Abstract
A whole-sediment test with the infaunal amphipod Monocorophium insidiosum has been developed to assess the long-term effects exerted by sediment contamination on survival, growth rates and attainment of sexual maturity. Juvenile amphipods were exposed for 28 days to a control sediment (native sediment) and three sediment samples collected in sites of the Venice Lagoon, characterized by contamination levels ranging from low to moderate, and absence of acute toxicity toward amphipods. Growth rate was estimated as daily length (μm d−1) and weight increments (μg d−1). The long-term exposure to the test sediments affected significantly both growth rate and attainment of sexual maturity of the females of M. insidiosum. In contrast, survival was high and uniform among all the samples, despite the contamination gradient. The results suggest growth to be the more reliable and statistically relevant endpoint. Attainment of sexual maturity, although allowed the identification of detrimental effects, was affected by a higher among-replicates variance as compared with growth rates, and thus less reliable than growth for the identification of impairments. The significant impairments observed both on growth and attainment of maturity evidenced the need to address the monitoring, also in the Lagoon of Venice, towards the assessment of the long-term effects on benthic species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the framework of the sediment quality assessment, the whole-sediment laboratory tests are demanded to provide information concerning the possible toxic effects exerted by sediment bound and dissolved contaminants on biological communities; as stated by Grapentine et al. (2002), whole-sediment test should support “evidence that responses observed in the resident community are associated with sediment rather than other potential stressors”. Therefore, abnormal mortality, impairments in the growth rate and in the attainment of sexual maturity, anomalous behaviours, and effects on the reproduction, are all possible outcomes of sediment contamination that should be investigated using suitable laboratory tools to assess the consistency of the stressor-effect correlation, in conjunction with benthic community analysis (Chapman 2007). Nevertheless, in most monitoring studies on marine and estuarine environments the whole-sediment tests are used rarely for evaluation of the survival of amphipods and polychaetes and seldom for the identification of chronic, sub-lethal effects (Kennedy et al. 2009). Short-term acute test are usually preferred, although 1) chronic exposure to contaminants is a more frequent condition in natural environments than acute exposure, 2) moderately contaminated sediments are more common than highly contaminated ones, and 3) sub-lethal effects may have greater ecological relevance than lethality to identify possible impairments due to the exposure to the contaminants (Costa et al. 2005).
Even if a standard method is available for polychaetes (ASTM 2013), most of the experiments concerning chronic sediment testing have been performed with amphipods, since these organisms are widely distributed, easy to collect and maintain in the laboratory, ecologically relevant and tolerant to wide ranges of sediment grain-size (Hyne et al. 2005; Peters and Ahlf 2005; Picone et al. 2008). The suitability of the amphipods as indicators led in the past decades to the development of a number of testing protocol with Ampelisca abdita and Leptocheirus plumulosus in North America (Redmond et al. 1994; U.S. EPA 2001; Ward et al. 2015; Lotufo et al. 2016), Gammarus locusta, Corophium multisetosum and C. volutator in Europe (Costa et al. 2005; Castro et al. 2006; Scarlett et al. 2007a, 2007b; van den Heuvel-Greve et al. 2007; Fox et al. 2014) and Melita plumulosa in Australia (Hyne et al. 2005). In most cases, the exploited endpoints were reproduction and growth rate.
In Italy, studies aimed to evaluate sub-lethal and chronic endpoints are scarce and have been performed only with adults specimens of the tube builder Monocorophium insidiosum Crawford, using survival and reproduction as endpoints (Bigongiari et al. 2001). Efforts have been focused on M. insidiosum as test species due to: (a) the availability of literature data concerning its biology, ecology and life-history (Casabianca 1967; Sheader 1978; Procaccini and Scipione 1992; Kevrekidis 2004; Prato and Biandolino 2006); (b) wide distribution along the coastal environments of the Ionian and Adriatic Sea (Brian 1935; Ceccherelli et al. 1994; Tagliapietra et al. 1998; Mancinelli et al. 2005); (c) well documented ability to reach sexual maturity and even reproduce within few weeks from the release from brood pouch, under proper temperature conditions (Casabianca 1975; Birklund 1977). These information were missing, or only partially available, for other eligible species occurring in Italian coastal environments such as Ampelisca diadema, Corophium orientale, Metaphoxus simplex and Melita palmata.
In the present work, we explored the suitability of sub-lethal endpoints measured on juvenile M. insidiosum for assessing chronic toxicity in sediments of the Venice Lagoon. In particular, the study focused on the evaluation of the possible occurrence of long term effects in some sites where acute toxicity toward amphipods (i.e. lethality after 10 days of exposure) was not observed during previous surveys (Picone et al. 2008, 2016). With this purpose, juvenile M. insidiosum were exposed for 28-days (as in the standard method available for L. plumulosus; U.S. EPA 2001) to test chronic sediment toxicity. The endpoints used for assessing the possible long-term impairments caused by sediment contamination were mortality, growth rate and attainment of sexual maturity of the females. Reproduction, even though it was recorded at the end of the test, was not considered as a candidate endpoint at this stage, because the early work of Bigongiari et al. (2001) evidenced a low reproducibility and a high among-replicates variation of the offspring production for M. insidiosum in the control (native sediment), suggesting that exposures significantly longer than 28-d could be needed to M. insidiosum population to reach maturity and release the first brood. The aims of this study were as follows: (1) to assess the discriminating ability and sensitivity of the long-term, sub-lethal endpoints, as compared with mortality, in moderately contaminated sediments, and (2) to evaluate possible correlation of the measured impairments with sediment chemistry.
Materials and methods
Sediment sampling and handling
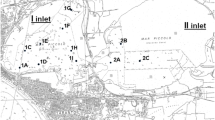
Sediments were collected in April 2008 in three previously investigated sites of the Venice Lagoon (Fig. 1), affected by low to moderate contamination, and characterized by absent to negligible acute effects towards amphipods (Picone et al. 2008, 2016).
Site San Francesco del Deserto (SFR) is located in a sandy-silt mudflat far from point sources of contamination and not affected by fishing or boating activities. Also site Valle Millecampi (VMI) is located in a sandy-silt mudflat and far from industrial plants and urban centres, but in this case the area is characterized by patchy occurrence of peaty substrate and is potentially affected by contamination from agricultural runoff. In contrast, site Canale delle Tresse (TRC) is a canal in nearness of the industrial area of Porto Marghera, characterized by silty-sand sediments and moderate contamination, due both to industrial activities and intense boat traffic.
Surface sediments (0–15 cm) were sampled using a 10 cm diameter Plexiglas® corer following the integrated design and QA/QC procedures reported in detail by Volpi Ghirardini et al. (2005). In the field, collected samples were immediately stored in a 2 L glass jar filled without leaving headspace, to minimize oxidation. In laboratory, the samples were thoroughly homogenized and press-sieved through a 250 µm mesh-size stainless steel screen to remove native amphipods or other organisms whose occurrence could interfere with amphipod survival (i.e. polychaetes and isopods).
Sediment chemistry
Sediment grain-size was determined following a gravimetric procedure (ICRAM 2001) and subsequently classified according to Shepard (1954). Total organic carbon (TOC) analyses were performed using a CHNS-O analyzer (mod. EA1110, CE Instruments, ThermoElectron, Milan, Italy), on aliquots of 10–20 mg of dry sediment acidified with 20-µL of 1 N HCl solution and dried at 105 °C for 15 min.
Dry-weight total-metal concentrations were measured using inductively coupled plasma—atomic emission spectrometry (ICP-AES) for Cu, Cr, Ni, Pb, V and Zn (EPA method 6010B), atomic absorption—furnace technique for As and Cd, (EPA methods 7060 and 7131 respectively) and atomic absorption spectrophotometry for Hg (EPA method 7473). Prior to analyses, samples were digested through microwave assisted acid digestion with aqua regia (EPA method 3051 A mod.). Simultaneously extracted metals (SEM) and acid-volatile sulphides (AVS) were determined following the procedure by Allen et al. (1993). Polynuclear aromatic hydrocarbons (PAHs) were analyzed using a reverse phase HPLC (EPA method 8310 mod.) after extraction through sonication (EPA method 3550 mod.). Polychlorobiphenyls (PCBs) and organochlorine pesticides were measured by gas chromatography (EPA method 8082 and 8081, respectively), after pressurized fluid extraction (PFE, EPA method 3545), cleanup for sulphur removal (EPA method 3660) and sulphuric acid/potassium permanganate cleanup (EPA method 3665).
Amphipods sampling and holding
Specimens of M. insidiosum were sampled in May 2008 in a brackish pond located inside a sandy-silt salt marsh of the northern Lagoon (N 45°28’436.1, E 12°25’315.7). The top 5 cm of sediment were hand-collected using a shovel and in situ wet-sieved through a stack of sieves with mesh-size of 1000, 500 and 250 µm, respectively. Only juvenile amphipods (<2.3 mm length from telson to tip of the rostrum) passing through the 500 μm mesh sieve and kept by the 250 μm sieve were selected for the 28-d chronic test. Young-adults (2.3–5.0 mm length) passed through 1000 μm mesh sieve and retained by 500 μm screen, were separately collected and brought to laboratory for performing the 96-h reference toxicant test (Cd), according with standard procedures (ISO 2005). Aliquots of native sediment were also collected, to be used both as substrate for acclimation/holding and as negative control in sediment test. Control sediment was handled and stored as test sediments.
Juvenile and young adults amphipods were held separately in 10 litres glass aquaria, filled with about 5 cm of press-sieved native sediment and 8 litres of natural seawater collected in nearness of the sampling site, to avoid sudden changes in environmental conditions (salinity and ionic strength) at the beginning of holding period. Holding aquaria were placed in a climatic room under a 16:8 light:dark cycle and continuous aeration, where amphipods were acclimated at testing conditions (T = 15 °C, S = 35 ppt) for 8 days, by changing salinity and temperature at a rate not exceeding 1 ppt and 1 °C per day. Acclimatization to testing conditions was accomplished by renewing at 48-h intervals about 2/3 of overlying water in the holding aquaria using artificial, filtered seawater at salinity of 35 ppt prepared by dissolving an artificial sea salt mixture (Ocean Fish®, PRODAC International, Cittadella, Italy) in Milli-Q® purified water.
Testing conditions were selected on the basis of previous works concerning sensitivity of M. insidiosum to non-contaminant factors (Prato and Biandolino 2006); moreover, these conditions resembled environmental temperature and salinity at time of sampling (T = 14 °C, S = 32 ppt). Amphipods were not fed during holding.
Toxicity testing
The 96-h reference toxicant tests (Cd) were performed for QA/QC purposes, to evaluate sensitivity of the autochthonous population of M. insidiosum. These tests were carried out according to ISO (2005), using young adult amphipods.
The 28-d whole-sediment test was performed in triplicate, using 1000 ml glass beaker as experimental unit, each with 30 juvenile amphipods. In each of the experimental units juvenile amphipods were exposed to 200 ml of sediment and 600 ml of artificial seawater prepared as described above. One litre glass beakers were used as test chambers. The exposure was performed under 16:8 light:dark cycle, continuous aeration and semi-static conditions, with water renewal on days 7, 14 and 21. Overlying water temperature, salinity and pH were measured at the beginning of test (day-0), prior to each water renewal and at the end of exposure. Amphipods were fed ad libitum with the cryptophycean Rhodomonas salina (Wislouch), Hill & Wetherbee, on day-0 and after each water renewal, in order to maintain a thin layer of algae on sediment surface. A mono-diet with R. salina was chosen since it has a very high nutritional quality, due to elevated content of polyunsaturated fatty acids (PUFAs) and highly unsaturated fatty acids (HUFAs) that have been shown to be essential for crustacean survival and growth (Gonzalez-Félix et al. 2003; Dahl et al. 2009).
On day-0, 50 randomly selected juvenile amphipods were individually measured under a dissecting microscope at ×10 magnification and weighed (in groupings of 10 specimens) to establish starting conditions (t = 0) for length and dry weight.
On day-28, the content of each beaker was sieved through a 250 μm mesh sieve and then poured into a sorting tray. Surviving amphipods isolated from each replicate were then counted, individually measured at the nearest 0.1 mm (from tip of the rostrum to end of the telson) and sexed. All the amphipods from each experimental unit were then pooled, blotted on adsorbent paper to remove excess of water, divided into groupings of 10 randomly selected specimens, weighted at the nearest 0.01 mg, dried overnight in oven at 80 °C and then weighed again for evaluation of dry weight (Scarlett et al. 2007a, 2007b). Females with fully developed oostegites, ovigerous females and females carrying embryos in brood pouch were all regarded as mature (Kevrekidis 2004).
Length measurements were performed on living amphipods anesthetized with a MgCl2 solution, to avoid possible impairments caused by dehydration or hydration due to preservation in alcohol, formaldehyde or other fixing agents.
Newborn amphipods eventually produced in each replicate were separately collected (clearly discernible from adults by their smaller size).
Statistical analysis
Survival of juvenile amphipods after 28-d was reported as mean of 3 replicates per sample. Growth rate of juvenile amphipods was expressed both as daily length increment (µm individual−1 d−1) and daily weight increment (µg individual−1 d−1).
Daily length increment within each experimental unit was calculated for the pooled population (males and females together) as well as separately for males and females. Daily weight increment was estimated only for the pooled population (males plus females), on dry weight (dw) basis. Daily increments were expressed as difference between length/weight at t = 28 and length/weight at t = 0, divided by the total exposure time (28 days).
Attainment of female maturity after 28-d of exposure to test sediments was calculated as percentage of mature females on surviving females in each replicate.
Analysis of the variance (ANOVA) and Fisher post-hoc test were used to check for differences among treatments; ANOVA was performed on arcsine square root transformed data for survival and log-transformed data for all the other parameters. Normality and homogeneity of variance were verified using Kolmogorov-Smirnov’s and Levene’s test respectively (α = 0.05). When normality and homogeneity of variance conditions were not met, Kruskal–Wallis test was performed to check for significant differences. Median lethal Concentration (LC50) with 95% confidence limits for the reference toxicant were calculated using the Trimmed Spearman–Karber method v1.5. All statistical analyses were performed using StatSoft® Statistica v7.0.
Results
Sediment chemistry
Results of sediment chemistry are summarized in Table 1. In general, sample SFR exhibited the lowest concentrations of chemicals, with only As (12.9 mg kg−1 dw) and Hg (0.75 mg kg−1 dw) concentrations exceeding effect range low (ERL) and effect range median (ERM), respectively (Long et al. 1995). With exception of Hg, sample VMI was characterized by metal and organic concentrations slightly higher than SFR; nevertheless in sample VMI only As (19.7 mg kg−1 dw), Hg (0.31 mg kg−1 dw), and Ni (31.1 mg kg−1 dw) concentrations exceeded ERL. As expected, site TRC showed high contamination regarding both metals and organics; concentrations above ERL were found for As (22.1 mg kg−1 dw), Cd (2.55 mg kg−1 dw), Cu (47.4 mg kg−1 dw), Ni (22.6 mg kg−1 dw), Pb (46.9 mg kg−1 dw), Zn (328 mg kg−1 dw), PAHs (6622 µg kg−1 dw) and DDTs (2110 ng kg−1 dw), while Hg (1.51 mg kg−1 dw) exceeded ERM too. The chemical analyses for the control sediment are reported in Table 1, all the metals and organics were below the ERL, except for mercury (0.37 mg kg−1 dw).
All samples were characterized by a mean ERM quotient (mERMq), a normalized chemical summary calculated by normalizing each chemical concentration to its respective effect range median (Long et al. 2006), less than 0.5, confirming that contamination levels are low to moderate (Long and Macdonald 1997). The index ΣSEM-AVS fOC−1 was negative in all samples, highlighting a surplus of AVS and hence a limited bioavailability of metals for uptake from water phase.
Amphipod sensitivity
Two distinct 96-h reference toxicant (Cd) tests were performed during the experimental period. The LC50 values were very similar, 2.0 and 2.1 mg l−1 respectively. Survival in water-only control was high in all the replicates (>90%).
Long term survival
Survival was high and homogeneous in all the tested samples (F3,8 = 0.6, p = 0.632, Fig. 2), with values ranging from 83% (SFR) and 91% (VMI).
Survival (%) of juveniles of M. insidiosum after 28 days of exposure to the test sediments. Whiskers indicated standard error. Uppercase letters indicate homogeneous groups according to one-way ANOVA and Fisher post-hoc test; samples marked with the same letter are not statistically different at α = 0.05
Growth rate
Daily length increment for pooled population was significantly higher in native sediment (62 µm individual−1 d−1) than in test sediments (31–46 µm individual−1 d−1) (F3,8 = 101.5, p < 0.001, Fig. 3); the trend highlighted by ANOVA (α = 0.05) was the following: Control > SFR > VMI > TRC (Table 2).
Daily length increments (μm d−1) measured for the pooled population (males plus female), males and females of M. insidiosum. Whiskers indicated standard error. Uppercase letters indicate homogeneous groups according to one-way ANOVA and Fisher post-hoc test; samples marked with the same letter are not statistically different at α = 0.05. Legend: A > B > C > D
A slightly different trend was highlighted by daily length increment of males (F3,8 = 45.6, p < 0.001, Fig. 3): control sediment (70 µm individual−1 d−1) was characterized by a significantly higher growth rate than the other samples (p < 0.001), but minor differences among samples were also evidenced (Table 2): sample TRC (34 µm individual−1 d−1) showed a weaker growth as compared with sample SFR (p < 0.001), but no significant difference with VMI was evidenced (p = 0.502). The trend of length increment in females was similar to that found for the pooled population (F3,8 = 65.1, p < 0.001, Fig. 3). In all samples there were more females than males (Table 2).
In all samples the individual weight increment (µg individual−1 d−1) was significantly lower than in control sediment (F3,8 = 43.44, p < 0.001, Fig. 4).
Daily weight increments (μg d−1) measured for the pooled population of M. insidiosum. Whiskers indicated standard error. Uppercase letters indicate homogeneous groups according to one-way ANOVA and Fisher post-hoc test; samples marked with the same letter are not statistically different at α = 0.05. Legend: A > B > C > D
Attainment of sexual maturity in the females
The higher percentage of mature females was observed in sample SFR (60%), followed by control sediment (Table 2). Significant reduction of mature females as compared with control was recorded only for sample TRC (F3,8 = 43.44, p < 0.01, Table 2). No significant differences were observed for the size of the mature females (H3,11 = 4.55, p = 0.208, Table 2).
Offspring production was observed in control and all tested samples, but was very low; offsprings per female ranged from 0.1 (control) to 0.5 (VMI) neonates per female.
Correlation between chemicals and effects
Spearman’s correlation showed that both male and female daily length increment were negatively correlated with all chemicals measured in sediments, except Hg and Cd (Table 3).
Daily weight increment showed a significant, negative Spearman correlation with all the measured organic contaminants and metals, except Cd, Cr and Ni (Table 3).
Positive correlation was found between all the growth parameters and TOC (Spearman’s R > 0.78; p < 0.001), whilst growth rates were negatively correlated with sand content (Spearman’s R < −0.78; p < 0.001). TOC content and grain size were negatively correlated (Spearman’s R = −1; p < 0.001).
Data concerning attainment of maturity were too few for the calculation of the Spearman’s correlation with an acceptable number of degrees of freedom.
Discussion
Amphipod sensitivity
The LC50 data showed that sensitivity to Cd in water-only exposure test of the autochthonous population of M. insidiosum could be compared to that reported for wild populations of Ionian Sea (0.70–2.11 mg l−1 at temperatures in the range 10–25 °C; Prato et al. 2008), Pacific Ocean (0.68–1.27 mg l−1 at 19 °C; Hong and Reish 1987; Reish 1993) and for laboratory cultured specimens (0.96 mg l−1 at 20 °C; Boese et al. 1997).
Furthermore, M. insidiosum was clearly more sensitive than autochthonous population of C. orientale; LC50 values found for C. orientale specimens collected in Spring (April-June) over a period of five years ranged in the interval 10.3–17.6 mg l−1 (Picone et al. 2008). All data concerning C. orientale refer to amphipods collected in two different sites of the Lagoon, far from point sources of contamination, including M. insidiosum collection site. However, the lack of comparative tests on contaminated or spiked sediments makes any sensitivity comparison between the two species not conclusive concerning sensitivity to contaminated sediments.
Long term survival
Survival in control sediments was very high and in agreement with the value suggested by U.S. EPA (2001) for L. plumulosus (mean survival of 80%; minimum survival in single replicate of 60%) and those reported for C. volutator (Scarlett et al. 2007a, 2007b; van den Heuvel-Greve et al. 2007); moreover, survival of M. insidiosum was significantly higher than the values reported by Costa et al. (2005) for G. locusta. Despite high survival, it was clearly the less sensitive endpoint, since there was no significant differences among the investigated sediments. The comparison with sub-lethal effects (Table 2) clearly shows that 28-d mortality is not sensitive enough for assessing and predicting the hazard caused by exposure to low and moderately contaminated sediments. A lower discriminating power of lethality as compared to sub-lethal endpoints was also observed by Scarlett et al. (2007a, 2007b) for juvenile C. volutator exposed to sediments spiked with crude oil.
Growth rate
Growth of juvenile amphipods was decreased in all the test sediments as compared with the control sediment. In particular, effects on length and weight increments, were more marked in the sample characterized by the highest level of contamination (TRC).
The data then corroborate the hypothesis that growth of M. insidiosum can be used as a reliable endpoint for assessing toxic effects when contamination levels are low and survival is not a sensitive endpoint for acute (i.e. 10 days) or long-term exposure (i.e. 28 or more days). This finding was expected, but not obvious. Exposure to contaminants may increase metabolic costs to maintain homeostasis, by triggering energetically expensive mechanisms of elimination, biomineralization, and metabolism of chemicals. Nevertheless, previous studies on L. plumulosus raised doubts about the enhanced sensitivity of the sub-lethal endpoints with respect to mortality, since significant reduction of growth (weight increment) was observed only in sediments that affected significantly survival (McGee et al. 2004). The data concerning M. insidiosum agree also with results obtained for C. volutator exposed to sediment contaminated by Unresolved Complex Mixtures (UCM) of hydrocarbons (Scarlett et al. 2007a, 2007b), suggesting that at least for these 2 species growth rate represents a sensitive and affordable endpoint.
Focusing on length increment, males of M. insidiosum exhibited in all samples a generally higher growth rate than females. These data are partially in contrast with the results reported by Nair and Anger (1979), who observed at 15 °C a generally higher growth rate for females of M. insidiosum (49–74 µm individual−1 d−1) than for males (37–71 µm individual−1 d−1). In any case, growth rates measured for amphipods kept in the control sediment (55 µm individual−1 d−1 and 70 µm individual−1 d−1 for females and males, respectively) are clearly within the ranges reported by Nair and Anger (1979). Growth rates of about 70 µm individual−1 d−1 were reported also for juveniles C. volutator (Peters and Ahlf 2005; Scarlett et al. 2007a, 2007b).
The measure of individual length, although it takes much more time than estimation of weight, can be performed on a large number of individuals per experimental unit (from 23 up to 30 individuals in the present work), allowing for minimization of variance and an accurate estimation of growth rate. The accurate determination of growth rate in terms of length increment in M. insidiosum, as well as in other species of family Corophiidae, is also facilitated by the straight body shape of these amphipods. In curve-shaped species the determination of total length is often difficult and less accurate; Gale et al. (2006), for instance, reported that growth measured as body length in curve-shaped M. plumulosa is not a sensitive endpoint, due to the high variability observed among specimens and low differences among treatments (sediments spiked with metals).
The results highlight that the inhibition of the growth can be ascribed to metals and organics. Marsden (2002) explored successfully the relations between sediment concentration of Cu and amphipod’s growth. The author observed a significant decrease of length of male and immature Paracorophium excavatum with increasing Cu concentration from 5 to 46 mg kg−1 dw, a range very close to that observed in the tested sediments (from 13.9 mg kg−1 dw in SFR to 47.4 mg kg−1 dw in TRC). These data show that low concentrations of chemicals can alter physiology of amphipods and impair their growth. Nevertheless, other authors observed effects on growth only at high concentrations of metals in sediments. Gale et al. (2006) investigated the effects of Cd, Cu and Zn on growth of M. plumulosa after a 42-d exposure to spiked sediments and observed significant effects at concentrations of 440 mg kg−1 for Cu and 1540 mg kg−1 dw for Zn. Ward et al. (2015) reported significant effects on growth of L. plumulosus at 418 mg kg−1 dw of Cu, while no effects have been observed for Cd up to concentrations of 600 mg kg−1 dw (Gale et al. 2006) and 1.627 mg kg−1 dw (Dewitt et al. 1996). No observed effect concentrations (NOECs) calculated for freshwater amphipods Hyalella azteca and Gammarus pseudolimneaus (139 and 228 mg kg−1 dw) were about one order of magnitude higher than the sediment concentrations measured in the test sediment (Vangheluwe et al. 2013).
Even if available data do not allow definite identification of possible detrimental effects due to metals, they allow for some speculation about the possible route of exposure; since AVS are in surplus with respect to SEM, metals are expected to be unavailable for uptake through the water phase, and it is more likely that toxic effects could be related to the ingestion of sediment-bound or particulate-bound metals, as noted by Gale et al. (2006) as well.
Concerning the organic contaminants measured in the present study, benchmark data for growth reduction are available for hydrocarbons and DDTs. Scarlett et al. (2007a, 2007b) reported growth reduction in C. volutator exposed to UCM, crude oil and water-accomodated fractions (WAF), whilst Lotufo et al. (2016) explored toxicity of total PAHs towards L. plumulosus. In particular, Lotufo et al. (2016) observed a consistent decrement of amphipod growth at total PAHs concentration of 2.6 mg kg−1, and provided an EC20 of 1.05 mg kg−1 PAHs; these data suggest that PAHs may be a key contributor to the toxicity observed in sample TRC, where a total PAHs concentration of 6.6 mg kg−1 was measured. DDTs effects on amphipod growth were studied by Lotufo et al. (2001a, b) both on estuarine (L. plumulosus) and freshwater amphipods (Hyalella azteca). Growth rate of L. plumulosus was not affected by DDT up to a concentration of 9.9 nmol g−1 dw (3.5 μg g−1 dw) (Lotufo et al. 2001a). In contrast, tests with H. azteca showed that growth may be inhibited at 5.3 nmol DDT g−1 dw (1.9 μg g−1 dw) (Lotufo et al. 2001b). In any case, both for L. plumulosus and H. Azteca, exposure to DDTs was performed at concentrations about 1000 times higher than that measured in the sample TRC in the present study; based on these data, contribution of DDTs to overall sediment toxicity might be considered negligible for the tested sediments. Concerning PCBs, in literature are available data on mortality and bioaccumulation after long term exposures to water-only solutions and/or spiked sediment (Landrum et al. 1989; Borgmann et al. 1990).
Besides action of toxicants, effects on growth may also be influenced by factors not related with sediment contamination; in particular, availability of food showed the potential to ameliorate toxic effects also in acute amphipod tests, and in chronic exposure amount of food can influence toxicological endpoints (Bridges et al. 1997; McGee et al. 2004). In the test with M. insidiosum, the food has been added at the same rate in all test chambers, so it is unlikely that the observed differences on growth rate could be related to a different feeding regime. On the other hand, the different TOC content observed in the samples indicates that endogenous food availability (i.e. availability of fungi, bacteria and labile organic matter) may differ among sediments. The higher concentration of TOC observed in control and SFR suggest a possible larger availability of food than in VMI and TRC, so that it cannot be excluded that differences in growth rates may be partially due to differences in food abundance in the sediment, not completely compensated by the feeding regime.
In the case of sample TRC, it should be noted that combination of low availability of endogenous food (less TOC) and high concentration of organic contaminants may also result in a higher exposure (and uptake) via ingestion than in other samples; the quantity of organic contaminants ingested per unit of organic carbon is, indeed, from about 2 times up to several orders of magnitude higher than in control and less contaminated samples (Table 1).
As observed by many authors, the quality of organic matter may influence both growth and uptake of contaminants in benthic invertebrates. Specifically, labile organic matter derived from phytoplankton turned out to be the substrate more efficiently converted into somatic growth and enhancing bioaccumulation, while organic matter derived from eelgrasses degradation and lignin provided both less growth and less bioaccumulation (Gunnarsson et al. 1999; Granberg and Forbes 2006; Granberg and Selck 2007; Thorsson et al. 2008). In any case, it seems necessary to dedicate further research on the study of possible influence of organic matter on amphipods growth; in particular, the characterization of the carbon sources in terms of lipid class composition, amino acid composition, total nitrogen and ratio C/N may provide valuable information for interpreting chronic test data.
Amphipods are usually tolerant to variations in grain size when studying sub-lethal endpoints (Marsden et al. 2000; U.S. EPA 2001); nevertheless, impairments in growth rates observed in coarse sediments supports the hypothesis that some substrate requirements are needed to allow optimal growth (Surtikanti and Hyne 2000; U.S. EPA 2001). However, since grain size and organic carbon are usually significantly correlated (as in the present study), it cannot be excluded that substrate requirements are linked to food availability/quality rather than to the proportion of sand-, silt- or clay-sized particles.
Attainment of sexual maturity
The determination of sexual maturity evidenced a clear decrease of mature females with increasing levels of contamination. However, the high among-replicate variance that was measured in most of the samples (Table 2) restricts the ability of this endpoint to provide statistically significant results; most probably a higher statistical power could be obtained by using a larger number of replicates, that should lead to a reduction of variance. In any case, effects on attainment of maturity seems to be not completely discernible from effects on growth; published data suggest that females of M. insidiosum need to achieve a critical size before attaining maturity and that this size is mainly dependent on temperature. Nair and Anger (1979) recovered mature females only at sizes larger than 3.2–3.7 mm at 15 °C and 3.0–3.3 mm at 20 °C, while Kevrekidis (2004) observed that mature females were rarely found at sizes below 2.1 mm in Monolimni Lagoon (Aegean Sea, Greece). Our data, even if obtained in the laboratory, are in full agreement with this latter observation, since no mature females were found at sizes lower than 2.2 mm in all the investigated samples. The sizes of the mature females show minor difference between control and testing samples: mean size in the control was 3.1 mm while it ranged from 2.9 mm (SFR and TRC) and 2.7 mm (VMI) in the samples. This close connection between growth and female maturity implies that the observation of morphological parameters does not allow to establish whether the “observed” effects on attainment of maturity are a direct consequence of the actions exerted by contaminants on sexual development of amphipods, or an indirect outcome on growth, or a co-occurrence of both these effects. Some authors pointed out that in crustaceans (including amphipods) lipid content and composition vary substantially between juvenile and mature stages (Clarke 1980; Quigley et al. 1989); characterization of lipids, coupled with determination of morphological parameters, may enhance the ability to provide information on the effects of contaminants on sexual development. Its application should be explored if attainment of sexual maturity is intended to be used as endpoint.
At the end of the 28-d of exposure, most of the mature females in control and samples SFR and VMI were still carrying embryos on their brood pouch. Even if first oviposition of M. insidiosum generally occurs within 20–25 days of age (Casabianca 1975, Birklund 1977), incubation time (i.e. period lasting from oviposition to the release of the neonates) reported by Sheader (1978) and Nair and Anger (1979) at 15 °C is about 9–11 days, so it is clearly possible that in this conditions of temperature the release of first brood could occur after the 28th day. Recent experiences with C. volutator confirmed that also for this species the time required to reach maturity and produce offspring can be significantly longer than 28-d (up to 72-d) (Peters and Ahlf 2005; Scarlett et al. 2007a, 2007b; van den Heuvel-Greve et al. 2007). Maturation times can be significantly shortened by increasing test temperature, as observed by Nair and Anger (1979) for their North Sea population (from 52 days at 15 °C to 29 days at 20 °C); however with an increase of 5 °C of temperature, the time needed to attain maturity and release first brood may exceed 28-d.
Conclusions
Impairments in growth rates and attainment of sexual maturity of juvenile M. insidiosum were observed in low contaminated sites where acute and long-term survival of the organism was not affected. This outcome underlines the need to address whole sediment monitoring towards assessment of long-term effects on benthic species rather than acute effects on survival. Effects on growth and attainment of maturation are first symptoms of pollution at population level and they can hardly be assessed using acute or short-term tests.
The sub-lethal endpoints measured after a 28-d exposure on M. insidiosum seem to be valuable tools for detecting these early signs of chemical stress. Although both endpoints provided a very good among-site discriminating ability, growth appears to be a more reliable and statistically relevant endpoint.
In contrast, attainment of sexual maturity was affected by a higher among-replicates variance and results were less reliable than growth for the identification of impairments; moreover, effects on growth and attainment of maturity were not completely discernible. The introduction of complementary measures (i.e. lipid characterization) as part of the testing procedure may enhance discriminating ability and reliability of this endpoint.
Time needed for maturation and incubation of embryos does not allow for implementation of offspring production within the framework of a 28-d chronic test with M. insidiosum; probably, effects on the reproduction of this species can be reliably assessed only by extending the exposure period over 28-d, in a life-cycle test, with consequent increase of costs in terms of personnel and materials.
The shortness of data concerning chronic toxicity of metals underline the need to address future research towards identification of sediment concentrations that can exert detrimental effects on growth, maturation and reproduction of benthic species (not only amphipods). The few available laboratory data indicate that low concentrations of chemicals in field (i.e. metals or organics) can affect and alter specific traits of the life-history of the amphipods, with possible detrimental consequences on population structure.
References
Allen HE, Fu G, Deng B (1993) Analysis of acid-volatile sulfide (AVS) and simultaneously extracted metals (SEM) for the estimation of potential toxicity in aquatic sediments. Environ Toxicol Chem 12:1441–1453. https://doi.org/10.1002/etc.5620120812
ASTM (2013) Standard Guide for Conducting Sediment Toxicity Tests with Polychaetous Annelids. E1611-00(2013).
Birklund J (1977) Biomass, growth and production of the amphipod Corophium insidiosum crawford, and preliminary notes on Corophium volutator (Pallas). Ophelia 16:187–203. https://doi.org/10.1080/00785326.1977.10425470
Bigongiari N, Braida T, Pasteris A (2001) Saggio biologico con l'anfipode Corophium orientale: metodiche ed esempi di applicazione ai sedimenti marini. Biol Mar Medit 8(2):60–71
Boese BL, Lamberson JO, Swartz RC, Ozretich RJ (1997) Photoinduced toxicity of fluoranthene to seven marine benthic crustaceans. Arch Environ Contam Toxicol 32:389–393. https://doi.org/10.1007/s002449900201
Borgmann U, Norwood WP, Ralph KM (1990) Chronic toxicity and bioaccumulation of 2,5,2’,5’- and 3,4,3’,4’-tetrachlorobiphenyl and Aroclor 1242 in the amphipod Hyalella azteca. Arch Environ Contam Toxicol 19:558–564
Brian A (1935) Gli Anfipodi della laguna di Venezia (nota preliminare). Boll dell’Istituto Zool di Anat Comp Univ di Genova 19:1–8
Bridges TS, Farrar JD, Duke BM (1997) The influence of food ration on sediment toxicity in Neanthes arenaceodentata (Annelida: Polychaeta). Environ Toxicol Chem 16:1659–1665. https://doi.org/10.1002/etc.5620160814
Casabianca ML (1967) Sur le biologie de Corophium insidiosum Crawford dans l’étang de Biguglia (Corse). Bulletin de la Societé Zoologique de France. Bull la Soc Zool Fr 91:401–405
Casabianca ML (1975) Méthode de calcul de la production par estimation de la mortalité. Application à une population à structure complexe du crustacé Corophium insidiosum Crawford (lagune de Biguglia, Corse). Comptes Rendues Des Séances l’Acadamie Sci Paris 280:1139–1142
Castro H, Ramalheira F, Quintino V, Rodrigues AM (2006) Amphipod acute and chronic sediment toxicity assessment in estuarine environmental monitoring: An example from Ria de Aveiro NW Portugal. Mar Pollut Bull 53:91–99. https://doi.org/10.1016/j.marpolbul.2005.09.029
Ceccherelli VU, Ferrari I, Viaroli P (1994) Ecological research on the animal communities of the Po River Delta lagoons. Bolletino di Zool 61:425–436. https://doi.org/10.1080/11250009409355916
Chapman PM (2007) Determining when contamination is pollution - Weight of evidence determinations for sediments and effluents. Environ Int 33:492–501. https://doi.org/10.1016/j.envint.2006.09.001
Clarke A (1980) The biochemical composition of krill, Euphausia superba Dana, from South Georgia. J Exp Mar Bio Ecol 43:221–236. https://doi.org/10.1016/0022-0981(80)90049-0
Costa FO, Neuparth T, Correia AD, Helena Costa M (2005) Multi-level assessment of chronic toxicity of estuarine sediments with the amphipod Gammarus locusta: II. Organism and population-level endpoints. Mar Environ Res 60:93–110. https://doi.org/10.1016/j.marenvres.2004.08.005
Dahl U, Lind CR, Gorokhova E et al. (2009) Food quality effects on copepod growth and development: Implications for bioassays in ecotoxicological testing. Ecotoxicol Environ Saf 72:351–357. https://doi.org/10.1016/j.ecoenv.2008.04.008
Dewitt TH, Swartz RC, Hansen DJ et al. (1996) Bioavailability and chronic toxicity of cadmium in sediment to the estuarine amphipod Leptocheirus plumulosus. Environ Toxicol Chem 15:2095–2101. https://doi.org/10.1002/etc.5620151205
Fox M, Ohlauson C, Sharpe AD, Brown RJ (2014) The use of a Corophium volutator chronic sediment study to support the risk assessment of medetomidine for marine environments. Environ Toxicol Chem 33:937–942. https://doi.org/10.1002/etc.2515
Gale SA, King CK, Hyne RV (2006) Chronic sublethal sediment toxicity testing using the estuarine amphipod, Melita plumulosa (Zeidler): evaluation using metal-spiked and field-contaminated sediments. Environ Toxicol Chem 25:1887–98
Gonzalez-Félix ML, Lawrence AL, Gatlin DM, Perez-Velazquez M (2003) Nutritional evaluation of fatty acids for the open thelycum shrimp, Litopenaeus vannamei: I. Effect of dietary linoleic and linolenic acids at different concentrations and ratios on juvenile shrimp growth, survival and fatty acid composition. Aquac Nutr 9:105–113. https://doi.org/10.1046/j.1365-2095.2003.00231.x
Granberg ME, Forbes TL (2006) Role of sediment organic matter quality and feeding history in dietary absorption and accumulation of pyrene in the mud snail (Hydrobia ulvae). Environ Toxicol Chem 25:995–1006
Granberg ME, Selck H (2007) Effects of sediment organic matter quality on bioaccumulation, degradation, and distribution of pyrene in two macrofaunal species and their surrounding sediment. Mar Environ Res 64:313–335. https://doi.org/10.1016/J.MARENVRES.2007.02.005
Grapentine L, Anderson J, Boyd D et al. (2002) A decision making framework for sediment assessment developed for the Great Lakes. Hum Ecol Risk Assess. Int J 8:1641–1655. https://doi.org/10.1080/20028091057538
Gunnarsson JS, Granberg ME, Nilsson HC et al. (1999) Influence of sediment-organic matter quality on growth and polychlorobiphenyl bioavailability in Echinodermata (Amphiura filiformis). Environ Toxicol Chem 18:1534–1543. https://doi.org/10.1002/etc.5620180728
Hong JS, Reish DJ (1987) Acute toxicity of cadmium to eight species of marine amphipod and isopod crustaceans from southern California. Bull Environ Contam Toxicol 39:884–888. https://doi.org/10.1007/BF01855870
Hyne RV, Gale SA, King CK (2005) Laboratory culture and life-cycle experiments with the benthic amphipod Melita plumulosa (Zeidler). Environ Toxicol Chem 24:2065. https://doi.org/10.1897/04-409R1.1
ICRAM (2001) Metodologie analitiche di riferimento. Ministero dell’Ambiente e della Tutela del Territorio, Servizio Difesa del Mare
ISO (International Standard Organization) (2005) Water quality – Determination of acute toxicity of marine or estuarine sediment to amphipods.
Kennedy AJ, Steevens JA, Lotufo GR et al. (2009) A comparison of acute and chronic toxicity methods for marine sediments. Mar Environ Res 68:118–127. https://doi.org/10.1016/j.marenvres.2009.04.010
Kevrekidis T (2004) Population dynamics, growth and reproduction of Corophium insidiosum (Crustacea: Amphipoda) at low salinities in Monolimni lagoon (Evros Delta, North Aegean Sea). Hydrobiologia 522:117–132. https://doi.org/10.1023/B:HYDR.0000029971.11713.41
Landrum P, Faust W, Eadie B (1989) Bioavailability and Toxicity of a Mixture of Sediment-Associated Chlorinated Hydrocarbons to the Amphipod. In: Aquatic Toxicology and HazardAssessment: 12th Volume. ASTM International, 100 Barr Harbor Drive, PO Box C700, West Conshohocken, PA19428-2959, pp 315-315–15
Long ER, Ingersoll CG, MacDonald DD (2006) Calculation and uses of mean sediment quality guideline quotients: A critical review. Environ Sci Technol 40:1726–1736. https://doi.org/10.1021/es058012d
Long ER, Macdonald DD (1997) Effects range low and median, threshold and probable effects levels. Interactive short course on “use of sediment quality guidelines in the assessment and management of contaminated sediments.” In: Proceedings of the 18th Annual Meeting of the Society of Environmental Toxicology and Chemistry (SETAC), San Francisco, CA.
Long ER, Macdonald DD, Smith SL, Calder FD (1995) Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ Manag 19:81–97. https://doi.org/10.1007/BF02472006
Lotufo GR, Farrar JD, Biedenbach JM et al. (2016) Effects of sediment amended with Deepwater Horizon incident slick oil on the infaunal amphipod Leptocheirus plumulosus. Mar Pollut Bull 109:253–258. https://doi.org/10.1016/j.marpolbul.2016.05.073
Lotufo GR, Farrar JD, Duke BM, Bridges TS (2001a) DDT toxicity and critical body residue in the amphipod Leptocheirus plumulosus in Exposures to Spiked Sediment. Arch Environ Contam Toxicol 41:142–150. https://doi.org/10.1007/s002440010231
Lotufo GR, Landrum PF, Gedeon ML (2001b) Toxicity and bioaccumulation of ddt in freshwater amphipods in exposures to spiked sediments. Environ Toxicol Chem 20:810–825. https://doi.org/10.1002/etc.5620200417
Mancinelli G, Sabetta L, Basset A (2005) Short-term patch dynamics of macroinvertebrate colonization on decaying reed detritus in a Mediterranean lagoon (Lake Alimini Grande,Apulia, SE Italy). Mar Biol 148:271–283. https://doi.org/10.1007/s00227-005-0091-5
Marsden ID (2002) Life-history traits of a tube-dwelling corophioid amphipod, Paracorophium excavatum, exposed to sediment copper. J Exp Mar Bio Ecol 270:57–72. https://doi.org/10.1016/S0022-0981(02)00010-2
Marsden ID, Wong CHT, Al-Mudaffar N (2000) Assessment of an estuatine amphipod (Paracorophium excavatum) as a bioindicator of contaminated sediment. Australas J Ecotoxicol 6:21–30
McGee BL, Fisher DJ, Wright DA et al. (2004) A field test and comparison of acute and chronic sediment toxicity tests with the estuarine amphipod Leptocheirus plumulosus in Chesapeake Bay, USA. Environ Toxicol Chem 23:1751–61
Nair KKC, Anger K (1979) Life cycle of Corophium insidiosum (Crustacea, Amphipoda) in laboratory culture. Helgoländer Wiss Meeresunters 32:279–294. https://doi.org/10.1007/BF02189586
Peters C, Ahlf W (2005) Reproduction of the estuarine and marine amphipod Corophium volutator (Pallas) in laboratory for toxicity testing. Chemosphere 59:525–536. https://doi.org/10.1016/j.chemosphere.2005.01.053
Picone M, Bergamin M, Losso C, et al (2016) Assessment of sediment toxicity in the Lagoon of Venice (Italy) using a multi-species set of bioassays. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2015.09.002
Picone M, Bergamin M, Novelli Alessandra A, et al (2008) Evaluation of Corophium orientale as bioindicator for Venice Lagoon: Sensitivity assessment and toxicity-score proposal. Ecotoxicol Environ Saf. https://doi.org/10.1016/j.ecoenv.2006.06.005
Prato E, Biandolino F (2006) Life history of the amphipod Corophium insidiosum (Crustacea: Amphipoda) from Mar Piccolo (Ionian Sea, Italy). Sci Mar 70:355–362. https://doi.org/10.3989/scimar.2006.70n3355
Prato E, Scardicchio C, Biandolino F (2008) Effects of temperature on the acute toxicity of cadmium to Corophium insidiosum. Environ Monit Assess 136:161–166. https://doi.org/10.1007/s10661-007-9672-8
Procaccini G, Scipione MB (1992) Observations on the spatio-temporal distribution of crustacean amphipods in the Fusaro coastal lagoon (Central Tyrrhenian Sea, Italy) and some notes on their presence in Mediterranean lagoons. Mar Ecol 13:203–224. https://doi.org/10.1111/j.1439-0485.1992.tb00351.x
Quigley MA, Cavaletto JF, Gardner WS (1989) Lipid composition related to size and maturity of the amphipod Pontoporeia hoyi. J Gt Lakes Res 15:601–610. https://doi.org/10.1016/S0380-1330(89)71514-8
Redmond MS, Jones JKP, Scott KJ, Swartz RC (1994) Preliminary culture and life-cycle experiments with the benthic amphipod Ampelisca abdita. Environ Toxicol Chem 13:1355–1365. https://doi.org/10.1002/etc.5620130817
Reish DJ (1993) Effects of metals and organic compounds on survival and bioaccumulation in two species of marine gammaridean amphipod, together with a summary of toxicological research on this group. J Nat Hist 27:781–794. https://doi.org/10.1080/00222939300770471
Scarlett A, Galloway TS, Rowland SJ (2007a) Chronic toxicity of Unresolved Complex Mixtures (UCM) of hydrocarbons in marine sediments. J Soils Sediment 7:200–206. https://doi.org/10.1065/jss2007.06.232
Scarlett A, Rowland SJ, Canty M et al. (2007b) Method for assessing the chronic toxicity of marine and estuarine sediment-associated contaminants using the amphipod Corophium volutator. Mar Environ Res 63:457–470. https://doi.org/10.1016/j.marenvres.2006.12.006
Sheader M (1978) Distribution and reproductive biology of Corophium insidiosum (Amphipoda) on the north-east coast of England. J Mar Biol Assoc U Kingd 58:585. https://doi.org/10.1017/S0025315400041242
Shepard FP (1954) Nomenclature based on silt-sand-clay ratios.
Surtikanti HK, Hyne RV (2000) Sediment toxicity testing using the amphipod Corophium sp: standardisation of test condition for an acute survival test and a sub-chronic growth test in freshwater. Australas J Ecotoxicol 6:11–20
Tagliapietra D, Pavan M, Wagner C (1998) Macrobenthic Community Changes Related to Eutrophication in Palude della Rosa (Venetian Lagoon, Italy). Estuar Coast Shelf Sci 47:217–226. https://doi.org/10.1006/ecss.1998.0340
Thorsson MH, Hedman JE, Bradshaw C et al. (2008) Effects of settling organic matter on the bioaccumulation of cadmium and BDE-99 by Baltic Sea benthic invertebrates. Mar Environ Res 65:264–281. https://doi.org/10.1016/j.marenvres.2007.11.004
U.S. EPA (2001) Method for assessing the chronic toxicity of marine and estuarine sediment-associated contaminants with the amphipod Letpocheirus plumulosus.
van den Heuvel-Greve M, Postma J, Jol J et al. (2007) A chronic bioassay with the estuarine amphipod Corophium volutator: Test method description and confounding factors. Chemosphere 66:1301–1309. https://doi.org/10.1016/j.chemosphere.2006.07.022
Vangheluwe MLU, Verdonck FAM, Besser JM et al. (2013) Improving sediment-quality guidelines for nickel: Development and application of predictive bioavailability models to assess chronic toxicity of nickel in freshwater sediments. Environ Toxicol Chem 32:n/a–n/a. https://doi.org/10.1002/etc.2373
Volpi Ghirardini A, Arizzi Novelli A, Tagliapietra D (2005) Sediment toxicity assessment in the Lagoon of Venice (Italy) using Paracentrotus lividus (Echinodermata: Echinoidea) fertilization and embryo bioassays. Environ Int 31:1065–1077. https://doi.org/10.1016/j.envint.2005.05.017
Ward TJ, Gaertner KE, Gorsuch JW, Call DJ (2015) Survival, reproduction and growth of the marine amphipod, Leptocheirus plumulosus, following laboratory exposure to copper-spiked sediment. Bull Environ Contam Toxicol 95:434–440. https://doi.org/10.1007/s00128-015-1638-x
Funding
This study has not received any funding
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Picone, M., Bergamin, M., Delaney, E. et al. Assessment of whole-sediment chronic toxicity using sub-lethal endpoints with Monocorophium insidiosum. Ecotoxicology 27, 1237–1248 (2018). https://doi.org/10.1007/s10646-018-1977-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-018-1977-6