Abstract
Arbuscular mycorrhizal fungi (AMF) are integral functioning parts of plant root systems and are widely recognized for enhancing contaminants uptake and metabolism on severely disturbed sites. However, the patterns of their influence on the phytoremediation of iron–cyanide (Fe–CN) complexes are unknown. Fe–CN complexes are of great common interest, as iron is one of the most abundant element in soil and water. Effect of ryegrass (Lolium perenne L.) roots inoculation, using mycorrhizal fungi (Rhizophagus irregularis and a mixture of R. irregularis, Funneliformis mosseae, Rhizophagus aggregatus, and Claroideoglomus etunicatum), on iron–cyanide sorption was studied. Results indicated significantly higher colonization of R. irregularis than the mixture of AMF species on ryegrass roots. Series of batch experiments using potassium hexacyanoferrate (II) solutions, in varying concentrations revealed significantly higher reduction of total CN and free CN content in the mycorrhizal roots, indicating greater cyanide decrease in the treatment inoculated with R. irregularis. Our study is a first indication of the possible positive contribution of AM fungi on the phytoremediation of iron–cyanide complexes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
From the early 1800s through the mid-1900s manufactured gas plants (MGPs) were operated nationwide to provide gas from coal or oil for lighting, heating and cooking (EPA 1999). The gas manufacturing and purification processes yielded contaminated residues (including petroleum hydrocarbons, polycyclic aromatic hydrocarbons, cyanides, etc.) that were spread in the vicinities or used as a filling material (Dzombak et al. 2006). The soils and groundwater on former MGPs are contaminated with various complex iron–cyanides (Fe–CN), mainly present in the form of Prussian Blue (ferric ferrocyanide, Fe4[Fe(CN)6]3) and its dissolution products (Theis et al. 1994). The potential risk to human health depends on Fe–CN complexes’ tendency to release toxic free cyanide, which is influenced by the kinetic stability of the complexes and by the presence of light (Kjeldsen 1998). Due to the complex chemistry, reactivity and toxicology of the cyanides, the management and remediation of former MGP sites can be very challenging.
Different reclamation technologies, including engineering and bioremediation approaches, have been applied to restore Fe–CN contaminated sites. However, considering operating costs, treatment time and volume, environmental issue etc. large-scale engineering concepts have not been very successful in cleaning up former MGPs (Zhang et al. 2013). Phytoremediation is a promising tool in stabilization and remediation of Fe–CN contaminated soils. Free cyanide can be metabolized by plants, and used by microorganisms as a nitrogen and carbon source (Knowles 1976). However, the strongly bonded nature of iron–cyanide complexes makes them less sensitive to degradation and transformation (Dzombak et al. 2006). According to the literature, uptake and transport of Fe–CN complexes via plants (Ebbs et al. 2003; Samiotakis and Ebbs 2004; Xiao-Zhang et al. 2006) reveal much lower rates are than when compared to free cyanide. According to Ebbs et al. (2003) diamond willow (Salix spp.) is capable of transporting and metabolizing Fe–CN complexes. However, only 8 % of the total Fe–CN mass was removed from the solution by willow tissues over the 20-day period. Similar results were obtained by Samiotakis and Ebbs (2004), who studied exposure of barley (Hordeum vulgare L.), oat (Avena sativa L.), and wild cane (Sorghum bicolor L.) to ferrocyanide solution. Based on own investigations, phytoremediation of former MGP sites leads to secondary accumulation of iron–cyanide complexes in the leaves of willow (Salix), poplar (Populus spp.) and robinia (Robinia pseudoacacia L.) trees. Another limitation is a potential effect of iron–cyanide coating on plant roots, which results in decreased plant water use and biomass (Reeves 2000). Most plants that survive in toxic soils are either avoiding the contamination, or hyper-accumulating them in their tissues (Khan et al. 2000).
Generally, shallow rotting, decreased biomass, poor growing, and the risk of the food chain contamination limits plant performance in land reclamation (Cunningham et al. 1995). However, phytoremediation may be enhanced via rhizosphere microbial activity, which may cooperate on the uptake, translocation and metabolism of contaminants (Hernández-Ortega et al. 2012). According to Dursun et al. (1999) degradation of iron–cyanide complexes was confined to a limited number of bacterial and fungal strains.
Plant-arbuscular mycorrhizal fungi (AMF) symbioses have positive effects on plant establishment and survival in contaminated soils through increased plant mineral nutrition, improved water supply and environmental stress tolerance (Gao et al. 2010; Soka and Ritchie 2014). Studies concerning AMF inoculation in plants growing on heavy metal (Pawlowska et al. 1996; Chaudhry et al. 1998) and polycyclic aromatic hydrocarbons (PAH) affected (Alarcón et al. 2006; Cheema et al. 2010) soils reveal upgraded contaminant uptake via extra-matrical fungal hyphae that explores rhizospheres, while increasing the absorptive surface area of the plant beyond the root hair zone (Khan et al. 2000). The influence of AMF on phytoremediation of iron–cyanide complexes is still unknown. No studies have been performed in order to enhance the uptake and metabolism of complexed cyanides via plants using symbiosis with AMF.
Thus, the aim of this study was to investigate the contribution of AMF-root symbiosis on the sorption of iron–cyanide complexes, using commercially available inoculant Rhizophagus irregularis (INOQ Sprint, INOQ GmbH, Germany) and a mixture of arbuscular mycorrhizal (Mykorrhiza Samenimpfstoff, Tyroler Glückspilze, Austria) species with the roots of the host plant ryegrass (Lolium perenne L.) in a batch experiment. Results provided by this study propose a new cost effective approach for the remediation of iron–cyanide contaminated sites.
Materials and methods
Study site
The research site is located in the central southwestern part of Cottbus (51°45,161′N; 14°18,529′E), in the south of Brandenburg State, in Germany. This study is related to the project “Stabilization of the DB AG locations of a former Manufactured Gas Plant in Cottbus using the Bioremediation (Phytoremediation)”, of which its main aim is to stabilize the contamination, in form of cyanide, at the MGP Cottbus site using different plants (Salix, Robinia pseudoacacia, Populus, Helianthus etc.) (Dimitrova et al. 2014). The production of manufactured gas was stopped in the middle of the twentieth century, which caused the cessation of the Cottbus MGP. The field covers an area of approximately 2500 m2. The groundwater table is situated at a depth of about 7 m below the surface. The top soil is composed of sand containing coal, slag, and organic matter (up to 0.5 m deep) (Fig. 1). The deeper soil (0.5–7.0 m) has a sandy texture (texture classes according to the German classification system) (Fig. 1). The soil pH varies between 3.2 in a depth of 0.5 m and 7.7 at 7 m depth (Sut et al. 2015). According to the Köppen classification system, Cottbus has a humid continental climate (Peel et al. 2007) with an annual average temperature of 8.8 °C and an average annual precipitation of 589 mm (Linder et al. 1997).
Field sampling, root and soil analysis
The site screening provided a spatial distribution of the CN concentration, where the investigation field was divided in six separate surface transects (A, B, C, E, G, I) (Fig. 1). Soil was collected within a sampling grid of 6 × 6 m spacing at the depth of 0.5 m. The highest CN contents where measured along the C profile in the central part of the site (up to 1600 mg kg−1). Soil analysis of a 7 m deep borehole (drilling point A-21) revealed the highest CN concentration in MGP waste layer (0.3–0.9 m deep) and sandy layer beneath it (0.9–1.4 m deep) (Fig. 1).
Core root samples were collected from the point A-21 and C-25 (Fig. 1) reaching the depth of 0.5 m (Fig. 1). Subsequently, roots were separated from the soil by sieving and washing with deionized water.
Roots were boiled in a 10 % potassium hydroxide solution for 5 min, rinsed twice with tap water and placed for 5 min in a 5 % solution of ink and acetic acid following the procedure of Vierheilig et al. (1998). The stained roots were cut into 1 cm pieces and mounted on glass slides (10 pieces of root per slide) for examination under a compound microscope (magnification:×100 to ×400), following the grid line intersection method (Giovannetti and Mosse 1980).
Colonization of AMF was determined for each sample by examining 1 cm long root segments using transmitted light microscope (Olympus BX51, Hamburg, Germany) equipped with a digital camera Axiocam ICc 5, controlled by ZEISS Microscopy Software and Imaging. Frequency and intensity of root mycorrhization was calculated according to the Trouvelot et al. (1986) method.
The total and free CN content in the soil samples were determined using the automated spectrophotometrical flow injection system FIA Compact (MLE, Medizin- und Labortechnik Engineering GmbH, Dresden, Germany). Injection Analyzer refers to the (DIN EN ISO 14 2002) D standard for determination of total and free cyanide with continuous flow analysis. The procedure was based on extracting 20 g of soil in 200 mL of 1 mol L−1 sodium hydroxide (1:10 ratio) for 12 h in an end-over-end shaker at 16 rpm. To force settling of soil particles, the extracts were centrifuged for 10 min at 3000 rpm. Subsequently, to liberate HCN from complexed cyanide, 5 mL of the extract were digested in an acidic environment using the micro dist system (Sut et al. 2015). The micro dist system refers to the method of the Hach Company (Loveland, CO, USA), US QuikChem Method 10-204-00-1-X approved by the USEPA (2008). The free cyanide concentration was obtained by directly measuring filtered (0.45 µm syringe filters) solutions using FIA system. Additionally, soil spectra were recorded using Fourier Transform Infrared (FTIR) Spectroscopy (Impact 410 Nicolet, SpectraLab Scientific Inc., Ontario, Canada, resolution 4 cm−1, eight scans per sample).
Experimental design
Plants were cultivated in the greenhouse to obtain mycorrhizal (M) and non-mycorrhizal (NM) root material for the sorption experiments using Fe–CN solutions. Plastic pots were filled with 2 kg of dry sandy substrate collected on an experimental site in the lignite mine Welzow Süd, located in Lower Lusatia, Germany. This substrate originates from Saale time glacial deposits brought to the surface during lignite mining (Boldt-Burisch et al. 2013). Prior to filling the pots, the substrate was sieved through a 2 mm sieve and sterilized by heating at 150 °C in a drying oven for 1 h.
Three variants were used in this experiment, NM as the control, and two mycorrhizal variants (M) with one inoculant of R. irregularis (INOQ Sprint, INOQ GmbH, Germany) and an inoculant consisting of a mixture of the different arbuscular mycorrhizal fungi R. irregularis (former Glomus intraradices), Funneliformis mosseae (former Glomus mosseae), Rhizophagus aggregatus (former Glomus aggregatum), and Claroideoglomus etunicatum (former Glomus etunicatum) (Mykorrhiza Samenimpfstoff, Tyroler Glückspilze, Austria).
Prior to planting, a third of the pots were inoculated with R. irregularis (INOQ Sprint, INOQ GmbH, Germany) by mixing the inoculum carefully with the grow medium (1:10 grow medium:inoculum ratio). A further third of the pots were inoculated with the AMF mixture by mixing 2 g of AMF powder (Mykorrhiza Samenimpfstoff, Tyroler Glückspilze, Austria) with ryegrass seeds that were subsequently planted into the growth medium. One third of the pots remained without AMF as a NM control. Each treatment was established in five replicates. The plant and AM fungal inoculum selection was based on previous results, where successful inoculation of ryegrass using above mentioned mycorrhiza species led to improved uptake of PAH and uranium by plant roots system (Chen et al. 2008; Gao et al. 2010; Yu et al. 2011). No studies have been performed to investigate the influence of AMF-plant symbiosis on the uptake of iron–cyanide complexes yet. However, ryegrass is known to produce more extensive root system, which justifies its usage for the purposes of this study (Chen et al. 2008).
In order to ensure uniform germination and sufficient moisture, in the first week of the experiment, the pots were covered with a plastic foil. All treatments were cultivated for 90 days in the greenhouse under controlled conditions: daylight (Ø 300 µmol m2 s−1) at 21 °C (day) and 16 °C (night). Pots containing NM treatment were placed in a separate room (under analogous conditions) to prevent spores dispersal.
After 90 days, experimental pots were disassembled and roots were carefully washed several times with deionized water to remove adhering soil particles. To collect sub-samples for the AMF frequency and intensity determination, each pot was randomly sampled in five spots. Obtained representative root material was stained and analyzed according to the procedure described in the “Field sampling, root and soil analysis” section.
Batch experiment
For the purposes of the sorption experiment, obtained root material was air-dried and 2 g were introduced into 50 mL polyethylene bottles. 20 mL of potassium hexacyanoferrate (II) (Merck, Darmstadt, Germany) was added, with a CN concentration equal to 13, 33 and 65 mg L−1. A batch experiment was conducted in order to determine Fe–CN sorption on NM and M ryegrass roots. Polyethylene bottles containing CN solutions (negative control), CN solution + NM roots and CN solution + N roots, in three replicates, were shaken for 24 h horizontally (150 rpm) to reach equilibrium (Rennert 2002). Subsamples (2 mL) were taken in the defined time intervals for further analysis (1 and 24 h). Polyethylene bottles were wrapped in aluminum foil to prevent light influence. In order to separate the phases, solutions were centrifuged and filtered through a 0.45 µm syringe filter. The CN content in the obtained samples was determined using FIA (DIN EN ISO 14 403 D) according to the procedure described in the “Field sampling, root and soil analysis” section.
Statistical analysis
Statistical analysis of the data was accomplished via one-way ANOVA with the Tukey test used to conduct pair wise contrast.
Results and discussion
AM colonization of root samples
Site screening performed prior to the root sampling provided identification of “CN hotspots”. Hence, core samples were obtained from points A-21 and C-25 (Fig. 1). In situ experiments were carried out to gain information concerning the AMF-root associations already existing on the site and capable to survive the toxic conditions.
Analysis of the soil, in the sampling point A-21, indicated total CN concentration reaching 970 mg kg−1 and the pH 6. The FTIR analysis revealed the infrared absorption bands at 2026 and 2087 cm−1 indicating ferricyanide adsorbed on goethite and ferric ferrocyanide (Rennert et al. 2007; Sut et al. 2015) respectively (data not shown). The microscopic analysis of the root samples collected from the point A-21 clearly identify root colonization demonstrating typical AM structures vesicles, arbuscules, internal and external hyphae (Fig. 2a). This investigation plot was planted in year 2011 with willow trees (Salix). Mycorrhizal infection frequency was generally low reaching 10–30 %. Mycorrhizal intensity stretched to 0.14–12.85 % of the total root length.
Roots stained with ink-vinegar solution originating from: a MGP site A-21 sampling point (30 cm deep); b MGP site C-25 sampling point (20 cm deep); c greenhouse experiment, ryegrass root sample colonized with R. irregularis; d greenhouse experiment, ryegrass root sample colonized with AM mixture; e greenhouse experiment, NM ryegrass root sample
Analysis of soil in the sampling point C-25 showed a CN concentration reaching 334 mg kg−1 and pH equal to 6.75. Analogous to sampling point A-21, the FTIR analysis revealed the presence of ferricyanide adsorbed on goethite and ferric ferrocyanide. In Fig. 2b AM structures (vesicles and internal hyphae) demonstrate colonization of the root tissue collected in the sampling point C-25. This investigation plot was planted in year 2011 with poplar trees (Populus). The microscopic analysis of the root samples revealed a mycorrhizal infection frequency of 10–30 %, comparable with those of roots from the willow plots. Mycorrhizal intensity reaching 0.14–0.3 % of the total root length was lower comparing to plots planted with willow.
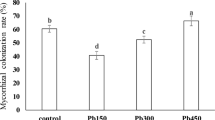
Greenhouse pot experiment revealed significant differences (p < 0.05) in mycorrhizal frequency and intensity of ryegrass roots (Fig. 3). AM frequency in roots colonized by R. irregularis reached 75–100 % and the intensity varied from 14.9 to 30.65 % of the total root length. Yu et al. (2011) reported colonization of ryegrass roots by R. irregularis in a pot experiment, ranging from 8 to 26 % of the total root length. Figure 2c shows R. irregularis structures (vesicles, arbuscules, internal and hyphae) in the ryegrass root tissue.
Colonization frequency of ryegrass roots by a mixture of AM (including G. mosseae) reached 40 %, with a maximum mycorrhization intensity amounting to 9.85 % of the total root length (Fig. 3). Gao et al. (2010) reported colonization of ryegrass roots by G. mosseae, in a pot experiment, to be increasing with time and reaching 17.8 %.
Figure 2d indicates AM structures (vesicles, arbuscules, internal and hyphae) in the ryegrass root tissue. It is assumed that the average low AM colonization, regardless of high mycorrhization in the individual sampling spots, is a result of using a mixture of spores. The symbiosis of ryegrass roots with G. mosseae (Corgié et al. 2006; Gao et al. 2010) and with G. intraradices (Nikbakht et al. 2013) was documented in the literature. However, no studies have been performed to determine the colonization of Glomus aggregatum and G. etunicatum on ryegrass roots, thus the potential symbiosis have not been proven. Even though the replicates in the pot experiment contained homogenized substrate, it is assumed that in the treatments, where the colonization of ryegrass roots was higher, the AMF inoculation contained more G. mosseae and G. intraradices spores. Another limitation, leading to such a low colonization, could be the manufacturing process of the commercial inoculum product. For future experiments, it is advised to purchase and use the isolated and propagated specific endomycorrhiza cultures.
Mycorrhizal colonization was only found in the treatments with AMF inoculation (Figs. 2e, 3). There was no contamination of controls.
Effect of root colonization on Fe–CN sorption
Until recently, iron–cyanide complexes were believed to be membrane impermeable for the plants tissues. However, within the last years numbers of studies concerning phytoremediation of Fe–CN complexes increased, as this is the predominant form of CN present in many environmental matrices. Several authors have stated that different plant species (Trapp et al. 2001; Larsen et al. 2004), including diamond willow (Salix), indian mustard (Brassica juncea L.), and various grasses (Samiotakis and Ebbs 2004), can uptake and transport complexed CN compounds, but with the lower removal rate when compared to free cyanide. Generally, a long duration is needed before remediation of Fe–CN complexes reaches an acceptable level. To allow remediation within a reasonable period (e.g. <5 year), the plant yield and Fe–CN uptake have to be enhanced dramatically (Khan et al. 2000). Protection and enhanced capability of greater uptake of minerals result in greater biomass production, a prerequisite for successful remediation (Khan et al. 2000). Since uptake and tolerance of iron–cyanide complexes depend on both plant and soil factors including soil microbes, investigating the influence of arbuscular mycorrhizal fungi interaction with roots is required. However, no studies have been performed to investigate this interaction.
In this study two AMF treatments, either containing single species R. irregularis or a mixture of R. irregularis, F. mosseae, R. aggregatus, and Claroideoglomus was used to inoculate ryegrass (L. perenne) in order to investigate a possible contribution of root mycorrhization on the sorption of iron–cyanide complexes.
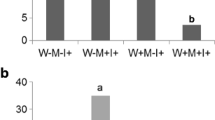
Figure 4 displays the results from the batch sorption experiment using M and NM roots. There were no significant differences in the total CN concentration, between the treatments, in the beginning of the experiment (0 h) (Fig. 4). In the solutions containing 13 mg L−1 CN (Fig. 4a), significant different in the CN concertation can be noticed between the potassium hexacyanoferrate (II) solution (no roots) and R. irregularis colonized roots (1 h, p < 0.05); and between potassium hexacyanoferrate (II) solution (no roots) and M (AM mixture) roots (24 h, p < 0.05). In the solutions containing 33 mg L−1 CN (Fig. 4b), significant different in the CN concertation can be noticed between the potassium hexacyanoferrate (II) solution (no roots) and M (R. irregularis) roots (24 h, p < 0.05). In the solutions containing 65 mg L−1 CN (Fig. 4c), significant different in the cyanide concertation can be noticed after 1 h between the potassium hexacyanoferrate (II) solution (no roots) and M (R. irregularis, AM mixture) roots (p < 0.05). After 24 h total CN concentration differed significantly between CN solution (no roots) and M (R. irregularis, AM mixture) roots (p < 0.05); and between NM roots and M (R. irregularis) roots (p < 0.05).
A general tendency of decreasing CN concentration in all treatments with time is noticeable (Fig. 4). For the samples containing no roots, after 24 h the reduction of total CN concentrations in the 13, 33 and 65 mg L−1 solutions were 14, 13 and 9 % respectively. This loss of CN from the solutions can be attributed to photodissociation. Ferro-cyanide complexes are quite stable in the dark, but they can dissociate rapidly, releasing free cyanide, when exposed to light. Meeussen et al. (1992) observed an initial decomposition rate of approximately 8 % per hour. In our experiment polyethylene bottles were wrapped in aluminum foil to reduce light penetration. Still, during the sampling procedure, the effect of UV radiation cannot be excluded. Additionally, the decrease in CN concentrations can be induced by the possible precipitation reaction of ferric ferrocyanide (Rennert 2002). However no precipitate was observed during the batch experiment.
In the NM root samples, the reduction of total CN concentration, in the 13, 33 and 65 mg L−1 solutions, reached on average 16, 14 and 16 % respectively. In Fig. 4 greater decrease and variation in total CN concentration in NM samples can be observed. Phytoremediation of cyanide-containing compounds is a relatively new research area. As with many new technologies, various mechanisms are either still unknown or poorly understood. Various organisms assimilate CN completely into primary metabolisms, which enable possible phytodegradation resulting in contaminant destruction. Plants and algae are protected to a degree from cyanide poisoning because their mitochondrial electron transport chain includes an alternative oxidase that minimizes the inhibition of electron transport, which is characteristic for cyanide intoxication. Cyanide can be metabolized by plants through the activity of enzymes such as β-cyanoalanine synthase and sulfur transferases. Additionally, cyanogenic plants produce cyanogenic glycosides, as a defensive compound and storage form for nitrogen free cyanide concentrations, which are subsequently maintained at low levels (Maruyama et al. 2001; Dzombak et al. 2006).
In Fig. 4 it can be noticed that both treatments containing M roots were more effective in reducing the total CN concentration in comparison to NM samples. Roots inoculated with R. irregularis decreased the total CN concentration in the 13, 33 and 65 mg L−1 solutions by average 18, 37 and 24 % respectively. For the 33 mg L−1 CN solution results are highly variable (Fig. 4b) and indicate a maximal CN reduction by 60 %. Roots inoculated with AM mixture revealed the reduction of total CN concentration in the 13, 33 and 65 mg L−1 solutions by 21, 19 and 21 % respectively. Stronger decrease in the CN concentration in the treatments inoculated with R. irregularis positively correlates with the higher mycorrhizal rate documented in the pot experiment. Greater reduction of the total CN concentration is most probably attributed to the expanded sorption surface area of the fungal hyphae in the M roots. Studies concerning AMF inoculation in plants growing on heavy metal (Pawlowska et al. 1996; Chaudhry et al. 1998) and PAH affected (Cheema et al. 2010) soils reveal upgraded contaminants accumulation and translocation via fungal hyphae that increase the absorptive surface area of plants beyond the root hair zone (Khan et al. 2000). Studies conducted by Barclay et al. (1998) demonstrated fungal (Fusarium solani, Trichodera polysporum, Fusarium oxysporum, Scytalidium thermophilum, and Penicillum miczynski) degradation of metal-cyanide complexes suggesting a role for cyanide hydratase. Formic acid and ammonia were reported as terminal degradation products, with a formamide intermediate detected in some instances. When hexacyanoferrate solution was used as a nitrogen sources for F. solani, 90 % loss of cyanide and 95 % loss of iron was observed within 34 days. In this study, the reduction of total CN concentration in the potassium hexacyanoferrate (II) solution by M roots can be possibly driven by assimilation of Fe–CN complexes as a carbon and nitrogen source.
According to O’Reilly and Turner (2003) the ability of cyanide hydratase/amidase to accept metal–cyanide complexes is limited to transformation of the cyanide liberated from the complex as it dissociates in solution. However, little loss of cyanide was observed in sterile systems, indicating that some biological component contributes to the removal of cyanide by fungi in systems containing metal–cyanide complexes (Dzombak et al. 2006). After completing the sorption experiment, concentration of free cyanide was measured for all the treatments. In Fig. 5 it can be noticed that significantly higher free CN content was documented in solutions containing no or NM roots. M roots revealed significantly greater (AM mixture) or complete (R. irregularis) reduction of free cyanide liberated from the complex as it dissociates in solution. According to the literature, direct biodegradation of the complex CN may be limited. However, if complexed cyanides cannot be degraded directly, then metal–cyanide complexes may have to dissociate first to free cyanide. Results of the free cyanide content, presented in the Fig. 5, imply a positive contribution of AM fungi in sorption of Fe–CN dissociation product.
Some plants (Hordeum vulgare, Avena sativa, Sorghum bicolor, Salix, Robinia pseudoacacia, Populus, Helianthus, etc.) have the capacity to uptake Fe–CN complexes and transport them through the cell membranes, most probably following the metal-cyanide complex dissociation. However, subsequently CN in form of Fe–CN complexes is often found in the plant’s tissues. This is apparently due to quicker complexation of CN with abundant elements, including Fe, within the plant cells, than metabolism through the activity of enzymes such as β-cyanoalanine synthase and sulfur transferases. Very little is known about the molecular, biochemical and physiological processes that characterize hyper-accumulation. It is still unclear whether Fe–CN complexes are themselves toxic, or whether the resulting adverse effects were indirectly based upon the blockage of water and solute movement across the root cell walls (Dzombak et al. 2006). Consequently, due to the dominance of complexed cyanide in the environment, it is likely that phytoremediation efforts will have to contend with this chemical species more frequently than free cyanide. AMF-plant symbiosis leads to the additional absorptive surface area that increases water, mineral uptake and plant biomass (Khan et al. 2000), which can result in the dilution of Fe–CN concentration in the plant tissues. Since cyanide is a valuable source of carbon and nitrogen, establishing plants-AMF symbiosis can potentially increase its utilization prior to reaching toxic level while storing or undergoing the complexation reaction in plants tissues. However, more studies need to be conducted to fully investigate the mechanisms supporting the phenomenon of iron–cyanide uptake and metabolism by roots inoculated with AM fungi.
Conclusion
Considering the abundance of Fe in many environmental matrices, it can be assumed that remediation activities will more frequently encounter complexed cyanide compounds, which until nowadays has proven to be energy and cost-intensive. Recently, increasing number of scientists report improved plants performance in the removal of toxic compounds with the support of mycorrhizae fungi. However, its role in remediation of iron–cyanide complexes is unknown. The presented study demonstrates AMF colonization of the roots at the former MGP site, implying the existence of species capable of surviving extremely toxic conditions. Furthermore, mycorrhizal root colonization caused significantly higher reduction of the total and free cyanide concentration in the solutions, most probably as a result of additional sorption surface area of the fungal hyphae increasing assimilation of Fe–CN complexes. Our study proposes the potential enhancement of phytoremediation efforts, for treating iron–cyanide contaminated soil, by establishing AMF-plant symbiosis.
References
Alarcón A, Delgadillo-Martínez J, Franco-Ramírez A, JrFT Davies, Ferrera- Cerrato R (2006) Influence of two polycyclic aromatic hydrocarbons on spore germination, and phytoremediation potential of gigaspora margarita-Echynochloa polystachya symbiosis in benzo[a]pyrene-polluted substrate. Rev int contam ambie 22:39–47
Barclay M, Tett VA, Knowles C (1998) Metabolism and enzymology of cyanide/metallocyanide biodegradation by Fusarium solani under neutral and acidic conditions. Enzyme Microb Technol 23:321–330
Boldt-Burisch KM, Gerke HH, Nii-Annang S, Schneider BU, Hüttl RF (2013) Root system development of Lotus corniculatus L. in calcareous sands with embedded finer-textured fragments in an initial soil. Plant Soil 368:281–296
Chaudhry TM, Hayes WJ, Khan AG, Khoo CS (1998) Phytoremediation focusing on accumulator plants that remediate metal contaminated soils. Aust J Ecotoxicol 4:37–51
Cheema A, Khan MI, Shen CF, Tang XJ, Farooq M, Chen L, Zhang CK, Chen YX (2010) Degradation of phenanthrene and pyrene in spiked soils by single and combined plants cultivation. J Hazard Mater 177:384–389
Chen B, Roos P, Zhu YG, Jakobsen I (2008) Arbuscular mycorrhizas contribute to phytostabilization of uranium in uranium mining tailings. J Environ Radioactiv 99:801–810
Corgié SC, Fons F, Beguiristain T, Leyval C (2006) Biodegradation of phenanthrene, spatial distribution of bacterial populations and dioxygenase expression in the mycorrhizosphere of Lolium perenne inoculated with Glomus mosseae. Mycorrhiza 16:207–212
Cunningham SD, Berti WR, Huang JW (1995) Phytoremediation of contaminated soils. Trends Biotechnol 13:393–397
Dimitrova T (2010) Determination of cyanides in contaminated coils using micro distillation- and spectrophotometric flow injection system. Dissertation, Brandenburg University of Technology Cottbus-Senftenberg
Dimitrova T, Repmann F, Freese D, Raab T (2014) Uptake of ferrocyanide in willow and poplar trees in a long term greenhouse experiment. Ecotoxicology. doi:10.1007/s10646-014-1398-0
DIN EN ISO 14 403 (2002) Bestimmung von gesamt Cyanid und freiem Cyanid mit der kontinuierlichen Fließanalytik-Teil D
Dursun AY, Calik A, Aksu Z (1999) Degradation of ferrous(II) cyanide complex ions by Pseudomonas fluorescens. Process Biochem 34:901
Dzombak DA, Ghosh RS, Young TC (2006) Physical–chemical properties and reactivity of cyanide in water and soil. In: Dzombak DA, Ghosh RS, Wong-Chong GM (eds) Cyanide in water and soil: chemistry, risk and management. CRC Press, Boca Raton, pp 57–88
Ebbs SD, Bushey JT, Poston S, Kosma D, Samiotakis M, Dzombak DA (2003) Transport and metabolism of free cyanide and iron cyanide complexes by willow. Plant Cell Environ 26:1467–1478
EPA (1999) A Resource for MGP site characterization and remediation expedited site characterization and source remediation at former manufactured gas plant sites. U.S. Environmental Protection Agency Office of Solid Waste and Emergency Response Technology Innovation Office Washington DC
Gao Y, Cheng Z, Ling W, Huang J (2010) Arbuscular mycorrhizal fungal hyphae contribute to the uptake of polycyclic aromatic hydrocarbons by plant roots. Bioresour Technol 10:6895–6901
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscula rmycorrhiza infection in roots. New Phytol 84:489–500
Hernández-Ortega HA, Alarcón A, Ferrera-Cerrato R, Zavaleta-Mancera H, López-Delgado HA, Mendoza-López MA (2012) Arbuscular mycorrhizal fungi on growth, nutrient status, and total antioxidant activity of Melilotus albus during phytoremediation of a diesel-contaminated substrate. J Environ Manage 95:319–324
Khan AG, Kuek C, Chaudhry TM, Khoo CS, Hayes WJ (2000) Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 41:197–207
Kjeldsen P (1998) Behavior of cyanides in soil and groundwater: a review. Water Air Soil Poll 115:279–307
Knowles CJ (1976) Microorganisms and cyanide. Bact Rev 40:652–680
Larsen M, Trapp S, Pirandello A (2004) Removal of cyanide by woody plants. Chemosphere 54:325–333
Linder M, Bugmann H, Lasch P, Fleschig M, Cramer W (1997) Regional impacts of climatic change on forests in the state of Brandenburg, Germany. Agric Forest Meteorol 84:123–135
Maruyama A, Saito K, Ishizawam K (2001) β-cyanoalanine synthase and cysteine synthase from potato: molecular cloning, biochemical characterization, and spatial and hormonal regulation. Plant Mol Biol 46:749–760
Meeussen JL, Keizer MG, de Haan FAM (1992) Chemical stability and decomposition rate of iron cyanide complexes in soil solutions. Environ Sci Techol 26:511–516
Nikbakht A, Pessarakli M, Daneshvar-Hakimi-Maibodi N, Kafi M (2013) Perennial ryegrass growth responses to mycorrhizal infection and humic acid treatments. Agron J 106(2):585–595
O’Reilly C, Turner PD (2003) The nitrilase family of CN hydrolyzing enzymes—a comparative study. J Appl Microbiol 95:1161–1174
Pawlowska TE, Blaszkowski J, Ruhling A (1996) The mycorrhizal status of plants colonizing a calamine spoil mound in southern Poland. Mycorrhiza 6:499–505
Peel MC, Finlayson BL, McMahon TA (2007) Updated world map of the Köppen Geiger climate classification. Hydrol Earth Syst Sc 11:1633–1644
Reeves M (2000) Treatment of fluoride and iron cyanides using willow: a greenhouse feasibility study. Dissertation, Cornell University
Rennert T (2002) Sorption of iron–cyanide complexes on iron oxides and in soils. Dissertation, Ruhr-Universität Bochum
Rennert T, Ufhold S, Mansfeldt T (2007) Identification of iron–cyanide complexes in contaminated soils and wastes by fourier transform infrared spectroscopy. Environ Sci Technol 41:5266–5270
Samiotakis M, Ebbs SD (2004) Possible evidence for transport of an iron cyanide complex by plants. Environ Poll 127:169–173
Soka G, Ritchie M (2014) Arbuscular mycorrhizal symbiosis and ecosystem processes: prospects for future research in tropical soils. Open J Ecol 4(1):11–22
Sut M, Repmann F, Raab T (2015) Retardation of iron–cyanide complexes in the soil of a former manufactured gas plant site. J Environ Sci Heal A. doi:10.1080/10934529.2015.981116
Theis TL, Young TC, Huang M, Knutsen KC (1994) Leachate characteristics and composition of cyanide-bearing wastes from manufactured gas plants. Environ Sci Technol 28:99–106
Trapp S, Koch I, Christiansen H (2001) Aufnahme von cyanid in pflanzen: risiko oder chance fuer die phytoremediation? Umwelt Schad Forsch 13:1–10
Trouvelot A, Kough J, Gianinazzi-Pearson V (1986) Evaluation of VA infection levels in root systems. Research for estimation methods having a functional significance. In: Gianinazzi-Pearson V, Gianinazzi S (eds) Physiological and genetical aspects of mycorrhizae. INRA, France, pp 217–221
USEPA, Method 10-204-00-1-X (2008) Lachat USEPA Approved and equivalent method revision 3 22.01
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microb 64(12):5004–5007
Xiao-Zhang Y, Pu-Hua Z, Yong-Miao Y (2006) The potential for phytoremediation of iron cyanide complex by willows. Ecotoxicology 15:461–467
Yu XZ, Wu SC, Wu FY, Wong MH (2011) Enhanced dissipation of PAHs from soil using mycorrhizal ryegrass and PAH-degrading bacteria. J Hazard Mater 186:1206–1217
Zhang FZ, Yu XZ, Gu JD (2013) Transport and assimilation of ferricyanide by three willow species. Water Air Soil Pollut. doi:10.1007/s11270-013-1522-4
Acknowledgments
This study is partially supported by the German Railways (Deutsche Bahn AG) and it contributes to the Virtual Institute of Integrated Climate and Landscape Evolution Analysis –ICLEA- of the Helmholtz Association.This study was supported by Deutsche Bahn (DB) AG within the project “Stabilisierung des DB AG-Standortes durch Verfahren der Bioremediation (Phytoremediation)”. The authors gratefully acknowledge great field and laboratory assistance provided by Neema Munuo, Obinna Duke, Folasade Olagoke and Sheeva Keshavarz. We would also like to acknowledge the help of Katharine Bendele for English language editing and anonymous reviewers for providing helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors declare that all the experiments conducted for this study were in compliance with the present German legislation.
Rights and permissions
About this article
Cite this article
Sut, M., Boldt-Burisch, K. & Raab, T. Possible evidence for contribution of arbuscular mycorrhizal fungi (AMF) in phytoremediation of iron–cyanide (Fe–CN) complexes. Ecotoxicology 25, 1260–1269 (2016). https://doi.org/10.1007/s10646-016-1678-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1678-y