Llusia J, Penuelas J (2000) Seasonal patterns of terpene content and emission from seven mediterranean woody species in field conditions. Am J Bot 87:133. https://doi.org/10.2307/2656691
Loreto F, Dicke M, Schnitzler J-P, Turlings TCJ (2014) Plant volatiles and the environment. Plant Cell Environ 37:1905–1908. https://doi.org/10.1111/pce.12369
Macfarlane MJ, Scott D, Jarvis P (1982) Allelopathic effects of white clover 2. Field investigations in tussock grasslands. N Z J Agric Res 25:511–518. https://doi.org/10.1080/00288233.1982.10425214
Marschner H (1995) Mineral Nutrition of Higher Plants, 2nd edn. Academic Press, London
Maurer D, Kolb S, Haumaier L, Borken W (2008) Inhibition of atmospheric methane oxidation by monoterpenes in Norway spruce and European beech soils. Soil Biol Biochem 40:3014–3020. https://doi.org/10.1016/J.SOILBIO.2008.08.023
Mesquita CS, Oliveira R, Bento F et al. (2014) Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem 458:69–71. https://doi.org/10.1016/j.ab.2014.04.034
Mirzoeva OK, Grishanin RN, Calder PC (1997) Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol Res 152:239–246. https://doi.org/10.1016/S0944-5013(97)80034-1
Mohamed Fahmy Gad El-Rab S, Abdel-Fattah Shoreit A, Fukumori Y (2006) Effects of Cadmium Stress on Growth, Morphology, and Protein Expression in Rhodobacter capsulatus B10. Biosci Biotechnol Biochem 70:2394–2402. https://doi.org/10.1271/bbb.60122
Monteiro C, Santos C, Pinho S et al. (2012) Cadmium-induced cyto- and genotoxicity are organ-dependent in lettuce. Chem Res Toxicol 25:1423–1434. https://doi.org/10.1021/tx300039t
Newman EI, Rovira AD (1975) Allelopathy among some British grassland species. J Ecol 63:727. https://doi.org/10.2307/2258598
Nies DH (1999) Microbial heavy-metal resistance. Appl Microbiol Biotechnol 51:730–750
Noma Y, Asakawa Y (2010) Biotransformation of monoterpenoids by microorganisms, insects, and mammals. In: Hüsnü Can Baser K, Buchbauer G (eds) Handbook of Essential Oils: Science, Technology, and Applications. CRC Press - Taylor & Francis Group, Boca Raton, pp 585–736
Nunes T, Cardoso P, Freitas R, Figueira E (2018) Protective effects of farnesol on a Rhizobium strain exposed to cadmium. Ecotoxicol Environ Saf 165:622–629. https://doi.org/10.1016/j.ecoenv.2018.07.125
Ohemeng KA, Schwender CF, Fu KP, Barrett JF (1993) DNA gyrase inhibitory and antibacterial activity of some flavones(1). Bioorg Med Chem Lett 3:225–230. https://doi.org/10.1016/S0960-894X(01)80881-7
Owen SM, Clark S, Pompe M, Semple KT (2007) Biogenic volatile organic compounds as potential carbon sources for microbial communities in soil from the rhizosphere of Populus tremula. FEMS Microbiol Lett 268:34–39. https://doi.org/10.1111/j.1574-6968.2006.00602.x
Pacheco CC, Passos JF, Castro R et al. (2008) Role of respiration and glutathione in cadmium-induced oxidative stress in Escherichia coli K-12. Arch Microbiol 189:271–278. https://doi.org/10.1007/s00203-007-0316-8
Pan Y, Zheng YM, Ho WS (2018) Effect of quercetin glucosides from Allium extracts on HepG2, PC‑3 and HT‑29 cancer cell lines. Oncol Lett. https://doi.org/10.3892/ol.2018.7893
Plaper A, Golob M, Hafner I et al. (2003) Characterization of quercetin binding site on DNA gyrase. Biochem Biophys Res Commun 306:530–536. https://doi.org/10.1016/S0006-291X(03)01006-4
Prévéral S, Gayet L, Moldes C et al. (2009) A common highly conserved cadmium detoxification mechanism from bacteria to humans: heavy metal tolerance conferred by the ATP-binding cassette (ABC) transporter SpHMT1 requires glutathione but not metal-chelating phytochelatin peptides. J Biol Chem 284:4936–4943. https://doi.org/10.1074/jbc.M808130200
Rahman I, Kode A, Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1:3159–3165. https://doi.org/10.1038/nprot.2006.378
Ravichandran R, Rajendran M, Devapiriam D (2014) Antioxidant study of quercetin and their metal complex and determination of stability constant by spectrophotometry method. Food Chem 146:472–478. https://doi.org/10.1016/J.FOODCHEM.2013.09.080
Registry A for TS and D Priority List of Hazardous Substances. https://www.atsdr.cdc.gov/spl/. Accessed 6 Apr 2017
Robinson HW, Hogden CG (1940) The biuret reaction in the determination of serum proteins. J Biol Chem 135:707–725
Schmidt R, Cordovez V, de Boer W et al. (2015) Volatile affairs in microbial interactions. ISME J 9:2329–2335. https://doi.org/10.1038/ismej.2015.42
Sharma R, Yang Y, Sharma A et al. (2004) Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal 6:289–300. https://doi.org/10.1089/152308604322899350
Shentu J, He Z, Yang X-E, Li T (2008) Accumulation properties of cadmium in a selected vegetable-rotation system of Southeastern China. J Agric Food Chem 56:6382–6388. https://doi.org/10.1021/jf800882q
Sikkema J, de Bont JA, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev 59:201–222
Silva ACR, da, Lopes PM, de Azevedo MMB et al. (2012) Biological activities of a-pinene and β-pinene enantiomers. Molecules 17:6305–6316. https://doi.org/10.3390/molecules17066305
Singh HP, Batish DR, Kaur S et al. (2006) alpha-Pinene inhibits growth and induces oxidative stress in roots. Ann Bot 98:1261–1269. https://doi.org/10.1093/aob/mcl213
Sinha S, Mukherjee SK (2009) Pseudomonas aeruginosa KUCd1, a possible candidate for cadmium bioremediation. Braz J Microbiol 40:655–662. https://doi.org/10.1590/S1517-83822009000300030
Siripornadulsil S, Thanwisai L, Siripornadulsil W (2014) Changes in the proteome of the cadmium-tolerant bacteria Cupriavidus taiwanensis KKU2500-3 in response to cadmium toxicity. Can J Microbiol 60:121–131. https://doi.org/10.1139/cjm-2013-0713
Somasegaran P, Hoben HJ (1994) Handbook for Rhizobia. Springer-Verlag, New York, NY, USA
Stepanović S, Antić N, Dakić I, Švabić-Vlahović M (2003) In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol Res 158:353–357. https://doi.org/10.1078/0944-5013-00215
Sybilska D, Asztemborska M, Kowalczyk J et al. (1994) Enantiomeric composition of terpenic hydrocarbons in essential oils from Juniperus communis L. J Chromatogr A 659:389–394. https://doi.org/10.1016/0021-9673(94)85081-X
Udenigwe CC, Udechukwu MC, Yiridoe C et al. (2016) Antioxidant mechanism of potato protein hydrolysates against in vitro oxidation of reduced glutathione. J Funct Foods 20:195–203. https://doi.org/10.1016/j.jff.2015.11.004
van Dijk C, Driessen AJ, Recourt K (2000) The uncoupling efficiency and affinity of flavonoids for vesicles. Biochem Pharmacol 60:1593–1600. https://doi.org/10.1016/S0006-2952(00)00488-3
Verdeguer M, Blázquez MA, Boira H (2012) Chemical composition and herbicidal activity of the essential oil from a Cistus ladanifer L. population from Spain. Nat Prod Res 26:1602–1609. https://doi.org/10.1080/14786419.2011.592835
Vig K, Megharaj M, Sethunathan N, Naidu R (2003) Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review. Adv Environ Res 8:121–135. https://doi.org/10.1016/S1093-0191(02)00135-1
Vokou D, Chalkos D, Karamanlidou G, Yiangou M (2002) Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J Chem Ecol 28:755–768. https://doi.org/10.1023/A:1015236709767
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250. https://doi.org/10.1021/bp00033a001
Wang S, Yao J, Zhou B et al. (2018) Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J Food Prot 81:68–78. https://doi.org/10.4315/0362-028X.JFP-17-214
White C (1991) The role of monoterpenes in soil nitrogen cycling processes in ponderosa pine. Biogeochemistry 12:43–68. https://doi.org/10.1007/BF00002625
White CS (1994) Monoterpenes: their effects on ecosystem nutrient cycling. J Chem Ecol 20:1381–1406. https://doi.org/10.1007/BF02059813
Xia J, Sinelnikov IV, Han B, Wishart DS (2015) MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res 43:W251–W257. https://doi.org/10.1093/nar/gkv380
Yang H, Woo J, Pae AN et al. (2016) α -Pinene, a major constituent of pine tree oils, enhances non-rapid eye movement sleep in mice through GABA A—benzodiazepine receptors. Mol Pharmacol 90:530–539. https://doi.org/10.1124/mol.116.105080
Yoo SK, Day DF (2002) Bacterial metabolism of α- and β-pinene and related monoterpenes by Pseudomonas sp. strain PIN. Process Biochem 37:739–745. https://doi.org/10.1016/S0032-9592(01)00262-X
Zhang M, Swarts SG, Yin L et al. (2011) Antioxidant properties of quercetin. Springer, Boston, MA, p 283–289
Funding
PC benefited from a Ph.D. grant (SFRH/BD/101140/2014) from the Portuguese Science Foundation (FCT), supported by the European Social Fund, Programa Operacional Capital Humano (POCH). Thanks are due, for the financial support, to CESAM (UID/AMB/50017/2019), to FCT/MEC through national funds, and the co-funding by FEDER, within the PT2020 Partnership Agreement and Compete 2020.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cardoso, P., Nunes, T., Pinto, R. et al. Rhizobium response to sole and combined exposure to cadmium and the phytocompounds alpha-pinene and quercetin. Ecotoxicology 29, 444–458 (2020). https://doi.org/10.1007/s10646-020-02184-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02184-6