Abstract
The objectives of this study were to assess the sediment contamination with heavy metals and to investigate accordingly the ecological risk posed in the SE of the Danube Delta. Sediments are important in assessing the contamination as they act as reservoirs, transporters and contamination sources. Sediment samples were collected and analysed for lead, cadmium, arsenic and mercury, revealing levels higher than the background, especially for cadmium and mercury (Pb > As > Cd > Hg). Concentrations exceeding the probable effect limit were noticed for arsenic and mercury. The contamination indexes describe the study area as having almost half of the samples as contaminated (pollution load index-PLI 1.04), however the contamination is mostly low-to moderate (modified contamination degree-mCd 1.36). The sediment contamination poses mostly a low ecological risk (RI 94.8). The sediment quality guideline quotient (SQG-Q 0.29) describes a moderate impact, while the probable effect concentration quotient (PEC-Q 0.16) confirms that there are no levels likely to affect the aquatic biota. In our study area, the main Branch of the Danube River and the Secondary Delta are the most affected by contamination, while the narrow, reed abundant channels as the preferred habitat of most aquatic organisms, have a low contamination level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The contamination of sediments with heavy metals is a matter of great concern, because of their persistence, bioaccumulative nature and toxicity. Among heavy metals, lead, cadmium, mercury, and arsenic, involve most often toxic effects on living organisms, as they have no known beneficial effects in living organisms as microelements (Banu et al. 2013). Arsenic is a metalloid included in the heavy metals group, as it has a similar behaviour in the environment. The three metals—lead, cadmium and mercury, and the metalloid—arsenic have caused significant health problems for human and non-human populations from various parts of the world. The toxicity of these elements has been recognized for many years, lead being known in ancient Greece as being toxic as far back as the 2nd century BC (Hutton 1987; Keil et al. 2010).
Heavy metals have a propensity to adsorb from aqueous phases to fine suspended particles and they are transported along the water course, where they can pose health risks to benthic organisms if toxic levels are reached, resulting in lower taxonomic diversity, lower reproduction rate, reduced growth or even death (Nasir and Harikumar 2011; Ogbeibu et al. 2014). According to Filgueiras et al. (2004), not even 1 % of the pollutants released in water remain in aqueous phases, the rest being deposited in sediment. Since sediment becomes an important sink for heavy metals that contaminate the water and acts as a habitat and an important food source for aquatic biota, its quality provides essential information for assessing the pollution status of an aquatic ecosystem, as it reflects the long term status (Nasir and Harikumar 2011). However, since a delta is mainly formed mainly of deposited sediments (Panin 1996), under changing water conditions, heavy metals can be released back to overlying waters through complex remobilisation processes, described in a previous paper (Gati et al. 2013).
The Danube River collects pollutants and heavy metals across its flow through Europe, forming a delta when reaching the Black Sea. The Danube Delta together with the Razim-Sinoe lagoon complex is the only delta stated as an UNESCO World Heritage Biosphere Reserve. It supports a high diversity of life—over 1600 plant species and over 3800 animal species, constituting a feeding ground of international importance for migrating birds (Wohl 2012). According to WWF reports it is one of the last habitats for some endangered species, like sturgeons, beavers, great white pelicans, and others.
The objectives of this research paper are to determine the level of lead (Pb), cadmium (Cd), arsenic (As) and mercury (Hg) in surface sediments from SE Danube Delta and to evaluate the ecological risks associated with the combined contamination of these metals using different SQG indexes.
Materials and methods
Study area and sampling
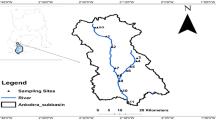
The sampling was performed in five different periods of the year during October 2012–September 2013 in the S–E of the Danube Delta, at the end sector of the Sf. Gheorghe Branch of the Danube River, near the locality of the same name (44º53′N and 29º36′E), between the Central and Turcesc Channels. The study area is known for having minimum local anthropogenic contamination sources in the Danube Delta, so it would describe the pollution generated upstream the Delta and support the theory of transportation of metals. We had 13 sampling sites (Fig. 1) and a total number of 55 samples. Some of the sampling sites were not available during the colder periods of the year. Sediment samples were collected at the margin of the river bed of the main branch and channels, at 5 cm under the river bed surface, in 50 ml polyethylene demineralised containers, and kept at 4 °C.
Analysis
Sediment samples were analysed for lead (Pb), cadmium (Cd), arsenic (As) and mercury (Hg). They were subjected to a microwave digestion, using a Mars 6 Microwave digester. One gram of sediment was digested in suprapure nitric acid, and after that it was diluted with ultrapure water to a 50 ml volume and analysed using Zeenit 700P atomic absorption spectroscopy (AAS).
For the analysis of Pb and Cd, the electrothermal (ET-AAS) method was used, and the hydride generation (HG-AAS) method for As, with amalgamation and cold vapour (CVAA) technology for Hg.
A soil Certified Reference Material (SRM 2709a) was examined using the same method. The results indicated, according to the quality certificate, an approximate recovery rate of 74 % for As, 110 % for Cd, 53 % for Pb and 95 % for Hg.
Temperature and pH of the overlying water was measured with a portable multiparameter analyzer (Waterproof pH tester HI 98127, Hannah Instruments).
Quality control and assurance
Quality control (QC) was made through specific methods, to evaluate the accuracy of our analyses, meeting the Romanian Accrediting Association (RENAR) standards and certifications. RENAR is a member of European Accreditation Organism (EA-MLA), certificated under SR EN ISO/CEI 17011.
An external standard curve method was used for the calculation of the concentrations, using control samples plotted between 0 and 10 µg/l, for Pb, Cd, As—0–4 µg/l, respectively 0–1 µg/l for Hg. Dilutions were done with 2 % v/v HNO3 in a volumetric flask. The calibration curve was plotted every day (with batch analyses not larger than 20 samples per day) using MRC ChemLab. The control sample was prepared with another standard solution than the one used for the calibration curve. The control and blank samples were analysed before, after every 10th, and after the last sample. The equivalent concentrations were calculated for sediment samples in mg/kg at 0–0.5 for Pb and As, 0–0.2 for Cd and 0–0.05 for Hg, and higher values were diluted to fit in the calibration curve.
The method detection limit (MDL) was 0.1, 0.025, 0.025 and 0.005 mg/kg for Pb, Cd, As and Hg. The uncertainty of the methods ranged between 13 % (Hg) and 18.56 % (As). Proficiency test samples for Cd and As indicated z scores of 0.01/0.04.
A Shewhard control diagram was computed using the results of the control samples, with daily and long-term interpretations. The laboratory participated in intercomparison analyses organised by ROLAB (accredited by RENAR).
Contamination and risk assessment
To evaluate the ecological status of the study area, following the contamination with heavy metals, a number of factors and indices were used. Some of them evaluate the contamination while the others assess the potential ecological risk posed by the pollutants toxicity. The more complex ecological risk indices are based on sediment guidelines or specific values given for each contaminant, according to their toxicity and impact.
The contamination factor (C f ) (Hakanson 1980; Tomlinson et al. 1980) describes the sediment contamination of toxic substances, being computed for each element at a time. It is a background enrichment index that stands as a basis for some of the following indexes. The contamination factor can be defined as:
In this study, the background values were set according to the mean concentration found at 50 cm depth in the Danube Delta lakes by Dinescu et al. (2004). To describe the contamination factor using a risk index approach, four thresholds are identified: Cf < 1; < 3; < 6 and > 6, representing low, moderate, considerable and very high contamination factor (Hakanson 1980).
The degree of contamination is a form of index that aggregates all the contaminants into one value, based on the contamination factor (C f ).We used a modified degree of contamination (mC d ) suggested by Abrahim and Parker (2008) that takes into account the number of elements (n), being defined as:
This index has a very detailed terminology, indicating values between seven thresholds: mC d < 1.5; < 2; < 4; < 8; < 16; < 32 and > 32, indicating nil to very low, low, moderate, high, very high, extremely high, respectively ultra-high degrees of contamination (Abrahim and Parker 2008).
Pollution load index (PLI) is an ecological risk index created by Tomlinson et al. (1980). It was widely used in different water systems around the world, allowing a comparison to be made. It is based on the contamination factor (C f ), being defined as:
If PLI is lower than 1 it indicates no pollution, while any value above 1 indicates pollution (Tomlinson et al. 1980).
The potential ecological risk index (RI), developed by Hakanson (1980), is also based on the contamination factor (C f ), but it is more complex, as it adds a toxicological factor (T if ) for each metal. (Pb-5, Cd-30, As-10, Hg-40). First, a risk factor (Er i) is computed for each metal, and then the potential ecological risk index (RI), as the sum of the risk factors:
The RI-values can be ranked following the terminology: RI < 150; < 300; < 600 and > 600; for low, moderate, considerable and very high ecological risk for the basin (Hakanson 1980).
The mean sediment quality guideline quotient (SQG-Q) is an ecological risk index developed by Long and MacDonald (1998) that evaluates toxicity according to the probable effect level (PEL), a concentration above which biological adverse effects can occur frequently (Pb-91.3, Cd-3.53, As-17, Hg-0.486). AEL quotient is computed for each contaminant (C), in accordance with the corresponding PEL value. Sediments are scored in three categories according to their impact (MacDonald et al. 2000): SQG-Q ≤ 0.1 for unimpacted, with the lowest potential for adverse biological effects to be observed; <1 for a moderate impact with potential for adverse biological effects and ≥1 for highly impacted and a high potential for observed adverse biological effects. The SQG-Q can be defined as:
For the evaluation of the effects on the ecosystem of heavy metals from the sediments, the probable effect concentration quotient (PEC-Q) values were computed for the metals, and then the mean PEC-Q value was calculated, according to Formula 7, for each sampling site. PEC values for assessing metal toxicity are 128, 4.98, 33, 1.06 for Pb, Cd, As and Hg. As PEC values are accounted as denominators in the formula, the lower the value, the higher the toxicity and the influence in the general result.
where, [Mc] i is the concentration of a metal in a sample and PEC i is the corresponding PEC value for that specific metal; n is the number of metals.
The mean PEC-Q value provides a basis for the evaluation of the potential toxic effects that heavy metals can have, in complex mixture conditions (MacDonald et al. 2000; USEPA 2000). The thresholds for this indicator are set according to toxicity tests assessing acute or chronic effects on the amphipod Hyalella azteca. Analyses conducted by USEPA (2000) on a large freshwater database showed that sediment samples with a mean PEC-Q higher than 0.7, respectively 4 are more likely to have acute or chronic toxic effects, especially on benthonic organisms. While the PEC-Q approach remains an empirically based guideline, it is better than comparing measured concentrations or PEC values for individual metals, for assessing toxicity, as it accounts for possible summing contributions in the overall toxicity. Long et al. (2006) reported PEC-Q values regarding two critical thresholds—0.25 and 0.34.
Results and discussions
Even if there aren’t any major contamination point sources identified in the sampling area, concentrations of studied heavy metals were found in sediments (Table 1). A study undertaken in North Carolina found that many sediments were contaminated in areas with no industrial activities or urban settlements (Pelley 1999). This is an evidence that sediments can act as a long-term source of contamination for the overlying water and the food chain, as pulses of polluted sediments can lead to the recontamination of the system, impacting the receiving system (Burton 2002).
The variability of lead showed higher levels in March and lower levels in October (2012) and September (2013). Arsenic had a cyclic seasonal variation, low in March and high in July. Cadmium and mercury had low levels in October, respectively high in July. According to national regulations (RN 2006), (Pb-85, As-29, Cd-0.8 and Hg-0.3, in mg/kg) cadmium and mercury surpassed maximum allowed concentrations in 13, respectively 20 samples.
By comparing the results with the most common sediment quality guidelines (SQGs): TEL (threshold effects limit) and PEL (probable effects limit), we can confirm that there are concentrations with the potential to impact the ecosystem. The smallest hazard comes from Pb contamination, with 5.5 % of the values exceeding TEL, followed by Cd and Hg with 32.7 % and toping with As, with 63.6 %. Furthermore, As and Hg registered 7.3 % of the values exceeding PEL. According to these two SQG levels, three ranges can be delineated. So, concentrations below TEL are in a minimal effect range; between TEL and PEL are in a possible effect range; and above PEL are in a probable effect range, being rarely, occasionally or frequently associated with adverse biological effects (Smith et al. 1996; Li 2014). These can be identified in Fig. 2.
The dataset was divided according to four predefined areas. These are, as in Fig. 1, the Danube’s main branch—Sf. Gheorghe Branch (2); the Secondary Delta and Melea Lagoon (1), formed in the SE; and the channels from the northern side of the Danube (3) and the southern side (4). These four areas are different when it comes to hydrological status, and have exchanges with various water systems, describing four ecosystem subtypes. Figure 2 presents the level of contamination in these areas. The most contaminated areas are the Danube’s Branch (2) and the Secondary Delta (1) with lead (mg/kg) [12.9–40.3] and [9.2–47.5]; cadmium (mg/kg) [0.07–0.99] and [0.07–1.34]; arsenic (mg/kg) [3.6–18.4] and [1.1–20.6]; mercury (mg/kg) [0.03–0.99] and [0.01–0.91]. The Channels from South (4) and North (3) are less contaminated with lead (mg/kg) [6.4–32.2] and [5.4–14.1]; cadmium (mg/kg) [0.04–1.2] and [0.04–0.07]; arsenic (mg/kg) [2.2–16.4] and [1.1–10.3]; mercury (mg/kg) [0.01–0.41] and [0.005–0.15]. The median values are close to the average values, indicating a uniform distribution.
The contamination factors reveal an enrichment for Cd and Hg, in relation to the average concentrations (Pb-24, Cd-0.2, As-11, Hg-0.1, all in mg/kg) found in the Danube Delta`s deep layer sediments dating back in the early 20th Century (Dinescu et al. 2004). The average Cf values for these metals exceed the contamination threshold, with 60 %, respectively 47.3 % of the samples being recorded over the threshold value of “1” (Table 2).
As a starting point, according to the PLI, we can distinguish 45.5 % of the samples with some degree of pollution (Fig. 3b). This is not a very detailed outcome, but allows a simple comparison between several rivers, estuaries, wetlands, as it is often used in studies. Our study area revealed an average PLI of 1.04, which in comparison with other water systems is slightly higher, although there are other protected wetlands with much higher levels (Table 3).
Results of the contamination and ecological risk indexes, differentiated by area: orange triangle—1, blue circle—2, purple diamond—3, green square—4; a modified contamination degree, b pollution load index, c potential ecological risk index, d probable effect concentration quotient (Color figure online)
Since PLI lacks in a detailed qualitative description, relating to mCd’s more complex terminology we can describe 61.8 % of the samples indicating nil to very low contamination, 12.7 % low contamination, 23.6 % moderate contamination, respectively 1.8 % indicating high contamination (Fig. 3a). The average mCd is 1.36, framed in the nil to very low contamination category.
Hakanson’s (1980) approach for assessing the ecological risk of a polluted aquatic system is often used in risk assessment studies. From RI results, of the RI, 81.8 % of the samples indicate a low risk, 12.7 % indicate a moderate risk, and 5.5 % indicate a considerable ecological risk for the basin (Fig. 3c). The average value for the ecological risk index is 94.8. This value designates a low average risk, although it is higher than Italian rivers, or Yellow River Delta, but still, lower than Yangtze River (Table 3).
In accordance to PEL values, the SQG-Q index revealed an average value of 0.29, describing 10.9 % of the samples as unimpacted, with the lowest potential for adverse biological effects and 89.1 % with a moderate impact and potential for adverse biological effects to be observed.
The mean PEC-Q is a toxicity index that is useful for assessing the potential effects of complex mixtures of sediment contaminants (MacDonald et al. 2000; USEPA 2000). Mean PEC-Q values did not exceed EPA’s value of 0.7, indicating that there are no acute or chronic toxic effects for benthonic organisms, with an average of 0.16. However, 18.2 % of the samples exceed the threshold of 0.25, associated with a less than 20 % incidence of toxicity for freshwater amphipods (USEPA 2000) and 5.5 % exceed 0.34, associated with a likely decrease in abundance of the amphipods, gastropods, polychaetes or other benthic organisms (Fig. 3d). The sediment-dwelling biota is very likely to be exposed to sediment contamination due to limited mobility, indirectly affecting also higher trophic organisms in the food chain.
The four predefined areas have different subtypes of ecosystems, since the water conditions vary from one to another. Many fish species tend to abide in the calmer waters of the channels and use them for spawning, nursing and foraging, rather than in the faster and higher flow rate of Danube’s Branch. Most of the Delta’s Channels have abounding reed plants towards the banks, creating perfect habitats for small aquatic organisms. Previous studies on fish habitat in this branch of the Danube River (Sfantu Gheorghe) observed the highest fish densities in the connected side waters, while the main branch had significantly bigger fish (Sindilariu et al. 2006). As it can be seen in Fig. 2, the concentration of heavy metals is higher in Danube’s Branch (2) and in the Secondary Delta (1) than in the Channels from the Southern side (4) and Northern side (3). It is clear that the Danube’s Branch is the most contaminated and poses the highest risk for the aquatic biota, followed by the Secondary Delta with the Lagoon and then the Southern and Northern Channels with lower contamination and risk levels (Table 4). While the Secondary Delta is unconditionally related to Danube’s Branch, which can be clearly observed regarding the sediments quality, the Southern Channels have some inflows from the Danube’s Branch, depending on the general water level in the area. The Northern Channels are linked to a large lake system—Rosu-Puiu-Rosulet, which probably acts as a buffer, being a sink for sediments, which explains the significantly lower levels of contamination and risk. Also, in the individual index values (Fig. 3) it can be observed that the highest values belong mainly to the Danube’s Branch (orange triangle) and the Secondary Delta (blue circle) areas.
Sediment becomes an important sink for the heavy metals that originally contaminate the water. However, it can also act as a potential contamination source for the overlying water in areas with no pollution sources, depending on the continuously changing water conditions. The changes in the physicochemical parameters of water alter the bioavailability of the heavy metals (Simpson and Batley 2003) and affect the release rate from sediments to the overlying water (Simpson et al. 2002; Peng et al. 2009). The complex processes which influence the metal concentrations in water and sediment are mainly influenced by pH, temperature, salinity, dissolved oxygen and organic matter content (Simpson et al. 2004), resulting in complex chemical reactivity and interactions between the solid and the solution phases of the trace metals (Guieu and Martin 2002; Peng et al. 2009). These physicochemical modifications that occur in water can lead to heterogeneous reactions like dissolution/precipitation, adsorption/desorption and coagulation/sedimentation, metals being precipitated in the sediments, dissolved from the suspended matter, or diluted in the seawater (Guieu and Martin 2002). Mercury and arsenic levels in sediment were found to have a positive correlation with temperature (0.587, p = 0.004, respectively 0.523, p = 0.013) and pH (0.518, p = 0.014, for Hg only) showing that the deposition process might be more intense in higher pH and temperature conditions. pH values varied in the [6–8.8] interval, six samples being <6.5, and three samples >8.5, compared to the national regulations (RN 2006). The concentration of mercury in sediments is influenced by the hyperoxic/hypoxic conditions of the overlying water. Hypoxic conditions, usually met in high temperature conditions, are associated with a high rate of deposition of total Hg and a low rate of speciation (Duong and Han 2011). The correlation of As in sediment with temperature might be due to the strong relation of the dissolution/precipitation processes of As with the hyperoxic/hypoxic environment (Smedley and Kinniburgh 2002). Only a small fraction of the total arsenic is available for exchanges between the sediment–water systems. Large quantities of As co-precipitates with iron and manganese or adsorbs to Fe(OH)3 surfaces, being accumulated within the top 5 cm of the sediment–water interface. So As concentration in sediment is highly related to those of Fe and Mn. Moreover, the arsenic in the form of As(III) has a small variability during pH conditions between 4 and 7 (Nikolaidis et al. 2004). The level of arsenic in water was recorded within normal values and it was observed to appear in contamination plumes (Gati et al. 2015). The fact that there is a high variability confirms that most of the arsenic originates upstream and less from sediment remobilisation processes.
Statistically, in our study, the sediment fractions of the metals show an important correspondence. Cadmium is correlated with all three metals—lead (0.568, p = 0.001), mercury (0.551, p = 0.001) and arsenic (0.439, p = 0.001). The correlation between arsenic and mercury (0.434, p = 0.002) and lead and arsenic (0.374, p = 0.007) can be added to complete the picture. Even though the metals in sediment react differently to the water conditions, on the long term, the release and deposition rates correspond for all the studied metals, and probably beyond, due to the seasonal cycles which are creating or influencing those water conditions.
Conclusions
Even without major contamination point sources in the study area, the studied heavy metals were found in sediment, due contamination and transportation from the Danube River’s Basin. As water velocity drops in the delta, towards the Black Sea, most of the sediment drops out of the flow and deposits.
All four metals have concentrations exceeding TEL, while arsenic and mercury are also exceed PEL. The order of the metals in sediment was (Pb > As > Cd > Hg).
Compared to the background levels, the studied area is contaminated with cadmium and mercury, according to the contamination factor. However, the specific indexes (mCd) quantify the overall contamination as low to moderate in over 95 % of the contaminated samples. The ecological status of the studied water system is described according to the SQG-Q as moderately impacted with a low to moderate risk for the aquatic organisms, according to RI. There were very few samples with high contamination level that pose a considerable risk and could decrease the taxonomic abundance of the sediment dwelling biota, even so, the health of the ecosystem could not be affected. According to the PEC-Q there are no levels that could produce any chronic or acute adverse effects to the benthonic biota.
The most suitable index for assessing the contamination level would be mCd, as it has a detailed qualitative description, while for assessing the ecotoxicological status, it would be the mean PEC-Q, as it describes potential effects based on complex studies. Even though PLI is not a complex index, it is more widely used, so it acts as a comparison method.
Sediment contamination affects primarily benthonic organisms but indirectly, also water-dwelling organisms, due to their higher trophic level or in cases of heavy metal remobilization to the overlying water.
The highest contamination was observed in the Danube’s Branch followed by the Secondary Delta and Lagoon, while the Southern and Northern Channels are less contaminated. The ecological risk follows the same principle. According to these results, the general ecosystem can be described as having favourable living conditions, since higher trophic organisms (e.g. fish) prefer the calmer and better water conditions found in channels.
Lead and arsenic concentrations in water varied inversely with the temperature and pH showing a higher remobilisation at lower values of pH and temperature, while mercury and arsenic levels in sediment varied along with the water temperature and pH showing that the deposition process is more intense in higher pH and temperature conditions.
References
Abrahim GMS, Parker PJ (2008) Assessment of heavy metal enrichment factors and the degree of contamination in marine sediment from Tamaki Estuary, Auckland, New Zeeland. Environ Monit Assess 136:227–238
Banu Z, Chowdhury SA, Hossain D, Nakagami K (2013) Contamination and ecological risk assessment of heavy metal in the sediment of Turag River, Bangladesh: an index analysis approach. J Water Res Prot 5:239–248
Burton GA Jr (2002) Sediment quality criteria in use around the world. Limnology 3:65–75
Chakravarty M, Patgiri AD (2009) Metal pollution assessment in sediments of the Dikrong River, NE India. J Hum Ecol 27(1):63–67
Dinescu LC, Steinnes E, Duliu OG, Ciortea C, Sjøbakk TE, Dumitru DE, Gugiu MM, Haralambie M (2004) Distribution of some major and trace elements in Danube Delta lacustrine sediments and soils. J Radioanal Nucl Chem 262(2):345–354
Duong HV, Han S (2011) Benthic transfer and speciation of mercury in wetland sediments downstream from sewage outfall. Ecol Eng 37(6):989–993
Filgueiras AV, Lavilla I, Bendicho C (2004) Evaluation of distribution, mobility and binding behaviour of heavy metals in surficial sediments of Louro River (Galicia, Spain) using chemo metric analysis: a case study. Sci Total Environ 330(1–3):115–129
Gati G, Pop C, Brudasca F, Gurzau AE, Spinu M (2013) Assessment of the heavy metal contamination in the Danube Delta from the bioaccumulation perspective. Glob J Hum Soc Sci 13(8):11–16
Gati G, Pop C, Brudasca F, Gurzau AE, Spinu M (2015) Hydrological modeling of arsenic in the Danube Delta. Environ Eng Manag J (in press)
Guieu C, Martin M (2002) The level and fate of metals in the Danube River Plume. Estuar Coast Shelf Sci 54:501–512
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hutton M (1987) Human health concerns of lead, mercury, cadmium and arsenic. In: Hutchinson TC, Meema KM (eds) Lead, mercury, cadmium and arsenic in the environment. Wiley, New York
Keil DE, Berger-Ritchie J, McMillin GA (2010) Testing for toxic elements: a focus on arsenic, cadmium, lead, and mercury. Lab Med 42(12):735–742
Kumar B, Kumar S, Mishra M, Prakash D, Singh SK, Sharma CS, Mukherjee DP (2011) An assessment of heavy metals in sediments from two tributaries of lower stretch of Hugli estuary in West Bengal. Arch Appl Sci Res 3(4):139–146
Li J (2014) Risk assessment pf heavy metals in surface sediments from the Yanghe river, China. Int J Environ Res Public Health 11:12441–12453
Liu ZJ, Zhang XL, Li P, Zhu LH (2012) Regional distribution and ecological risk evaluation of heavy metals in surface sediments from coastal wetlands of the Yellow River Delta. Huan Jing Ke Xue 33(4):1182–1188
Long ER, MacDonald DD (1998) Recommended uses of empirically derived, sediment quality guidelines for marine and estuarine ecosystems. Hum Ecol Risk Assess 4:1019–1039
Long ER, Ingersoll CG, MacDonald DD (2006) Calculation and uses of mean sediment quality guideline quotient: a critical review. Environ Sci Technol 40:1726–1736
Lu SC, Zhang H, Shan BQ, Li LQ (2013) Spatial distribution and ecological risk assessment of heavy metals in the estuaries surface sediments from the Haihe River Basin. Huan Jing Ke Xue 34(11):4204–4210
MacDonald DD, Ingersoll CG, Berger TA (2000) Development and evaluation of consensus based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Nasir UP, Harikumar PS (2011) Ecotoxicology and ecosystem health of Ramsar Wetland System of India. J Environ Prot 2:710–719
Nikolaidis NP, Dobbs GM, Chen J, Lackrovic JA (2004) Arsenic mobility in contaminated lake sediments. Environ Pollut 129(3):479–487
Ogbeibu AE, Omoigberale MO, Ezenwa IM, Eziza JO, Igwe JO (2014) Using pollution load index and geoaccumulation index for the assessment of heavy metal pollution and sediment quality of the Benin River, Nigeria. Nat Environ 2(1):1–9
Ong MC, Menier D, Shazili NAM, Kamaruzzaman BY (2013) Geochemical characteristics of heavy metals concentration in sediments of Quiberon Bay Waters, South Brittany, France. Orient J Chem 29(1):39–45
Panin N (1996) Danube Delta genesis, evolution, geological setting and sedimentology, Geo-Eco-Marina, RCGGM, 1/1996: 7–23
Pelley J (1999) North Carolina considers controls to protect contaminated waters. Environ Sci Technol 33(1):10A
Peng JF, Song YH, Cui XY, Qiu GL (2009) The remediation of heavy metals contaminated sediment. J Hazard Mater 161:633–640. doi:10.1016/j.jhazmat.2008.04.061
Protano C, Zinna L, Giampaoli S, Spica VR, Chiavarini S, Vitali M (2014) Heavy metal pollution and potential ecological risks in river: a case study from southern Italy. Bull Environ Contam Toxicol 92:75–80
Rabee AM, Al-Fatlawy YF, Najim H, Nameer M (2011) Using pollution load index (PLI) and geoaccumulation index (I-Geo) for the assessment of heavy metals pollution in Tigris River sediment in Baghdad Region. J Al-Nahrain Univ 14(4):108–114
Rahman MS, Saha N, Molla AH (2013) Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ Earth Sci. doi:10.1007/s12665-013-2631-5
RN (2006) Romanian Normative framework 161/2006 regarding the rating of the water surfaces for setting the ecological status of water bodies, Romanian Official Monitor, no 511
Seshan BRR, Natesan U, Deepthi K (2010) Geochemical and statistical approach for evaluation of heavy metal pollution in core sediments in southeast coast of India. Int J Environ Sci Tech 7(2):291–306
Simpson SL, Batley GE (2003) Disturbances to metal partitioning during toxicity testing of iron(II)-rich estuarine pore waters and whole sediments. Environ Toxicol Chem 22(2):424–432
Simpson SL, Rochford L, Birch GF (2002) Geochemical influences on metal partitioning in contaminated estuarine sediments. Mar Freshw Res 53(1):9–17
Simpson SL, Maher EJ, Jolley DF (2004) Processes controlling metal transport and retention as metal-contaminated groundwaters efflux through estuarine sediments. Chemosphere 56(9):821–831
Sindilariu PD, Freyhof J, Wolter C (2006) Habitat use of juvenile fish in the lower Danube and the Danube Delta: implications for ecotone connectivity. Hydrobiologia 571:51–61
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17(5):517–568
Smith SL, MacDonald DD, Keenleyside KA, Ingersoll CG, Field LJ (1996) A preliminary evaluation of sediment quality assessment values for freshwater ecosystems. J Great Lakes Res 1996(22):624–638
Tomlinson DL, Wilson JG, Harris CR, Jeffrey DW (1980) Problems in the assessment of heavy metal levels in estuaries and the formation of a pollution index. Helgolander Meeresuntersuchungen 33(1–4):566–575
USEPA (United States Environmental Protection Agency) (2000) Prediction of sediment toxicity using consensus-based freshwater quality guidelines
Wohl E (2012) A world of rivers. The University of Chicago Press, Chicago. ISBN: 9780226007601
Yu R, Ji J, Yuan X, Song Y, Wang C (2012) Accumulation and translocation of heavy metals in the canola (Brassica napus L.)-soil system in Yangtze River Delta, China. Plant Soil 353:33–45
Acknowledgements
The current study was accomplished with the support of the DeltaBioTox research project, supported by the PN-II-PT-PCCA-2011-3-61/2012 financed by the Romanian Ministry of Education, Research, Youth and Sports.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Gati, G., Pop, C., Brudaşcă, F. et al. The ecological risk of heavy metals in sediment from the Danube Delta. Ecotoxicology 25, 688–696 (2016). https://doi.org/10.1007/s10646-016-1627-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-016-1627-9