Abstract
Mercury concentrations in Lake Champlain fish increased (2011–2017) for the first time in more than two decades. The increase, however, was not consistent among species or throughout the lake. Mercury concentrations in smallmouth bass and yellow perch from the three Main Lake segments increased significantly while concentrations in the eastern portions of the lake (Northeast Arm and Malletts Bay) remained unchanged or decreased; mercury concentrations in white perch remained unchanged. Factors examined to explain the increase included: atmospheric deposition, lake temperature, chlorophyll-a, fishery dynamics, lake flooding and loading of total suspended solids (TSS). This paper examines how each factor has changed between study periods and the spatial variability associated with the change. We hypothesize fishery dynamics, flooding and TSS loading may be partially responsible for the increase in fish mercury. Both growth efficiency and biomass of fish suggest mercury concentrations would increase in the Main Lake segments and decrease in the eastern portion of the lake. Additionally, two extreme climate events in 2011 resulted in extensive flooding and a four-fold increase in annual TSS loading, both potentially increasing biotic mercury with the impact varying spatially throughout the lake. Changes to the fishery and disturbance caused by extreme climatic events have increased biotic mercury and the processes responsible need further study to identify possible future scenarios in order to better protect human and wildlife health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mercury became a global pollutant with the rise of industrialization and is responsible, at least in part, for 81% of all fish consumption advisories in the US (USEPA 2013). Ignoring re-emissions, mercury is primarily emitted to the atmosphere through the burning of coal and mining (UNEP 2013). Mercury can remain in the atmosphere anywhere from hours to a year depending on the form (Driscoll et al. 2013). Once deposited on the landscape, mercury can be sequestered in soils and vegetation, re-emitted to the atmosphere or transported to the aquatic environment. The majority of mercury exported from the terrestrial to aquatic system is particulate mercury via streams (Grigal 2002). Once in the aquatic environment, mercury can be methylated by bacteria to a more toxic form, methylmercury. This occurs in low oxygen environments, which include but are not limited to: wetlands, sediment–water interface and periphytic biofilms (Paranjape and Hall 2017). Methylmercury is bioavailable and can accumulate within an organism and biomagnify throughout the food web to levels that are dangerous to humans and wildlife consuming piscivorous fish. To help minimize health risks, the US EPA set a health criterion of 300 ng Hg per g of fish that suggests reducing the number of meals each month and the US FDA set an action limit that restricts the commercial sale of fish with mercury levels greater than 1000 ng Hg per g.
Mercury studies have been carried out extensively throughout the northeastern United States because it receives some of the highest rates of mercury deposition in the country (Lin et al. 2012) and is a mercury-sensitive ecosystem (Driscoll et al. 2007). Multiple assessments of biotic mercury throughout the Northeast using 26 studies that included over 20,000 mercury measurements agree that low pH (<6.0), acid neutralizing capacity (ANC, <100 µeq/L), productivity (TP < 30 µg/g) along with high dissolved organic carbon (DOC, >4 mg C/L) and, to a lesser degree, area (basin or lake) result in elevated biotic mercury (Evers et al. 2007, Driscoll et al. 2007, Chen, Folt 2005, Kamman et al. 2005). Lake Champlain does not meet the typical mercury sensitive landscape; pH rarely falls below 7 (https://dec.vermont.gov/watershed/lakes-ponds/monitor/lake-champlain) and DOC is <4 mg C/L in all lake segments except Missisquoi Bay and South Lake (Miller et al. 2012). However, total phosphorus is typically below 30 mg/L in most areas of the lake (Smeltzer et al. 2012) and Lake Champlain is the largest lake in the Northeast with a watershed ~18 times larger than the lake itself.
The lower nutrient status of Lake Champlain along with its large size can promote elevated biotic mercury. Chen et al. (2012) measured higher mercury concentrations in zooplankton from the oligotrophic Malletts Bay compared to the eutrophic Missisquoi Bay in all bloom years illustrating the impact of biomass on mercury bioaccumulation. Likewise, the large lake and watershed receives substantial mercury loading from atmospheric deposition and both the lake and watershed act as a sink for mercury. Shanley and Chalmers (2012) estimated that ~13% of the mercury deposited to the landscape is flushed to the lake through tributaries and only 16% of the mercury reaching the lake is exported. Tributaries are the primary source of mercury loading to Lake Champlain, delivering ~2.5 times more mercury than atmospheric deposition directly to the lake surface. Tributary loading of mercury occurs primarily during high flow events because total mercury (THg) is strongly and consistently related to total suspended solids (TSS) which is positively correlated to tributary discharge. This makes stream discharge via precipitation events an important component to mercury loading and uncertainty in a changing climate (Shanley and Chalmers 2012).
The elevated deposition of atmospheric mercury and the mercury-sensitive landscape in the Northeast has resulted in all states in the Northeast to implement statewide fish consumption advisories for mercury (https://fishadvisoryonline.epa.gov/Advisories.aspx last accessed 29 Jan 2019) with Lake Champlain issuing two additional fish consumption advisories specifically for walleye (NYS DOH, https://www.health.ny.gov/publications/2800.pdf last accessed 29 Jan 2019) and smallmouth bass (VT DOH, http://www.healthvermont.gov/health-environment/recreational-water/mercury-fish last accessed 29 Jan 2019). This study was designed as a re-examination of mercury concentrations in indicator fish taxa to aid in fish consumption advisories and to determine if any lake segments pose elevated risk. However, an unexpected finding indicated lake-wide mercury concentrations in some species increased since the 2011 assessment. Here we evaluate lake-wide changes in fish mercury concentrations for all available studies and an assessment by lake segment for the 2011 and 2016–2017 studies. Additionally, we examined changes in atmospheric mercury deposition, lake temperature, chlorophyll-a, fishery dynamics, flooding and loading of total suspended solids (TSS) as possible factors impacting the spatial and temporal changes identified in fish mercury.
Methods
Study site
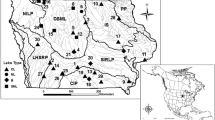
Lake Champlain is the 13th largest lake in the United States at 1269 km2 (U.S. Census Bureau 2011) situated between New York, Vermont and Quebec (Fig. 1). Water flows south to north throughout the southern and western portions of the lake to the outlet, the Richelieu River. The eastern portion of the lake flows south from Missisquoi Bay through the Northeast Arm and Malletts Bay to enter into the Main Lake (Fig. 1).
Lake Champlain separated into the five lake segments with each watershed outlined. Numbers indicate sampling location, refer to Table 1 for data. Dark areas represent the area inundated during the flood in spring 2011. The Main Lake Extended included the South Main Lake, Main Lake and North Main Lake segments
Sample collection and methodology described here is for the 2016–2017 study only. Methodology for the 2011 study is detailed in Johnson (2012). Studies prior to 2011 were carried out by Vermont Department of Environmental Conservation (VT DEC) and Vermont Department of Fish and Wildlife (VT DFW) following the methodology described in Kamman et al. (2004). All studies analyzed samples from a standard fillet.
Sample Collections
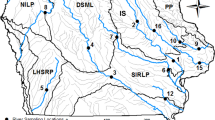
The goal was to collect a minimum of 10 specimens from each of the five indicator taxa (walleye, lake trout, smallmouth bass, yellow perch and white perch) in each of the seven lake segments (Fig. 1). Fish samples were collected by Lake Champlain International (LCI), an advocacy organization addressing water quality threats to the lake, during 2016 and 2017 with ~1/3 of the samples collected during the 2016 Father’s Day Fishing Derby. All derby captains and volunteers were trained on clean sampling techniques. Tissue plugs were collected from the mid-dorsal region of larger specimens that were to be released while dead specimens had small sections of fillet removed. All samples were stored in glass scintillation vials and frozen until analyses could be performed. Each fish was weighed on a certified scale and measured for total length using a measuring board with a standard ruler attached. Outside of the fishing derby, specimens were collected by LCI employees or select anglers with all fish measurements and samples taken by LCI employees following the fishing derby protocols. Samples previously frozen were thawed and flexed gently for a more accurate length measurement.
Methodology
All samples were analyzed for total mercury (THg) using the Milestone Direct Mercury Analyzer, DMA-80 (Shelton, CT). Best-fit curves (second order polynomial) used to determine sample concentrations were based on analyses of aqueous standards (dilutions of 0.020, 0.100, 0.400, 1.000, 2.000 and 4.000 µg/ml from 100 µg/ml stock Spex Certi-Prep PLHG2-1Y) along with soil and sediment Standard Reference Materials (NIST SRM 2702, 2704, and 2709) and fish muscle tissue (NIST SRM 1946). Blanks, duplicates and SRMs were run every ten samples with reanalysis if blanks were elevated or duplicates/SRMs exceeded 10% variance. Average percent variance (standard deviation) for fish (N = 49), sediments (N = 78) and duplicates (N = 61) were 3.7 ± 3.0, 5.4 ± 4.2 and 5.2 ± 4.7, respectively. Specimens identified as outliers from linear regression were re-analyzed to confirm mercury measurement, only validated measurements were included.
Statistical analyses
The relationship between mercury in fish tissue and fish length for each species in each lake segment or combined across lake segments was analyzed using linear regression and ANCOVA on log-transformed THg data in Sigmaplot. Linear regressions identified significant trends in fish mercury concentrations and detected outliers. Only significant regressions were included in the ANCOVA analysis. Outliers were identified as exceeding the 95% prediction interval of the linear regression. When the ANCOVA indicated the factor being tested had a significant effect on fish mercury concentrations, all pairwise multiple comparisons were performed utilizing the Holm–Sidak method. Adjusted means were used to illustrate the difference in fish mercury concentrations between groups, which is the predicted mercury concentration for a fish of average length using the data from all groups being compared. The 2011 and 2016–2017 studies had adequate sample sizes to conduct linear regressions in each lake segment, if a regression was not significant for a taxa in a specific lake segment, the data was not included in subsequent analyses. Excluded indicator taxa included walleye and lake trout because the trends were not significant in 2011. Excluded lake segments include South Lake and Missisquoi Bay because either specimens were not collected, trends were not significant or bioaccumulation rates varied significantly between studies which violated a basic assumption of the analysis. The ANCOVA analysis has the same assumptions as ANOVA with three additions (1) mercury must have a linear relationship with length (data was log-transformed), (2) the bioaccumulation rates cannot vary significantly between study periods and (3) fish length was not influenced by study period.
Ancillary data
Additional data, not collected as part of this study, were obtained from a number of sources. Atmospheric deposition of mercury at Underhill, VT, ~30 km east of Lake Champlain, was obtained from the Mercury Deposition Network (http://nadp.slh.wisc.edu/data/mdn/ last accessed 10 Jan 2019). Lake temperature and chlorophyll-a data were obtained from the Vermont Department of Environmental Conservation (VT DEC) website (http://dec.vermont.gov/watershed/lakes-ponds/monitor/lake-champlain#Monitoring%20Activities last accessed 10 Jan 2019). TSS data (LChamp_2014_datarelease_daily_valuesTSS.csv) was obtained from USGS website (https://www.sciencebase.gov/catalog/item/57bf06a7e4b0f2f0ceb733ab last accessed 4 Feb 2019). Map of inundated area from spring flood of 2011 was developed by Lake Champlain Basin Program based on lake level. All available fish mercury data prior to the 2011 study were obtained from Neil Kamman (VT DEC). Sampling began in the early 1980s; some of the early studies were combined due to low sample size.
Hg Deposition at Underhill, VT
Mercury deposition is reported as average daily deposition during each season. Collections with fewer than 20 days in a month were removed. Mercury deposition was summed and then divided by the number of days collected for each month which ranged from 20 to 35 and averaged for each season: Spring (April–June), Summer (July–September), Fall (October–December) and Winter (January–March) from 2005 to 2017.
Lake Champlain Temperature and Chlorophyll-a
Monthly values were averaged for the time period 2007–2011 and 2012–2016 to represent the values that would have influenced the 2011 and 2016–2017 studies, respectively. The difference between the study periods is graphed along with standard deviation for each of the lake sections to illustrate the change between periods and not the absolute values, no error bars are included for Malletts Bay because only one sampling location was present.
Tributary Total Suspended Solids (TSS)
Eighteen tributaries to Lake Champlain which account for ~80% of the land catchment were monitored by the VT DEC (Troy et al. 2007). The average annual TSS was calculated for 1995–2014 without using the 2011 data to avoid overinflating the typical average annual load. Annual TSS loading and average annual TSS load were graphed for the 1995–2014 period along with the daily TSS loading for 2011.
Results
Lake-wide THg concentrations rose significantly in smallmouth bass and yellow perch between the 2011 and 2016–2017 studies while no change was observed in white perch (Fig. 2). Mercury concentrations in smallmouth bass varied significantly among studies (ANCOVA factor: study p < 0.001, covariate: length p < 0.001) with concentrations during the 2016–2017 study being significantly greater than 2011 (Holm–Sidak p < 0.001) while prior studies exhibited intermediate concentrations. Mercury concentrations in yellow perch were significantly different among studies (ANCOVA factor: study p < 0.001, covariate: length p < 0.001) with the highest THg concentrations measured during the initial samplings (1988–1998) which decreased in 2011 with a rebound to an intermediate level in the 2016–2017 study. All three studies were significantly different from one another (Holm–Sidak p ≤ 0.001), the 2003–2004 study was not included because the regression was not significant. Mercury concentrations in white perch did not exhibit any significant differences among studies.
Lake-wide mercury concentrations for a smallmouth bass, b yellow perch and c white perch. Significant regressions are represented by line graphs, non-significant trends are identified by asterisk in legend. The 2016–2017 is indicated by solid lines, the 2011 study by long dash, studies including 2003–2004 by short dash and studies conducted solely in 1990s by the dash-dot. Significant differences between studies are indicated by different letters in the legend
Fish sample sizes prior to the 2011 study were insufficient to compare mercury concentration among lake segments, therefore the comparison among lake segments only included the 2011 and the 2016–2017 studies. The three Main Lake segments (North Main Lake, Main Lake and South Main Lake) are grouped together because no significant differences in mercury concentrations existed among these three segments in the 2016–2017 study and the 2016–2017 mercury concentrations were consistently higher than 2011 concentrations in all three lake segments for smallmouth bass and yellow perch. The combined segments will be referred to as Main Lake Extended.
Trends in mercury concentrations between the 2011 and 2016–2017 studies in Main Lake Extended, Northeast Arm and Malletts Bay vary depending on the indicator taxa being assessed, but in general, mercury concentrations in the Main Lake Extended increased, concentrations in the Northeast Arm remained unchanged and concentrations in Malletts Bay continued to decrease. The percent change between study periods is based on adjusted mercury concentrations which are the predicted mercury concentrations for a fish of average length based on the linear regressions from both studies.
Smallmouth bass mercury concentrations in 2011 did not differ significantly among the three lake sections, but mercury concentrations in the Main Lake Extended during the 2016–2017 study were significantly greater than those from Malletts Bay (Holm–Sidak p = 0.002) and the Northeast Arm (Holm–Sidak p = 0.019; S1). The change was due to a 50% mercury increase in the Main Lake Extended (Holm–Sidak p < 0.001) while mercury concentrations in the Northeast Arm decreased by 1.6% (Holm–Sidak p = 0.830) and in Malletts Bay decreased by 11% (Holm–Sidak p = 0.558; Fig. 3). All lake segments had specimens exceeding the US EPA 300 ng/g mercury advisory limit with specimens exceeding the US FDA mercury action limit of 1000 ng/g in the Main Lake Extended beginning when specimens reached ~450 mm, Northeast Arm ~500 mm and the only specimen to exceed 1000 ng/g in Malletts Bay was 520 mm (Fig. 3). The increased mercury bioaccumulation in the Main Lake Extended is reflected in smaller size specimens reaching the US FDA action limit before other lake segments.
Mercury concentrations of smallmouth bass during the 2011 and 2016–2017 studies in the a Main Lake Extended, b Northeast Arm, and c Malletts Bay. The Main Lake extended consists of North Main Lake, Main Lake and South Main Lake. Significant differences between studies are indicated by different letters in the legend
Yellow perch mercury concentrations in 2011 were significantly elevated in Malletts Bay relative to Main Lake Extended (Holm–Sidak p < 0.001) and the Northeast Arm (Holm–Sidak p < 0.001; S2). Yellow perch collected from the Northeast Arm in the 2016–2017 study exhibited a significantly different THg bioaccumulation rate from the Main Lake Extended and Malletts Bay which limited the ANCOVA. Removing the Northeast Arm data from the analysis resulted in mercury concentrations being significantly greater in the Main Lake Extended compared to Malletts Bay (ANCOVA factor: location p = 0.047, covariate: length p < 0.001; S2).
Between the 2011 and 2016–2017 studies, mercury concentrations in yellow perch increased significantly in the Main Lake Extended (ANCOVA factor: study p < 0.001, covariate: length p < 0.001) and significantly decreased in Malletts Bay (ANCOVA factor: study p = 0.030, covariate: length p < 0.001; Fig. 4). These changes were a result of a 96% increase in adjusted mercury in the Main Lake Extended and a 20% decrease in Malletts Bay. However, caution should be taken when assessing changes in Malletts Bay. The small sample size results in the ANCOVA being heavily influenced by the single measurement above 300 ppb. When this point was removed, the analysis was no longer significant (Holm–Sidak p = 0.172). Yellow perch mercury concentrations in the Northeast Arm exhibited a 1.6% increase when an outlier was removed; the outlier limited direct comparisons between study periods because of significantly different bioaccumulation rates. Overall, specimens from all lake segments exceeded the US EPA advisory limit but no specimens exceeded the US FDA action limit (Fig. 4). Similar to smallmouth bass, yellow perch from the Main Lake Extended began exceeding the US EPA advisory limit (300 ng/g) at smaller sizes (175–200 mm) relative to the Northeast Arm and Malletts Bay which reached 300 ng Hg/g at ~275 mm.
Mercury concentrations of yellow perch during the 2011 and 2016–2017 studies in the a Main Lake Extended, b Northeast Arm, and c Malletts Bay. Main Lake Extended consists of North Main Lake, Main Lake and South Main Lake. Significant differences between studies are indicated by different letters in the legend. Outlier in 2011 from the Northeast Arm removed for statistical analyses identified by circle
In 2011, white perch mercury concentrations were close to being significantly different among lake segments (ANCOVA factor: study p = 0.051, covariate: length p < 0.001) with Malletts Bay exhibiting elevated mercury concentrations (S3). In the 2016–2017 study, mercury concentrations were significantly elevated in the Northeast Arm compared to Main Lake Extended (Holm–Sidak p = 0.012) but not Malletts Bay (Holm–Sidak p = 0.091). However, there were no significant differences between studies in any of the lake sections (Fig. 5) even though mercury concentrations increased by 13% and 55% in the Main Lake Extended and Northeast Arm, respectively while Malletts Bay concentrations decreased by 6% (Fig. 5). Additionally, specimens began exceeding the US EPA advisory limit of 300 ng/g in all lake segments when fish length was between 220 mm and 235 mm, illustrating similar bioaccumulation rates in white perch throughout the lake.
Mercury concentrations of white perch during the 2011 and 2016–2017 studies in the a Main Lake Extended, b Northeast Arm, and c Malletts Bay. Main Lake Extended consists of North Main Lake, Main Lake and South Main Lake. Significant differences between studies are indicated by different letters in the legend
During the 2011 study, mercury concentrations in all three indicator taxa studied were lower in the Main Lake Extended relative to the Northeast Arm and Malletts Bay, although only significant for yellow perch. However, in the 2016–2017 study mercury concentrations in smallmouth bass and yellow perch were significantly greater in the Main Lake Extended compared to the Northeast Arm and Malletts Bay. This was the result of large increases in fish mercury concentrations from the Main Lake Extended while THg in the Northeast Arm remained relatively similar between studies and mercury concentrations in Malletts Bay remained similar or decreased. White perch did not exhibit a large mercury increase in the Main Lake Extended but rather the increase was greater in the Northeast Arm, albeit the variation was high. The trends observed indicate that mercury bioavailability is increasing in the Main Lake Extended relative to other lake segments but the impact does not affect white perch to the same extent as smallmouth bass and yellow perch.
Discussion
Fish mercury concentrations increased significantly in the Main Lake Extended between the 2011 and the 2016–2017 studies, but the trend was not observed in the Northeast Arm or Malletts Bay. This raises two main questions: (1) what was the cause of the mercury increase and (2) why was the impact isolated to the Main Lake Extended? To identify possible causes, we examine factors known to impact mercury concentrations in fish over the time span of the two studies and assess their impacts spatially; these factors include: atmospheric deposition, lake temperature, chlorophyll-a, fishery dynamics, flooding and TSS loading. Data for DOC and pH are not examined here because the values reported do not fall within the range known to impact mercury bioaccumulation (see Introduction).
Since 2005 atmospheric deposition rates of mercury have remained low and steady during fall and winter while the higher deposition rates in spring and summer exhibited a decreasing trend which was significant in summer (linear regression p = 0.044; Fig. 6a). The elevated values during spring and summer were associated with greater precipitation rates and the decreasing trend is presumably a result of stricter emission regulations. A significant decrease in mercury deposition during summer is important because deposition directly onto a lake’s surface is quickly incorporated into the food web (Harris et al. 2007) and methylation rates are highest during warm summer months (Korthals and Winfrey 1987). This would presumably lead to a decrease in biotic mercury throughout the entire lake.
Ancillary data from Lake Champlain and surrounding area. a Mercury deposition obtained from Underhill, VT and presented as average daily deposition during each season from 2005 to 2017. The regression line indicates the trend was significant during Summer. b Lake temperature and c chlorophyll-a data represent the average monthly difference between 2007–2011 and 2012–2016, standard deviation is the variability among sampling sites within the lake segment. d Daily Total Suspended Solids (TSS) loaded to Lake Champlain during 2011 with the 67-day spring flood identified by the box and Tropical Storm Irene in late August. Insert shows the average annual TSS load calculated from daily fluxes of 18 tributaries monitored between 1995 and 2014, average annual loading without 2011 indicated by dashed line
Lake temperature has increased slightly between study periods with the greatest increase observed at the surface in Spring (Fig. 6b). Surface temperatures (0–2 m), in all three lake sections, have increased on average by >1 °C during May with the increase diminishing until mid-summer when a rebound is experienced; the Main Lake Extended experienced the greatest fluctuation. The temperature increase is positive in most locations, only a few instances in the Main Lake exhibit negative trends (Table 1). Water temperatures directly above the sediment demonstrate more varied trends between the lake segments (Fig. 6b). The Main Lake extended, on average, experienced only moderate temperature increases with the individual sites exhibiting average increases ranging from 0 °C to 1.0 °C (Table 1). The Northeast Arm experienced similar trends at the surface and directly above sediments with the spring peak remaining until summer while the initial peak in Malletts Bay quickly disappeared and remained slightly cooler throughout the remainder of the sampling season. Increases in water temperatures can enhanced bioaccumulation through increased metabolic rates (Dijkstra et al. 2013) and methylation rates (Fagerstrӧm and Jernelӧv 1972). The majority of methylation will occur at the sediment-water interface (Ramal et al. 1993) and an increase of 1 °C is likely to only enhance rates minimally because methylation rates generally follow the Q10 rule which states rates will double with an increase of 10 °C (Fagerstrӧm and Jernelӧv 1972). The change in metabolic rates will depend on the organism in question and the temperature range. Dijkstra et al. (2013) found killifish MeHg increased when temperatures increased from 21 °C to 27 °C but not from 15 °C to 21 °C. However, if temperature was a major driver of the mercury increase in fish, we would expect the effect to be prevalent throughout other lakes in the region experiencing increases in water temperature.
The greatest increase in lake productivity, based on chlorophyll-a measurements, occurred in the Northeast Arm during late summer/early fall (Fig. 6c). The increase in chlorophyll-a over the study periods will likely have a negligible impact on fish mercury. Chen et al. (2012) determined that zooplankton mercury concentrations only varied significantly between the most productive (Missisquoi Bay) and least productive lake segments (Malletts Bay) only in years when Missisquoi Bay experienced algal blooms. But if the slight increase in chlorophyll-a had a detectable impact on biotic mercury, it would likely result in a decrease due to biodilution. Large variation in biomass at any trophic level can impact mercury bioaccumulation.
The Lake Champlain fishery has experienced a multitude of dramatic shifts in recent years with many being associated with the invasion of the non-native alewife. These changes have the potential to both increase and decrease fish mercury concentrations and can be categorized into three main areas, (1) prey replacement, (2) growth dilution and (3) biodilution. The alewife invasion has been associated with a >95% reduction of smelt in the Northeast Arm and Malletts Bay, disappearance of cisco (lake herring), replacement of smelt as the primary food source of walleye and salmon and an expanded depth range of alewife (Pientka and Staats 2018, Simonin et al. 2012). As the alewife encroach on and displace smelt and other prey fish to become the primary food source for piscivorous fish, mercury concentrations have the potential to decrease because alewife are an energy dense food source, exceeding that of Rainbow Smelt (Rand and Stewart 1998) and feed at a lower trophic level (Simonin et al. 2018). Alewife reside in the epilimnion and metalimnion of Lake Champlain (Simonin et al. 2012, Pientka and Staats 2018) and thus the shift in walleye and salmon prey coincides with habitat overlap. Because smallmouth bass and yellow perch habitat overlap with alewife, adults could also shift to alewife as a primary prey item. Additionally, it appears alewife have seriously impacted the cisco population because they have not been observed in monitoring efforts since 2014. Alewife are also expanding their range, residing in waters deeper than 10 m since 2009 (Pientka and Staats 2018) which could allow for further changes in the fishery and biotic mercury concentrations. However, alewife have the potential to increase biotic mercury through competition that results in less efficient growth (Ward et al. 2010a). Pientka and Staats (2018) documented the growth (length-at-age) of smelt declined in the Main Lake Extended since the mid-2000s with alewife growth declining since about 2010, in contrast to the slightly enhanced growth of the smelt remaining in the Northeast Arm and Malletts Bay. Additionally, the Main Lake experienced an abrupt decrease in growth of smelt and alewife in 2012 which persisted in that class during subsequent years. This abrupt decline in growth may be a secondary consequence of the extreme climatic events in 2011 creating a more disturbed environment, illustrating how the effects of extreme events cascade through the lake and food web. And finally, multiple spikes in fish biomass around the lake can reduce mercury bioaccumulation through biodilution (Ward et al. 2010b, Trudel and Rasmussen 2006). The Main Lake Extended experienced peak biomass before the 2011 study with a sharp decline in 2012 which could have decreased fish mercury concentrations for the 2011 study and increased concentrations for the 2016–2017 study. The greatest fish biomass in the Northeast Arm occurred in 2014 and 2015 while Malletts Bay fish biomass increased by roughly an order of magnitude in 2012, both peaks have the potential to reduce fish mercury concentrations in the 2016–2017 study.
Lake Champlain was impacted by two extreme climatic events in 2011 that resulted in extensive flooding and a four-fold increase in TSS loading. Heavy spring rain and a substantial snowpack resulted in a record high lake level (USGS 04294500) that remained above flood stage for 67 days inundating an estimated 171 km2 (Fig. 1) and delivered 525,000 tonnes of suspended solids to the lake (Fig. 6d). Four months later, Tropical Storm Irene delivered 260,000 tonnes of sediment in a single day and >400,000 tonnes in total (Fig. 6d). These events have the potential to increase biotic mercury because flooding creates an ideal environment for methylation to occur and the strong association between mercury and organic matter (Yu et al. 2011, Marvin-DiPasquale et al. 2009, Gabriel and Williamson 2004) suggests mercury loading will increase with TSS loading. A newly flooded reservoir increased methylmercury concentrations by 3.4-fold after one week with concentrations rising more than a 10-fold after 10 weeks (Hall et al. 2005), roughly the duration of flood stage in Lake Champlain. However, the majority of flooding in Lake Champlain occurred near the Northeast Arm where fish mercury levels were similar between studies; this may be explained by biodilution. Pientka and Staats (2018) documented fish biomass in the Northeast Arm increased dramatically in 2014 and 2015, roughly 3-fold greater than an average year. The increase in fish biomass was not observed in any other sites monitored and may offset the presumed increase in mercury methylation from the flooding. In addition to the flooding, each of these extreme climatic events delivered more than an entire year worth of sediment to the lake. Because mercury preferentially binds to organic matter, mercury loading coincides with TSS loading (Shanley et al. 1999). Dissolved MeHg would likely be incorporated into the food web through facilitated transport and passive diffusion by phytoplankton (Le Faucheur et al. 2014) while particulate mercury would settle to the lake bottom. Surficial sediments provide an ideal environment for bacteria to methylate mercury (Ramlal et al. 1993) which can then be incorporated into the food web. Dissolved MeHg can diffuse into the water column and be incorporated by algal species while particulate MeHg or the bacteria themselves can be ingested by benthic organisms. Mysis relicta, which reside in both the benthic and pelagic environment, have been documented as a link between sediment contaminants (PCBs) and the pelagic food web in Lake Champlain (Lester and McIntosh 1994) and could transport mercury in a similar manner.
Examining the change in each of the factors between the 2011 and 2016–2017 studies and the presumed impact on biotic mercury in each of the three lake areas suggest the increase in fish mercury concentrations are, at least in part, due to changes in the fishery and possibly the extreme climatic events of 2011 (Table 2). Mercury deposition is clearly decreasing and should result in a decrease of biotic mercury, especially because the significant decrease occurs in summer when methylation rates are highest. While temperature has increased slightly, it is presumed the effect would be seen throughout the lake and other lakes in the region if temperature was a strong driver. The invasion of the alewife has potential to decrease fish mercury because it is an energy dense food source and feeds at a lower trophic level but it is unclear to what extent, and if species other than walleye and salmon have shifted prey preference. The growth efficiency and biomass of the fishery has changed substantially over the last decade with different patterns emerging among the three lake areas. The Main Lake Extended has exhibited diminished growth of prey fish and shifts in biomass that would result in increased fish mercury while growth and biomass in the Northeast Arm and Malletts Bay would result in a decrease. The extreme climatic events of 2011 were responsible for extensive flooding and excessive TSS loading. The majority of the flooding was isolated to the Northeast Arm with the majority of TSS loading impacting the Main Lake Extended. The spatial variability of TSS loading can help explain the spatial variability in fish mercury concentrations. The Main Lake Extended drains more than half of the watershed and receives roughly an equivalent TSS load on an annual basis, between 51% and 65% from 1993 to 2017. The excessive TSS loading in 2011would provide ample mercury for methylation and subsequent incorporation into the food web, as long as, mercury availability was a limiting factor. The Northeast Arm does not have any major tributaries draining into it and therefore the loading of TSS would not likely have had a substantial impact on fish mercury concentrations. While Malletts Bay received excessive TSS loading from the Lamoille tributary in 2011, fish mercury concentrations continued to decrease or remain unchanged. Mercury loading from this tributary has been found to be low relative to other tributaries with the assumption being upstream impoundments have helped attenuate loading to the lake (Shanley and Chalmers 2012). Additionally, the roughly 10-fold increase in fish biomass in 2012 may overshadow the increase due to mercury loading.
Lake Champlain is not the first lake to experience an increase in fish mercury in recent decades. Top predatory fish, including walleye, northern pike and largemouth bass, have experienced increases in mercury throughout the Great Lakes Region and Ontario with potential causes including climate change, invasive species, global emissions and local geochemistry (Gandhi et al. 2014, Monson et al. 2011, Bhavsar et al. 2010, Monson 2009). These increases are all documented to begin in the 1990s with the concentrations generally increasing a few percent a year at most. In contrast to the 50% and 96% mercury increase observed in smallmouth bass and yellow perch, respectively, in a 5-year period isolated to the Main Lake Extended.
Conclusions
The dramatic and isolated mercury increase experienced in Lake Champlain is likely due to changes to the fishery and possibly the extreme climatic events of 2011. It is currently unclear to what extent each factor contributed to the increase in fish mercury concentrations and demonstrates the need to better understand how these changes will impact mercury methylation and bioaccumulation. Accomplishing this goal would ideally utilize a standardized protocol after extreme climatic events to sample multiple trophic levels within the pelagic and benthic food webs. Likewise, sampling efforts should be increased and expanded to multiple trophic levels upon the arrival of invasive species, isolating sampling efforts to fish provides little information on the factors responsible for the increase in fish mercury.
References
Bhavsar SP, Gewurtz SB, McGoldrick, Keir MJ, Backus SM (2010) Changes in mercury levels in Great Lakes fish between 1970s and 2007. Environ Sci Technol 44:3273–3279
Chen CY, Folt CL (2005) High plankton densities reduce mercury biomagnification. Environ Sci Technol 39:115–121
Chen C, Kamman N, Williams J, Bugge D, Taylor V, Jackson B, Miller E (2012) Spatial and temporal variation in mercury bioaccumulation by zooplankton in Lake Champlain (North America). Environ Pollut 161:343–349
Dijkstra JA, Buckman KL, Ward D, Evans DW, Dionne M, Chen CY (2013) Experimental and natural warming elevates mercury concentrations in estuarine fish. PLoS ONE 8(3):e58401
Driscoll CT, Han YJ, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK (2007) Mercury contamination in forest and freshwater ecosystems in the northeastern United States. BioScience 57:17–28
Driscoll CT, Mason RP, Chan HM, Jacob DJ, Pirrone N (2013) Mercury as a global pollutant: Sources, pathways, and effects. Environ Sci Technol 47:4967–4983
Evers DC, Han Y, Driscoll CT, Kamman NC, Goodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T (2007) Biological mercury hotspots in the Northeastern United States and Southeastern Canada. Bioscience 57:29–43
Fagerstrӧm T, Jernelӧv A (1972) Some aspects of the quantitative ecology of mercury. Water Res 6:1193–1202
Gabriel MC, Williamson DG (2004) Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environ Geochem Hlth 26:421–434
Gandhi N, Tang RWK, Bhavsar SP, Arhonditsis (2014) Fish mercury levels appear to be increasing lately: A report from 40 years of monitoring in the Province of Ontario, Canada. Environ Sci Technol 48:5404–5414
Grigal DF (2002) Inputs and outputs of mercury from terrestrial watesheds: a review. Environ Rev 10:1–39
Hall BD, St. Louis VL, Rolfhus KR, Bodaly RA, Beaty KG, Paterson MJ, Peech Cherewyk KA (2005) Impacts of reservoir creation on the biogeochemical cycling of methyl mercury and total mercury in boreal upland forests. Ecosystems 8:248–266
Harris RC, Rudd JWM, Amyot M, Babiarz CL, Beaty KG, Blanchfield PJ, Bodaly RA, Branfireun BA, Gilmour CC, Graydon JA, Heyes A, Hintelmann H, Hurley JP, Kelly CA, Krabbenhoft DP, Lindberg SE, Mason RP, Paterson MJ, Podemski CL, Robinson A, Sandilands KA, Southworth GR, St. Louis VL, Tate MT (2007) Whole-ecosystem study shows rapid fish-mercury response to changes in mercury deposition. PNAS 104:16586–16591
Johnson I (2012) A synoptic assessment of mercury and re-evaluation of PCB’s in Lake Champlain Fishes. Lake Champlain Basin Program Technical Report No. 66 p. 63
Kamman NC, Burgess NM, Driscoll CT, Simonin HA, Goodlae W, Linehan J, Estabrook R, Hutcheson, Major A, Scheuhammer AM, Scruton D (2005) Mercury in freshwater fish of Northeast North America – A geographic perspective based on fish tissue monitoring databases. Ecotoxicology 14:163–180
Kamman N, Driscoll CT, Estabrook B, Evers DC, Miller EK (2004). Biogeochemistry of mercury in Vermont and New Hampshire lakes: An assessment of mercury in water, sediment and biota of Vermont and New Hampshire lakes. Prepared for USEPA p89
Korthals ET, Winfrey MR (1987) Seasonal and spatial variation in mercury methylation and demethylation in an oligotrophic lake. Appl Environ Micro 53:2397–2404
Le Faucheur S, Campbell PGC, Fortin C, Slaveykova VI (2014) Interactions between mercury and phytoplankton: speciation, bioavailability, and internal handling. Environ Toxicol Chem 33:1211–1224
Lester DC, McIntosh A (1994) Accumulation of polychlorinated biphenyl congeners from Lake Champlain sediments by Mysis relicta. Environ Toxicol Chem 13:1825–1841
Lin CJ, Shetty SK, Pan L, Pongprueska P, Jang C, Chu H (2012) Source attribution for mercury deposition in the contiguous United States: Regional difference and seasonal variation. J Air Waste Manag 62:52–63
Marvin-DiPasquale M, Lutz MA, Brigham ME, Krabbenhoft DP, Aiken GR, Orem WH, Hall BD (2009) Mercury cycling in stream ecosystems. 2.Benthic methylmercury production and bed sediment-pore water partitioning. Environ Sci Technol 43:2726–2732
Miller EK, Chen C, Kamman N, Shanley J, Chalmers A, Jackson B, Taylor V, Smeltzer E, Stangel P, Shambaugh A (2012) Mercury in the pelagic food web of Lake Champlain. Ecotoxicology 21:705–708
Monson BA, Staples DF, Bhavsar SP, Holsen TM, Schrank CS, Moses SK, McGoldrick DJ, Backus SM, Williams KA (2011) Spatiotemporal trends in mercury in walleye and largemouth bass from the Laurentian Great Lakes Region. Ecotoxicology 20:1555–1567
Monson BA (2009) Trend reversal of mercury concetrations in piscivorous fish from Minnesota lakes: 1982-2006. Environ Sci Technol 43:1750–1755
Paranjape AR, Hall BD (2017) Recent advances in the study of mercury methylation in aquatic systems. FACETS 2:85–119
Pientka B, Staats NR (2018) Lake Champlain forage fish assessment. Vt Fish Wildl Dep Annu Rep. 2018:p133
Ramlal PS, Kelly CA, Rudd JWM, Furutani A (1993) Sites of methyl mercury production in remote Canadian Shield lakes. Can J Fish Aquat Sci 50:972–979
Rand PS, Stewart DJ (1998) Dynamics of salmonine diets and foraging in Lake Ontario, 1983-1993: a test of a bioenergetics model prediction. Can J Fish Aquat Sci 55:307–317
Shanley JB, Chalmers AT (2012) Streamwater fluxes of total mercury and methylmercury into and out of Lake Champlain. Environ Pollut 161:311–320
Shanley JB, Donlon AF, Scherbatskoy T, Keeler GJ (1999) Mercury cycling and transport in the Lake Champlain Basin. In: Manley TO, Manley PL (eds) Lake Champlain in Transition: From Research Toward Restoration. American Geophysical Union, Washington, DC, p 277–299
Simonin PW, Rudstam LG, Parrish DL, Pientka B, Sullivan PJ (2018) Piscivore diet shifts and trophic level change after Alewife establishment in Lake Champlain. T Am Fish Soc 147:939–947
Simonin PW, Parrish DL, Rudstam LG, Sullivan PJ, Pientka B (2012) Native rainbow smelt and nonnative alewife distribution related to temperature and light gradients in Lake Champlain. J Gt Lakes Res 38:115–122
Smeltzer E, Shambaugh AD, Stangel P (2012) Environmental change in Lake Champlain revealed by long-term monitoring. J Gt Lakes Res 38:6–18
Troy A, Wang D, Capen D, O’Niel-Dunne J, MacFaden S (2007) Updating the Lake Champlain Basin land use data to improve prediction of phosphorus loading. Lake Champlain Basin Program Tech Rep. No. 54:121
Trudel M, Rasmussen JB (2006) Bioenergetics and mercury dynamics in fish: A modelling perspective. Can J Fish Aquat Sci 63:1890–1902
U.S. Census Bureau (2011) Statistical Abstract of the United States: Largest Lakes in the United States
US EPA (2013) 2011 national listing of fish advisories technical report. EPA-820-F-13-058, US EPA, Washington, DC p8
UNEP (2013) Global mercury assessment: Sources, emissions, release and environmental transport. United Nations Environment Programme Chemicals Branch, Geneva, Switzerland p.44
Ward DM, Nislow KH, Flot CL (2010a) Bioaccumulation syndrome: identifying factors that make some stream food webs prone to elevated mercury bioaccumulation. Ann NY Acad Sci 1195:62–83
Ward DM, Nislow KH, Chen CY, Folt CL (2010b) Rapid, efficient growth reduces mercury concentrations in stream-dwelling Atlantic salmon. Trans Am Fish Soc 139:1–10
Yu X, Driscoll CT, Montesdeoca M, Evers D, Duron M, Williams K, Schoch N, Kamman NC (2011) Spatial patterns of mercury in biota of Adirondack, NY lakes. Ecotoxicology 20:1543–1554
Acknowledgements
We would like to thank Dr. Richard Bopp for his guidance and assistance analyzing fish samples. Neil Kamman, Bernie Pientka, James Shanley, Angela Shambaugh and Fred Dunlap for comments and advice on the project. And anonymous reviewers for comments and suggestions on the manuscript.
Funding
This project was selected for funding by the Lake Champlain Basin Program (LCBP) Steering Committee and it has been supported directly by an agreement or sub-award issued by the New England Interstate Water Pollution Control Commission (NEIWPCC). NEIWPCC manages LCBP’s personnel, contracts, grants, and budget tasks through a partnership with the LCBP Steering Committee.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of Rensselaer Polytechnic Institute.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Swinton, M.W., Nierzwicki-Bauer, S.A. Mercury increase in Lake Champlain fish: links to fishery dynamics and extreme climatic events. Ecotoxicology 29, 1750–1761 (2020). https://doi.org/10.1007/s10646-019-02148-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-019-02148-5