Abstract
Gene expression studies could provide insight into the physiological mechanisms and strategies used by plants under stress conditions. Selection of suitable internal control gene(s) is essential to accurately assess gene expression levels. For the mangrove plant, Aegiceras corniculatum, reliable reference genes to normalize real-time quantitative PCR data have not been previously investigated. In this study, the expression stabilities of five candidate reference genes [glyceraldehydes-3-phosphate dehydrogenase (GAPDH), 18SrRNA, β-Actin, 60S ribosomal protein L2, and elongation factor-1-A] were determined in leaves of A. corniculatum treated by cold, drought, salt, heavy metals, and pyrene and in different tissues of A. corniculatum under normal condition. Two software programs (geNorm and NormFinder) were employed to analyze and rank the tested genes. Results showed that GAPDH was the most suitable reference gene in A. corniculatum and the combination of two or three genes was recommended for greater accuracy. To assess the value of these tested genes as internal controls, the relative quantifications of CuZnSOD gene were also conducted. Results showed that the relative expression levels of CuZnSOD gene varied depending on the internal reference genes used, which highlights the importance of the choice of suitable internal controls in gene expression studies. Furthermore, the results also confirmed that GAPDH was a suitable reference gene for qPCR normalization in A. corniculatum under abiotic stresses. Identification of A. corniculatum reference gens in a wide range of experimental samples will provide a useful reference in future gene expression studies in this species, particularly involving similar stresses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mangroves are a group of plant communities that distributed in tropical and subtropical coasts with characteristics of high salinity and anaerobic soil (Tomlinson 1994). With the intervention of human activities, mangrove plants are more and more threatened by pollutants such as heavy metals and polycyclic aromatic hydrocarbon (PAH) (Marchand et al. 2006; Tam et al. 2009), as well as global climate change (e.g., sea-level rising and cold events) (Gilman et al. 2008). Numerous investigations have focused on identification and expression analyses of stress-responsive genes from mangroves, with the aim of better understanding the stress-resistant mechanisms of mangroves (Fu et al. 2005; Jithesh et al. 2006; Zhang et al. 2007; Huang and Wang 2009, 2010; Basyuni et al. 2010; Peng et al. 2013).
Quantitative real-time PCR (qPCR) is currently one of the most powerful and sensitive techniques for rapid and reliable quantification of gene expression. Despite qPCR has many advantages, such as high sensitivity, high specificity and broad quantification range, qPCR also has its pitfalls. The accurate and reliable results quantified by qPCR rely on the use of appropriate normalization techniques. Since the use of internal reference gene takes into account variation introduced by initial sample amount, RNA integrity and enzymatic efficiencies, it has become one of the most popularly used methods for normalization (Remans et al. 2008). The expression of reference gene(s) used as internal control should not be affected by experimental conditions or tissue types; otherwise, it may lead to erroneous results (Udvardi et al. 2008). However, several studies have demonstrated that the expression levels of many so-called reference genes, such as the 18S rRNA, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), β-Actin, cyclophilin (Cyp), elongation factor-1-A (EF1A) and the 60S ribosomal protein L2 (rpl2) may differ greatly among different tissue types, species or in response to various stresses (Czechowski 2005; Migocka and Papierniak 2010; Xu et al. 2011). Recognizing the importance of reference genes in normalization of qPCR data, valuation of suitable reference genes has been conducted in various plants, such as Arabidopsis thaliana (Remans et al. 2008), Cucumis sativus (Migocka and Papierniak 2010), and Populous (Xu et al. 2011), under specific conditions. Hitherto suitable internal controls for gene expression studies have not been defined for mangroves.
Aegiceras corniculatum is a dominant mangrove species growing in the tidal zone of South China, and has been found to be highly tolerant to various abiotic stresses, such as salt and heavy metals (Burchett et al. 2006; Tam et al. 2009). Since A. corniculatum was considered to represent an excellent model system to study the stress-resistant mechanism of mangroves, many studies focus on gene expression analyses based on qPCR have been conducted in A. corniculatum (Fu et al. 2005; Huang and Wang 2010). In these studies the 18S rRNA or β-actin was commonly used as internal control. Since fluctuant stabilities of 18S rRNA or β-actin have been reported in many other plants under abiotic stresses (Remans et al. 2008; Migocka and Papierniak 2010), it is necessary to validate suitable reference genes for A. corniculatum.
With the aim to identify suitable internal reference gene(s) for normalization of qPCR data, the present study report the validation of five candidate reference genes (GAPDH, 18S rRNA, β-Actin, rpl2, and EF1A) in A. corniculatum under various abiotic stresses (cold, drought, salt, heavy metals, and pyrene) and among different tissue types. Two software programs (geNorm and NormFinder) were employed to analyze and rank the tested genes. And in order to assess the value of these genes as internal controls in expression studies, the relative quantifications of Cu–Zn superoxide dismutase (CuZnSOD) gene were also conducted.
In the paper, it was the first attempt to validate a set of reference genes of mangroves. The results of this study will contribute to accurate and reliable normalization of qPCR data in this species in the future.
Materials and methods
Plant materials and treatments
Propagules of A. corniculatum were collected from Dongchong, Shenzhen, China. Seedlings were planted in clean sand and fertilized with 1/2 Hoagland nutritive medium every day. Four-month-old plantlets were subjected to experiments. All the results were obtained from at least two independent experiments on three plantlets. To evaluate the expression stabilities of genes among different tissues (leaves, stems, and roots) were randomly harvested on three plantlets and immediately frozen in liquid nitrogen until RNA extraction. To evaluate the expression stabilities of genes in response to cold, leaves were collected on three plantlets at 0, 2, 24 and 48 h after chilling (5 °C). To evaluate the expression stabilities of genes in response to drought, leaves were collected at 3 days, on three plantlets grown in solutions containing 10 or 20 % (w/v) PEG 6000, respectively. To evaluate the expression stabilities of genes in response to salt, leaves were collected at 3 days, on three plantlets grown in solutions containing NaCl (0, 15 or 30 g l−1). To evaluate the expression stabilities of genes in response to PAH, leaves were collected at 3 days, on three plantlets grown in sand media containing pyrene (10 or 30 μg g−1). To evaluate the expression stabilities of genes in response to heavy metals, leaves were collected at 3 days, on three plantlets grown in solutions with heavy metals (Cd 20 mg l−1 or Pb 60 mg l−1). Harvested leaves were dropped immediately into liquid nitrogen and stored at −80 °C until RNA extraction.
Primer design
The sequences of 18S rRNA, β-Actin, GAPDH and CuZnSOD were obtained from GenBank database (Table 1). The EST sequence of EF1A and rpl2 in A. corniculatum were obtained by homologous clone using degenerate primers (Table 2). Multi-alignment showed that the two genes display high similarity to their homologous genes in Arabidopsis. The sequences of the two genes were deposited to GenBank (Accession number: AcEF1A, JQ396426 and Acrpl2, JQ396427). Six primer pairs for real-time qPCR were designed by Oligo 7.0. The primer sequences, sizes of the amplified bands and the expected melting temperature of the six genes were listed in.
Two step quantitative real-time RT-PCR
Total RNA was isolated using plant total RNA extract kit (Tiangen, Invitrogen). The concentration and purity of total RNA was determined using a NanoDrop ND-2000C spectrophotometer (Thermo Scientific, USA). Retrotranscription of RNA (1 μg) was performed with PrimeScript® RT reagent Kit (Takara, Dalian, China). The concentration of cDNA was determined and then diluted to 20 ng·μl−1. qPCR was performed in a 15 µl of 2× SYBR® Premix Ex Taq™ II (Takara, Dalian, China) mixture, with 200 ng of cDNA and 350 nM of each primer. Three replicates of each PCR were run in iCycler iQ5 real-time PCR detection system (Bio-Rad, CA, USA) using a program as the following: 1 min denaturation at 95 °C, then 45 cycles of 5 s at 95 °C, 15 s at 57/60 °C and 20 s at 72 °C. All PCRs displayed with efficiencies between 95 and 105 %. Specific amplified products were confirmed by the melting curves (Fig. 1).
Data analysis
Expression levels were determined as the number of cycles needed for the amplification to reach a threshold fixed in the exponential phase of PCR reaction (Cp). To evaluate the stability of these candidate reference gene(s), two free Excel-based tools, geNorm and Normfinder, were used in this study. NormFinder (Andersen et al. 2004) evaluate the systematic error (SE) introduced when a gene is used for normalization. GeNorm (Vandesompele et al. 2002) determined the expression stability as well as the optimal number of reference genes. The relative expression of CuZnSOD was calculated according to Pfaffl (2001) using a unique reference gene or the combination of reference genes suggested by geNorm.
Results
Variations of housekeeping genes
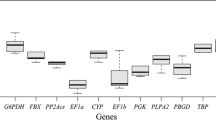
The RNA transcription profiles of the five housekeeping genes and CuZnSOD were shown in Fig. 2. The crossing point (Cp) values were fluctuated from 12.25 to 31.26. The β-Actin and rpl2 showed relatively low expression levels, while 18S rRNA showed the highest expression level. These genes displayed relative stable expression levels except in leaves treated by heavy metals Cd and Pb (Table 3).
Expression stability of candidate reference genes
NormFinder analysis
As shown in Table 4, NormFinder evaluate the SE introduced when a gene is used for normalization; and the gene with the lowest SE is assigned as the best reference gene. According to NormFinder analysis, the GAPDH gene had a lowest SE value in drought (0.194) and salt experiments (0.267). The EF1A gene was ranking as the most stable gene in Pyrene treatments with a lowest value of 0.148 and in heavy metal experiments (0.335). The rpl2 was ranking as the most stable gene among tissue types under normal condition and in cold experiment. The 18S rRNA was ranking as the least stable gene among different tissues, and in cold or heavy metal treatments. When all samples were considered, the EF1A had the lowest SE value (0.344), followed by GAPDH (0.399) and rpl2 (0.407).
geNorm analysis
geNorm provides a measure of gene expression stability (M), which is the mean pairwise variation between an individual gene and all other tested control genes. The gene with lowest M is the best reference gene for a given set of samples. According to geNorm analysis (Table 4), the GAPDH was ranking as the most stable gene among different tissues, and under cold and pyrene treatments. The EF1A had the lowest M value in drought, salt and heavy metal experiments. The rpl2 had the lowest M value when all samples were involved, followed by EF1A and GAPDH. The Actin was always ranking as the least stable gene except in the cold or heavy metal experiments. The 18S rRNA was always ranking as the least stable gene except in the salt or drought experiments.
GeNorm also measured the pairwise variation to determine the optimal number of reference genes. The value of “Vn/n + 1” represents the effect of adding additional reference genes on the normalization factor for these treatments (Table 5). There was no need to add another control gene if the “Vn/n + 1” value is <0.15. For Pyrene stress, both the V2/3 and V3/4 were <0.15, which means that two reference genes should be used as control. For different tissue types, as well as the cold and salt stresses, the V3/4 was less than 0.15, which means that three reference genes should be used as internal control. For heavy metal and drought stresses, the V values were all more than 0.15, which means that the stabilities of all these candidate references genes may largely fluctuated under these conditions.
CuZnSOD gene expression
When GAPDH, β-Actin and the combination of three genes (GAPDH, β-Actin and rpl2) were used for normalization, the CuZnSOD gene expression increased quickly after 2-h of cold, and then decreased to the control level after 48 h of cold (Fig. 3a). When 18S rRNA, EF1A and rpl2 were used as a unique internal control separately, obvious increase of CuZnSOD transcripts after 2 and 24 h of cold were not detected. Moreover, when 18S rRNA was used as a unique control, the CuZnSOD transcripts decreased to below the control level after 48 h of cold (Fig. 3a). Under drought, salt and pyrene stresses, the CuZnSOD expression levels were declined to various extents when the best reference genes recommended by geNorm were used as internal control (see Fig. 3b–d). The expression levels were obviously overestimated or underestimated when the least two stable genes (β-Actin and rpl2 in drought treatment, β-Actin and GAPDH in salt treatment, β-Actin and 18S rRNA in PAH treatment) were used as unique control (see Fig. 3b–d). For heavy metal treatments, the CuZnSOD gene expression were seriously inhibited in Pb treated plants and strongly induced in Cd treated plants when the combination of GAPDH, β-Actin and EF1A was used for normalization (Fig. 3e). The use of 18S rRNA as unique control obviously underestimated the CuZnSOD expression level under Cd stress, while the use of rpl2 significantly overestimated the CuZnSOD mRNA transcripts abundance. Under normal condition, the minimum of CuZnSOD mRNA transcripts was observed in roots except using 18S rRNA for normalization (Fig. 3f).
Relative expression levels of CuZnSOD gene in leaves after cold (a), drought (b), salt (c), Pyrene (d) or heavy metals (e) treatments using different internal controls. Relative expression levels of CuZnSOD gene among different tissues under normal condition (f) using different internal controls. Asterisks indicate significant differences between expression levels of CuZnSOD calculated using a unique reference gene ore the combination of the best two or three genes, significance was defined as p < 0.05
Discussions
Although 18S rRNA has been frequently used as an internal control in mangroves A. corniculatum (Fu et al. 2005; Huang and Wang 2009), our results indicated that 18S rRNA may be not suitable as reference gene for qPCR normalization in A. corniculatum under various stresses. Due to the high abundance of 18S rRNA compared with target mRNA transcripts, it was difficult to accurately subtract the baseline value in qPCR data analysis (Vandesompele et al. 2002). The expression level of CuZnSOD was obviously overestimated when 18S rRNA was used as a unique internal control under cold and heavy metal experiments (Fig. 3). According to geNorm and NormFinder analysis, the β-Actin gene of A. corniculatum have poor stability under various abiotic stresses (e.g. drought, salinity and PAH treatment). Qi et al. (2010) also demonstrated that the expression level of β-Actin gene may fluctuate largely in Cabbage.
The EF1A gene has been evaluated to be stably expressed in cucumber under salt, osmotic and metal stresses (Migocka and Papierniak 2010) and in cabbage under drought stress (Qi et al. 2010). In this study, EF1A was suggested to be the most stable gene under pyrene and metal experiments by NormFinder and the most stable gene under salt, heavy metal and drought experiment by geNorm. Our results also demonstrated that the rpl2 was the most stable reference gene under cold stress by NormFinder and under salt stress by geNorm, such as found in cabbage (Qi et al. 2010). The small inconsistency between different softwares was due to their different statistical algorithms (Migocka and Papierniak 2010).
Taken together, GAPDH gene was suggested to be better internal control than the other four candidate reference genes. GAPDH was ranked among the top three best reference genes except for salt treatment according to geNorm. When NormFinder was used, GAPDH was identified as the most stable gene under salt and drought stresses. CuZnSOD expression analyses also suggested that GAPDH was a suitable reference gene in A. corniculatum. Although variations of GAPDH expression were observed among tissue types of human (Barber et al. 2005), GAPDH has been proved to be a suitable reference gene in many plants, such as cabbage (Qi et al. 2010), Swingle citrumelo (Carvalho et al. 2010) and Coffea arabica (Barsalobres-Cavallari et al. 2009).
Since using single gene could lead to relatively large error, it has been suggested that at least two or three housekeeping genes should be used for normalization (Vandesompele et al. 2002). According to the pairwise variation (V) analysis from geNorm, the geometric mean of multiple reference genes was proposed to normalize the qPCR data. For different tissue types, the geometric mean of three genes (GAPDH, EF1A and rpl2) was suggested to be internal control. Similar results were detected in a study of Barsalobres-Cavallari et al. (2009), which identified the stable expression levels of GAPDH, 14-3-3 and rpl7 mRNA in different C. arabica tissue types. For the cold, salt or pyrene experiments, geNorm suggested the geometric mean of two or three genes for normalization. The sets of suitable reference genes for the three experiments are as follows: (1) cold stress—EF1A, rpl2 and GAPDH; (2) salt stress—EF1A, rpl2 and 18S rRNA; (3) Pyrene stress—GAPDH and EF1A. Former studies have reported similar suitable reference genes in other plants. For example, EF1A and rpl2 were recommended to normalize qPCR data in potato under cold stress, while EF1A, cyclophilin and rpl2 were suggested for salt stress (Nicot et al. 2005). In Swingle citrumelo, EF1A, ADP ribosylation factor (ADP) and GAPDH were suggested to be internal control under drought stress (Carvalho et al. 2010). For heavy metal and drought experiments, the V values were all more than 0.15, which means that all these tested genes may be fluctuated largely under these conditions. Therefore, it is necessary to explore metal-stable and drought-stable reference genes in A. corniculatum in the future. Relatively higher metal-induced variations of traditional reference gene (e.g. 18S rRNA, β-Actin and EF1A) were also observed in A. thaliana (Remans et al. 2008).
In the last part of this study, we compared the relative transcript level of a target gene (CuZn-SOD) normalized by each single reference gene and that normalized by the geometric mean of multiple reference genes. As shown in the Fig. 3a, e and f, when 18S rRNA was used as a unique internal control, the transcript levels of target gene were significantly underestimated. And as shown in the Fig. 3b–d, the target gene expression levels were obviously overestimated when β-Actin was used as a unique control. As shown in Fig. 3a–f, the target gene expression levels quantified by GAPDH were similar with that quantified by the geometric mean of multiple reference genes. Therefore, taken together of the geNorm and Normfinder analysis, the GAPDH gene was suggested as a suitable reference gene for qPCR normalization in A. corniculatum under abiotic stresses.
Conclusion
The present work evaluated the suitability of five housekeeping genes as internal control with two Excel-based tools, geNorm and Normfinder. The results evidenced the stable expression levels of GAPDH gene under several abiotic stresses, such as cold, salt and pyrene. Moreover, in order to get more accurate quantification of qPCR date, it is better to use the geometric mean of multiple reference genes as internal control. Finally, the heavy metal-stable and drought-stable reference genes in A. corniculatum remain to be explored in the future. Moreover, with the development and use of gene chip technology and transcriptome analysis, more and more novel reference genes that are more stable than traditional reference genes will be identified in further studies.
References
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64:5245–5250
Barber RD, Harmer DW, Coleman RA, Clark BJ (2005) GAPDH as a housekeeping gene: analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol Genomics 21:389
Barsalobres-Cavallari CF, Severino FE, Maluf MP, Maia IG (2009) Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Mol Biol 10:1
Basyuni M, Kinjo Y, Baba S, Shinzato N, Iwasaki H, Siregar EBM, Oku H (2010) Isolation of salt stress tolerance genes from roots of mangrove plant, Rhizophora stylosa Griff., using PCR-based suppression subtractive hybridization. Plant Mol Bio Rep 29:533–543
Burchett M, Clarke C, Field C, Pulkownik A (2006) Growth and respiration in two mangrove species at a range of salinities. Physiol Plant 75:299–303
Carvalho K, de Campos MKF, Pereira LFP, Vieira LGE (2010) Reference gene selection for real-time quantitative polymerase chain reaction normalization in “Swingle” citrumelo under drought stress. Anal Biochem 402:197–199
Czechowski T (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139:5–17
Fu X, Huang Y, Deng S, Zhou R, Yang G, Ni X, Li W, Shi S (2005) Construction of a SSH library of Aegiceras corniculatum under salt stress and expression analysis of four transcripts. Plant Sci 169:147–154
Gilman EL, Ellison J, Duke NC, Field C (2008) Threats to mangroves from climate change and adaptation options: a review. Aquat Bot 89:237–250
Huang G-Y, Wang Y-S (2009) Expression analysis of type 2 metallothionein gene in mangrove species (Bruguiera gymnorrhiza) under heavy metal stress. Chemoshpere 77:1026–1029
Huang GY, Wang YS (2010) Expression and characterization analysis of type 2 metallothionein from grey mangrove species (Avicennia marina) in response to metal stress. Aquat Toxicol 99:86–92
Jithesh MN, Prashanth SR, Sivaprakash KR, Parida A (2006) Monitoring expression profiles of antioxidant genes to salinity, iron, oxidative, light and hyperosmotic stresses in the highly salt tolerant grey mangrove, Avicennia marina (Forsk.) Vierh. by mRNA analysis. Plant Cell Rep 25:865–876
Marchand C, Lallier-Vergès E, Baltzer F, Albéric P, Cossa D, Baillif P (2006) Heavy metals distribution in mangrove sediments along the mobile coastline of French Guiana. Mar Chem 98:1–17
Migocka M, Papierniak A (2010) Identification of suitable reference genes for studying gene expression in cucumber plants subjected to abiotic stress and growth regulators. Mol Breed 3:343–357
Nicot N, Hausman JF, Hoffman L, Evers D (2005) Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J Exp Bot 56:2907–2914
Peng YL, Wang YS, Cheng H, Sun CC, Wu P, Wang LY, Fei J (2013) Characterization and expression analysis of three CBF/DREB 1 transcriptional factor genes from mangrove Avicennia marina. Aquat Toxicol 140–141:68–76
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:2004–2007
Qi J, Yu S, Zhang F, Shen X, Zhao X, Yu Y, Zhang D (2010) Reference gene selection for real-time quantitative polymerase chain reaction of mRNA transcript levels in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Plant Mol Biol Rep 28:597–604
Remans T, Smeets K, Opdenakker K, Mathijsen D, Vangronsveld J, Cuypers A (2008) Normalization of real-time RT-PCR gene expression measurements in Arabidopsis thaliana exposed to increased metal concentrations. Planta 227:1343–1349
Selvey S, Thompson E, Matthaei K, Lea RA, Irving MG, Griffiths L (2001) Beta-actin: an unsuitable internal control for RT-PCR. Mol Cell Probe 15:307
Tam N, Wong Y, Wong M (2009) Novel technology in pollutant removal at source and bioremediation. Ocean Coast Manag 52:368–373
Tomlinson PB (1994) The botany of mangroves. Cambridge University Press, Cambridge
Udvardi MK, Czechowski T, Scheible WR (2008) Eleven golden rules of quantitative RT-PCR. Plant Cell Online 20:1736
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:research0034
Xu M, Zhang B, Su X, Zhang S, Huang M (2011) Reference gene selection for quantitative real-time polymerase chain reaction in Populus. Anal Biochem 408:337–339
Zhang F-Q, Wang Y-S, Lou Z-P, Dong J-D (2007) Effect of heavy metal stress on antioxidative enzymes and lipid peroxidation in leaves and roots of two mangrove plant seedlings (Kandelia candel and Bruguiera gymnorrhiza). Chemosphere 67:40–50
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Nos. 41430966 and 41176101), the Projects of Guangzhou Science and Technology (No. 15020024), the key projects in the National Science and Technology Pillar Program in the Eleventh Five-year Plan Period (No. 2012BAC07B0402), and the projects of the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCX2-SW-132).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, YL., Wang, YS. & Gu, JD. Identification of suitable reference genes in mangrove Aegiceras corniculatum under abiotic stresses. Ecotoxicology 24, 1714–1721 (2015). https://doi.org/10.1007/s10646-015-1487-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1487-8